Abstract
Streptococcus pneumoniae is a leading cause of invasive bacterial infections, with nasal colonization an important first step in disease. While cigarette smoking is a strong risk factor for invasive pneumococcal disease, the underlying mechanisms remain unknown. This is partly due to a lack of clinically relevant animal models investigating nasal pneumococcal colonization in the context of cigarette smoke exposure. We present a model of nasal pneumococcal colonization in cigarette smoke-exposed mice and document, for the first time, that cigarette smoke predisposes to invasive pneumococcal infection and mortality in an animal model. Cigarette smoke increased the risk of bacteremia and meningitis without prior lung infection. Mechanistically, deficiency in interleukin 1α (IL-1α) or platelet-activating factor receptor (PAFR), an important host receptor thought to bind and facilitate pneumococcal invasiveness, did not rescue cigarette smoke-exposed mice from invasive pneumococcal disease. Importantly, we observed cigarette smoke to attenuate nasal inflammatory mediator expression, particularly that of neutrophil-recruiting chemokines, normally elicited by pneumococcal colonization. Smoking cessation during nasal pneumococcal colonization rescued nasal neutrophil recruitment and prevented invasive disease in mice. We propose that cigarette smoke predisposes to invasive pneumococcal disease by suppressing inflammatory processes of the upper respiratory tract. Given that smoking prevalence remains high worldwide, these findings are relevant to the continued efforts to reduce the invasive pneumococcal disease burden.
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) is a leading cause of invasive infections, including bacteremia and meningitis (1–3). S. pneumoniae colonizes the nasal mucosa of up to 18% of adults as a commensal bacterium (4). While nasal pneumococcal colonization alone is asymptomatic, it is considered a requirement for the development of infectious disease (5). Cigarette smoking is a strong risk factor for invasive pneumococcal disease (IPD) (6–8), although the underlying mechanisms remain unclear. Recent estimates indicate that over 1 billion people continue to smoke worldwide (World Health Organization), placing a considerable proportion of the global population at increased risk of IPD.
Nasal colonization with pneumococci induces rapid neutrophil recruitment, followed by prolonged macrophage accumulation, ultimately leading to the control and clearance of colonizing bacteria (9–11). Cigarette smoking impacts the respiratory host defense in ways that may increase the risk of acquiring infection (12). Smoke exposure has been documented to impair both mucociliary flow and epithelial barrier integrity at the nasopharynx (13). In addition, cigarette smoke suppresses the phagocytic activity of macrophages, contributing to impaired pneumococcal clearance from the lower respiratory tract (14). Cigarette smoke has also been shown to increase bacterial adherence to host cells via the platelet activating factor receptor (PAFR) (15–17). Activation of host cells by cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin 1α (IL-1α) further contributes to bacterial invasion through the PAFR. Moreover, IL-1 administration to rabbits increased the lung pneumococcal burden in a PAFR-dependent manner (16). However, the effects of cigarette smoke on host responses to S. pneumoniae in the upper respiratory tract remain to be elucidated.
The current study investigated the impact of cigarette smoke exposure on pneumococcal nasal colonization in mice. Similar to clinical observations, cigarette smoke exposure predisposed mice to IPD, followed by sepsis and meningitis. To our knowledge, this is the first report of cigarette smoke exposure predisposing to IPD in mice. Mechanistically, we found cigarette smoke to suppress the expression of nasal inflammatory mediators normally elicited by pneumococcal colonization. The effects of cigarette smoke on the host response may be reversible, as smoking cessation following nasal colonization fully rescued mice from IPD. These data suggest that compromised host responses in the nasopharynx predispose to IPD following cigarette smoke exposure. These findings have increased our understanding of the effects of cigarette smoke on the nasal bacterial host defense and may help guide future efforts to reduce the incidence of IPD.
(Part of this work has been previously presented in abstracts at the American Thoracic Society Conference, San Diego, CA, May 2014, and the Upstate New York Immunology Conference, Bolton Landing, NY, October 2015.)
MATERIALS AND METHODS
Animals.
C57BL/6 mice were from The Jackson Laboratory (Bar Harbor, ME). PAFR knockout (KO) mice from Elaine Tuomanen (18) and IL-1α KO mice from Yoichiro Iwakura (University of Tokyo) (19) were also on a C57BL/6 background. The Animal Research Ethics Board of McMaster University approved all experimental procedures.
Bacterial preparation.
S. pneumoniae P1547, a serotype 6A virulent clinical isolate, was grown in tryptic soy broth (BD Biosciences, Franklin Lakes, NJ) (20, 21).
Cigarette smoke exposure.
Mice were exposed to the mainstream smoke from 12 3R4F reference cigarettes (Tobacco and Health Research Institute, University of Kentucky, Lexington, KY), with filters removed, for 50 min, twice daily for 5 days per week (unless otherwise stated) using a whole-body cigarette smoke exposure system (SIU-48; Promech Lab AB, Vintrie, Sweden) as reported previously (22). Prior to cigarette smoke exposure, the mice were acclimatized to the restrainers for the whole-body cigarette smoke exposure system for 20 min on the first day, 30 min on the second day, and 50 min on the third day. Control mice were exposed to room air only.
Nasal colonization.
One-week smoke-exposed mice were intranasally inoculated with pneumococci (10 μl) in the absence of anesthesia and continued to be exposed to smoke without interruption. The mice were given HydroGel and placed on heating pads following daily cigarette smoke exposure for the first 3 days post-nasal colonization.
Endpoint monitoring.
According to the Canadian Council on Animal Care Guidelines, the death of experimental animals is considered unethical. Therefore, the mice were subjected to endpoint monitoring as described previously (23), and endpoint mice were immediately euthanized during survival studies.
Determination of cellular inflammation and bacterial burden.
Nasal wash samples; bronchoalveolar lavage (BAL) fluid; and homogenates of the lung, brain, and spleen were generated. BAL fluid cytospins and blood smears were generated to determine the proportions of mononuclear cells and neutrophils. Total cell numbers were determined using a hemocytometer. The bacterial burden was quantified by plating on blood agar.
Pathology.
Mice reaching endpoint were anesthetized and perfused through the heart with lactated Ringer's solution, followed by 10% formalin. Organs were collected and fixed in formalin. In a separate experiment, the lungs were inflated with formalin at 30 cm H2O pressure. Tissues were paraffin embedded, sectioned, and stained with hematoxylin and eosin (H&E). Gram staining was also performed based on the Brown and Hopps method as described in the supplemental methods.
Serum cytokine measurement.
Serum TNF-α and IL-6 levels were determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Real-time quantitative PCR.
Nasal washes were performed with lysis buffer RLT (Qiagen, Mississauga, ON, Canada) as described previously (24). Lung tissue was homogenized in lysis buffer RLT. RNA was isolated using an RNeasy minikit with DNase step (Qiagen, Mississauga, ON, Canada). cDNA was synthesized with Superscript II reverse transcriptase (Invitrogen, Grand Island, NY) and TaqMan real-time reverse transcription (RT)-quantitative PCR (qPCR) was performed with the StepOnePlus real-time PCR system (Life Technologies Inc., Burlington, ON, Canada). Gene expression was determined using the ΔΔCT method. Target gene expression was normalized to the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) housekeeping gene and expressed as the fold change over the relevant control group, as indicated in the figure legends.
Flow cytometry.
Nasal wash samples were stained with fluorescent antibodies by incubation at 4°C and assayed with a BD LSRII flow cytometer on the same day. Data were gathered with FACSDiva software (BD) and analyzed with FlowJo software (TreeStar).
Imaging.
Room air- or smoke-exposed mice were anesthetized by intraperitoneal injection with ketamine/xylazine and nasally inoculated with 99mtechnetium-diethylene triamine penta-acetic acid (99mTc-DTPA) (Lantheus Medical Imaging, North Billerica, MA) at a time point corresponding to day 3 post-nasal colonization. Dynamic images of mice were obtained by single-photon emission computed tomography (SPECT) every 30 s for 20 min to observe the distribution of the radioligand (X-SPECT system; GammaMedica-Ideas, Northridge, CA). The mean count from the nasal region of interest was converted to a percentage of the highest count (obtained in the first frame) for each mouse and expressed as percent maximal activity. A whole body scan was performed at 30 min, followed by whole body computed tomography (CT). The upper airway doses of 99mTC-DTPA as a percentage of the whole body distribution were compared between room air- and smoke-exposed mice.
Statistical analysis.
Data are expressed as means and standard errors of the mean (SEM). GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA) and SPSS software (IBM, Armonk, NY) were utilized for statistical analyses. Levene's test for equality of variances was used to account for differences in data variability between groups. Independent t tests were used for comparisons between two groups. A log rank test was used for comparison of Kaplan-Meier survival curves. Differences with P values of <0.05 were considered statistically significant.
RESULTS
Cigarette smoke exposure compromises the nasal host response following pneumococcal colonization.
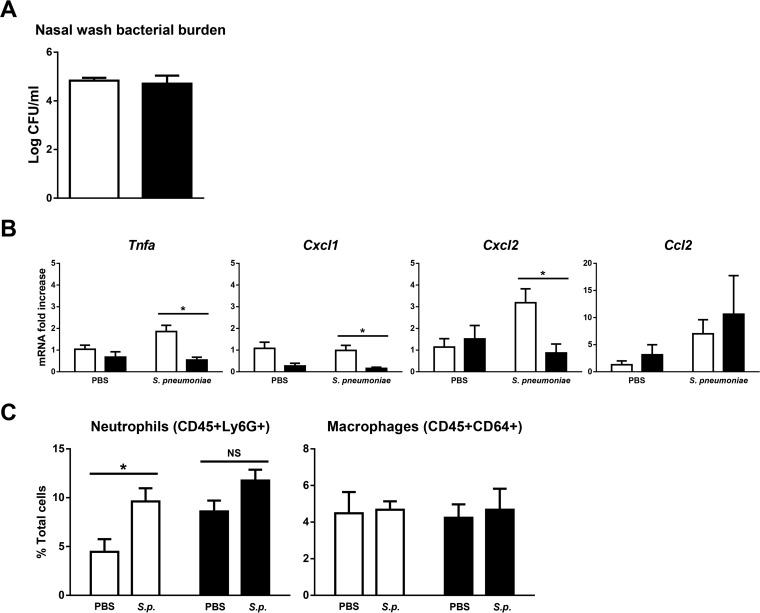
We investigated the effect of cigarette smoke exposure on day 2 post-nasal pneumococcal colonization. While the nasal bacterial burden was comparable to that of the room air-exposed control (Fig. 1A), we observed attenuation of nasal TNF-α, CXCL-1, and CXCL-2 expression in smoke-exposed mice (Fig. 1B). Corresponding to this, room air-exposed control mice had a significant increase in nasal neutrophils following pneumococcal colonization, as assessed by flow cytometry (Fig. 1C). There was no significant increase in response to pneumococcal colonization in smoke-exposed mice, suggesting that the ability to recruit additional neutrophils may be impaired. Lastly, cigarette smoke did not impact nasal CCL2 expression (Fig. 1B) or the proportion of macrophages (Fig. 1C). Our findings suggest that cigarette smoke may impair the host response to S. pneumoniae at the nasal mucosa.
FIG 1.
Cigarette smoke exposure compromises the nasal host response following nasal S. pneumoniae colonization. Room air-exposed control mice or 1-week cigarette smoke-exposed mice were nasally colonized with approximately 106 CFU of S. pneumoniae. The mice continued to be exposed to cigarette smoke and were euthanized on day 2 postcolonization. (A) Nasal wash sample bacterial burden. n = 4 or 5 mice per group. The data are representative of 3 independent experiments. (B) mRNA was isolated from lysis buffer RLT nasal wash samples. Gene expression was determined by real-time RT-qPCR and normalized to that of the room air-exposed vehicle control group. n = 3 to 5 mice per group. The Tnfa, Cxcl1, and Cxcl2 data are representative of 3 independent experiments; the Ccl2 data are representative of 2 independent experiments. (C) Neutrophil and macrophage proportions in the nasal wash samples were determined using flow cytometry. Open bars, room air; solid bars, smoke; S.p., S. pneumoniae; PBS, phosphate-buffered saline. The data are shown as means ± SEM. n = 4 or 5 mice per group. *, P < 0.05 compared to the corresponding control as assessed by an independent t test; NS, not significant.
Cigarette smoke exposure predisposes mice to IPD and mortality following nasal pneumococcal colonization.
To investigate the consequences of a compromised nasal host response, the health status of the mice was monitored for 16 days post-nasal colonization. Cigarette smoke exposure increased the frequency of mice reaching endpoint (60%) compared to room air-exposed controls (10%) (Fig. 2A). Rapid weight loss was observed in endpoint mice (Fig. 2A). Additionally, mortality occurred in smoke-exposed mice regardless of the initial bacterial-inoculation dose (see Fig. S1 in the supplemental material). Although the low-bacterial-dose group appeared to have higher mortality rates than the intermediate- and high-dose groups, the survival curves of cigarette smoke-exposed mice at these three doses were not significantly different from each other as assessed by a log rank test (P = 0.7294).
FIG 2.
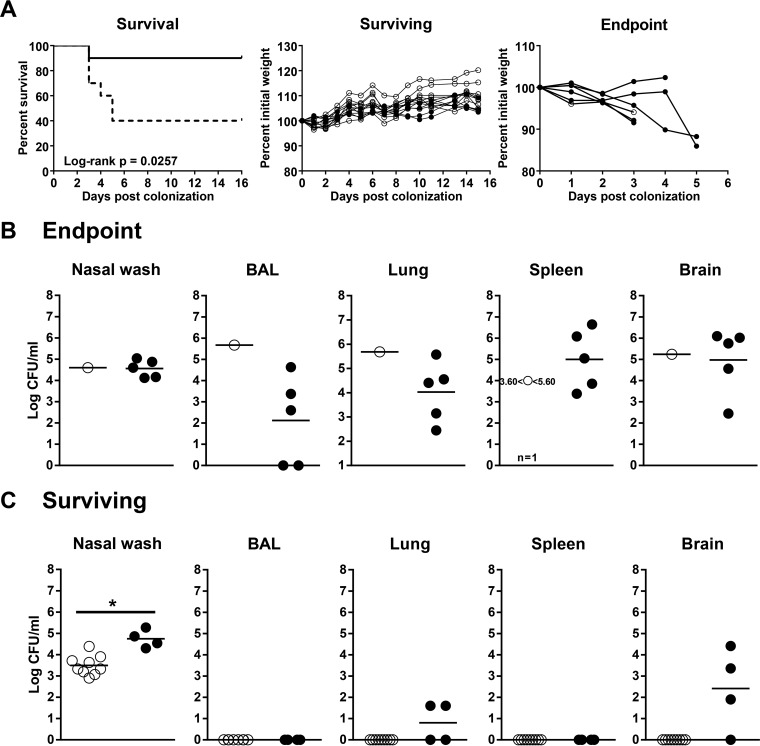
Cigarette smoke exposure predisposes mice to IPD and mortality following nasal pneumococcal colonization. Room air-exposed control mice or 1-week cigarette smoke-exposed mice were nasally colonized with approximately 106 CFU of S. pneumoniae. The mice continued to be exposed to cigarette smoke for 16 days post-nasal colonization and were euthanized as they reached endpoint. (A) (Left) Survival curve showing room air-exposed control mice (solid line) and smoke-exposed mice (dotted line). (Middle and right) Individual weights of surviving and endpoint mice are shown as percentages of initial weights on the day of bacterial colonization (day 0). Open circles, control mice; solid circles, smoke-exposed mice. (B and C) Bacterial burdens in various tissues of mice at endpoint (B) and of mice surviving to day 16 (C). Open circles, control mice; solid circles, smoke-exposed mice. n = 10 per group of control or smoke-exposed mice. The survival curves were compared by log rank test; a P value of <0.05 was considered significant. *, P < 0.05 compared to the corresponding control as assessed by an independent t test.
To determine if mortality was associated with IPD, we cultured for pneumococci in the lungs, spleen, and brain. Nasal wash cultures indicated successful colonization (Fig. 2B and C). In addition, we detected pneumococci in the BAL fluid, lungs, spleen, and brain in endpoint mice (Fig. 2B). Interestingly, two endpoint mice had pneumococci in the lung homogenate, but not the BAL fluid. While surviving room air-exposed control mice remained nasally colonized, no bacteria were cultured from other tissues (Fig. 2C). Smoke-exposed mice surviving to day 16 had higher nasal bacterial burdens than controls. Of note, some surviving smoke-exposed mice also had pneumococci in the brain, and IPD might have developed if the study had continued longer (Fig. 2C). Overall, these data provide evidence that cigarette smoke exposure following nasal pneumococcal colonization increases the incidence of IPD and mortality in mice.
Endpoint mice showed characteristics of sepsis and meningitis.
Given the presence of pneumococcal bacteremia in endpoint mice, we looked for abnormalities characteristic of sepsis. Total blood leukocyte numbers were lower in all endpoint mice than in both vehicle control and surviving mice (Fig. 3A). Differential cell counting showed reduced numbers of mononuclear cells, but not neutrophils (Fig. 3A). Moreover, we detected IL-6 in the sera of endpoint mice (Fig. 3B). The presence of serum IL-6, combined with blood leukopenia and evidence of bacteremia (Fig. 2B), indicated that endpoint mice developed sepsis.
FIG 3.
Endpoint mice show features of sepsis and meningitis. Room air-exposed control mice or 1-week cigarette smoke-exposed mice were nasally colonized with approximately 106 CFU of S. pneumoniae. The mice continued to be exposed to cigarette smoke post-nasal colonization and were euthanized as they reached endpoint. (A) Blood leukocyte counts were determined in mice surviving to day 16, in endpoint mice, and in vehicle control mice. (B) IL-6 was measured by ELISA in the sera of surviving and endpoint mice. Open circles, room air-exposed mice; solid circles, smoke-exposed mice. (C to H) Mice reaching endpoint were perfused with 10% formalin. Tissues were fixed in formalin, sectioned, and stained with H&E. Representative images are shown. (C) Inflammatory cell infiltrate at the meninges (arrow). Bar, 100 μm. (F) Higher magnification of panel C. Bar, 60 μm. (D) Endomyocarditis (arrow). Bar, 600 μm. (G) Higher magnification of panel D. Bar, 60 μm. (E) Cardiac microlesion (arrow). Bar, 60 μm. (H) Gram-positive stain of cardiac microlesion (arrow). Bar, 60 μm. n = 5 to 10 per group of control or smoke-exposed mice.
We next assessed the histopathology of endpoint mice and observed neutrophilic inflammation at the meninges, characteristic of meningitis (Fig. 3C and F). Endomyocarditis was also noted, with an area of intense neutrophil accumulation in one case (Fig. 3D and G). Notably, microlesions were present within the myocardium (Fig. 3E), which stained Gram positive (Fig. 3H), likely due to pneumococcal presence. Strikingly, the lungs of smoke-exposed endpoint mice were without evidence of overt inflammation and tissue consolidation (see Fig. S2A in the supplemental material) and comparable to those of surviving smoke-exposed mice (see Fig. S2B in the supplemental material). The lack of inflammation was also reflected in the BAL fluid cell counts of endpoint mice (see Fig. S2C in the supplemental material). Our observations suggest that endpoint mice developed IPD in the absence of pneumonia, leading to meningitis, sepsis, endomyocarditis, and cardiac damage.
Invasive infection occurred rapidly in the blood and brain of cigarette smoke-exposed mice.
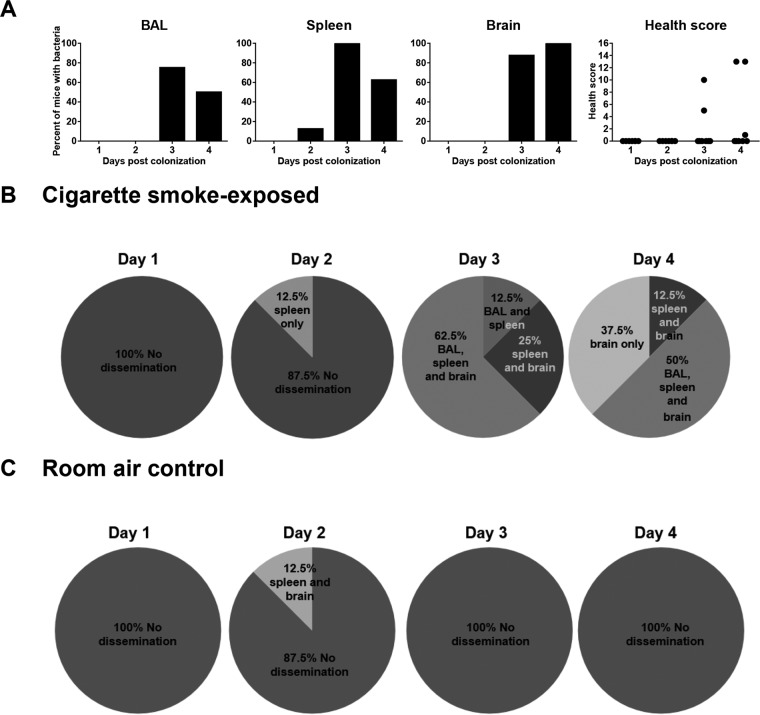
To characterize the pattern of bacterial dissemination from nasal colonization, we euthanized smoke-exposed mice daily post-nasal colonization and assessed the pneumococcal burden in the BAL fluid, spleen, and brain. Mice reaching endpoint outside the indicated time points were excluded from the study. Widespread bacterial dissemination occurred on days 3 and 4, as shown by the percentage of mice with pneumococci in various tissues (Fig. 4A). Of note, the majority of mice showed no obvious change in health status (Fig. 4A). In some mice, bacteria were detected in the spleen only, the brain only, or simultaneously in the brain and spleen without presence in the BAL fluid (Fig. 4B). Interestingly, there was no case of pneumococcal detection in the BAL fluid only, and we observed no increased BAL fluid cellular inflammation in mice with detectable BAL fluid bacteria compared to mice without bacterial dissemination (see Fig. S3A in the supplemental material). In addition, the nasal bacterial burden was not increased despite widespread bacterial dissemination (see Fig. S3B in the supplemental material). Finally, we observed no bacterial dissemination in room air-exposed control mice, except in one case (Fig. 4C). Overall, these data suggest that more bacterial dissemination occurs in smoke-exposed mice than in control mice and that the point of dissemination may be the nasopharynx, rather than the lungs.
FIG 4.
Invasive infection occurred rapidly in the blood and brain of cigarette smoke-exposed mice. Room air-exposed control or 1-week cigarette smoke-exposed mice were nasally colonized with approximately 106 CFU of S. pneumoniae. The mice continued to be exposed to cigarette smoke, and the bacterial burden was assessed daily for 4 days in the indicated tissue samples. (A) Percentages of cigarette smoke-exposed mice with detectable S. pneumoniae in each tissue sample over 4 days. The health status of the mice was also assessed prior to euthanization each day. (B) Breakdown of bacterial dissemination patterns in cigarette smoke-exposed mice by day. (C) Breakdown of bacterial dissemination patterns in room air-exposed mice by day. n = 8 mice per time point.
Cigarette smoke exposure did not compromise nasal clearance or epithelial barrier integrity.
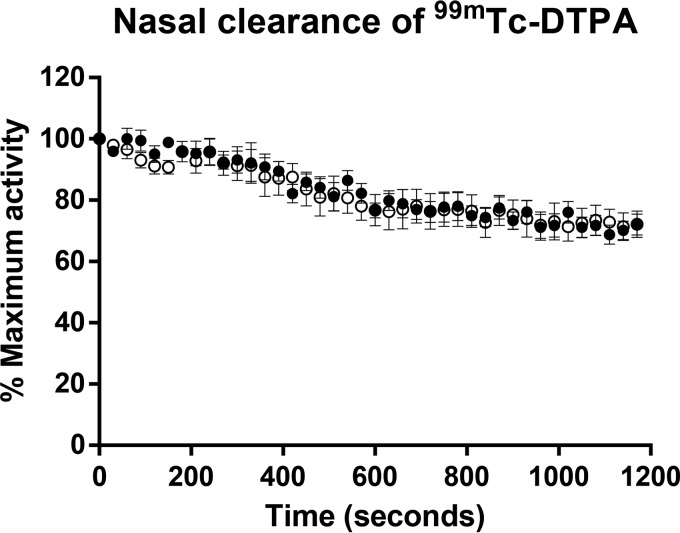
To investigate whether cigarette smoke exposure predisposed to IPD by compromising nasal clearance or epithelial barrier integrity, we administered 99mTc-DTPA intranasally to room air- and smoke-exposed mice at a time point corresponding to day 3 post-nasal colonization, when bacterial dissemination is first observed. Dynamic images were obtained by SPECT to observe nasal clearance of the radioligand (see Fig. S4A in the supplemental material). The activities of 99mTc-DTPA in the upper airways, measured every 30 s, were comparable between room air- and smoke-exposed mice (Fig. 5). To determine whether cigarette smoke exposure compromised nasal epithelial barrier function, we assessed the upper airway dose of 99mTC-DTPA as a percentage of the whole body distribution (outlined in Fig. S4B in the supplemental material). At 30 min post-nasal delivery, the 99mTc-DTPA was still mainly confined to the upper respiratory tract (see Fig. S4C in the supplemental material), and there was no significant difference between the upper airway doses of 99mTC-DTPA in room air-exposed (75.2% ± 1.3%) and smoke-exposed (73.9% ± 0.34%) animals as a percentage of the whole body distribution. These data suggest that short-term cigarette smoke exposure alone did not impact either nasal clearance or epithelial barrier integrity in the upper airways.
FIG 5.
Cigarette smoke exposure did not compromise nasal clearance of 99mTc-DTPA. Room air-exposed control mice or cigarette smoke-exposed mice were anesthetized and nasally inoculated with 99mTc-DTPA at a time point corresponding to day 3 post-nasal colonization. Dynamic images were acquired every 30 s using SPECT to observe the distribution of the radioligand. A standard region of interest was positioned in the nasal region, and the activity of 99mTc-DTPA was measured every 30 s and expressed as percent maximal activity (obtained in the first frame). The data are shown as means ± SEM. Open circles, control mice; solid circles, smoke-exposed mice. n = 5 or 6 mice per group.
PAFR or IL-1α deficiency did not rescue smoke-exposed mice from mortality following nasal pneumococcal colonization.
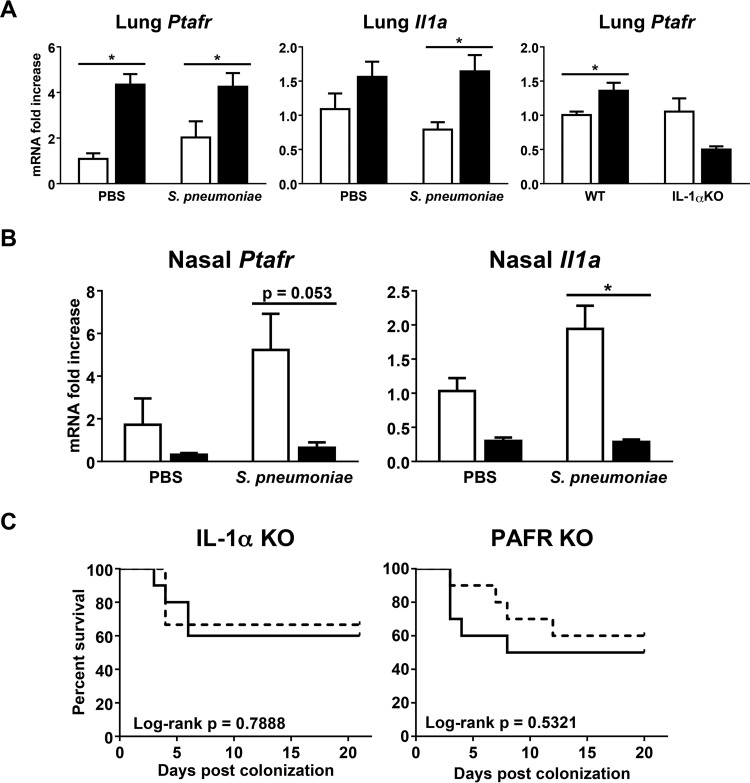
S. pneumoniae is known to bind several host receptors, including the PAFR (16, 25, 26), which may facilitate bacterial invasion independently of epithelial barrier function. Of particular interest, S. pneumoniae has been shown to adhere to and invade host cells activated by thrombin or cytokines, such as TNF-α and IL-1α, in a PAFR-dependent manner (16). Moreover, both PAFR and IL-1α are increased in the lungs of smokers and chronic obstructive pulmonary disease (COPD) patients (17, 27, 28). Therefore, cigarette smoke exposure may predispose to IPD by inducing PAFR expression in an IL-1α-dependent manner. In support of this, we found that cigarette smoke exposure significantly upregulated lung mRNA expression of both PAFR and IL-1α. Furthermore, the smoke-induced increase in lung PAFR mRNA expression was IL-1α dependent (Fig. 6A). In contrast, cigarette smoke exposure attenuated PAFR and IL-1α expression at the nasopharynx (Fig. 6B). Functionally, IL-1α or PAFR deficiency did not rescue smoke-exposed mice from mortality following nasal pneumococcal colonization (Fig. 6C). PAFR or IL-1α deficiency did not significantly affect the survival of corresponding room air-exposed controls compared to wild-type (WT) mice following nasal pneumococcal colonization (data not shown). Our findings show differential regulation of PAFR and IL-1α by cigarette smoke between the upper and lower respiratory tracts. Furthermore, the increased susceptibility to IPD from nasal pneumococcal colonization caused by cigarette smoke occurs independently of PAFR and IL-1α.
FIG 6.
PAFR or IL-1α deficiency did not rescue smoke-exposed mice from mortality following nasal pneumococcal colonization. Room air-exposed control mice or 1-week cigarette smoke-exposed mice were nasally colonized with approximately 106 CFU of S. pneumoniae. The mice continued to be exposed to cigarette smoke post-nasal colonization. (A) Lung mRNA expression of PAFR (Ptafr) and IL-1α at day 3 post-nasal colonization. Lung PAFR mRNA expression was also assessed in IL-1α KO mice following 4 days of cigarette smoke exposure. Gene expression was determined by real-time RT-qPCR and normalized to that of the room air-exposed vehicle control group of the appropriate mouse strain. n = 5 mice per group. (B) Lysis buffer RLT nasal wash sample mRNA expression of PAFR and IL-1α at day 2 post-nasal colonization. Gene expression was determined by real-time RT-qPCR and normalized to that of the room air-exposed vehicle control group. Open bars, room air-exposed mice; solid bars, smoke-exposed mice. The data are shown as means ± SEM. n = 3 to 5 mice per group. The data are representative of 2 independent experiments. *, P < 0.05 compared to the corresponding control as assessed by an independent t test. (C) Survival curves of smoke-exposed IL-1α KO and PAFR KO mice post-nasal pneumococcal colonization. Solid lines, wild-type mice; dashed lines, KO mice. n = 9 or 10 mice per group. The data are representative of 2 independent experiments. The survival curves were compared by log rank test. A P value of <0.05 was considered significant.
Smoking cessation fully protected mice from mortality following nasal pneumococcal colonization.
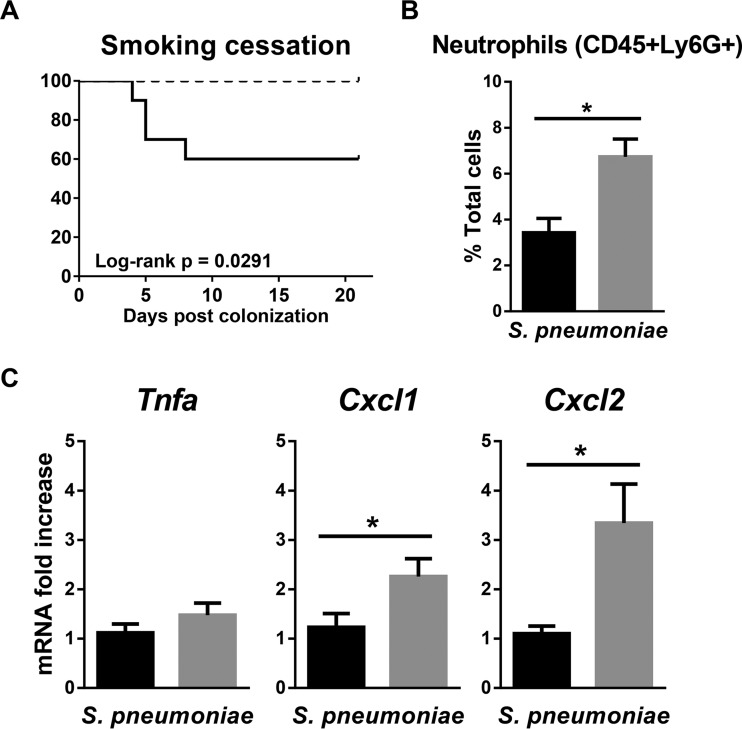
Notably, mice were completely rescued from IPD if cigarette smoke exposure was stopped following nasal pneumococcal colonization, suggesting that smoking cessation may be the most effective intervention strategy (Fig. 7A). The increased survival rate was associated with an increase in nasal neutrophils in the cessation group (Fig. 7B). Although smoking cessation did not impact the nasal expression of TNF-α compared to smoke-exposed mice, we did observe an increase in CXCL-1 and CXCL-2 expression (Fig. 7C). These data suggest that the increased incidence of IPD following cigarette smoke exposure is reversible and that smoking cessation may recover nasal CXCL-2 expression and neutrophil recruitment.
FIG 7.
Smoking cessation fully protected mice from mortality following nasal pneumococcal colonization. One-week cigarette smoke-exposed mice were nasally colonized with S. pneumoniae, and cigarette smoke exposure was either continued or stopped following colonization. (A) Survival curves of mice that continued to be exposed to cigarette smoke (solid line) or that underwent smoking cessation (dashed line). n = 10 mice per group. The survival curves were compared by log rank test. A P value of <0.05 was considered significant. (B) Neutrophil proportions in the nasal wash samples from the smoking and cessation groups were determined at day 2 post-nasal colonization using flow cytometry. n = 5 mice per group. (C) mRNA was isolated from lysis buffer RLT nasal wash samples from the smoke-exposed and cessation groups at day 2 post-nasal colonization. Gene expression was determined by real-time RT-qPCR and normalized to that of the smoke-exposed group. Black bars, smoke-exposed group; gray bars, cessation group. The data are shown as means ± SEM. n = 8 to 10 mice per group. *, P < 0.05 compared to the corresponding control as assessed by an independent t test.
DISCUSSION
Despite the well-documented association between cigarette smoking, as well as exposure to secondhand smoke, and IPD (6–8), no animal models to date have investigated the underlying mechanisms by which cigarette smoke predisposes to the progression of IPD from nasal colonization, an asymptomatic carrier state. Moreover, there are multiple reports that secondhand smoke increases the incidence of nasal pneumococcal colonization in children (29–31), further emphasizing the need for additional mechanistic studies (6). In the current study, we sought to establish a mouse model of nasal pneumococcal colonization and cigarette smoke exposure. Doing so has allowed us to characterize the extent of bacterial dissemination following cigarette smoke exposure while providing a tool to investigate the mechanisms that drive IPD.
Mice were exposed to cigarette smoke using an established whole-body smoke exposure system (22). Cigarette smoke is well tolerated, with carboxyhemoglobin and cotinine levels comparable to those of human smokers (22, 32). The mice were colonized nasally with S. pneumoniae as previously described (33, 34). By using a volume of 10 μl in the absence of anesthesia, bacteria were delivered to the nose only, as shown previously by Southam et al. (35) and Miller et al. (36). Our imaging data using 99mTc-DTPA confirm that nasal delivery of the bacteria was limited to the upper respiratory tract. Similar to nasopharyngeal carriage in humans, colonization is asymptomatic in adult mice and lasts several weeks with few cases of IPD (33, 37). Mice continued to be exposed to smoke postcolonization without interruption to mimic the fact that smokers continue smoking during asymptomatic nasal pneumococcal colonization.
Cigarette smoke had profound effects on the development of IPD; we observed increased mortality in mice associated with meningitis and bacteremia. Pneumococci were cultured from brain tissue associated with neutrophilic inflammation at the meninges. Pneumococcal bacteremia in mice was also associated with leukopenia, an observation that is consistent with the lymphopenia and splenic lymphocyte apoptosis described in septic patients and mouse models of sepsis (38–40). Moreover, all endpoint mice had detectable serum IL-6, which is thought to predict organ dysfunction and mortality in sepsis (41, 42). These findings mirror clinical observations of cigarette smoking as a risk factor for IPD; smokers are overrepresented in the population of IPD patients at 58%, compared to 24% for control subjects (6). To our knowledge, we are the first to describe, in the context of cigarette smoke, the progression from an asymptomatic carrier state to IPD in mice. Clinically, cardiac complications occur frequently with IPD (43). We observed bacterial endocarditis, where 15% of all cases were associated with pneumococcal infection in the preantibiotic era (44). In addition, we found microlesions in the heart, likely as a consequence of bacteremia, filled with Gram-positive bacteria, as recently described by Brown et al. (45). These microlesions were reported to correlate with markers of cardiac damage and collagen deposition, which may lead to cardiac scarring over time (45). Taken together, our model presents multiple characteristics of IPD, validating the use of our animal model to investigate disease mechanisms in the context of cigarette smoke.
Mechanistically, we found that cigarette smoke exposure predisposes to IPD in association with attenuated nasal expression of TNF-α and the neutrophil-recruiting chemokines CXCL-1 and CXCL-2. While neutrophils do not directly impact the nasal bacterial burden, they play a role in antigen priming of the adaptive immune response, which impacts later macrophage-mediated clearance of colonizing pneumococci (10, 11). Although the baseline proportion of nasal neutrophils was high in smoke-exposed mice, we did not see a significant increase in neutrophils in response to pneumococcal colonization compared to room air-exposed control mice. Hence, we postulate that the impaired ability to recruit additional neutrophils in response to S. pneumoniae may predispose to IPD in smoke-exposed mice. In support of this, the effect of cigarette smoke is similar to previous observations of neutrophil depletion, which had no immediate impact on the nasal bacterial burden but predisposed mice to IPD (11). Furthermore, we show that increased survival in nasally colonized mice following smoking cessation was associated with an increase in neutrophils. Cigarette smoke may thus predispose to IPD by impairing the neutrophilic response induced by nasal pneumococcal colonization.
S. pneumoniae has been shown to adhere to and enter host cells through the PAFR in an IL-1α-dependent manner (16). Both PAFR and IL-1α are increased with cigarette smoke exposure (17, 28) and may facilitate bacterial invasion. Here, we show that deficiency in PAFR or IL-1α did not rescue smoke-exposed mice from higher rates of IPD. This may be due to differential regulation of PAFR and IL-1α in the upper and lower respiratory tracts. Our findings confirm an increase in lung PAFR due to cigarette smoke exposure consistent with previous findings in both human smokers and our mouse model (17). While it is possible that PAFR contributes to pneumococcal infection and bacterial dissemination in the lungs of smokers, it may have little effect in the upper respiratory tract, since expression of both nasal PAFR and IL-1α is decreased by cigarette smoke exposure. Thus, although PAFR may potentially be involved in pneumococcal dissemination from the lungs following cigarette smoke exposure, and in heart microlesion formation and translocation across the blood-brain barrier (45, 46), it played little role in promoting IPD from nasal colonization.
There are approximately 90 pneumococcal serotypes, with the invasive potential and bacterial dissemination pattern highly dependent on both the serotype and the clonal type (47–49). Therefore, cigarette smoke exposure may affect IPD, depending on the virulence potential and organ-specific propensity of the nasally colonizing S. pneumoniae strain. The current study used a clinical blood isolate of serotype 6A (20, 21), which has been associated with a higher risk of acquiring meningitis (50). Since invasive infection had already progressed to the lungs, blood, and brain in the majority of cigarette smoke-exposed mice examined in our time course, we cannot rule out the possibility that we might have missed the very early phase of disease and that bacterial dissemination from the lungs did occur. However, the propensity of the S. pneumoniae strain we utilized and the observation that some mice acquire pneumococci only in the spleen or brain post-nasal pneumococcal colonization suggest there may be a predisposition toward bacteremia and meningitis, rather than pneumonia, in our model. Similarly, Basilico et al. recently reported that cigarette smoke exposure predisposed mice to ear infection following pulmonary delivery of a clinical isolate of S. pneumoniae from a patient with otitis media (51). Of note, occult pneumococcal bacteremia, the direct spread of S. pneumoniae from the nasopharynx into the blood, as well as direct S. pneumoniae translocation to the brain from the nasopharynx, is well documented (52, 53). Our data suggest that, in addition to pneumonia, smokers may be at increased risk of occult bacteremia, which warrants further attention.
Importantly, we found smoking cessation post-nasal colonization fully rescued mice from mortality. This observation likely explains why nasal colonization with a clinical isolate of the same serotype (6A) did not cause IPD or attenuate nasal inflammation in a recent study by Voss et al. (54), where mice did not continue to be exposed to smoke following colonization. These differences in experimental approach should be taken into consideration when investigating mechanisms of nasal pneumococcal colonization and IPD, especially given how quickly nasal CXCL-1 and CXCL-2 expression, as well as neutrophil recruitment, increased following smoking cessation in colonized mice. Our data suggest that the immunosuppressive effects of smoke at the nasopharynx are highly reversible. Epidemiological studies also support a reversible effect in that the odds ratio of acquiring IPD is lower in former smokers than in current smokers (6, 55). Our findings suggest that smoking cessation may be the most effective intervention strategy to reduce the incidence of IPD.
Our model demonstrates that cigarette smoke exposure predisposes to IPD by suppressing nasal inflammatory mediator expression. A similar effect of cigarette smoke was observed in humans, where nasal IL-6 expression was decreased in young healthy smokers following nasal inoculation with live attenuated influenza virus (56). In contrast, we have previously shown that alveolar macrophage responses to nontypeable Haemophilus influenzae (NTHi) in the lungs are skewed by cigarette smoke, suppressing the expression of some inflammatory mediators while augmenting that of others, including IL-1α, which contributed to excessive lung neutrophilia in response to bacterial infection (57). This could be attributed to differential regulation of inflammatory processes by cigarette smoke in the upper and lower respiratory tracts, as suggested by Huvenne et al. (58).
In conclusion, we present a novel animal model of cigarette smoke-associated IPD. Our data suggest that cigarette smoke exposure may increase the risk of not only pneumonia, but also occult pneumococcal bacteremia. Mechanistically, we propose that cigarette smoke attenuates a proinflammatory environment at the nasopharynx normally induced by S. pneumoniae, which hinders recruitment of crucial cellular effectors required for the prevention of IPD. Smoking cessation may be the most effective intervention strategy to reduce the incidence of IPD, emphasizing the need for more supportive smoking prevention and cessation programs. However, given the addictive nature and high prevalence of cigarette smoking, future studies are also warranted to understand and restore inflammatory processes at the nasal mucosa.
Supplementary Material
ACKNOWLEDGMENTS
We thank Joanna Kasinska, Rod Rhem, and Iris Wang for expert technical support and Marie Bailey for secretarial assistance.
Carla M. T. Bauer and Christopher S. Stevenson were employees of Hoffmann-La Roche at the time of the study.
Funding Statement
This work was supported by the Canadian Institutes of Health Research (CIHR) (MOP-64390 and MOP-87517) and Hoffmann-La Roche. Pamela Shen was supported by an Ontario Graduate Scholarship. Funders did not contribute to experimental design, data collection, data interpretation, or the decision to submit this study for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01504-15.
REFERENCES
- 1.Anonymous. 2007. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec 82:93–104. [PubMed] [Google Scholar]
- 2.Levine OS, O'Brien KL, Knoll M, Adegbola RA, Black S, Cherian T, Dagan R, Goldblatt D, Grange A, Greenwood B, Hennessy T, Klugman KP, Madhi SA, Mulholland K, Nohynek H, Santosham M, Saha SK, Scott JA, Sow S, Whitney CG, Cutts F. 2006. Pneumococcal vaccination in developing countries. Lancet 367:1880–1882. doi: 10.1016/S0140-6736(06)68703-5. [DOI] [PubMed] [Google Scholar]
- 3.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendley JO, Sande MA, Stewart PM, Gwaltney JM. 1975. Spread of Streptococcus pneumoniae in families. I. Carriage rates and distribution of types. J Infect Dis 132:55–61. [DOI] [PubMed] [Google Scholar]
- 5.Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 6.Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, Breiman RF. 2000. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 342:681–689. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Vidal C, Ardanuy C, Tubau F, Viasus D, Dorca J, Linares J, Gudiol F, Carratala J. 2010. Pneumococcal pneumonia presenting with septic shock: host- and pathogen-related factors and outcomes. Thorax 65:77–81. doi: 10.1136/thx.2009.123612. [DOI] [PubMed] [Google Scholar]
- 8.Grau I, Ardanuy C, Calatayud L, Schulze MH, Liñares J, Pallares R. 2014. Smoking and alcohol abuse are the most preventable risk factors for invasive pneumonia and other pneumococcal infections. Int J Infect Dis 25:59–64. doi: 10.1016/j.ijid.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 9.van Rossum AMC, Lysenko ES, Weiser JN. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun 73:7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthias KA, Roche AM, Standish AJ, Shchepetov M, Weiser JN. 2008. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J Immunol 180:6246–6254. doi: 10.4049/jimmunol.180.9.6246. [DOI] [PubMed] [Google Scholar]
- 12.Stämpfli MR, Anderson GP. 2009. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 13.Sherman CB. 1992. The health consequences of cigarette smoking. Pulmonary diseases. Med Clin North Am 76:355–375. [DOI] [PubMed] [Google Scholar]
- 14.Phipps JC, Aronoff DM, Curtis JL, Goel D, O'Brien E, Mancuso P. 2010. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infect Immun 78:1214–1220. doi: 10.1128/IAI.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piatti G, Gazzola T, Allegra L. 1997. Bacterial adherence in smokers and non-smokers. Pharmacol Res 36:481–484. doi: 10.1006/phrs.1997.0255. [DOI] [PubMed] [Google Scholar]
- 16.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 17.Grigg J, Walters H, Sohal SS, Wood-Baker R, Reid DW, Xu CB, Edvinsson L, Morissette MC, Stampfli MR, Kirwan M, Koh L, Suri R, Mushtaq N. 2012. Cigarette smoke and platelet-activating factor receptor dependent adhesion of Streptococcus pneumoniae to lower airway cells. Thorax 67:908–913. doi: 10.1136/thoraxjnl-2011-200835. [DOI] [PubMed] [Google Scholar]
- 18.Radin JN, Orihuela CJ, Murti G, Guglielmo C, Murray PJ, Tuomanen EI. 2005. β-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun 73:7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. 1998. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med 187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura S, Davis KM, Weiser JN. 2011. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 121:3657–3665. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JO, Weiser JN. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis 177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 22.Botelho FM, Gaschler GJ, Kianpour S, Zavitz CC, Trimble NJ, Nikota JK, Bauer CM, Stampfli MR. 2010. Innate immune processes are sufficient for driving cigarette smoke-induced inflammation in mice. Am J Respir Cell Mol Biol 42:394–403. doi: 10.1165/rcmb.2008-0301OC. [DOI] [PubMed] [Google Scholar]
- 23.Drannik AG, Pouladi MA, Robbins CS, Goncharova SI, Kianpour S, Stämpfli MR. 2004. Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 170:1164–1171. doi: 10.1164/rccm.200311-1521OC. [DOI] [PubMed] [Google Scholar]
- 24.Beisswenger C, Lysenko ES, Weiser JN. 2009. Early bacterial colonization induces Toll-like receptor-dependent transforming growth factor beta signaling in the epithelium. Infect Immun 77:2212–2220. doi: 10.1128/IAI.01224-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang JR, Mostov KE, Lamm ME, Nanno M, Shimida S, Ohwaki M, Tuomanen E. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102:827–837. doi: 10.1016/S0092-8674(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 26.Orihuela CJ, Mahdavi J, Thornton J, Mann B, Wooldridge KG, Abouseada N, Oldfield NJ, Self T, Ala'Aldeen DAA, Tuomanen EI. 2009. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest 119:1638–1646. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shukla SD, Sohal SS, Mahmood MQ, Reid D, Muller HK, Walters EH. 2014. Airway epithelial platelet-activating factor receptor expression is markedly upregulated in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 9:853–861. doi: 10.2147/COPD.S67044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botelho FM, Bauer CM, Finch D, Nikota JK, Zavitz CC, Kelly A, Lambert KN, Piper S, Foster ML, Goldring JJ, Wedzicha JA, Bassett J, Bramson J, Iwakura Y, Sleeman M, Kolbeck R, Coyle AJ, Humbles AA, Stampfli MR. 2011. IL-1alpha/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice. PLoS One 6:e28457. doi: 10.1371/journal.pone.0028457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardozo DM, Nascimento-Carvalho CM, Andrade A-LSS, Silvany-Neto AM, Daltro CHC, Brandão M-AS, Brandão AP, Brandileone M-CC. 2008. Prevalence and risk factors for nasopharyngeal carriage of Streptococcus pneumoniae among adolescents. J Med Microbiol 57:185–189. doi: 10.1099/jmm.0.47470-0. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg D, Givon-Lavi N, Broides A, Blancovich I, Peled N, Dagan R. 2006. The contribution of smoking and exposure to tobacco smoke to Streptococcus pneumoniae and Haemophilus influenzae carriage in children and their mothers. Clin Infect Dis 42:897–903. doi: 10.1086/500935. [DOI] [PubMed] [Google Scholar]
- 31.Lee C, Middaugh N, Howie S, Ezzati M. 2010. Association of secondhand smoke exposure with pediatric invasive bacterial disease and bacterial carriage: a systematic review and meta-analysis. PLoS Med 7:e1000374. doi: 10.1371/journal.pmed.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domino EF, Kadoya C, Matsuoka S, Ni L, Fedewa KS. 2003. Comparative American and Japanese tobacco smoke uptake parameters after overnight tobacco deprivation. Prog Neuropsychopharmacol Biol Psychiatry 27:973–984. doi: 10.1016/S0278-5846(03)00157-X. [DOI] [PubMed] [Google Scholar]
- 33.Wu HY, Virolainen A, Mathews B, King J, Russell MW, Briles DE. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb Pathog 23:127–137. doi: 10.1006/mpat.1997.0142. [DOI] [PubMed] [Google Scholar]
- 34.McCool TL, Weiser JN. 2004. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect Immun 72:5807–5813. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Southam DS, Dolovich M, O'Byrne PM, Inman MD. 2002. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol 282:L833–L839. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 36.Miller MA, Stabenow JM, Parvathareddy J, Wodowski AJ, Fabrizio TP, Bina XR, Zalduondo L, Bina JE. 2012. Visualization of murine intranasal dosing efficiency using luminescent Francisella tularensis: effect of instillation volume and form of anesthesia. PLoS One 7:e31359. doi: 10.1371/journal.pone.0031359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekdahl K, Ahlinder I, Hansson HB, Melander E, Mölstad S, Söderström M, Persson K. 1997. Duration of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae: experiences from the South Swedish Pneumococcal Intervention Project. Clin Infect Dis 25:1113–1117. doi: 10.1086/516103. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD. 2006. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am J Respir Crit Care Med 174:1003–1010. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felmet KA, Hall MW, Clark RSB, Jaffe R, Carcillo JA. 2005. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol 174:3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 40.Inoue S, Suzuki-Utsunomiya K, Okada Y, Taira T, Iida Y, Miura N, Tsuji T, Yamagiwa T, Morita S, Chiba T, Sato T, Inokuchi S. 2013. Reduction of immunocompetent T cells followed by prolonged lymphopenia in severe sepsis in the elderly. Crit Care Med 41:810–819. doi: 10.1097/CCM.0b013e318274645f. [DOI] [PubMed] [Google Scholar]
- 41.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. 2007. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care 11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gentile LF, Cuenca AG, Vanzant EL, Efron PA, McKinley B, Moore F, Moldawer LL. 2013. Is there value in plasma cytokine measurements in patients with severe trauma and sepsis? Methods 61:3–9. doi: 10.1016/j.ymeth.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrales-Medina VF, Suh KN, Rose G, Chirinos JA, Doucette S, Cameron DW, Fergusson DA. 2011. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med 8:e1001048. doi: 10.1371/journal.pmed.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thayer WS. 1931. Bacterial or infective endocarditis; Gibson lectures for 1930. Edinb Med J 38:237–265, 307–334. [PMC free article] [PubMed] [Google Scholar]
- 45.Brown AO, Mann B, Gao G, Hankins JS, Humann J, Giardina J, Faverio P, Restrepo MI, Halade GV, Mortensen EM, Lindsey ML, Hanes M, Happel KI, Nelson S, Bagby GJ, Lorent JA, Cardinal P. 2014. Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLoS Pathog 10:e1004383. doi: 10.1371/journal.ppat.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ring A, Weiser JN, Tuomanen EI. 1998. Pneumococcal trafficking across the blood-brain barrier: molecular analysis of a novel bidirectional pathway. J Clin Invest 102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, Donohoe K, Harris D, Murphy L, Quail MA, Samuel G, Skovsted IC, Kaltoft MS, Barrell B, Reeves PR, Parkhill J, Spratt BG. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet 2:e31. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sandgren A, Sjostrom K, Olsson-Liljequist B, Christensson B, Samuelsson A, Kronvall G, Henriques Normark B. 2004. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J Infect Dis 189:785–796. doi: 10.1086/381686. [DOI] [PubMed] [Google Scholar]
- 49.Orihuela CJ, Gao G, McGee M, Yu J, Francis KP, Tuomanen E. 2003. Organ-specific models of Streptococcus pneumoniae disease. Scand J Infect Dis 35:647–652. doi: 10.1080/00365540310015854. [DOI] [PubMed] [Google Scholar]
- 50.van Hoek AJ, Andrews N, Waight PA, George R, Miller E. 2012. Effect of serotype on focus and mortality of invasive pneumococcal disease: coverage of different vaccines and insight into non-vaccine serotypes. PLoS One 7:e39150. doi: 10.1371/journal.pone.0039150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basilico P, Cremona T, Oevermann A, Piersigilli A, Benarafa C. 2015. Cigarette smoke exposure increases myeloid cell production and lung bacterial clearance in mice. Am J Respir Cell Mol Biol 54:424–435. [DOI] [PubMed] [Google Scholar]
- 52.Joffe MD, Alpern ER. 2010. Occult pneumococcal bacteremia: a review. Pediatr Emerg Care 26:448–454. doi: 10.1097/PEC.0b013e3181e15e36. [DOI] [PubMed] [Google Scholar]
- 53.van Ginkel FW, McGhee JR, Watt JM, Campos-Torres A, Parish LA, Briles DE. 2003. Pneumococcal carriage results in ganglioside-mediated olfactory tissue infection. Proc Natl Acad Sci U S A 100:14363–14367. doi: 10.1073/pnas.2235844100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voss M, Wonnenberg B, Honecker A, Kamyschnikow A, Herr C, Bischoff M, Tschernig T, Bals R, Beisswenger C. 2015. Cigarette smoke-promoted acquisition of bacterial pathogens in the upper respiratory tract leads to enhanced inflammation in mice. Respir Res 16:41. doi: 10.1186/s12931-015-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Almirall J, González CA, Balanzó X, Bolíbar I. 1999. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest 116:375–379. doi: 10.1378/chest.116.2.375. [DOI] [PubMed] [Google Scholar]
- 56.Noah TL, Zhou H, Monaco J, Horvath K, Herbst M, Jaspers I. 2011. Tobacco smoke exposure and altered nasal responses to live attenuated influenza virus. Environ Health Perspect 119:78–83. doi: 10.1289/ehp.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikota JK, Shen P, Morissette MC, Fernandes K, Roos A, Chu DK, Barra NG, Iwakura Y, Kolbeck R, Humbles AA, Stampfli MR. 2014. Cigarette smoke primes the pulmonary environment to IL-1/CXCR-2-dependent nontypeable Haemophilus influenzae-exacerbated neutrophilia in mice. J Immunol 193:3134–3145. doi: 10.4049/jimmunol.1302412. [DOI] [PubMed] [Google Scholar]
- 58.Huvenne W, Pérez-Novo CA, Derycke L, De Ruyck N, Krysko O, Maes T, Pauwels N, Robays L, Bracke KR, Joos G, Brusselle G, Bachert C. 2010. Different regulation of cigarette smoke induced inflammation in upper versus lower airways. Respir Res 11:100. doi: 10.1186/1465-9921-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.