Abstract
Borrelia burgdorferi, a Lyme disease agent, makes different major outer surface lipoproteins at different stages of its mouse–tick infectious cycle. Outer surface protein A (OspA) coats the spirochetes from the time they enter ticks until they are transmitted to a mammal. OspA is required for normal tick colonization and has been shown to bind a tick midgut protein, indicating that OspA may serve as a tick midgut adhesin. Tick colonization by spirochetes lacking OspA is increased when the infecting blood meal is derived from mice that do not produce antibody, indicating that OspA may protect the spirochetes from host antibody, which will not recognize tick-specific proteins such as OspA. To further study the importance of OspA during tick colonization, we constructed a form of B. burgdorferi in which the ospA open reading frame, on lp54, was replaced with the ospC gene or the ospB gene, encoding a mammal-specific or tick-specific lipoprotein, respectively. These fusions yielded a strain that produces OspC within a tick (from the fusion gene) and during early mammalian infection (from the normal ospC locus) and a strain that produces OspB in place of OspA within ticks. Here we show that the related, tick-specific protein OspB can fully substitute for OspA, whereas the unrelated, mammal-specific protein OspC cannot. These data were derived from three different methods of infecting ticks, and they confirm and extend previous studies indicating that OspA both protects spirochetes within ticks from mammalian antibody and serves an additional role during tick colonization.
INTRODUCTION
The Lyme disease agent Borrelia burgdorferi is an obligate parasite that cycles between Ixodes ticks and small mammals (1, 2). The spirochete varies its gene expression during the course of this infectious cycle, allowing adaptation to these distinct and changing environments (3). Different major and minor outer surface lipoproteins are made at different stages of the cycle and represent points of interaction (recognition) between the bacteria and their hosts and vectors. Outer surface protein A (OspA) is synthesized and coats the bacterial surface when spirochetes are acquired by larval ticks feeding on an infected small mammal, and it continues to be made as the B. burgdorferi-infected ticks molt to the nymphal stage (4, 5). OspB, a related protein encoded by a gene adjacent to and cotranscribed with ospA, is also produced on spirochetes within ticks (5). When nymphs feed on a mammal, OspC synthesis begins (5), and spirochetes that successfully colonize a naïve animal produce OspC and no longer exhibit OspA on their surfaces (6). However, OspC is an antigenic protein (7, 8), so only spirochetes that subsequently downregulate OspC production can persist as the mammalian antibody response to B. burgdorferi develops (9). Synthesis of a third lipoprotein, VlsE (10), increases as OspC production wanes (11–13). The VlsE protein is antigenically variable, with new coding sequences generated by gene conversion of vlsE, using adjacent silent cassette sequences located on the lp28-1 plasmid (10, 14, 15). Antigenic variation of the VlsE protein is required for persistent infection of immunocompetent hosts by B. burgdorferi (16, 17).
Although appreciation of the changing pattern of lipoprotein synthesis has emerged over time, the specific functions of those proteins remain mysterious. OspA is required for normal tick colonization (18–20). Previous studies have demonstrated that OspA binds to a tick midgut receptor (TROSPA) (21) and protects spirochetes from the tick midgut environment, including antibodies against invariant B. burgdorferi surface components entering with a blood meal from an infected animal (18). OspB mutants have also been reported to be defective in tick colonization (22), but other data indicate that OspB serves a minor role in the infectious cycle, at least in a laboratory setting (18). OspC is required for early mammalian infection (23–25), and a recent study indicates that OspC blocks the phagocytosis of spirochetes by macrophages in the skin (26). VlsE is required for persistent infection, except in immunocompromised mice (16, 17). We and others (27–29) have begun to address the possibility that the three major lipoproteins perform overlapping functions at the different stages of the infectious cycle. To do so, we demonstrated that OspC can take the place of VlsE during the infection of an immunodeficient animal, which will not make antibodies that would lead to the clearance of OspC-producing spirochetes (29). In contrast, VlsE and OspA could not replace OspC during early mammalian infection, although others have found conditions in which they partially substituted for OspC (27). Our conclusion was that OspC and VlsE carry out similar basic functions but are specialized to the time and context in which they are normally produced. Because OspC is made only during early infection, prior to specific-antibody production, it may have an optimal structure that allows successful infection by the small number of spirochetes transmitted by a feeding tick. VlsE has a broadly similar structure (30–32), with variable loops, which may provide adequate function for low-level persistence after colonization of multiple tissues has been established.
VlsE and OspC are both normally synthesized during mammalian infection, albeit at different times, with acquired immunity representing the major difference in an otherwise similar mammalian environment. In this study, we extended our earlier studies by testing whether OspC produced in a distinct environment, i.e., during tick infection, can functionally replace OspA. We found that OspC production does not increase tick colonization in the absence of OspA, either when the blood meal is derived from an immunodeficient mouse or when the blood meal contains an anti-OspC antibody. In contrast, OspB could substitute for OspA during tick colonization. Our data provide further evidence supporting the conclusion of Battisti et al. (18) that a significant role of OspA is to protect spirochetes in the tick midgut from ingested mammalian antibodies, while we also demonstrate that OspA plays a significant role in tick colonization in the absence of specific antibody.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All B. burgdorferi strains were derived from infectious clone A3 of the type strain B31 (33). The derivative S9, previously designated A3-68Δbbe02 (34), served as the direct progenitor for strains with ospA promoter fusions (Table 1). S9 is called wild type (WT) in this study because it has no observable defects in the laboratory mouse–tick infectious cycle. A3 and S9 produce a truncated version of OspB because of a stop codon within the open reading frame (ORF), a defect that appears to correlate with increased transformability by electroporation (unpublished results). S9 and its derivatives lack lp56, lp5, and cp9. The ospA1 deletion-insertion mutant (called the ΔospA mutant and derived from B31-A3, parent of S9) has been described elsewhere (18). A joint reisolate (uncloned) of the ospA1 mutant, whose infectivity was confirmed by reinoculating mice and which is missing lp21, lp5, cp9, cp32-9, and cp32-3, was used in these studies. B. burgdorferi was grown in Barbour-Stoenner-Kelly II (BSK II) medium (35) with 6% rabbit serum (Pel-Freez, Rogers, AR, USA).
TABLE 1.
B. burgdorferi strains used in this study
Constructing B. burgdorferi strains with the ospA promoter fused to various genes.
Plasmids for creating fusions of the ospA promoter to the ospC, ospA, and intact ospB open reading frames at the endogenous ospAB locus on lp54 were constructed by similar strategies. The ospA promoter and upstream flanking sequences were amplified (all PCR amplifications used DreamTaq from Thermo Fisher Scientific, Waltham, MA, USA) from A3 genomic DNA using primers ospAup and ospA5out (all oligonucleotides are described in Table S1 in the supplemental material) and were cloned into Topo XL (Thermo Fisher Scientific). The ospC gene was amplified from A3 genomic DNA with primers 5′ospC-NdeI and ospC3′X and was cloned into pCR2.1 Topo (Thermo Fisher Scientific). A flgBp-aacC1 fusion construct was amplified from A3chbC1 DNA (36) using primers flg5′-XhoI and aacC13′XhoI and was cloned into Topo XL. The ospB gene (encoding a spontaneously truncated OspB protein) and flanking sequences were amplified from A3 genomic DNA with primers ospB5′ and ospBdown and were cloned into Topo XL. The ospA gene was amplified from A3 genomic DNA with primers ospA5′NdeI and ospA3′XhoI and was cloned into pCR2.1 Topo. An intact ospB gene with downstream flanking sequences was amplified from B31 clone 4A (37) with primers ospB5′NdeI and ospBdownXhoI and was cloned into pCR2.1 Topo. The ospA upstream sequences were moved from TopoXLospAup into NotI-HF- and NdeI-digested pOK12, yielding pOK12ospAup. pOK12ospAup was digested with NdeI and XhoI and was ligated with ospA, intact ospB, and ospC fragments derived from the plasmids described above, yielding plasmids pOK12ospAup-ospA, pOK12ospAup-ospC, and pOK12ospAup-ospB. A fragment containing ospB (with a nonsense mutation) and downstream sequences was ligated into pOK12ospAup-ospA and pOK12ospAup-ospC, yielding pOK12ospAup-ospAB and pOK12ospAup-ospCB, respectively. The flgBp-aacC1 fragment was ligated into the XhoI sites of these plasmids, yielding pOK12ApospC and pOK12ApospA (Fig. 1A). The flgBp-aacC1 fusion construct was ligated into the AfeI site of pOK12ospAup-ospB. Plasmid BG5 had the flgBp-aacC1 fusion construct pointing in the same orientation as intact ospB and was used in subsequent experiments (Fig. 1A). All plasmids were checked by restriction digestion (all restriction enzymes purchased from New England BioLabs, Ipswich, MA), PCR, and sequencing.
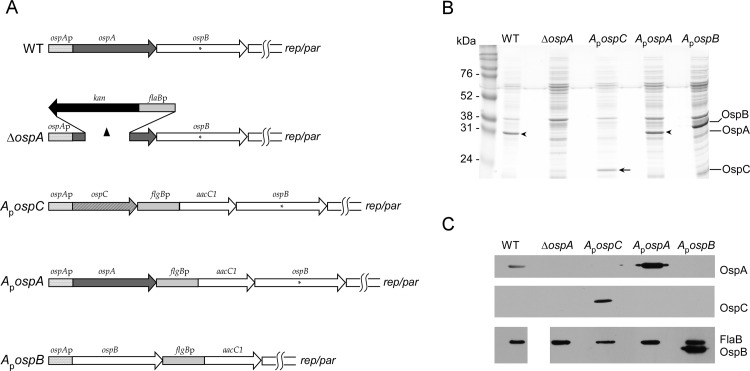
FIG 1.
Gene arrangement and protein production by various strains. (A) Graphical depiction of ospAB loci (on lp54) of strains used in this study. ospAp, ospA promoter. A capital delta indicates deleted sequences. An asterisk indicates the position of the stop codon in the ospB ORF, yielding truncated OspB. rep/par indicates the nearby position of ORFs thought to be involved in the replication and partition of lp54. flgBp-aacC1 denotes a selectable marker (the flgB promoter linked to the aacC1 gene) conferring gentamicin resistance. (B) Coomassie blue-stained gel of protein lysates of strains used in this study. The molecular masses of standards are given (in kilodaltons) on the left. The arrow indicates OspC produced by the ApospC strain but absent in all other strains. All strains carry the WT ospC gene on cp26 and will produce OspC from that gene within a mammal but not when grown in culture medium. Arrowheads indicate the position of OspA protein, produced by the WT and ApospA strains but absent in the other strains. (C) Immunoblots of protein lysates of the strains used in this study. The anti-OspA and anti-OspC antibodies were hybridized sequentially to the same blot, whereas the anti-FlaB and anti-OspB antibodies were both hybridized to a blot from a different gel of the same lysates, in which no lane was skipped between the WT and ΔospA lysates.
Plasmids pOK12ApospC, pOK12ApospA, and BG5 were used to transform B. burgdorferi S9 (34) by electroporation (38), and colonies were obtained in each transformation. After screening colonies by PCR for the selectable marker (using primers flg5′ and aacC13′), positive colonies were picked into liquid medium. DNA and protein were extracted from the resulting cultures. The presence and absence (as predicted) of OspA, OspB, and OspC in protein lysates were demonstrated by SDS gel electrophoresis and Coomassie blue staining (Fig. 1B). The identities of the proteins produced by the strains were confirmed by immunoblotting with monoclonal antibodies against OspA (H5332 [39]), OspB (H4610 [40]), and FlaB (H9724 [41]) (all of which were gifts from Tom Schwan, Rocky Mountain Laboratories, and were used at a dilution of 1:100), and a polyclonal anti-OspC antibody (42) (used at a dilution of 1:500) (Fig. 1C). DNA preparations (Wizard genomic DNA extraction kit; Promega, Madison, WI) from these strains were tested by PCR to confirm the presence of the expected fusions and to confirm that the plasmid content was unaltered. The ApospA7, ApospB7 (which is also missing lp21), and ApospC2 transformants, with the ospAp-ospA, ospAp-ospB, and ospAp-ospC fusions (referred to below as the ApospA, ApospB, and ApospC fusion strains) were used in subsequent experiments.
Mouse infection by needle inoculation and tick transmission.
All animal experiments were performed using protocols approved by the Animal Care and Use Committee of the Rocky Mountain Laboratories and according to the guidelines of the National Institutes of Health. The Rocky Mountain Laboratories are accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). RML mice (an outbred strain derived from Swiss-Webster mice and raised at the Rocky Mountain Laboratories; called WT or immunocompetent mice in this study) or C.B-17/IcrHsd-Prkdcscid mice (called SCID mice; Harlan Laboratories, Livermore, CA, USA) were inoculated with approximately 104 spirochetes, receiving 80% of the inoculum intraperitoneally and the remainder subcutaneously. Inocula were checked by plating diluted cultures to confirm the concentration and by screening cultures and colonies for plasmid content. Mice were retro-orbitally bled and euthanized 3 to 4 weeks after inoculation. The ear, bladder, and ankle joint were cultured in BSK II medium for spirochete isolation, and the other ear, the other ankle joint, and the heart were frozen for subsequent DNA extraction. Seroconversion was assayed by immunoblotting (23), using WT spirochete lysate as the antigen. To assess tick transmission, 20 infected nymphs were placed on mice and were allowed to feed to repletion. Three weeks after tick infestation, the mice were bled and euthanized. Spirochete isolation was attempted from ears, bladders, and ankle joints.
Tick infection by immersion.
Tick infection by immersion was carried out by the method of Policastro and Schwan (43). Briefly, larval ticks were partially dehydrated for 2 days by placing them in a bell jar under an atmosphere of saturated (NH4)2SO4. About 100 dehydrated ticks were immersed in log-phase B. burgdorferi cultures for an hour at 35°C. The medium was removed, and the ticks were rinsed twice in phosphate-buffered saline (PBS), allowed to dry at ambient humidity for a few hours, and allowed to recover at 98% humidity for 2 days, before feeding on naïve mice.
Assessment of tick colonization by plating and culture.
Tick colonization was assessed either by plating diluted triturate for single B. burgdorferi colonies or by culturing individual or pooled ticks. Prior to either of these procedures, the ticks were surface sterilized by immersion in 3% hydrogen peroxide, followed by immersion in 70% ethanol. After removal of the ethanol and brief drying, ticks were crushed with disposable pestles in 0.5 ml BSK-H medium (Sigma) in disposable snap-cap tubes. Subsequently, 0.5 ml BSK-H medium was added, and aliquots were plated in BSK II medium with agarose and rifampin (50 μg ml−1)-phosphomycin (20 μg ml−1)-amphotericin B (2.5 μg ml−1) (Borrelia antibiotics). Alternatively, some or all of the triturate was left in the snap-cap tubes (for individual ticks) or brought to 10 ml with BSK II medium (containing Borrelia antibiotics) for tick pools.
DNA isolation from mouse tissues and TaqMan analysis.
We isolated DNA from mouse ears by use of a previously described protocol (44, 45) that involves collagenase A, proteinase K, and RNase digestion, followed by phenol-chloroform and chloroform extractions and ethanol precipitation. The DNA pellets were resuspended in 400 μl of water, and 200 μl of each was further purified using a QIAquick kit (Qiagen). The number of B. burgdorferi genome copies per mouse genome was determined by TaqMan analysis using flaB and mouse nidogen primer-probe sets and the standard curve method (45) (see Table S1 in the supplemental material).
Statistics.
Tick colonization data were analyzed by the Kruskal-Wallis test, followed by Dunn's multiple-comparison test, using Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). The prevalence of infection was analyzed by pairwise comparison of all combinations using Fisher's exact test (Prism 6).
RESULTS
Constructing and characterizing B. burgdorferi strains that replace OspA with another lipoprotein.
As part of our effort to understand the functions of the B. burgdorferi major lipoproteins and to assess their interchangeability, we constructed a B. burgdorferi strain that stably produced OspC instead of OspA in ticks. To do so, we linked the ospC gene to the ospA promoter (ospAp) in place of ospA at the native ospAB locus on linear plasmid 54 (lp54) (Fig. 1A) (see Materials and Methods for the details of strain construction). This strain (called the ApospC strain) retains the native copy of the ospC gene and promoter on cp26, which would be normally expressed during early mammalian infection. We also made two similar strains, the first with ospAp driving ospA (with a selectable marker downstream) (called the ApospA strain). The second strain (the ApospB strain) has ospAp driving an ospB gene that directs the synthesis of full-length OspB in place of OspA (Fig. 1A), in contrast to our wild-type strain, which encodes a truncated form of OspB. We first confirmed by PCR that the gene arrangement was as expected after allelic exchange.
To determine if the appropriate ospAp-driven gene products were produced, we then used SDS-polyacrylamide gel electrophoresis and Coomassie blue staining to examine protein production by the various strains during growth in culture. As anticipated, the WT and ApospA strains made OspA but no OspC. In contrast, the ApospC strain made no OspA but now made OspC (Fig. 1B). Finally, the ApospB strain made neither OspA nor OspC but made full-length OspB (Fig. 1B). The identities of these proteins were confirmed by immunoblot analysis with antibodies against OspC, OspA, OspB, and flagellin (FlaB) as a loading standard (Fig. 1C).
Mouse infection with ospA promoter fusion strains.
The ospA promoter is normally not expressed during mammalian infection, so we anticipated that the promoter fusions would not affect mammalian infection by the strains containing them. To determine if our expectation was correct (and to infect ticks by the natural route), we inoculated immunocompetent mice with the WT, ApospC, and ApospA strains, using an inoculum of 104 spirochetes per animal. In a separate experiment, immunocompetent mice were infected with the same dose of WT, ΔospA, and ApospB spirochetes. Three weeks after inoculation, the mice were bled, and seroconversion assessed by immunoblotting. One WT-inoculated mouse was seronegative but all other mice were seropositive (Table 2). The mice were euthanized (in many cases, after feeding larval ticks), and ears, bladders, and ankle joints were collected for spirochete isolation. All seropositive mice were also culture positive for the B. burgdorferi strains (Table 2). We assessed spirochete loads in infected mouse ear skin using quantitative PCR and found no significant differences among strains (see Fig. S1 in the supplemental material). Therefore, all strains were demonstrated to be equally competent for mammalian infection.
TABLE 2.
Mouse infection after needle inoculation of B. burgdorferi strains with ospA promoter fusionsa
| B. burgdorferi strain genotype | No. of mice infected/total no. inoculated |
|
|---|---|---|
| WT mice | SCID mice | |
| WT | 9/10 | 5/5 |
| ΔospA | 5/5 | 4/5 |
| ApospC | 5/5 | 4/7 |
| ApospA | 5/5 | 4/7 |
| ApospB | 5/5 | 5/5 |
Infection after intraperitoneal and subcutaneous inoculation of 104 B. burgdorferi bacteria was assessed by serology (for WT mice only) and by ear, bladder, and ankle culture of all mice. Infected animals were seropositive (for WT mice) and had at least two tissues positive out of three tissues assessed. Negative animals were seronegative (for WT mice) and had no positive tissues.
Since we anticipated that tick colonization by ApospC spirochetes would be impacted by anti-OspC antibodies in the incoming blood meal when the spirochetes were derived from immunocompetent mice, we also infected SCID mice (which lack B and T cells and do not mount an acquired immune response) with WT, ApospC, and ApospA bacteria and, in a separate experiment, with the same three strains plus the ΔospA and ApospB strains. All five of the mice inoculated with the WT or ApospB strain were infected, whereas fewer of the mice inoculated with the ApospC, ApospA, or ΔospA strain were infected (Table 2), a difference that was not statistically significant. We again found no significant differences between the loads of the different spirochete strains in infected SCID mouse ears, as measured by quantitative PCR (see Fig. S1 in the supplemental material) (data not shown), and all strains were acquired by feeding larvae, as described below.
Tick colonization by B. burgdorferi derived from SCID mice.
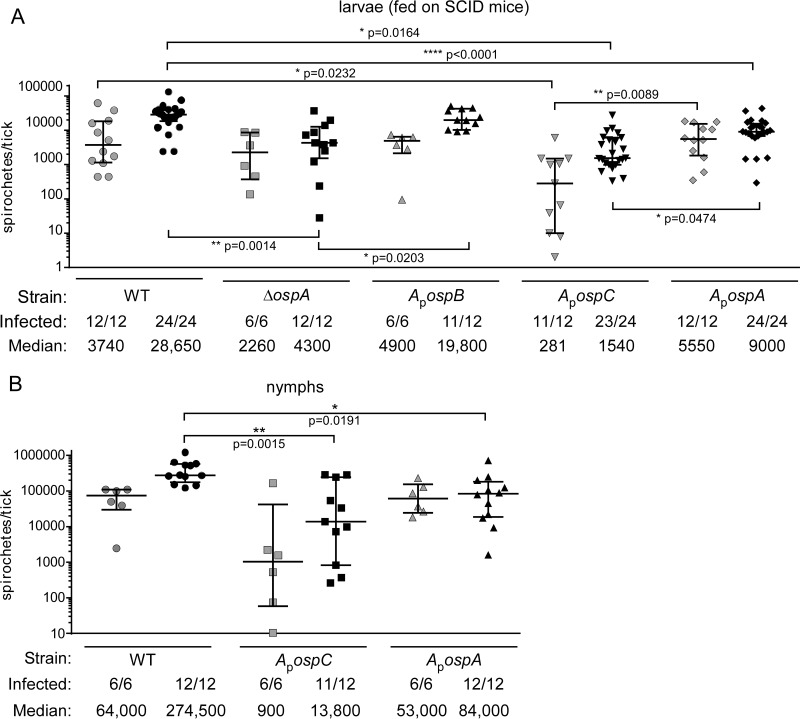
As the first step in determining whether OspC or OspB could take the place of OspA during tick colonization by B. burgdorferi, we fed naïve larval ticks on SCID mice infected with WT, ΔospA, ApospC, ApospB, or ApospA spirochetes (2 to 4 mice were infected per B. burgdorferi strain, in two separate experiments). As mentioned above, the blood of these mice would not contain anti-OspC antibody, which would recognize ApospC spirochetes and potentially diminish tick colonization. We then assessed the spirochete loads soon after dropoff and 7 to 10 days after dropoff (Fig. 2) by determining the CFU count per tick. At early times, the spirochete loads in ticks infected with the ApospC strain were significantly lower than those in ticks infected with the WT or ApospA strain (Fig. 2A). At the late time point, the spirochete loads in ticks infected with the ApospC strain still differed significantly from the loads in ticks infected with the ApospA strain, and the loads in these ticks and in ticks infected with the ΔospA mutant were significantly lower than those in WT-infected ticks (by Dunn's multiple-comparison test). Most or all of the ticks tested were infected, regardless of the strain with which they were infected.
FIG 2.
Spirochete loads in ticks infected by feeding on needle-inoculated SCID mice. Symbols represent CFU counts in individual ticks, as determined by plating of tick homogenates on solid medium. Medians and interquartile ranges are indicated. (A) Spirochete loads in larvae infected by feeding on SCID mice, at dropoff (shaded symbols) or 7 to 10 days after dropoff (filled symbols). (B) Spirochete loads in nymphs infected by feeding as larvae on SCID mice, shown at dropoff (shaded symbols) or 7 to 10 days after dropoff, after feeding on WT mice (filled symbols). Significant differences between samples at a particular time point (as determined by Dunn's multiple-comparison test) are marked by asterisks, and probabilities are given. The proportion of ticks infected (number of infected ticks per total ticks plated) and the median spirochete load per tick are given below each graph.
The larvae infected with the WT, ApospC, or ApospA strain were allowed to molt and were fed on naïve mice, and spirochete loads in fed nymphs were assessed. At this stage, loads of ApospC and ApospA spirochetes were not significantly different from each other at either time point but were significantly lower than those of WT spirochetes at the later time point (by the Kruskal-Wallis test) (Fig. 2), at least in part because of the wide spreads in spirochete loads per tick. These data indicate that production of OspC in place of OspA within ticks does not increase spirochete loads in fed larvae over those found for spirochetes that make neither protein, but that OspB production allowed normal colonization. The spirochete numbers in ticks infected with the ApospC strain recovered somewhat by the nymphal stage but remained lower than found for spirochetes producing OspA. Again, most, if not all, ticks within each cohort contained spirochetes. All nymphal tick feedings resulted in transmission to the mice that were fed upon, which again became infected (Table 3).
TABLE 3.
Mouse infection by nymphal tick transmission of B. burgdorferi strains with ospA promoter fusionsa
| Route of larval tick infection | B. burgdorferi strain genotype | No. of mice infected/total no. exposed |
|---|---|---|
| SCID mouse blood meal | WT | 2/2 |
| ApospC | 2/2 | |
| ApospA | 2/2 | |
| Immersion | WT | 2/2 |
| ApospC | 2/2 | |
| ApospA | 2/2 | |
| WT mouse blood meal | WT | 4/4 |
| ΔospA | 0/2 | |
| ApospC | 0/2 | |
| ApospA | 2/2 | |
| ApospB | 2/2 |
Twenty nymphs per tick cohort were applied to WT mice and were allowed to feed to repletion. Three weeks after the application of ticks, mouse infection was assessed by seroconversion and by isolation from ears, bladders, and joints. Infected animals were seropositive and had at least two tissues positive out of three tissues assessed. Negative animals were seronegative and had no positive tissues.
Artificial infection with B. burgdorferi ApospC and B. burgdorferi ApospA.
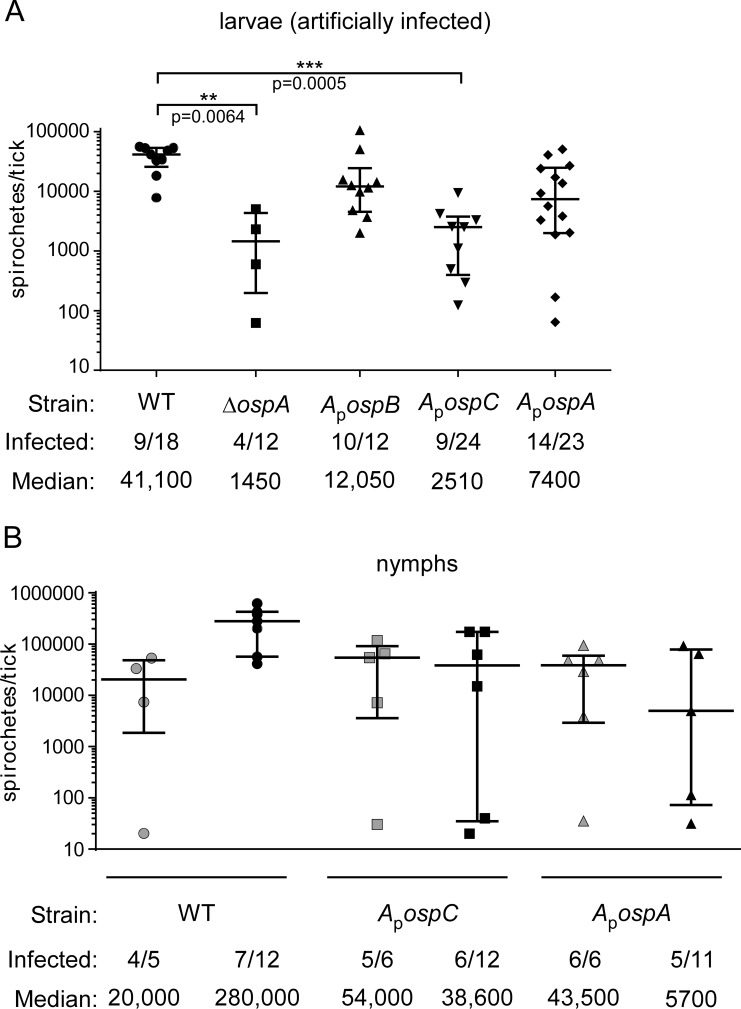
Artificial infection by immersion provides another method for infecting ticks with B. burgdorferi without exposing the incoming bacteria to antibody elicited during mammalian infection. In this method, larval ticks are immersed in spirochete cultures and are then fed on naïve WT animals, which produce natural antibody but no B. burgdorferi-specific antibody. For this method of infection, we immersed larval ticks in cultures of the WT, ΔospA, ApospC, ApospB, and ApospA strains (in two separate experiments), and then the larvae were fed on naïve mice. Seven to 10 days later, ticks were crushed and the CFU count per tick assessed. In this case, spirochete loads in ticks infected with the ΔospA or ApospC strain were statistically significantly lower than those in WT-infected ticks, but the differences from loads in ticks infected with the ApospA or ApospB strain were not significant. Also, the proportions of infected ticks were low after artificial infection, and there was a large spread in the data (Fig. 3).
FIG 3.
Spirochete loads in ticks artificially infected by immersion. Symbols represent CFU counts in individual ticks, as determined by plating of tick homogenates on solid medium. Medians and interquartile ranges are indicated. (A) Spirochete loads in artificially infected larvae 7 to 10 days after feeding. Significant differences (as determined by a Kruskal-Wallis test) are marked by asterisks, and probabilities are given. (B) Spirochete loads in fed nymphs artificially infected as larvae, shown at dropoff (shaded symbols) or 7 to 10 days after dropoff (filled symbols). Spirochete loads at dropoff did not differ significantly between strains. Seven to 10 days after dropoff, the set included significant differences (P, 0.0265 by Dunn's multiple-comparison test), but no statistically significant pairwise differences were observed (by Dunn's multiple-comparison test). The proportion of ticks infected (number of infected ticks per total ticks plated) and the median spirochete load per tick are given below each graph.
The larval ticks from one experiment (infected with the WT, ApospC, or ApospA strain) were allowed to molt, and the resultant nymphs were fed on naïve mice. The CFU count per tick was assessed soon (0 to 2 days) after dropoff and again 7 to 10 days after dropoff. At both of these time points, the spirochete loads in infected ticks and the proportion of ticks infected were not significantly different for the WT, ApospC, and ApospA strains (Fig. 3B). Because few of the ticks analyzed were infected, and because of the large variation in B. burgdorferi loads in ticks, the implications of these data are unclear. All the mice on which these nymphs were fed became infected (Table 3), demonstrating that the CFU counts within the ticks (for every strain) were adequate for transmission to a naïve animal.
Tick infection by feeding on immunocompetent mice infected with ospAp fusion strains.
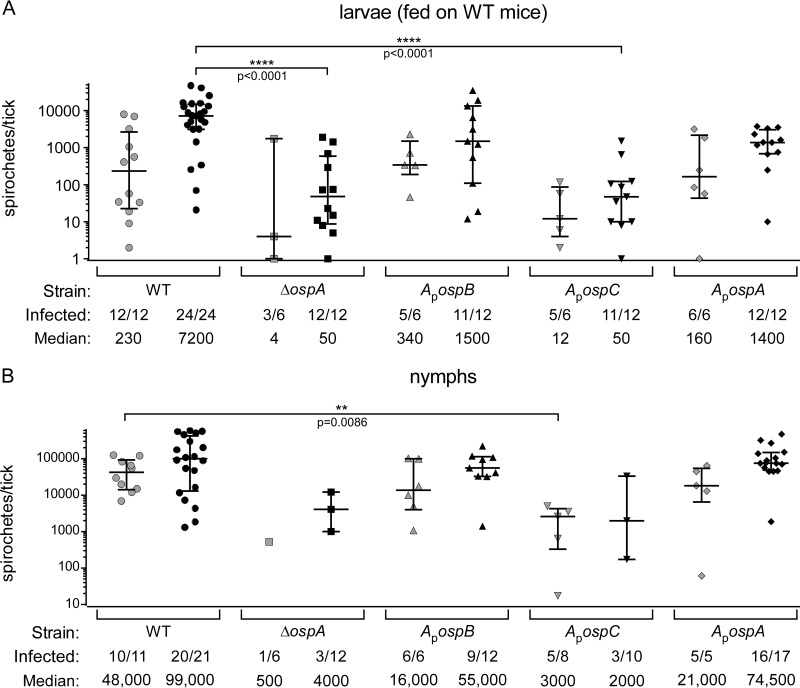
Ticks normally become infected by feeding on immunocompetent mice, in which case the spirochetes enter the ticks along with host anti-B. burgdorferi antibodies, in addition to the other components of a blood meal. To determine if OspC could facilitate tick colonization in this context, we fed naïve larval ticks on immunocompetent RML mice that were infected with B. burgdorferi WT, ApospC, ApospA, ApospB, or ΔospA spirochetes (in two separate experiments). Several ticks per mouse were used to assess spirochete loads by plating for CFU. All strains were acquired by ticks, but ΔospA and ApospC spirochete loads were significantly lower than loads of other strains, whether measured soon (1 to 2 days) after dropoff or a week later (Fig. 4A shows pooled data from the two experiments). In these experiments, we found that OspB fully substituted for OspA, since there was no difference in the CFU count per tick between ticks infected with the ApospB strain and those infected with the ApospA strain (Fig. 4A). The proportions of ticks infected were similar and high for all tick cohorts at the later time point (Fig. 4A). Our data are consistent with those of Battisti et al. (18), demonstrating that ΔospA spirochetes have difficulty surviving an incoming blood meal derived from immunocompetent mice, which contains antibodies recognizing invariant components of the spirochete surface. Likewise, we hypothesized that the ApospC spirochetes would have entered the ticks along with blood that contained an anti-OspC antibody, which could have killed many of the spirochetes and reduced the load in ticks infected with the ApospC strain. Antibodies persisting within the midgut after blood meal digestion and molting may have contributed to the reduced prevalence of infection found for ticks infected with the ΔospA or ApospC strain (compare Fig. 4B and A).
FIG 4.
Spirochete loads in ticks infected by feeding on needle-inoculated immunocompetent mice. Symbols represent CFU counts in individual ticks, as determined by plating of tick homogenates on solid medium. Medians and interquartile ranges are indicated. (A) Spirochete loads in larvae infected by feeding on immunocompetent mice, shown at dropoff (shaded symbols) or 7 to 10 days after dropoff (filled symbols). At dropoff, spirochete loads did not differ significantly among strains (by the Kruskal-Wallis test). At 7 to 10 days after dropoff, WT-infected ticks had significantly higher spirochete loads than ticks infected with the ΔospA or ApospC strain (by Dunn's multiple-comparison test). (B) Spirochete loads in fed nymphs infected as larvae by feeding on immunocompetent mice, shown at dropoff (shaded symbols) or 7 to 10 days after dropoff (filled symbols), as determined by plating of tick homogenates on solid medium. The medians differed significantly at both time points (by the Kruskal-Wallis test), but only one data set was significantly different from any other by Dunn's multiple-comparison test. The load of ΔospA bacteria at dropoff could not be included in this comparison, because only 1 of 6 ticks plated was infected. The prevalences of infection for the ΔospA and ApospC strains were significantly lower than those for the rest, except that the difference between the ApospC and ApospB strains was not significant (by Fisher's exact test) (see the text for more details). The proportion of ticks infected (number of infected ticks per total ticks plated) and the median spirochete load per tick are given below each graph.
As mentioned above, we excluded the possibility that mice infected with the ApospC strain had lower spirochete levels in their tissues by using quantitative PCR to measure spirochete genomes per mouse genome in infected ears. These values did not differ among mice infected with the WT, ApospC, or ApospA strain in one experiment or among mice infected with the WT, ΔospA, or ApospB strain in another (see Fig. S1 in the supplemental material).
The larval tick cohorts were allowed to molt to the nymphal stage and were fed on naïve mice. The CFU count per nymph was assessed soon (1 to 2 days) after dropoff as well as 7 to 10 days after dropoff (Fig. 4B). The loads of ΔospA and ApospC spirochetes in ticks were lower at both time points, although the prevalences of infection by those strains were so low, especially at the later time point, that only one pairwise comparison resulted in a statistically significant difference (Kruskal-Wallis test [P, 0.0228 for the set], followed by Dunn's multiple-comparison test [P, 0.1532 to >0.9999]) (Fig. 4B). When the prevalences of infection were compared, those for the ΔospA and ApospC strains were significantly lower than those for the rest (P, <0.0001 to 0.0391 by Fisher's exact test), except that the difference between the ApospC and ApospB strains was not significant (P = 0.0836). These data indicate that OspC cannot facilitate tick colonization in place of OspA when the spirochetes enter the tick in the context of a blood meal from an immunocompetent animal. Since these spirochetes also make OspC during the mammalian phase of infection (directed by the normal ospC gene on cp26), the animals have developed an immune response to that protein, and anti-OspC antibodies would enter the tick along with the bacteria. Our data are consistent with the idea that OspA protects spirochetes within the tick because it is an abundant surface protein that is not produced within the mammal, which is not true for OspC. The similar spirochete loads per tick for the ApospB and ApospA strains (Fig. 4A and B) were expected, since OspB is not produced within the mammal and is structurally more similar to OspA than is OspC.
In accord with the relative spirochete loads in ticks, WT, ApospA, and ApospB spirochetes were transmitted to mice by infected nymph feeding, whereas ApospC and ΔospA spirochetes were not transmitted (Table 3).
DISCUSSION
B. burgdorferi OspA is the major outer surface lipoprotein produced by the spirochete in the tick vector, but several different functions have been proposed for this protein. Spirochetes lacking the protein are clearly deficient in tick colonization (18, 20). One series of experiments indicated that the protein was required for binding a tick midgut receptor (21, 46). Another demonstrated impaired colonization by ospA mutant spirochetes, which was partially alleviated when the spirochetes were derived from mice unable to mount an antibody response, a finding consistent with OspA protecting the bacteria from antibodies present in the incoming blood meal (18). Since the ospA promoter is not expressed in the mammalian host, OspA would not be synthesized in that environment, and no anti-OspA antibodies would be produced. Therefore, OspA could shield invariant outer surface components from antibodies and other components of the blood meal or midgut milieu. These data are consistent with those of Bunikis and Barbour (47), who demonstrated that OspA (and other lipoproteins) can shield P66, an integral outer membrane protein, from antibody and protease access in cultured spirochetes.
The experiments carried out in the present study indicate that the OspC protein, when produced in the tick by expression from the ospA promoter, does not increase colonization over that for spirochetes lacking OspA. OspC failed to compensate for the absence of OspA even when anti-OspC antibodies were not present, as when the ticks acquired spirochetes by feeding on infected SCID mice. The further reduced levels of tick colonization by ApospC spirochetes derived from WT animals can be attributed to anti-OspC antibodies in the spirochete-containing blood meal, since the ApospC strain would produce OspC from its native promoter during early mammalian infection. Consistent with this explanation is our finding that the relative increase in colonization when the infectious blood meal was derived from immunodeficient rather than immunocompetent mice was greater for ApospC spirochetes than for WT or ApospA spirochetes, in which cases the major lipoprotein OspA was not produced in a mammal (Fig. 2 and 4; Table 4).
TABLE 4.
Relative spirochete loads in larvae fed on WT versus SCID micea
| B. burgdorferi strain genotype | Median spirochete load in infected larvae fed on: |
Ratio of loads in SCID mice to loads in WT mice | |
|---|---|---|---|
| WT mice | SCID mice | ||
| WT | 7,200 | 28,650 | 4 |
| ΔospA | 50 | 4,300 | 86 |
| ApospC | 50 | 1,540 | 30.8 |
| ApospA | 1,400 | 9,000 | 6.4 |
| ApospB | 1,500 | 19,800 | 13.2 |
Our data address the mechanism by which OspA is likely to facilitate the colonization of ticks by B. burgdorferi and are in accord with a relatively nonspecific role for OspA, such as shielding invariant surface proteins of the spirochete from the tick environment, including mammalian blood components (18). The findings of Bunikis and Barbour (47), mentioned above, are also consistent with OspA functioning to mask invariant B. burgdorferi surface components from incoming antibodies and other blood meal and midgut molecules. Our data also suggest that OspA has a second function, since ΔospA spirochetes do not colonize normally, even in the absence of host antibodies. This second function could be receptor binding, as suggested previously (21, 46).
The B. burgdorferi strain that makes OspB in place of OspA (the ApospB strain) was able to infect immunocompetent mice and colonize ticks that fed on those infected mice as well as WT or ApospA spirochetes. In this case (and in WT spirochetes), OspB is not normally made in the mammal, so incoming antibodies would not recognize OspB and would not clear ApospB spirochetes from ticks. Also, the OspB sequence (48) and structure (49, 50) are very similar to those of OspA, making them readily interchangeable. Although OspB does not bind to TROSPA (21), it has been shown to bind other components of tick gut extract (22), which may be significant in allowing ApospB spirochetes to colonize ticks.
One puzzle is that in many experiments, the data for one control (i.e., the ApospA strain) are different from those for the WT control. Although we have not pinpointed the reason for this difference, the fusion construct strains may differ from the WT due to effects of the constitutive selectable marker inserted downstream of the ospAp fusion constructs. The constitutive promoter driving the selectable marker may direct increased levels of truncated OspB protein in the ApospA and ApospC strains or may influence lp54 replication or partition by directing transcription through the adjacent rep-par region of the plasmid in the ApospA, ApospC, and ApospB strains. We found no major difference in lp54/chromosome ratios (assessed by qPCR) between S9 spirochetes and ApospC or ApospA spirochetes (data not shown), making it unlikely that lp54 stability is adversely affected by the fusions, but we do not know if altered production of truncated OspB could affect tick colonization by the ApospA and ApospC strains. In the ApospB strain, the selectable marker is inserted in a different position than in the other fusion strains and the OspB protein encoded is full-length, both of which features could influence surface properties. One surprising consequence of this fusion is that the strain is very poorly resuspended in electroporation solution, making it difficult to transform by electroporation (unpublished results). This characteristic may explain why many transformable strains carry mutations that lead to truncation of the ospB ORF. The ApospC strain is filamentous and has altered surface properties, leading to clumps and intertwined bacteria. We do not know the basis for these morphological changes. This phenotype correlates with a somewhat reduced plating efficiency for the ApospC strain and may affect our ability to recover ApospC spirochetes from tissues or ticks, but these in vitro properties are not sufficient to explain the tick colonization defects that we have observed for the ApospC strain.
Over the past several years, a body of data has accumulated that indicates that the major outer surface proteins of B. burgdorferi perform functionally overlapping roles at different points in the mouse–tick infectious cycle (27, 29). The ability of OspC to substitute for VlsE, albeit imperfectly, demonstrates that the role within mammals is not dependent on protein sequence specificity. Rather, a large lipoprotein tethered to the outer surface of the spirochete seems to be the crucial element. In immunocompetent mice, the ability to vary antigenically, thereby evading acquired immunity, is also crucial. The inability of OspC to substitute for OspA indicates that the requirements for tick colonization may include a sequence-specific or structural component. Our data are consistent with previous findings (18, 29, 47) and support the model that (i) OspA is functional within the tick, because it is not normally made within the mammal and so is not recognized by antibodies in the incoming blood meal and may also bind to a midgut receptor (46); (ii) OspC is functional during early mammalian infection, when a few spirochetes must defend themselves against the host innate immune system in order to establish themselves in the foreign host environment (26); and (iii) VlsE functions while varying to evade host immunity during persistent infection within a mammal, when the spirochetes have disseminated to multiple tissues. Each protein has unique features that optimize it to the time and place at which it is synthesized. Further studies will continue to define the tick and mammalian defenses targeting the spirochete and the mechanisms by which the surface lipoproteins block the action of those defenses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tom Schwan for the gift of monoclonal antibodies to B. burgdorferi proteins. We are very grateful to Anita Mora and Ryan Kissinger for expert figure preparation. Laszlo Kari, Tregei Starr, and Philip Stewart provided helpful comments on the manuscript. We thank Chad Hillman, Valentina Carracoi, Bharti Bhatia, and Tyler Evans for help on some experiments.
This research was supported by the Intramural Research Program of the NIAID, NIH.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00063-16.
REFERENCES
- 1.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. 1983. The spirochetal etiology of Lyme disease. N Engl J Med 308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 3.Iyer R, Caimano MJ, Luthra A, Axline D Jr, Corona A, Iacobas DA, Radolf JD, Schwartz I. 2015. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Mol Microbiol 95:509–538. doi: 10.1111/mmi.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fingerle V, Hauser U, Liegl G, Petko B, Preac-Mursic V, Wilske B. 1995. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J Clin Microbiol 33:1867–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A 92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohnishi J, Piesman J, de Silva AM. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci U S A 98:670–675. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilske B, Preac-Mursic V, Schierz G, Busch KV. 1986. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl Bakteriol Mikrobiol Hyg A 263:92–102. [DOI] [PubMed] [Google Scholar]
- 8.Wilske B, Preac-Mursic V, Schierz G, Kuhbeck R, Barbour AG, Kramer M. 1988. Antigenic variability of Borrelia burgdorferi, p 126–143. In Benach JL, Bosler EM (ed), Lyme disease and related disorders. New York Academy of Sciences, New York, NY. [DOI] [PubMed] [Google Scholar]
- 9.Xu Q, Seemanapalli SV, McShan K, Liang FT. 2006. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect Immun 74:5177–5184. doi: 10.1128/IAI.00713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang JR, Hardham JM, Barbour AG, Norris SJ. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275–285. doi: 10.1016/S0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 11.Crother TR, Champion CI, Whitelegge JP, Aguilera R, Wu X-Y, Blanco DR, Miller JN, Lovett MA. 2004. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect Immun 72:5063–5072. doi: 10.1128/IAI.72.9.5063-5072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, Fikrig E. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun 72:5759–5767. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang FT, Jacobs MB, Bowers LC, Philipp MT. 2002. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med 195:415–422. doi: 10.1084/jem.20011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JR, Norris SJ. 1998. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun 66:3689–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coutte L, Botkin DJ, Gao L, Norris SJ. 2009. Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection. PLoS Pathog 5:e1000293. doi: 10.1371/journal.ppat.1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrenz MB, Wooten RM, Norris SJ. 2004. Effects of vlsE complementation on the infectivity of Borrelia burgdorferi lacking the linear plasmid lp28-1. Infect Immun 72:6577–6585. doi: 10.1128/IAI.72.11.6577-6585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bankhead T, Chaconas G. 2007. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol Microbiol 65:1547–1558. doi: 10.1111/j.1365-2958.2007.05895.x. [DOI] [PubMed] [Google Scholar]
- 18.Battisti JM, Bono JL, Rosa PA, Schrumpf ME, Schwan TG, Policastro PF. 2008. Outer surface protein A protects Lyme disease spirochetes from acquired host immunity in the tick vector. Infect Immun 76:5228–5237. doi: 10.1128/IAI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal U, Fikrig E. 2003. Adaptation of Borrelia burgdorferi in the vector and vertebrate host. Microbes Infect 5:659–666. doi: 10.1016/S1286-4579(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 20.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med 199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, de Silva AM, Bao F, Yang X, Pypaert M, Pradhan D, Kantor FS, Telford S, Anderson JF, Fikrig E. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Neelakanta G, Li X, Pal U, Liu X, Beck DS, Deponte K, Fish D, Kantor FS, Fikrig E. 2007. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog 3:e33. doi: 10.1371/journal.ppat.0030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A 101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart PE, Wang X, Bueschel DM, Clifton DR, Grimm D, Tilly K, Carroll JA, Weis JJ, Rosa PA. 2006. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect Immun 74:3547–3553. doi: 10.1128/IAI.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Stewart P, Rosa P. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrasco SE, Troxell B, Yang Y, Brandt SL, Li H, Sandusky GE, Condon KW, Serezani CH, Yang XF. 2015. Outer surface protein OspC is an anti-phagocytic factor that protects Borrelia burgdorferi from phagocytosis by macrophages. Infect Immun 83:4848–4860. doi: 10.1128/IAI.01215-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Q, McShan K, Liang FT. 2008. Essential protective role attributed to the surface lipoproteins of Borrelia burgdorferi against innate defenses. Mol Microbiol 69:15–29. doi: 10.1111/j.1365-2958.2008.06264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Q, McShan K, Liang FT. 2008. Modification of Borrelia burgdorferi to overproduce OspA or VlsE alters its infectious behaviour. Microbiology 154:3420–3429. doi: 10.1099/mic.0.2008/019737-0. [DOI] [PubMed] [Google Scholar]
- 29.Tilly K, Bestor A, Rosa PA. 2013. Lipoprotein succession in Borrelia burgdorferi: similar but distinct roles for OspC and VlsE at different stages of mammalian infection. Mol Microbiol 89:216–227. doi: 10.1111/mmi.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. 2002. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem 277:21691–21696. doi: 10.1074/jbc.M201547200. [DOI] [PubMed] [Google Scholar]
- 31.Eicken C, Sharma V, Klabunde T, Owens RT, Pikas DS, Höök M, Sacchettini JC. 2001. Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi. J Biol Chem 276:10010–10015. doi: 10.1074/jbc.M010062200. [DOI] [PubMed] [Google Scholar]
- 32.Kumaran D, Eswaramoorthy S, Luft BJ, Koide S, Dunn JJ, Lawson CL, Swaminathan S. 2001. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. EMBO J 20:971–978. doi: 10.1093/emboj/20.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, Bono JL, Akins DR, Radolf JD, Schwan TG, Rosa P. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun 70:2139–2150. doi: 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rego RO, Bestor A, Rosa PA. 2011. Defining the plasmid-encoded restriction-modification systems of the Lyme disease spirochete Borrelia burgdorferi. J Bacteriol 193:1161–1171. doi: 10.1128/JB.01176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbour AG. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 36.Tilly K, Grimm D, Bueschel DM, Krum JG, Rosa P. 2004. Infectious cycle analysis of a Borrelia burgdorferi mutant defective in transport of chitobiose, a tick cuticle component. Vector Borne Zoonotic Dis 4:159–168. doi: 10.1089/1530366041210738. [DOI] [PubMed] [Google Scholar]
- 37.Tilly K, Elias AF, Bono JL, Stewart P, Rosa P. 2000. DNA exchange and insertional inactivation in spirochetes. J Mol Microbiol Biotechnol 2:433–442. [PubMed] [Google Scholar]
- 38.Samuels DS. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol 47:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbour AG, Tessier SL, Todd WJ. 1983. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun 41:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosa PA, Schwan T, Hogan D. 1992. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol 6:3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 41.Barbour AG, Hayes SF, Heiland RA, Schrumpf ME, Tessier SL. 1986. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun 52:549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tilly K, Bestor A, Jewett MW, Rosa P. 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect Immun 75:1517–1519. doi: 10.1128/IAI.01725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Policastro PF, Schwan TG. 2003. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J Med Entomol 40:364–370. doi: 10.1603/0022-2585-40.3.364. [DOI] [PubMed] [Google Scholar]
- 44.Seiler KP, Vavrin Z, Eichwald E, Hibbs JB Jr, Weis JJ. 1995. Nitric oxide production during murine Lyme disease: lack of involvement in host resistance or pathology. Infect Immun 63:3886–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jewett MW, Lawrence K, Bestor AC, Tilly K, Grimm D, Shaw P, VanRaden M, Gherardini F, Rosa PA. 2007. The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol Microbiol 64:1358–1374. doi: 10.1111/j.1365-2958.2007.05746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal U, de Silva AM, Montgomery RR, Fish D, Anguita J, Anderson JF, Lobet Y, Fikrig E. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J Clin Invest 106:561–569. doi: 10.1172/JCI9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunikis J, Barbour AG. 1999. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun 67:2874–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergström S, Bundoc VG, Barbour AG. 1989. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol 3:479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Dunn JJ, Luft BJ, Lawson CL. 1997. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc Natl Acad Sci U S A 94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker M, Bunikis J, Lade BD, Dunn JJ, Barbour AG, Lawson CL. 2005. Structural investigation of Borrelia burgdorferi OspB, a bactericidal Fab target. J Biol Chem 280:17363–17370. doi: 10.1074/jbc.M412842200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.