Abstract
The Yersinia high-pathogenicity island (HPI) is common to multiple virulence strategies used by Escherichia coli strains associated with urinary tract infection (UTI). Among the genes in this island are ybtP and ybtQ, encoding distinctive ATP binding cassette (ABC) proteins associated with iron(III)-yersiniabactin import in Yersinia pestis. In this study, we compared the impact of ybtPQ on a model E. coli cystitis strain during in vitro culture and experimental murine infections. A ybtPQ-null mutant exhibited no growth defect under standard culture conditions, consistent with nonessentiality in this background. A growth defect phenotype was observed and genetically complemented in vitro during iron(III)-yersiniabactin-dependent growth. Following inoculation into the bladders of C3H/HEN and C3H/HeOuJ mice, this strain exhibited a profound, 106-fold competitive infection defect in the subgroup of mice that progressed to high-titer bladder infections. These results identify a virulence role for YbtPQ in the highly inflammatory microenvironment characteristic of high-titer cystitis. The profound competitive defect may relate to the apparent selection of Yersinia HPI-positive E. coli in uncomplicated clinical UTIs.
INTRODUCTION
Urinary tract infection (UTI) is one of the most common bacterial infections, affecting nearly 9 million individuals every year in the United States (1). Uropathogenic Escherichia coli (UPEC) is responsible for over 80% of all community-acquired UTI cases and in some patients progresses from a localized bladder infection to an infection of the kidneys and bloodstream (2, 3). Over 50% of women acquire a UTI over their lifetimes, with 30% suffering from recurrent UTIs (4, 5). Uncomplicated UTIs appear to follow an ascending route of infection in which the bladder is exposed to a polymicrobial inoculum of intestinal bacteria that colonize the vaginal vestibule and urethra (6–10). This early colonization event may precede patients' visits to physicians by several days. Our understanding of the molecular mechanisms that UPEC uses to emerge from this early inoculum and dominate the urinary microbiome at the time of clinical UTI diagnosis remains incomplete.
When an uncomplicated UTI patient's rectal and urinary E. coli strains are compared, the urinary strain is more likely to carry the 30-kb Yersinia high-pathogenicity island (HPI) (11). The Yersinia HPI is nearly ubiquitous among model uropathogenic E. coli strains, is associated with fluoroquinolone resistance when present in combination with the aerobactin siderophore system, and is typically present in over 70% of clinical urinary isolates (3, 11, 12). The Yersinia HPI encodes the yersiniabactin (Ybt) siderophore system, including yersiniabactin biosynthetic enzymes, in addition to outer and inner membrane transporters associated with metal-yersiniabactin complex import (13–16). Yersiniabactin is one of three genetically nonconserved E. coli siderophore types (yersiniabactin, salmochelin, and aerobactin) that may be expressed in addition to enterobactin, the conserved E. coli siderophore (3, 11, 12). The Yersinia HPI is central to multiple E. coli urovirulence strategies, raising the possibility that it is functionally distinct from other siderophore systems (12). In uropathogenic E. coli, the yersiniabactin system has been appreciated to facilitate importer-independent copper and reactive oxygen species resistance (17, 18). Furthermore, in Yersinia pestis, the yersiniabactin system is linked to zinc import (19). E. coli virulence may be enhanced in strains that use multiple siderophore types, including yersiniabactin, to overcome the metal ion limitation characteristic of nutritional immunity (20, 21). These observations raise the question of whether importer-dependent functions associated with the Yersinia HPI, such as metal ion acquisition, are pathophysiologically important during a UTI (14, 16).
Seminal studies with Yersinia pestis identified several proteins that are specifically necessary for iron(III)-yersiniabactin-dependent growth (14, 15). Within the Yersinia HPI, the inner membrane transporter genes (ybtP and ybtQ) are present in an operon that is separate from the outer membrane transporter gene (denoted psn in Y. pestis and fyuA in other bacteria) operon. Both operons are regulated in part by the AraC-like transcription factor YbtA (see Fig. S1 in the supplemental material) (13, 14, 22, 23). Typical of most outer membrane siderophore transporters, FyuA uses the TonB complex to import its cognate iron(III)-siderophore [Fe(III)-yersiniabactin] complex into the periplasmic space (15, 16, 24). Unlike most siderophore-associated inner membrane transporters, ybtP and ybtQ are overlapping genes (overlapping by 40 nucleotides); they each encode half of an inner membrane ATP-binding cassette (ABC) transporter, and they each have unusual amino-terminal membrane-spanning domains and carboxy-terminal ATPases (14). It is unclear whether these unusual structural features correspond to atypical or complex functionality. While siderophore-associated ABC transporters are typically implicated in iron(III)-siderophore transport, some members of this family possess additional signaling roles (25). Metabolomic comparisons also raise the possibility that YbtP and YbtQ may exert functions distinct from iron(III)-yersiniabactin utilization in UPEC (26).
The overall expression of the yersiniabactin system can be inferred by evidence of yersiniabactin biosynthesis as well as serological and transcriptional evidence of ybtP, ybtQ, and fyuA expression during mouse and human cystitis (17, 27–29). Beyond UTIs, ybtPQ mutants of Yersinia pestis and Klebsiella pneumoniae exhibit diminished virulence in mouse models of bubonic plague and pneumonia, respectively (14, 30). ybtP and ybtQ homologues in Pseudomonas aeruginosa and Mycobacterium tuberculosis also affected virulence in a burned mouse model of sepsis and a mouse aerosol infection model (31, 32). The broad spectrum of pathogens associated with these distinctive bacterial proteins makes their associated functions an intriguing target for future virulence-associated therapies.
In this study, we sought to determine whether YbtP and YbtQ affect urinary tract infection pathogenesis. Using a combined bacteriologic and liquid chromatography-mass spectrometry approach, we found that YbtPQ is necessary for iron(III)-Ybt-dependent growth but not Ybt synthesis in a UPEC strain. We next compared isogenic wild-type and ybtPQ mutant strains in an experimental murine cystitis model using C3H/HeN and C3H/HeOuJ mice, which are known to develop distinctive high-titer, persistent infections with UPEC. A uropathogenic E. coli strain lacking ybtPQ exhibited a profound competitive fitness defect during high-titer infections but not under low-iron liquid culture conditions. These results are consistent with an important role for YbtP and YbtQ in inflamed bladder microenvironments during UTI pathogenesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Uropathogenic E. coli isolate UTI89, the genome of which encodes the enterobactin, salmochelin, and yersiniabactin siderophore systems, was used in this study (33, 34). Strains were grown in lysogeny broth (LB) agar (Becton, Dickson and Company) or M63 minimal medium supplemented with 0.2% (vol/vol) glycerol and 10 mg ml−1 niacin (Sigma) with antibiotics as appropriate (11). Ampicillin (100 μg ml−1; GoldBio) and kanamycin (50 μg ml−1; GoldBio) were used for strain selection. The UTI89 mutant strains used in this study are listed in Table 1. In-frame deletions in the UTI89 genome were made using the bacteriophage lambda Red recombinase method with pKD4 or pKD13 as the template (35). PCR with flanking primers was used to confirm the deletions. Antibiotic resistance insertions were removed by transforming strains with pCP20 expressing FLP recombinase. A YbtPQ-expressing plasmid was constructed with the pTrc99a vector (36) using standard PCR and recombination techniques.
TABLE 1.
UTI89 mutant strains used in this study
| Strain | Relevant properties | Reference or source |
|---|---|---|
| UTI89 ΔybtS | Salicylate synthase deletion, yersiniabactin biosynthesis mutant | 11 |
| UTI89 ΔybtPQ | ATP-binding cassette transporter deletion, yersiniabactin transport mutant | 26 |
| UTI89 ΔybtPQ::Kanr | Kanamycin resistance insert in place of the ATP-binding cassette transporter, yersiniabactin transport mutant | 26, this study |
Yersiniabactin and 13C-yersiniabactin preparation.
Apo-Ybt was generated from UTI89 ΔentB grown in M63 minimal medium supplemented with 0.2% (vol/vol) glycerol and 10 mg ml−1 niacin (Sigma) as previously described (17). 13C-Ybt was generated by growing UTI89 Δfur in medium supplemented 13C-labeled glycerol as previously described (17). Iron(III)-Ybt complexes were produced by adding iron(III) chloride (Sigma) to apo-Ybt. Iron(III)-Ybt was applied to a methanol-conditioned C18 silica column (Sigma) and eluted with 80% methanol as previously described (16). The eluates were lyophilized overnight. Dried samples were resuspended in deionized water and purified through high-performance liquid chromatography (HPLC) using a C18 silica column (Partisil; Whatman). Iron(III)-Ybt-containing fractions were collected, dried using a lyophilizer, and resuspended in deionized water. Stock iron(III)-Ybt quantification was carried out using the previously described extinction coefficient (16). Sample iron(III)-Ybt levels were quantified in multiple reaction monitoring mode using known collision-induced dissociation fragmentations and 13C-labeled Fe(III)-Ybt internal standards.
Fe(III)-Ybt-dependent growth.
After overnight growth in M63 minimal medium, strains were normalized for the starting optical density at 600 nm (OD600) in M63 minimal medium with 2 mM ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid) (EDDHA; Complete Green Company). HPLC-purified Fe(III)-Ybt (1 μM) was added to the strains, and the strains were grown for 18 h in 37°C with shaking, as previously described (16). Bacterial growth was measured using OD600 readings.
Mouse infections.
The bacteria used for infection were prepared as previously described (37). Six- to 7-week-old female C3H/HeN mice (Harlan) and C3H/HeOuJ mice (The Jackson Laboratory) were transurethrally infected with a 50-μl suspension containing 1 × 107 CFU of UTI89 or UTI89 ΔybtPQ in phosphate-buffered saline (PBS) while they were under 4% isoflurane anesthesia (37, 38). All animal experiments received prior review and approval of the Animal Studies Committee at Washington University School of Medicine. For competitive infections, the mice were infected with a 50-μl combined suspension containing 5 × 106 CFU each of UTI89 and UTI89 ΔybtPQ::Kanr in PBS while they were under 4% isoflurane anesthesia. At the time points indicated below, they mice were sacrificed by cervical dislocation while they were under isoflurane anesthesia, and their bladders and kidneys were aseptically removed. The organs were homogenized in PBS and were then serially diluted, and a total of 50 μl of each dilution was spotted onto LB agar plates and LB agar plates with 50 μg/ml kanamycin where appropriate. Competitive indices (CIs) were calculated as follows by using UTI89 as the reference strain: CI = (UTI89CFU out/UTI89CFU in)/(UTI89 ΔybtPQ::KanrCFU out/UTI89 ΔybtPQ::KanrCFU in), where UTI89CFU out is the number of CFU of UTI89 recovered, UTI89CFU in is the number of CFU of UTI89 inoculated, UTI89 ΔybtPQ::KanrCFU out is the number of CFU of UTI89 ΔybtPQ::Kanr recovered, and UTI89 ΔybtPQ::KanrCFU in is the number of CFU of UTI89 ΔybtPQ::Kanr inoculated (39).
Bladder IBCs.
Bladders were aseptically removed at 6 h postinfection to count the number of intracellular bacterial communities (IBCs). Harvested bladders were bisected, splayed on silicone plates, and fixed in 2% paraformaldehyde. IBCs were quantified by LacZ staining of whole bladders as previously described (40).
In vitro cogrowth experiments.
The bacteria used for cogrowth experiments were prepared in the same way that the bacteria were prepared for infection of mouse described above. Five microliters of a combined suspension containing 5 × 106 CFU each of UTI89 and UTI89 ΔybtPQ::Kanr in PBS was inoculated into 5 ml M63 minimal medium, and the bacteria were grown at 37°C statically or with shaking, as indicated below. At the time points indicated below, the cultures were removed and serially diluted, and a total of 50 μl of each dilution was spotted onto LB agar plates and LB agar plates with 50 μg/ml kanamycin where appropriate.
Statistical analysis.
Student's t test was used to compare yersiniabactin biosynthesis levels between pairs of strains. A two-tailed Mann-Whitney U test was used to compare bladder and kidney colonization levels and IBC counts between UTI89 and UTI89 ΔybtPQ. The Wilcoxon signed-rank test was used to compare the log of the competitive indices to a theoretical median of 0. Statistics and graphs were generated using GraphPad Prism (version 5) software (GraphPad Software).
RESULTS
ybtPQ deletion does not affect growth under iron(III)-Ybt-independent conditions.
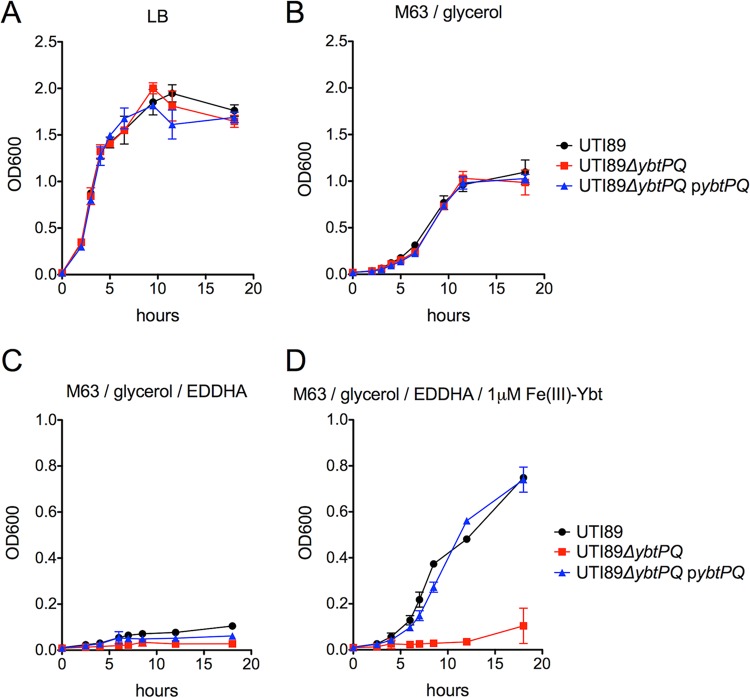
To investigate the role of YbtPQ in uropathogenic E. coli growth, we first measured the growth of the model UPEC strain UTI89 in nutrient-rich LB medium as well as M63 minimal medium. The growth of UTI89 ΔybtPQ was indistinguishable from that of wild-type strain UTI89 in both media (Fig. 1A and B). Addition of the ferric ion chelator EDDHA (2 mM), which sequesters Fe(III) and renders it poorly accessible to siderophores, to M63 minimal medium prior to inoculation diminished the growth of both strains to a similar degree. Addition of 1 μM purified Fe(III)-Ybt reversed the EDDHA-mediated inhibition of wild-type strain UTI89 but not that of UTI89 ΔybtPQ. Genetic complementation of UTI89 ΔybtPQ with ybtPQ expressed on a plasmid restored growth to wild-type levels under this EDDHA/Fe(III)-Ybt condition (Fig. 1C and D). Insertion of an empty vector did not restore UTI89 ΔybtPQ growth to wild-type levels under this EDDHA/Fe(III)-Ybt condition (data not shown). These results are consistent with ybtP and ybtQ being nonessential genes that permit Fe(III)-Ybt to be used as an iron source by UPEC.
FIG 1.
YbtPQ is required for Fe(III)-Ybt-dependent growth in UPEC. (A and B) The growth of UTI89, UTI89 ΔybtPQ, and UTI89 ΔybtPQ/pybtPQ was indistinguishable in nutrient-rich LB medium (A) and nutrient-limiting M63 minimal medium supplemented with 0.2% (vol/vol) glycerol and 10 mg ml−1 niacin (B). (C) In M63 minimal medium chelated with 2 mM EDDHA, all strains show decreased growth. (D) This growth defect was rescued for UTI89 and UTI89 ΔybtPQ/pybtPQ upon addition of exogenous 1 μM Fe(III)-Ybt to the chelated medium. Results are shown as means ± SDs (n = 3).
ybtPQ is not required for yersiniabactin synthesis in UPEC.
ybtPQ shares an operon with the yersiniabactin biosynthetic gene ybtS (see Fig. S1 in the supplemental material). To determine whether ybtPQ deletion exerts pleiotropic effects upon Ybt synthesis in UPEC, we compared the levels of Ybt secretion between wild-type strain UTI89 and strains UTI89 ΔybtPQ::Kanr (Fig. 2) and UTI89 ΔybtPQ (see Fig. S2 in the supplemental material) in M63 minimal medium. Yersiniabactin production by UTI89 ΔybtS was undetectable, while that by UTI89 ΔybtPQ::Kanr was comparable to that by wild-type strain UTI89. UTI89 ΔybtPQ::Kanr complemented with ybtPQ expressed on a plasmid showed a small but significant increase in Ybt synthesis compared to that of UTI89. UTI89 ΔybtPQ also showed no difference in Ybt biosynthesis compared to that of wild-type strain UTI89. These results show that yersiniabactin biosynthesis is preserved in UTI89 ybtPQ deletion mutants, similar to previous results seen in studies with Y. pestis and Yersinia enterocolitica (14, 23). These results also support a function for ybtPQ in UPEC that is distinct from yersiniabactin biosynthesis.
FIG 2.
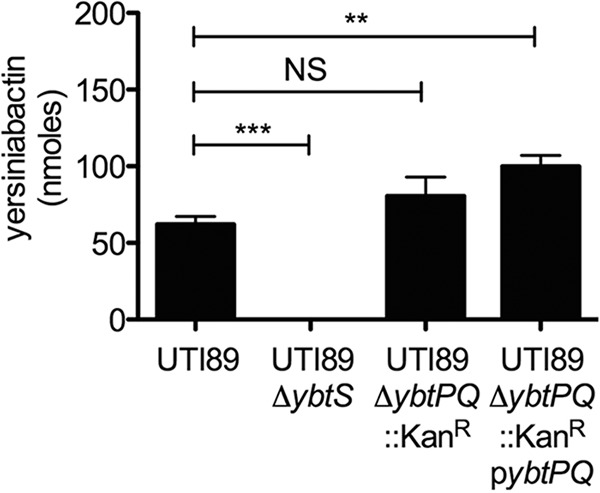
YbtPQ is not required for Ybt synthesis in UPEC. Yersiniabactin production was determined from the culture supernatants of UTI89 strains using quantitative liquid chromatography-tandem mass spectrometry methods. While salicylate synthesis-deficient strain UTI89 ΔybtS showed a decreased level of Ybt production, the level of Ybt production by strain UTI89 ΔybtPQ::Kanr showed no difference from that by the wild type. UTI89 ΔybtPQ::Kanr/pybtPQ showed an increased level of Ybt synthesis compared to that of the wild type. Results are shown as means ± SDs (n = 3). **, P < 0.005; ***, P < 0.0005; NS, not significant.
Individual infections by the wild type and ybtPQ mutants in C3H/HeN mice.
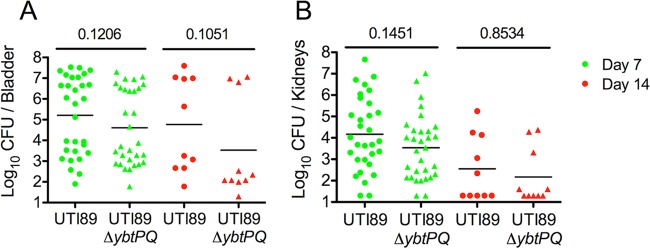
We hypothesized that the loss of ybtPQ would affect in vivo fitness during urinary tract infection. We tested this in C3H/HeN mice (homozygous for the retinal degeneration allele Pde6brd1), where a 107-CFU E. coli transurethral inoculum causes an infection involving epithelial cell invasion followed by a bifurcation to high and low numbers of CFU that are evident as soon as 3 days postinfection (dpi) (38). By 4 weeks postinfection, the mice with high numbers of CFU exhibit high-titer (>104 CFU per organ) bladder colonization that is characterized by urothelial hyperplasia and high levels of inflammation (37, 38, 41). We first conducted single-strain inoculations with UTI89 or UTI89 ΔybtPQ. The bladder and kidneys were harvested at 7 and 14 days postinfection, and the numbers of CFU of both strains were determined (Fig. 3). As was observed previously (38), mice infected with wild-type strain UTI89 exhibited a bimodal distribution of the numbers of CFU in the bladder at 7 and 14 dpi, with one group exhibiting greater than 105 CFU per bladder and the other group exhibiting less than 104 CFU per bladder. Mice infected with UTI89 ΔybtPQ showed no statistically significant differences in the numbers of CFU in either the bladder or kidneys compared with those in the bladder or kidneys of mice infected with wild-type strain UTI89 (Fig. 3A and B). These results indicate that YbtPQ is not required for the development of acute and persistent high-titer colonization in infections with a single strain.
FIG 3.
In vivo single-strain infections in a C3H/HeN mouse background. Bacterial titers at 7 days and 14 days following inoculation with 107 CFU UTI89 or UTI89 ΔybtPQ in the bladders (A) and kidneys (B) from C3H/HeN mice are shown. Statistical significance was assessed using the two-tailed Mann-Whitney U test. The numbers above the bars indicate the mean values.
Bladder IBC assessment.
To further assess the role of YbtPQ in acute cystitis, we quantified the formation of bladder intracellular bacterial communities (IBCs). IBCs are biofilm-like aggregations of UPEC that have invaded and subsequently replicated in the cytoplasm of bladder superficial umbrella cells (42, 43). During acute bladder infection, IBCs have been observed at up to 24 h postinoculation (27, 43). C3H/HeN mice were infected with 107 CFU of UTI89 ΔybtPQ or wild-type strain UTI89. The bladders were harvested at 6 h postinfection, and IBCs were quantified by LacZ staining (see Fig. S3 in the supplemental material). IBCs were observed during UTI89 ΔybtPQ infections, and the number of IBCs per bladder was not significantly different from the number observed during wild-type strain UTI89 infections. These results indicate that YbtPQ is not required for IBC development.
Competitive infections and in vitro cogrowth with the wild type and the ybtPQ mutant.
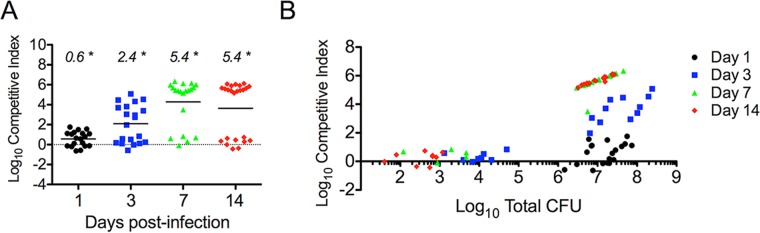
Previous observations suggest that uncomplicated UTIs originate with the polymicrobial inoculation of the urinary tract and that patient rectal E. coli strains with the Yersinia high-pathogenicity island are preferentially recovered from patient urine once the infections become symptomatic (10, 11). To determine if the loss of ybtPQ function would affect fitness during mixed inoculation with a wild-type strain with an intact ybtPQ, we measured the levels of wild-type strain UTI89 and UTI89 ΔybtPQ::Kanr in competitive infections. We infected C3H/HeN mice with a total inoculum of 107 CFU consisting of a 1:1 ratio of the wild type and mutant UTI89. In both the bladder and kidneys, we saw a significant (P < 0.05) increase in mean competitive index (CI) values over time, demonstrating that wild-type strain UTI89 outcompetes the mutant strain (Fig. 4A and B). By 3 days postinfection, a markedly bimodal CI distribution became apparent in the bladders, with one group demonstrating CIs of greater than 104 (a 10,000-fold increase in wild-type selection) and the other demonstrating CIs of less than 101 (a 10-fold increase in wild-type selection). This separation was widened by 7 and 14 days postinfection. A similar progression toward a bimodal CI distribution was observed in the kidneys. Neither a high CI nor a bimodal distribution was observed in a competitive inoculation of wild-type strain UTI89 and UTI89 ΔybtPQ::Kanr into M63 minimal medium under static or shaking conditions at 37°C (Fig. 4C and D). UTI89 ΔybtPQ::Kanr showed only a small competitive defect against the wild-type strain under both static and shaking conditions near 7 days postinoculation. Together, these results suggest that UPEC strains lacking YbtPQ are at a marked competitive disadvantage in the distinctive host environment of the mouse cystitis model.
FIG 4.
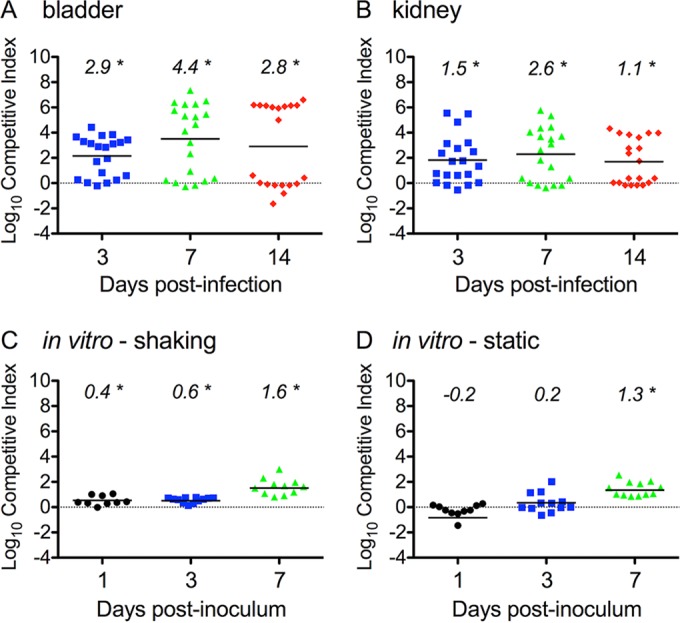
In vivo competitive infection in C3H/HeN mice and in vitro coculture growth. UTI89 showed a significant competitive advantage over UTI89 ΔybtPQ::Kanr during in vivo competitive infections compared to that seen during coculture growth in vitro. UTI89 and UTI89 ΔybtPQ::Kanr were mixed 1:1 to a total of 107 CFU prior to coinfection and coculture. (A and B) Competitive infections were performed in C3H/HeN mice, and bacterial titers in the bladders (A) and kidneys (B) were determined. (C and D) UTI89 strains were cocultured in M63 minimal medium under shaking (C) and static (D) conditions. Each symbol represents a datum from an individual mouse or culture. Statistical significance was assessed using the Wilcoxon signed-rank test, with a log10 CI of 0 (dotted line) being used as the theoretical median. Numbers at the top indicate median values. *, P < 0.05. The bars indicate mean values.
The unusual bimodal CI distribution observed here resembles the bimodal distribution of the numbers of CFU in the bladder seen in this C3H/HeN mouse model of cystitis (38) (Fig. 3). To determine whether the CI distribution is related to infection severity, we compared the CI values with the total number of CFU (Fig. 5A and B). The group with high CIs corresponded to mice with high titers in the bladder, while the group with low CIs corresponded to mice with low titers in the bladder. The CI values within the high-titer group progressively increased over the course of 3-, 7-, and 14-day infections until they were near the maximum resolvable CI limit (on the basis of the numbers of CFU). A similar relationship was observed in the kidneys, although the numbers of CFU were more evenly distributed and CI values appeared to approach the maximum resolvable limit for all values of the numbers of CFU. Together, these results suggest that the persistent high-titer but not low-titer bladder infection environment strongly favors UPEC strains with an intact yersiniabactin import system. This may relate to the apparent selection of E. coli strains with the Yersinia HPI in UTI patients (11).
FIG 5.
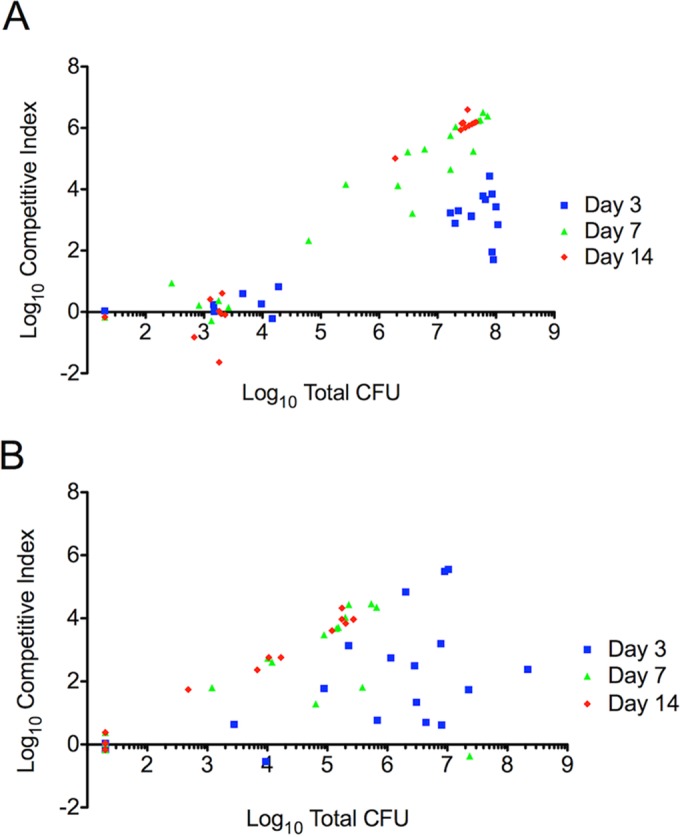
In vivo competitive infections in C3H/HeN mice show a correlation with bacterial levels. Presentation of the competitive index values against the total bacterial titers from the bladders (A) and kidneys (B) of C3H/HeN mice revealed that mice with high competitive indices had high bacterial titers in each organ, while mice with low competitive indices had low bacterial burdens in each organ.
Competitive infections by the wild type and ybtPQ mutants in C3H/HeOuJ mice.
To determine whether the loss of YbtPQ has similar effects during infection in other mouse backgrounds, we competitively inoculated C3H/HeOuJ mice (homozygous for the retinal degeneration allele Pde6brd1) with wild-type strain UTI89 and UTI89 ΔybtPQ::Kanr as described above for C3H/HeN mice. C3H/HeOuJ mice have previously been shown to be highly susceptible to persistent bladder and kidney infections caused by UPEC (38, 44). Similar to the results seen in C3H/HeN mice, we saw a significant increase (P < 0.05) in mean CI values over time in both the bladder (Fig. 6A) and the kidneys (see Fig. S4A in the supplemental material). When we compared CI values with the total number of CFU, we again observed that the group with high CIs corresponded to mice with high titers and the group with low CIs corresponded to mice with low titers in both the bladder (Fig. 6B) and the kidneys (see Fig. S4B), similar to the findings for C3H/HeN mice.
FIG 6.
In vivo competitive infection with C3H/HeOuJ mice. UTI89 showed a significant competitive advantage over UTI89 ΔybtPQ::Kanr during in vivo competitive infections in C3H/HeOuJ mice. UTI89 and UTI89 ΔybtPQ::Kanr were mixed 1:1 to total of 107 CFU prior to coinfection. (A) Competitive indices were calculated from the bacterial titers collected from the bladders. (B) Competitive index values against total bacterial titers from the bladders are presented. Each symbol represents a datum from an individual mouse. Statistical significance was assessed using the Wilcoxon signed-rank test, with a log10 CI of 0 (dotted line) being the theoretical median. Numbers at the top indicate median values. *, P < 0.05. The bars indicate mean values.
We also conducted single-strain inoculations with UTI89 or UTI89 ΔybtPQ. Similar to the results seen in C3H/HeN mice, mice infected with wild-type strain UTI89 exhibited a bimodal distribution of the numbers of CFU detected in the bladder. In mice infected with UTI89 ΔybtPQ, by 14 days postinfection there was a significant decrease in the mean number of CFU per bladder compared to the number seen in mice infected with wild-type strain UTI89 (see Fig. S5A in the supplemental material). Even though significantly lower numbers of CFU were observed in the kidneys at 3 days postinfection with UTI89 ΔybtPQ, there were no statistically significant differences in the numbers of CFU per kidney at later time points (see Fig. S5B in the supplemental material). These results suggest that UPEC strains with an intact yersiniabactin import system have greater fitness during persistent high-titer bladder infections.
DISCUSSION
In this study, we show that the UPEC genome-encoded ATP-binding cassette transporters YbtP and YbtQ promote bacterial fitness during urinary tract infections. YbtPQ most profoundly affected fitness in the highly inflammatory infection environment of high-titer mouse cystitis. While wild-type UPEC exhibited up to a 106-fold greater survival than the coinoculated ybtPQ deletion mutants in murine infections, no such competitive defect was noted during in vitro cultures. This report is the first to describe a role for YbtPQ during E. coli urinary tract infection, in which ybtPQ causes a defect that is among the strongest competitive defects reported to be caused by a nonconserved, nonessential gene during experimental cystitis. It is notable that this phenotype is evident in strain UTI89, where in vitro iron uptake by yersiniabactin is redundant with that of the enterobactin and salmochelin siderophore systems. Together, these data suggest that YbtPQ contributes distinctive functions that facilitate the urovirulence of E. coli carrying the Yersinia high-pathogenicity island.
Previous work has found that in patients with intestinal colonization by multiple E. coli strains, strains possessing the Yersinia HPI are preferentially isolated from their urine at the time of UTI diagnosis (11). Here, the profound competitive disadvantage for YbtPQ-null UPEC strains during high-titer infections, but not in vitro culture suggests a similar advantage for Yersinia HPI-expressing E. coli strains in the polymicrobial inoculum preceding uncomplicated UTIs in humans. Features of the high-titer infection state in C3H/HeN and C3H/HeOuJ mice suggest aspects of the host response that may select against YbtPQ-null UPEC. In these infections, up to 20% of bladder UPEC isolates are intracellular and exist within urothelial cells, macrophages, and neutrophils. The high-titer infection state is also characterized by a robust inflammatory response, which was shown to be required for the development of chronic cystitis (38, 45). If these aspects of the host response do select against YbtPQ-null UPEC, a virulence defect during single-strain infections may be more difficult to resolve if the mutant strain elicits an inflammatory response less vigorous than that which is present during a mixed infection with wild-type UPEC. If so, a potentially greater inflammatory response during a mixed infection may favor wild-type UPEC survival. These inflammatory responses may resemble those present during the 2 to 3 days of the preclinical UTI that predate the patient's decision to seek treatment for UTI symptoms (5). The current study thus raises the possibility that the yersiniabactin import pathway equips UPEC to better overcome barriers to growth imposed by inflammatory microenvironments in the infected host.
With the notable exception of Y. pestis YbtP and Salmonella enterica serovar Typhimurium IroC (14, 46), experimental infection studies have tended to focus upon the TonB-dependent outer membrane siderophore transporters, rather than siderophore-associated inner membrane ABC protein components. A recent study using a fyuA-deficient UPEC strain found that it had a competitive defect in the bladder weaker than that observed for the ybtPQ-deficient UPEC strain in the present study. In contrast, the defect in infections with a single strain was somewhat more pronounced for fyuA mutants than ybtPQ mutants (29, 47–50). A previous study using a tonB-deficient UPEC strain yielded results similar to those obtained with the fyuA mutant (51). The notable differences between fyuA and ybtPQ mutants are interesting and may derive from different strain backgrounds or distinctive features of the C3H background mice used in this study. It is possible that the robust inflammation in C3H mice increases UPEC outer membrane permeability (through antimicrobial peptides), making outer membrane transporters relatively less critical for metal-yersiniabactin import (52–54). Lastly, the marked competitive advantage observed with YbtPQ may derive from additional virulence functions that are not directly related to yersiniabactin import. For example, Lv and Henderson have shown that a UTI89 ybtP mutant exhibits a significant change in cellular cysteine levels compared to those found in an isogenic fyuA mutant (26). More direct experimental functional comparisons of outer and inner membrane siderophore import proteins will be necessary to understand how functionally distinct these proteins may be.
Structurally diverse ABC cassette transporters such as YbtPQ have been the subject of extensive pharmacologic study in both eukaryotes and prokaryotes, making this an appealing class of targets for new anti-infectives (55, 56). Structural differences in ABC transporters between humans and bacteria may enable antibiotics with specificity to be identified. Numerous functions of the bacterial ATP-binding cassette transporter superfamily have been described in both Gram-positive and Gram-negative pathogens (25, 55–57). Further investigation of the mechanisms of action of ABC transporters will provide insight into their role during pathogenesis as well as allow the development of countermeasures targeting these transporters.
Supplementary Material
ACKNOWLEDGMENTS
We thank Scott Hultgren for reagents and helpful discussion and Jennifer Walker and Samara Levine for technical assistance.
We have no conflicts of interest to declare.
J.P.H. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund and acknowledges the grants from the National Institute of Diabetes and Digestive and Kidney Diseases.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01211-15.
REFERENCES
- 1.Foxman B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 113(Suppl 1A):5S–13S. [DOI] [PubMed] [Google Scholar]
- 2.Foxman B. 2010. The epidemiology of urinary tract infection. Nat Rev 7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 3.Marschall J, Zhang L, Foxman B, Warren DK, Henderson JP. 2012. Both host and pathogen factors predispose to Escherichia coli urinary-source bacteremia in hospitalized patients. Clin Infect Dis 54:1692–1698. doi: 10.1093/cid/cis252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foxman B, Gillespie B, Koopman J, Zhang L, Palin K, Tallman P, Marsh JV, Spear S, Sobel JD, Marty MJ, Marrs CF. 2000. Risk factors for second urinary tract infection among college women. Am J Epidemiol 151:1194–1205. doi: 10.1093/oxfordjournals.aje.a010170. [DOI] [PubMed] [Google Scholar]
- 5.Czaja CA, Stamm WE, Stapleton AE, Roberts PL, Hawn TR, Scholes D, Samadpour M, Hultgren SJ, Hooton TM. 2009. Prospective cohort study of microbial and inflammatory events immediately preceding Escherichia coli recurrent urinary tract infection in women. J Infect Dis 200:528–536. doi: 10.1086/600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamey TA, Timothy M, Millar M, Mihara G. 1971. Recurrent urinary infections in adult women. The role of introital enterobacteria. Calif Med 115:1–19. [PMC free article] [PubMed] [Google Scholar]
- 7.Stamey TA. 1973. The role of introital enterobacteria in recurrent urinary infections. J Urol 109:467–472. [DOI] [PubMed] [Google Scholar]
- 8.Stamey TA, Sexton CC. 1975. The role of vaginal colonization with Enterobacteriaceae in recurrent urinary infections. J Urol 113:214–217. [DOI] [PubMed] [Google Scholar]
- 9.Marsh FP, Murray M, Panchamia P. 1972. The relationship between bacterial cultures of the vaginal introitus and urinary infection. Br J Urol 44:368–375. doi: 10.1111/j.1464-410X.1972.tb10093.x. [DOI] [PubMed] [Google Scholar]
- 10.Buckley RM, McGuckin M, MacGregor RR. 1978. Urine bacterial counts after sexual intercourse. N Engl J Med 298:321–324. doi: 10.1056/NEJM197802092980607. [DOI] [PubMed] [Google Scholar]
- 11.Henderson JP, Crowley JR, Pinkner JS, Walker JN, Tsukayama P, Stamm WE, Hooton TM, Hultgren SJ. 2009. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog 5:e1000305. doi: 10.1371/journal.ppat.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker KS, Wilson JD, Marschall J, Mucha PJ, Henderson JP. 2015. Network analysis reveals sex- and antibiotic resistance-associated antivirulence targets in clinical uropathogens. ACS Infect Dis 1:523–532. doi: 10.1021/acsinfecdis.5b00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fetherston JD, Bearden SW, Perry RD. 1996. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol Microbiol 22:315–325. doi: 10.1046/j.1365-2958.1996.00118.x. [DOI] [PubMed] [Google Scholar]
- 14.Fetherston JD, Bertolino VJ, Perry RD. 1999. YbtP and YbtQ: two ABC transporters required for iron uptake in Yersinia pestis. Mol Microbiol 32:289–299. doi: 10.1046/j.1365-2958.1999.01348.x. [DOI] [PubMed] [Google Scholar]
- 15.Perry RD, Fetherston JD. 2011. Yersiniabactin iron uptake: mechanisms and role in Yersinia pestis pathogenesis. Microbes Infect Inst Pasteur 13:808–817. doi: 10.1016/j.micinf.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh E-I, Hung CS, Parker KS, Crowley JR, Giblin DE, Henderson JP. 2015. Metal selectivity by the virulence-associated yersiniabactin metallophore system. Metallomics 7:1011–1022. doi: 10.1039/C4MT00341A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE, Henderson JP. 2012. The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol 8:731–736. doi: 10.1038/nchembio.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi KS, Hung CS, Giblin DE, Urushidani S, Austin AM, Dinauer MC, Henderson JP. 2014. Cupric yersiniabactin is a virulence-associated superoxide dismutase mimic. ACS Chem Biol 9:551–561. doi: 10.1021/cb400658k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobrov AG, Kirillina O, Fetherston JD, Miller MC, Burlison JA, Perry RD. 2014. The Yersinia pestis siderophore, yersiniabactin, and the ZnuABC system both contribute to zinc acquisition and the development of lethal septicaemic plague in mice. Mol Microbiol 93:759–775. doi: 10.1111/mmi.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hood MI, Skaar EP. 2012. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subashchandrabose S, Hazen TH, Brumbaugh AR, Himpsl SD, Smith SN, Ernst RD, Rasko DA, Mobley HL. 2014. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A 111:18327–18332. doi: 10.1073/pnas.1415959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry RD, Balbo PB, Jones HA, Fetherston JD, DeMoll E. 1999. Yersiniabactin from Yersinia pestis: biochemical characterization of the siderophore and its role in iron transport and regulation. Microbiology 145(Pt 5):1181–1190. doi: 10.1099/13500872-145-5-1181. [DOI] [PubMed] [Google Scholar]
- 23.Brem D, Pelludat C, Rakin A, Jacobi CA, Heesemann J. 2001. Functional analysis of yersiniabactin transport genes of Yersinia enterocolitica. Microbiology 147:1115–1127. doi: 10.1099/00221287-147-5-1115. [DOI] [PubMed] [Google Scholar]
- 24.Lukacik P, Barnard TJ, Keller PW, Chaturvedi KS, Seddiki N, Fairman JW, Noinaj N, Kirby TL, Henderson JP, Steven AC, Hinnebusch BJ, Buchanan SK. 2012. Structural engineering of a phage lysin that targets Gram-negative pathogens. Proc Natl Acad Sci U S A 109:9857–9862. doi: 10.1073/pnas.1203472109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Z, Jacobsen FE, Giedroc DP. 2009. Metal transporters and metal sensors: how coordination chemistry controls bacterial metal homeostasis. Chem Rev 109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv H, Henderson JP. 2011. Yersinia high pathogenicity island genes modify the Escherichia coli primary metabolome independently of siderophore production. J Proteome Res 10:5547–5554. doi: 10.1021/pr200756n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reigstad CS, Hultgren SJ, Gordon JI. 2007. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem 282:21259–21267. doi: 10.1074/jbc.M611502200. [DOI] [PubMed] [Google Scholar]
- 28.Hagan EC, Lloyd AL, Rasko DA, Faerber GJ, Mobley HLT. 2010. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog 6:e1001187. doi: 10.1371/journal.ppat.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brumbaugh AR, Smith SN, Subashchandrabose S, Himpsl SD, Hazen TH, Rasko DA, Mobley HLT. 2015. Blocking yersiniabactin import attenuates extraintestinal pathogenic Escherichia coli in cystitis and pyelonephritis and represents a novel target to prevent urinary tract infection. Infect Immun 83:1443–1450. doi: 10.1128/IAI.02904-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawlor MS, Hsu J, Rick PD, Miller VL. 2005. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol Microbiol 58:1054–1073. doi: 10.1111/j.1365-2958.2005.04918.x. [DOI] [PubMed] [Google Scholar]
- 31.Choi JY, Sifri CD, Goumnerov BC, Rahme LG, Ausubel FM, Calderwood SB. 2002. Identification of virulence genes in a pathogenic strain of Pseudomonas aeruginosa by representational difference analysis. J Bacteriol 184:952–961. doi: 10.1128/jb.184.4.952-961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez GM, Smith I. 2006. Identification of an ABC transporter required for iron acquisition and virulence in Mycobacterium tuberculosis. J Bacteriol 188:424–430. doi: 10.1128/JB.188.2.424-430.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen SL, Hung CS, Xu J, Reigstad CS, Magrini V, Sabo A, Blasiar D, Bieri T, Meyer RR, Ozersky P, Armstrong JR, Fulton RS, Latreille JP, Spieth J, Hooton TM, Mardis ER, Hultgren SJ, Gordon JI. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc Natl Acad Sci U S A 103:5977–5982. doi: 10.1073/pnas.0600938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulvey MA, Schilling JD, Hultgren SJ. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amann E, Ochs B, Abel KJ. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 37.Hung C-S, Dodson KW, Hultgren SJ. 2009. A murine model of urinary tract infection. Nat Protoc 4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. 2010. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog 6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freter R, Allweiss B, O'Brien PC, Halstead SA, Macsai MS. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun 34:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Justice SS, Lauer SR, Hultgren SJ, Hunstad DA. 2006. Maturation of intracellular Escherichia coli communities requires SurA. Infect Immun 74:4793–4800. doi: 10.1128/IAI.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. 2012. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 43.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. 2004. Differentiation and development pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A 101:1333–1338. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hopkins WJ, Gendron-Fitzpatrick A, Balish E, Uehling DT. 1998. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect Immun 66:2798–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz DJ, Chen SL, Hultgren SJ, Seed PC. 2011. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun 79:4250–4259. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crouch M-LV, Castor M, Karlinsey JE, Kalhorn T, Fang FC. 2008. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol Microbiol 67:971–983. doi: 10.1111/j.1365-2958.2007.06089.x. [DOI] [PubMed] [Google Scholar]
- 47.Hancock V, Ferrieres L, Klemm P. 2008. The ferric yersiniabactin uptake receptor FyuA is required for efficient biofilm formation by urinary tract infectious Escherichia coli in human urine. Microbiology 154:167–175. doi: 10.1099/mic.0.2007/011981-0. [DOI] [PubMed] [Google Scholar]
- 48.Brumbaugh AR, Smith SN, Mobley HLT. 2013. Immunization with the yersiniabactin receptor, FyuA, protects against pyelonephritis in a murine model of urinary tract infection. Infect Immun 81:3309–3316. doi: 10.1128/IAI.00470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia EC, Brumbaugh AR, Mobley HLT. 2011. Redundancy and specificity of Escherichia coli iron acquisition systems during urinary tract infection. Infect Immun 79:1225–1235. doi: 10.1128/IAI.01222-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spurbeck RR, Dinh PC, Walk ST, Stapleton AE, Hooton TM, Nolan LK, Kim KS, Johnson JR, Mobley HLT. 2012. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect Immun 80:4115–4122. doi: 10.1128/IAI.00752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres AG, Redford P, Welch RA, Payne SM. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect Immun 69:6179–6185. doi: 10.1128/IAI.69.10.6179-6185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canny G, Levy O, Furuta GT, Narravula-Alipati S, Sisson RB, Serhan CN, Colgan SP. 2002. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proc Natl Acad Sci U S A 99:3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elsbach P, Weiss J. 1998. Role of the bactericidal/permeability-increasing protein in host defence. Curr Opin Immunol 10:45–49. doi: 10.1016/S0952-7915(98)80030-7. [DOI] [PubMed] [Google Scholar]
- 54.Kim B, Richards SM, Gunn JS, Slauch JM. 2010. Protecting against antimicrobial effectors in the phagosome allows SodCII to contribute to virulence in Salmonella enterica serovar Typhimurium. J Bacteriol 192:2140–2149. doi: 10.1128/JB.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higgins CF. 1992. ABC transporters: from microorganisms to man. Annu Rev Cell Biol 8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 56.Davidson AL, Chen J. 2004. ATP-binding cassette transporters in bacteria. Annu Rev Biochem 73:241–268. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- 57.Noinaj N, Buchanan SK. 2014. Structural insights into the transport of small molecules across membranes. Curr Opin Struct Biol 27:8–15. doi: 10.1016/j.sbi.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.