SUMMARY
Normal platelet function is critical to blood hemostasis and maintenance of a closed circulatory system. Heightened platelet reactivity, however, is associated with cardiometabolic diseases and enhanced potential for thrombotic events. We now show gut microbes, through generation of trimethylamine N-oxide (TMAO), directly contribute to platelet hyperreactivity and enhanced thrombosis potential. Plasma TMAO levels in subjects (N>4000) independently predicted incident (3 yr) thrombosis (heart attack, stroke) risk. Direct exposure of platelets to TMAO enhanced submaximal stimulus-dependent platelet activation from multiple agonists through augmented Ca2+ release from intracellular stores. Animal model studies employing dietary choline or TMAO, germ-free mice, and microbial transplantation, collectively confirm a role for gut microbiota and TMAO in modulating platelet hyperresponsiveness and thrombosis potential, and identify microbial taxa associated with plasma TMAO and thrombosis potential. Collectively, the present results reveal a previously unrecognized mechanistic link between specific dietary nutrients, gut microbes, platelet function, and thrombosis risk.
INTRODUCTION
Cardiovascular arterial thrombotic events, such as myocardial infarction and stroke, are a leading cause of disability and mortality. Platelet activation, aggregation and the subsequent generation of an occlusive intra-arterial thrombus are essential steps in atherothrombotic disease, and enhanced platelet reactivity is associated with both the extent of end organ injury and adverse prognosis (Frossard et al., 2004; Tantry et al., 2013). Platelet hyperreactivity and thrombosis risk are enhanced in the setting of numerous conditions associated with atherosclerotic heart disease, such as hyperlipidemia, oxidant stress, and hyperglycemia (Podrez et al., 2007; Chen et al., 2008; Zhu et al., 2012a). Understanding the mechanisms through which platelets become hyper-responsive and more prone to clot formation is therefore of considerable importance (Jennings, 2009).
The past decade has witnessed a rapidly growing awareness of the involvement of gut microbial organisms in the development of numerous cardiometabolic phenotypes (Bäckhed et al., 2004; Turnbaugh et al., 2006; Cox et al., 2014). For example, recent studies reveal participation of gut microbes in a metaorganismal pathway that contributes to the development of atherosclerosis (Wang et al., 2011; Koeth et al., 2013; Tang et al., 2013; Gregory et al., 2015). Briefly, specific trimethylamine (TMA) containing dietary nutrients, such as phosphatidylcholine, choline, and carnitine, can be used by gut microbes as a carbon fuel source. A waste product produced is TMA, which is carried via the portal circulation to the liver where it is rapidly converted by a family of enzymes, host hepatic flavin monooxygenases (FMOs), into TMA N-oxide (TMAO) (Wang et al., 2011). Animal model studies show direct provision of TMAO within the diet is pro-atherogenic, and similarly, provision of its dietary precursors (e.g. choline, carnitine, gamma butyrobetaine) also accelerates atherosclerosis development, but only in the setting of intact gut microbiota and TMA/TMAO generation (Wang et al., 2011; Koeth et al., 2013; Koeth et al, 2014). Recent studies involving genetic manipulation of FMO3, the major FMO responsible for converting TMA into TMAO (Bennett et al., 2013), further confirm the involvement of this meta-organismal pathway in both atherosclerosis development and regulation of whole body cholesterol and sterol metabolism (Miao et al., 2015; Shih et al., 2015; Warrier et al., 2015). Moreover, an obligatory role for gut microbes in TMAO generation in humans was affirmed by two distinct studies involving ingestion of either isotope labeled phosphatidylcholine or isotope labeled carnitine as tracer before versus following exposure to an oral cocktail of poorly absorbed antibiotics to suppress intestinal microbes (Tang et al., 2013; Koeth et al., 2013). Finally, an association between plasma TMAO levels and both the extent of coronary atherosclerotic plaque burden and CVD risks has been observed in multiple distinct clinical studies (Wang et al., 2011; Tang et al., 2013; Tang et al., 2014; Lever et al., 2014; Tang et al., 2015).
Insights into the mechanisms through which the meta-organismal pathway responsible for TMAO production are associated with enhanced CVD risks have thus far focused on the involvement of TMAO and FMO3 in atherosclerotic plaque development, altered sterol and glucose metabolism, and changes in macrophage phenotype (Wang et al., 2011 ; Koeth et al., 2013; Miao et al., 2015 ; Shih et al., 2015; Warrier et al., 2015). The involvement of gut microbes and TMAO in modulation of platelet function and thrombosis risks in vivo has not yet been reported.
RESULTS
Plasma TMAO levels predict incident thrombotic event risk (myocardial infarction and stroke)
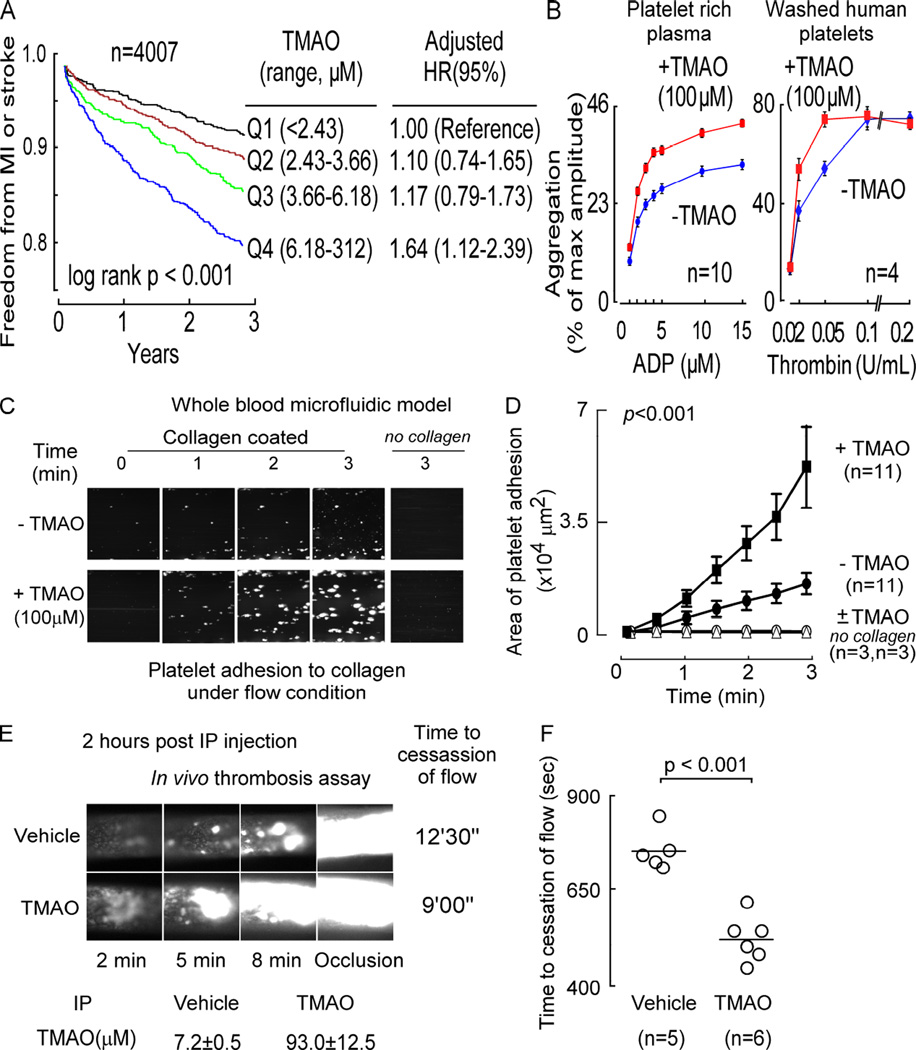
We previously reported an association between plasma levels of TMAO and incident risk for the composite of major adverse cardiovascular events in a cohort of sequential stable subjects (n=4007) presenting to a cardiology clinic for elective diagnostic cardiac evaluations and for whom adjudicated longitudinal follow-up (3 year) for incident development of myocardial infarction (MI), stroke, death, or need for revascularization were collected (Tang et al., 2013). Initially, we therefore sought to reanalyze the clinical dataset and test whether an association between plasma levels of TMAO and incident risk for thrombotic events (MI or stroke) was observed. Plasma TMAO levels within the cohort showed a large dynamic range (0.06 to 312 µM; Figure 1A). TMAO showed a dose-dependent association with increased cumulative burden of incident thrombotic events as illustrated by Kaplan-Meier survival analyses (Figure 1A). Importantly, the association between TMAO and incident thrombosis risk was observed even following adjustments for CVD history, traditional CVD risk factors, renal function and medication use (4th quartile level versus 1st quartile, adjusted 1.64-fold risk, p<0.001; Figure 1A).
Figure 1. TMAO is associated with thrombosis potential.
(A) Relationship between plasma TMAO level and incident (3 year) thrombotic event (MI or stroke) risk in sequential subjects undergoing elective diagnostic coronary angiography. Kaplan–Meier survival analyses and Cox proportional hazards regression analyses were used for time-to-event analysis to determine adjusted Hazard ratio (HR) and 95% confidence intervals (95%CI). (B) Effect of TMAO on dose response curves for ADP- (left) or thrombin- (right) induced platelet activation monitored using platelet aggregometry. (C and D) Platelet adhesion in whole blood to a microfluidic chip surface (± collagen coating) under physiological sheer conditions ± TMAO, with (C) representative images of platelet adhesion at the indicated times; and (D) adherent platelet area per µm2 of chip surface. (E and F) In vivo thrombosis potential using the FeCl3-induced carotid artery injury model in response to TMAO (vs. normal saline) injected i.p. into mice. Plasma TMAO levels at time of thrombosis model are indicated. (E) Shown are representative vital microscopy images of carotid artery thrombus formation at the indicated time points following arterial injury, and TMAO levels (n≥5 each group); and (F) time to cessation of blood flow in mice from the indicated dietary groups. Bar represents mean time to cessation of blood flow within the indicated group. Significance was measured with either log rank or unpaired Student’s t test. The number of independent biological replicates (n = subjects or animals per group) in all panels are shown. Data are presented as mean ± SEM (B,D,E,F). Linear mixed effect models were used to analyze the repeated measure data in panel D. A Mann-Whitney test was used to compare the time to cessation of flow between the TMAO and control groups. See also Figure S1.
TMAO directly enhances human platelet responsiveness to multiple agonists
The robust association observed between increasing TMAO levels and incident risk of thrombotic events in subjects served as impetus for further studies aimed at testing the hypothesis that TMAO may directly modulate platelet function. Initially, platelet-rich plasma was isolated from healthy volunteers with low levels of TMAO (all in first quartile, < 2.4 µM), and the effect of physiologically relevant levels of exogenous TMAO (100 µM vs. vehicle) on platelet activation by the agonist ADP, as monitored by platelet aggregometry, was examined. While inter-individual variations in the magnitude of the effect of TMAO on platelet responsiveness to ADP stimulation was seen (Figure S1), overall, a leftward shift (p<0.0001) in the dose-response curve in the presence of high TMAO levels was observed (Figure 1B, left). Upon further examination, the enhancing effect of TMAO on platelet function was seen to be more generalizable, since similar results were observed using washed human platelets briefly incubated in the presence vs. absence of TMAO (100 µM) and varying doses of thrombin instead of ADP as agonist (Figure 1B, right).
We next examined the effect of TMAO on platelet adhesion to collagen, a highly thrombogenic component of the subendothelium (Furie and Furie, 2008). Platelets within whole blood were fluorescently labeled, and adherence to the collagen surface of a microfluidic device under physiological shear forces was monitored in real time by microscopy, as described under Experimental Procedures. Figure 1C shows representative images of the collagen coated biochip under flow with whole blood from a typical subject at initial (t=0) and subsequent 1 minute interval periods of exposure, and illustrates the visible increase in fluorescent platelet adhesion noted in the presence of TMAO. Computer image assisted quantification of adherent platelets was employed, and cumulative data from multiple subjects (n=11) is shown in Figure 1D. TMAO significantly (p<0.0001) increased platelet adhesion within whole blood to immobilized collagen under physiological shear force. In the absence of collagen coating (±TMAO), platelet adhesion to the biochip surface was negligible (Figure 1D). Cumulatively, the above data demonstrate that physiological levels of TMAO directly enhance platelet responsiveness to multiple distinct agonists (ADP, thrombin and collagen).
TMAO enhances in vivo thrombosis
Since the enhancing effect of TMAO on platelet responsiveness is both rapid and direct, we reasoned that we should be able to induce a relatively acute rise in plasma TMAO levels within the physiologically observed range and simultaneously enhance in vivo thrombosis potential. To test this, plasma TMAO levels in mice were raised using intraperitoneal (i.p.) TMAO injection (vs. normal saline), and we quantified in vivo thrombosis potential using a carotid artery injury (FeCl3) model using fluorescently tagged platelets and intravital microscopy (we aimed to perform the thrombosis model when plasma TMAO was ~100 µM, as described under Experimental Procedures). Figure 1E shows representative images of thrombus development within the internal carotid artery at various time points following FeCl3 injury in a representative mouse injected with either TMAO or vehicle (normal saline). At the time of the carotid artery injury (2h following TMAO i.p. injection), a >10-fold increase in plasma TMAO was observed that is well within the (patho)physiological range observed in humans (Figure 1E; 93.0±12.5 µM vs. 7.2±0.5 µM TMAO in TMAO vs. vehicle, respectively; p<0.001; n≥5 per group). As can be seen in Figure 1E, the rate of fluorescent thrombus formation is increased by TMAO. Further, the occlusion time, monitored by direct visualization of when platelets cease to pass downstream of the growing thrombus, is significantly (p<0.001) shorter in TMAO injected mice (Figure 1F).
TMAO promotes platelet hyperresponsiveness by enhancing stimulus-dependent release of Ca2+ from intracellular Ca2+ stores
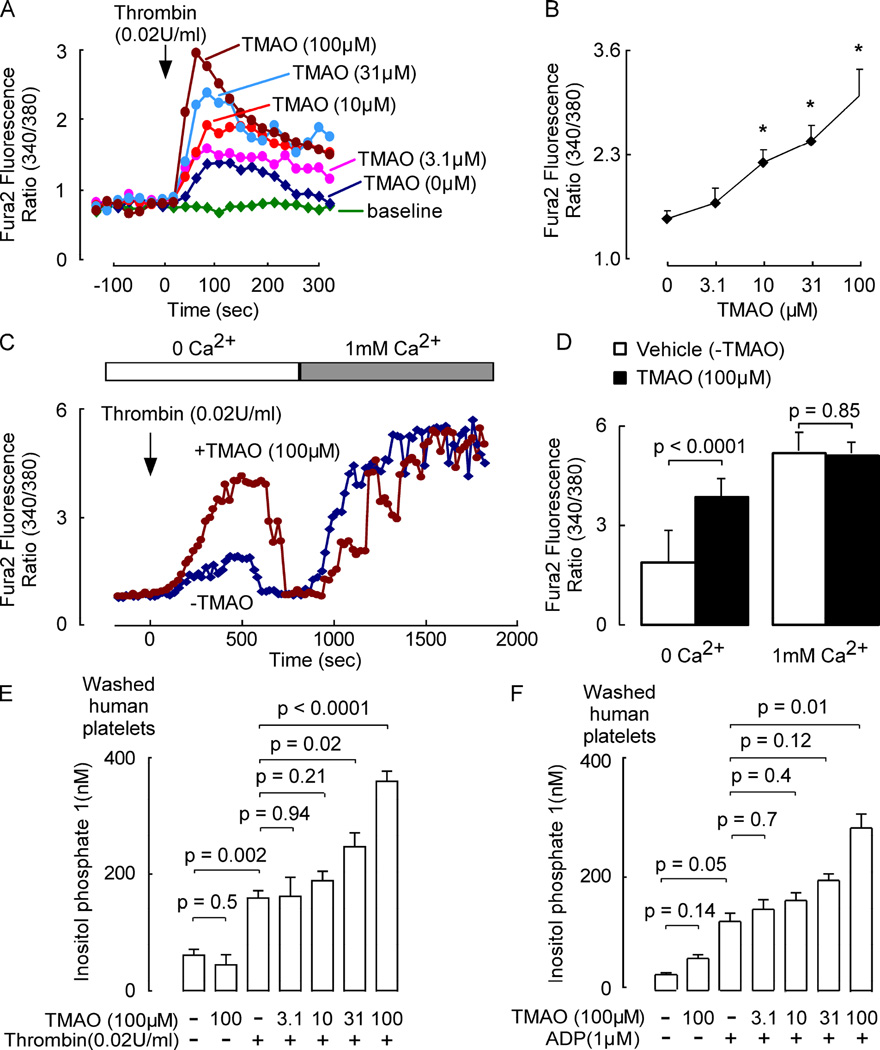
Under resting conditions, platelets maintain a low intracellular cytosolic calcium ([Ca2+]i) level as they circulate through healthy vessels. However, at sites of vessel injury, platelets are rapidly activated by stimulus-dependent mobilization of [Ca2+]i, a precursor to thrombus formation and hemostasis (Rink and Sage, 1990; Furie and Furie, 2008). To explore how TMAO influences platelet [Ca2+]i washed platelets from healthy donors were loaded with Fura 2-AM, a calcium selective dye, to permit real time monitoring of [Ca2+]i at rest and following stimulus-dependent activation. Pre-incubation with TMAO showed no effect on resting platelet [Ca2+]i (Figure 2A). However, exposure to physiological levels of TMAO enhanced submaximal thrombin-evoked augmentation of platelet [Ca2+]i in a dose-dependent manner (Figure 2A and 2B). In parallel studies TMAO similarly increased submaximal ADP (1 µM) - triggered rise in platelet [Ca2+]i (Figure S2, panel A and B). The stimulatory effect of TMAO on platelet responsiveness to agonist was observed to occur rapidly, since only brief exposure (10 min) to TMAO significantly enhanced stimulus triggered rise in [Ca2+]i (data not shown) and platelet aggregation (Figure S2, panel C). To determine whether TMAO affects the release of Ca2+ from platelet stores or the entry of Ca2+ from extracellular media, we first examined [Ca2+]i in Fura 2-AM loaded platelets incubated with submaximal thrombin dose in media in the absence of free Ca2+ (and 0.1 mM EGTA was added to the medium) in the presence vs. absence of TMAO. As seen in Figure 2C (left), under these conditions, platelet activation in the presence of TMAO resulted in an initial several-fold enhancement in rise of [Ca2+]i, indicating an intracellular source of Ca2+ is sufficient for TMAO induced augmentation in stimulus dependent rise in [Ca2+]i. With sustained incubation in media containing excess Ca2+ chelator, platelet [Ca2+]i eventually plummets, consistent with depletion of intracellular stores triggering plasma membrane Ca2+ channel opening. Subsequent addition of excess Ca2+ to the external media reveals sustained increase in [Ca2+]i, but no significant effect of TMAO on Ca2+ entry rate from the extracellular pool (Figure 2C and 2D). Finally, TMAO was seen to augment inositol-1,4,5-trisphosphate (IP3) signaling pathways in platelets with either submaximal thrombin (Figure 2E) or ADP (Figure 2F) as agonists by quantifying inositol phosphate levels.
Figure 2. TMAO augments stimulus-dependent release of Ca2+ from intracellular stores in human platelets.
(A and B) Thrombin evoked changes in intracellular calcium concentration [Ca2+]i in Fura 2-AM loaded washed human platelets incubated with the indicated amounts of TMAO. (A) Representative fluorescent signal from a single subject’s platelet preparation; and (B) mean ± SEM results from n=4 subjects are shown (*, p<0.05 for comparison with vehicle). (C and D) Fluorescence ratio of Fura-2 loaded platelets incubated in medium supplemented with TMAO or vehicle. Cells were first stimulated at the indicated time with a sub-maximal thrombin dose in a Ca2+-free medium, and then where indicated, Ca2+ was added to the medium. Representative (C) fluorescent signal and (D) mean fluorescence ± SEM (n=4 donors) are shown. (E and F) Washed human platelets pre-incubated (30 min, 22°C) with the indicated concentrations of TMAO wer e stimulated with sub-maximal levels of either (E) thrombin or (F) ADP, and then platelet IP1 concentrations determined. Data shown represent mean ± SEM (n=4 donors each). Linear mixed effect models were used to analyze the repeated measure data. A Wilcoxon Signed-Rank Test was used to conduct pairwise comparisons. See also Figure S2.
Dietary choline enhances platelet responsiveness and in vivo thrombosis potential
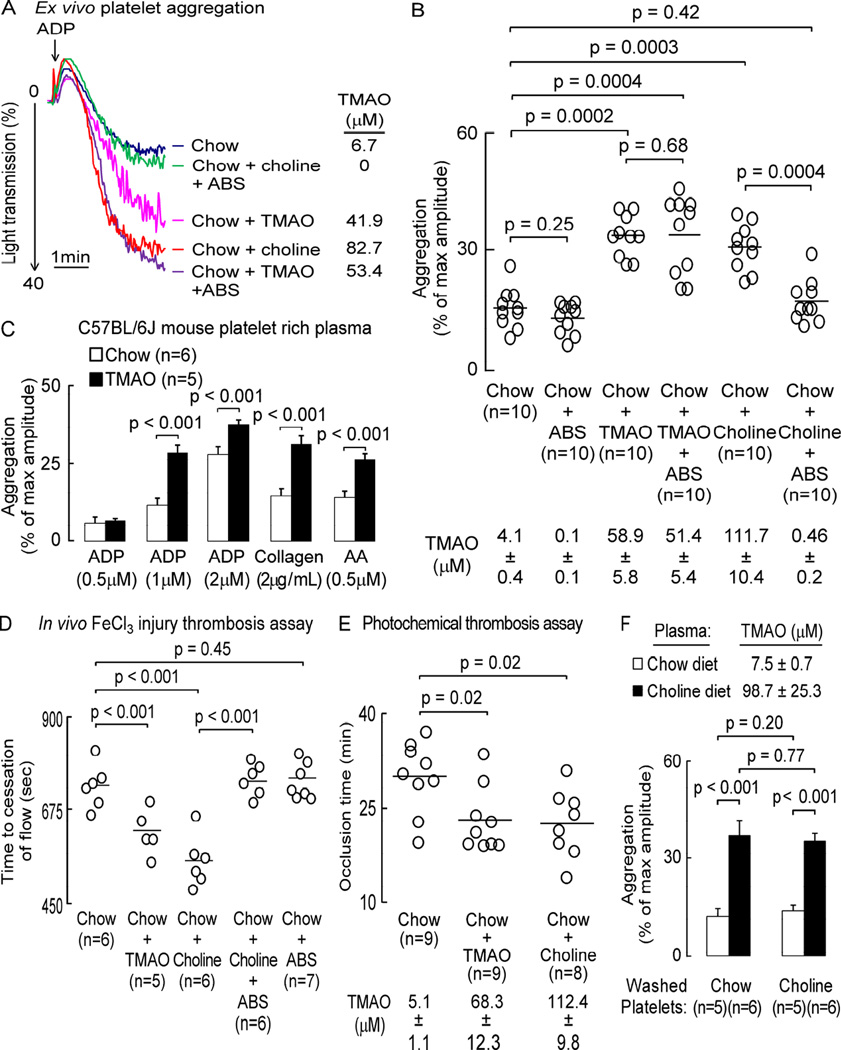
Demonstration of a direct effect of TMAO on platelet function suggests that gut microbiota and specific dietary nutrients that enhance TMAO generation should similarly modulate platelet function and thrombosis potential in vivo. To explore this possibility, mice were placed on a chemically defined diet comparable to normal chow (0.08% total choline) versus the same chemically defined diet supplemented with either TMAO (0.12%) or choline (1%). In addition, the effect of gut microbial suppression with a cocktail of oral broad-spectrum, poorly-absorbed antibiotics (ABS) (Wang et al., 2011) was examined with both diets (Figure 3). After 6 weeks, platelet-rich plasma was isolated from mice and both platelet responsiveness to ADP stimulation determined using ex vivo platelet aggregometry, and TMAO levels quantified. Figure 3A shows a representative tracing from the platelet aggregometry studies of a mouse from each dietary group using 1µM ADP as agonist, while data in Figure 3B represents scatter plots and cumulative statistical analyses of % maximal aggregation results at the sub-maximal ADP dose as agonist for each of the dietary +/− ABS groups of mice. Examination of plasma TMAO revealed elevated levels in mice provided dietary supplementation with either choline or TMAO. In parallel, ex vivo platelet aggregation induced by ADP stimulation was significantly increased in these groups (Figure 3B). Moreover, the enhancing effect of dietary choline on platelet aggregation was suppressed in mice receiving oral ABS, which markedly reduces plasma TMAO levels (Figure 3A and 3B). Importantly, provision of ABS to the TMAO supplemented group did not have a significant effect on either plasma TMAO levels, or platelet aggregation (Figure 3B). Figure 3C illustrates parallel independent studies examining platelet activation in response to different concentrations of ADP or different agonists (collagen or arachidonic acid). Notably, TMAO supplementation similarly enhanced ex vivo platelet aggregometry responses to submaximal levels of each of the agonists examined (Figure 3C).
Figure 3. Dietary choline, via gut microbe-generated TMAO, enhances platelet responsiveness and promotes a prothrombotic phenotype.
Mice were fed the indicated diets ± oral broad-spectrum antibiotics (ABS). (A, B) Platelet aggregometry was monitored in platelet-rich plasma following addition of sub-maximal (1µM final) ADP; or (D) in vivo thrombosis potential was quantified using the FeCl3-induced carotid artery injury model. (A) Representative aggregation tracing in response to sub-maximal ADP dose from a mouse within each group, along with the corresponding plasma TMAO level. (B) Aggregation (% of max amplitude of aggregation) in response to ADP, along with plasma TMAO levels, for each group (n>9 each). (C) Aggregation responses to the indicated submaximal concentrations of agonist (ADP, collagen or arachidonic acid, AA) in platelet-rich plasma recovered from mice on the indicated diets (n≥5). (D and E) Time to occlusive thrombus formation in either the (D) FeCl3-induced carotid artery injury model, or (E) photochemical injury-induced carotid artery injury model. Bar (B,D,E) represents mean value for the indicated group. TMAO levels are reported as mean ± SEM. (F) Aggregation responses to ADP (1µM) within the indicated mixtures of washed platelets and platelet-poor plasma prepared from mice on the indicated diets. Plasma TMAO levels (mean± SEM) of mice on the indicated diets is also shown. Data shown are % of max amplitude of aggregation (± SEM; for n≥=5 each). For significance, a Kruskal Wallis test was used for multiple group comparison and a Mann Whitney test was used for two group comparisons. See also Table S1.
In further studies the impact of dietary choline, TMAO and gut microbiota on in vivo thrombosis potential were examined using the carotid artery FeCl3 injury model using an overall study design identical to that used for the ex vivo platelet aggregometry studies above (Figure 3D). Consistently, in vivo thrombosis potential, as monitored by time to blood flow cessation in the carotid artery following FeCl3 injury, was shortened (indicating a more prothrombotic phenotype) in mice with heightened TMAO levels (choline or TMAO supplemented diet groups) (Figure 3D). Importantly, the effect of dietary choline on in vivo thrombosis was inhibited by concurrent oral ABS administration, which suppresses the gut microbiota and TMAO levels (Figure 3D; TMAO levels in Figure 3B). We also examined the impact of each dietary intervention on platelet counts, along with other metabolic parameters (Table S1), and saw no differences to account for the observed effects on in vivo thrombosis potential. In a complementary set of studies, we employed an alternative trigger of platelet activation and thrombosis in vivo, photochemical injury using Rose Bengal as a photosensitizing agent (Figure 3E) (Furie and Furie, 2008). Mice were placed on the chemically defined chow diet (0.08% total choline) versus the same diet supplemented with either TMAO (0.12%) or choline (1%) for 1 week, at which point the Rose Bengal was injected, the mid portion of the common carotid artery was illuminated with a 1.5 mW green light laser and blood flow was continuously monitored by doppler, as described under Experimental Procedures. Notably, dietary TMAO or choline supplementation each markedly increased plasma TMAO levels, and were accompanied by significant shortening of occlusion time (Figure 3E).
We next sought to determine whether the enhancing effect of dietary choline supplementation on platelet function was due to a factor(s) that was contained within platelet-poor plasma, as anticipated for TMAO, or whether the platelets themselves showed altered responsiveness when recovered from mice chronically on the choline supplemented diet. Platelet-rich plasma from each group of mice was recovered and then further fractionated into a washed platelet versus platelet-poor plasma fraction under ultracentrifugation conditions that also removed microparticles and exosomes, as described under Experimental Procedures. As shown in Figure 3F, enhanced platelet aggregation responses in add-back/mixing studies revealed that the hyperresponsive phenotype followed the choline diet derived platelet-poor plasma. In contrast, the source of the washed platelets (chow vs. choline diet fed animals) failed to impact overall extent of platelet aggregation monitored.
Gut microbes play an obligatory role in choline diet dependent enhancement in thrombosis
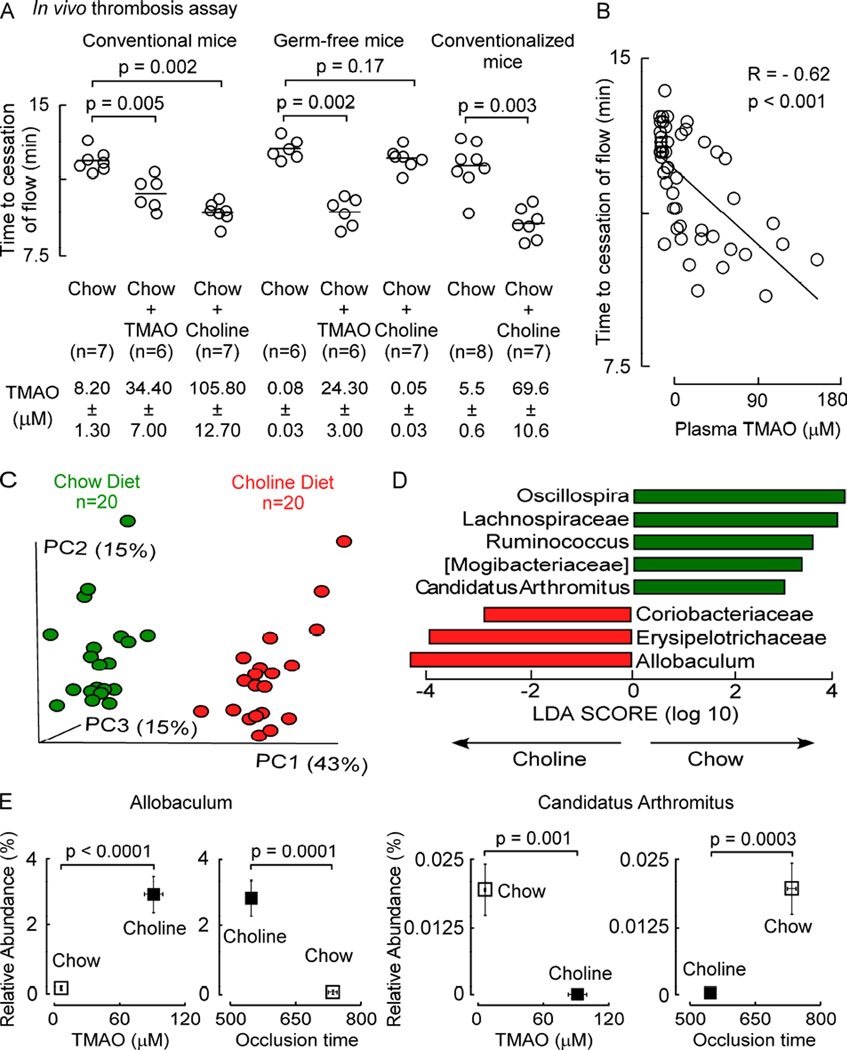
To confirm a role for gut microbes in choline diet-dependent enhancement of platelet responsiveness in vivo, we performed in vivo thrombosis studies (FeCl3 induced carotid artery injury model) using germ-free C57BL/6J mice housed under sterile conditions, their conventional (microbe colonized) counterparts, or germ-free mice after conventionalization (housing in conventional cages to permit microbe colonization). All mice were maintained on the indicated diets for 6 weeks, and plasma was recovered for TMAO analyses on the day of the in vivo thrombosis assay. As previously observed, conventional mice supplemented with either dietary choline or TMAO (relative to the control (chow) group) showed significant increases in plasma TMAO levels, and in parallel, enhanced thrombosis (reductions in carotid artery time to cessation of blood flow following FeCl3 injury; Figure 4A). In contrast, within the germ-free mice, supplementation with choline no longer produced TMA (and hence TMAO) or the prothrombotic phenotype. Moreover, provision of dietary TMAO within the germ-free mice (bypassing the need for gut microbes to elevate TMAO) resulted in significant shortening in the time to cessation of blood flow (Figure 4A). Notably, germ-free mice that were subsequently conventionalized (placed in conventional cages allowing microbe colonization) at the start of the 6 week dietary exposure period, showed choline diet-dependent enhancement in plasma TMAO levels and accompanying pro-thrombotic phenotype compared with chow fed conventionalized mice (Figure 4A). Examination of the relationship between plasma TMAO levels and a quantitative measure of thrombosis potential (time to cessation of blood flow using the carotid artery injury model) across all groups of mice studied revealed a significant negative correlation (R=−0.62; p<0.001; Figure 4B).
Figure 4. Microbial taxa associated with choline diet-induced TMAO generation and a prothrombotic phenotype.
(A) Germ-free mice, conventionally reared mice, and germ-free mice subsequently conventionalized (all groups female, 4 week old, C57BL/6J), were fed the indicated sterile diets for 6 weeks and then both in vivo thrombosis potential (FeCl3 carotid artery injury model) and plasma TMAO levels were assessed. TMAO data represent mean ± SEM. (B) Correlation (Spearman) between plasma TMAO levels and time to cessation of blood flow among mice in all groups. (C) Microbial DNA encoding 16S rRNA was analyzed from cecum of mice in the indicated diet groups. Principal coordinates analyses demonstrate distinct cecal microbial composition between groups (p < 0.001 for Student’s t test with 1,000 Monte Carlo simulations). Each data point represents a sample from a distinct mouse projected onto the first three principal coordinates (percent variation explained by each PCo is shown in parentheses). (D) Linear Discriminant Analysis (LDA) Effect Size (LEfSe) identified taxa most characteristic (increased abundance) in chow (green) and choline (red) fed groups. (E) Example of choline diet group characteristic taxon (Allobaculum) and chow characteristic taxon (Candidatus Arthromitus) whose proportions are associated with both plasma TMAO levels and time to cessation of blood flow (occlusion time). A Kruskal Wallis test was used for multiple group comparison and a Mann Whitney test was used for two group comparison. See also Figure S3, Figure S4, Figure S5 and Tables S2–S4.
Specific gut microbial taxa are associated with both TMAO levels and thrombosis potential
To identify microbial genera associated with dietary choline-induced enhancement in in vivo thrombosis potential, mice were maintained on either the chow (0.08% total choline; n=20) or choline supplemented diet (1.0% total choline; n=20) for 6 weeks and then plasma TMAO levels, in vivo thrombosis potential using the carotid artery injury model, and cecal microbial composition examined. Principal Coordinates Analysis of microbial DNA encoding 16S ribosomal RNA was initially used to visualize whether community differences were observed between cecal microbes in mice on the chow versus choline supplemented diets. Distinct (nonoverlapping) clusters were observed (Figure 4C, p<0.001 for Student’s t test with 1,000 Monte Carlo simulations) suggesting chronic exposure to supplemental dietary choline induced a significant rearrangement in the overall cecal microbial composition. Linear Discriminant Analysis (LDA) coupled with effect size measurements (LEfSe) further identified cecal microbial taxa that accounted for the greatest differences observed in mice on the distinct diets. In total, 8 taxa were identified through LEfSe analyses as being characteristic of choline vs. chow diet (Figure 4D), where choline diet significantly increased proportions of 3 genera (Figure S3, panels A, B and C) and decreased 5 genera (Figure S3, panels D, E, F,G and H).
In further analyses of the detected cecal genera within all groups of mice, 9 taxa were identified whose proportions were significantly associated with plasma TMAO levels (FDR adjusted p≤0.1), and 15 taxa were identified whose proportions were significantly associated with in vivo thrombosis potential (i.e. occlusion time; FDR adjusted p≤0.1) (Table S2 and Figure S4, panel A). The proportions of 8 taxa were significantly associated with both plasma TMAO levels and occlusion time, and in every case, microbial taxa positively associated with higher plasma TMAO levels were associated with shorter time to blood flow cessation (more prothrombotic), whereas taxa significantly associated with reduced TMAO levels were associated with longer time to blood flow cessation (Table S2 and Figure S4, panel A). Interestingly, the proportions of most of the identified bacterial taxa characteristic of a high choline diet were also positively associated with a pro-thrombotic phenotype (shorter occlusion time). For example, the proportion of Allobaculum, a high choline diet characteristic taxa, was significantly positively associated with TMAO levels and shortened internal carotid artery occlusion times (Figure 4E, left; Figure S4). In contrast, alternative bacterial taxa that showed significant reduction in proportion such as Candidatus Arthromitus or Lachnospiraceae were associated with both lower TMAO levels and an anti-thrombotic phenotype (longer occlusion times) (Figure 4E, right; Figure S4).
Metagenomic analyses were also performed following shotgun sequencing of the cecal microbiota within mice from each of the three diets. Over 6000 species and strains were detected altogether. Table S3 lists the KEGG pathways that were present, indicating those that were identified in one or more samples and the significance of differences in abundance between the diets, and Figure S5 summarizes microbial pathways significantly different between cecal microbes recovered from the different dietary groups of mice. In addition to the choline utilization gene cluster identified in Desulfovibrio desulfuricans (Cracium and Balskus, 2012), several human gut microbiota reference species associated with TMA production, encoding the CutC/CutD, YeaW/X and CntA/B enzymes recently identified (Falony et al., 2015; Koeth et al., 2014) were also observed. Table S4 lists all of those detected in one or more samples in our study, as well as differences in abundance between the diets. In recent studies Romano and colleagues (Romano et al., 2015) examined 79 common gut microbe isolates and tested these in vitro for choline consumption and TMA production. Of these, 8 were found to be TMA producers, and all 8 were present in the metagenomic data from the mice in our study: Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, Clostridium sporagenes, Escherichia fergusonii, Proteus penneri, Providencia rettgeri, and Edwardsiella tarda. Of these, only the first three differed significantly between the chow and choline diets; however, surprisingly, these tended to be more abundant in mice from the chow vs. high choline diet groups (Table S4). These results underscore the complex community dynamic operating in vivo is more relevant to global TMA/TMAO production, as opposed to the in vitro ability of an individual microbial species to produce TMA from choline. Moreover, additional microbial TMA lyases no doubt exist that can contribute to TMA/TMAO production besides those already reported.
Cecal microbial transplantation studies demonstrate thrombosis potential is a transmissible trait
We next sought to test whether the effect of dietary choline on thrombosis potential is transmissible with microbial transplantation (i.e. fulfillment of an essential Koch’s postulate (Koch, 1880). We recently examined TMAO levels in a mouse diversity panel comprised of over 20 different inbred strains of mice, and identified C57BL/6J as a “high TMAO” producing atherosclerotic disease-prone strain, and NZW/LacJ as a “low TMAO” producing disease-resistant strain (Gregory et al., 2015). In addition, we successfully used cecal contents from these inbred strains of mice as donors of functionally distinct stable microbial communities in transplantation studies, revealing that both atherosclerosis susceptibility and TMA/TMAO production are transmissible traits (Gregory et al., 2015). Before further using these inbred strains as donors in cecal microbial transplantation studies exploring thrombosis potential, we thought it important to first confirm that the high TMAO producing strain (C57BL/6J) showed shorter occlusion time (more pro-thrombotic phenotype) than the low TMAO producing strain (NZW/LacJ), particularly on a high choline diet. Conventional C57BL/6J and NZW/LacJ mice were maintained for 6 weeks on chemically defined chow (0.08% total choline) versus choline supplemented (1.0% total choline) diets. On the day of in vivo thrombosis assay, plasma TMAO levels in the C57BL/6J mice were over twice as high as those observed in the NZW/LacJ mice on the corresponding diet in both normal chow and choline supplemented diet arms of the study (Figure S6, panel A). Interestingly, whereas a choline supplemented diet fed to C57BL/6J mice provoked a significant prothrombotic phenotype (p=0.0002 relative to chow), the NZW/LacJ mice on the choline supplemented (versus chow) diet exhibited only a trend (p=0.07) toward reduced carotid artery occlusion time following FeCl3-induced injury (Figure S6, panel A).
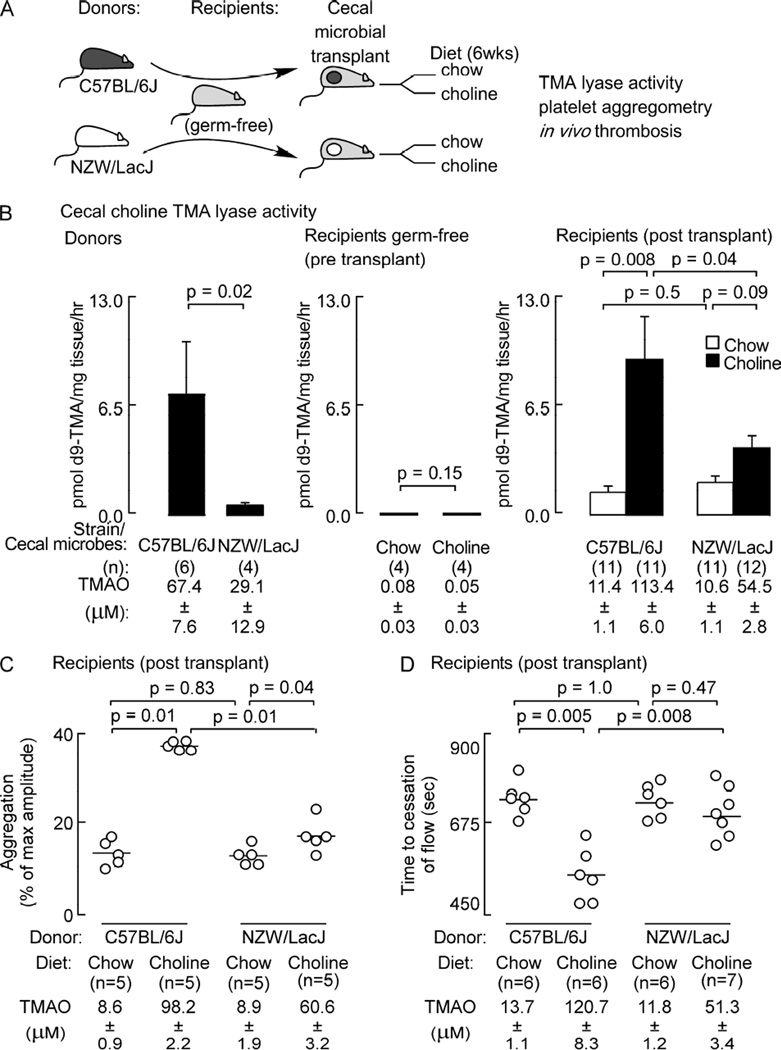
Based upon the above results, we proceeded with cecal microbial transplantation studies using C57BL/6J (“high TMAO”, “pro-thrombotic”) and NZW/LacJ (“low TMAO”, “low thrombotic”) mice as cecal microbial donor strains, and germ-free mice (C57BL/6J) as recipients. For 6 weeks preceding the start of the study, all mice (both donors and germ-free recipients) were maintained on the chemically defined (sterile) chow diet. Following cecal microbial transplantation, recipients were individually housed in separate microisolators and maintained on sterile (irradiated) chow vs. choline supplemented diets, as described under Experimental Procedures (overall scheme is shown in Figure 5A). Successful transplantation of differential microbial choline TMA lyase enzyme activity in the donors (measured by conversion of d9-choline into d9-TMA in cecum) was observed in the recipients following transplantation (Figure 5B). In contrast, examination of hepatic FMO enzyme activity, which is responsible for converting TMA into TMAO (Wang et al., 2011; Bennett et al., 2013), revealed no significant differences between both donor mouse strains, and among the various recipient groups post cecal microbial transplantation (Figure S6, panel B). Results of plasma TMAO levels on day of sacrifice, platelet function (ex vivo platelet aggregometry) and in vivo thrombosis potential (FeCl3 carotid artery injury model) in each group of mice are shown in Figure 5C and D. First, as previously observed, germ-free recipients following microbial transplantation with C57BL/6J cecal contents showed a significant ~ 2-fold increase in plasma TMAO levels compared to recipients on the comparable diets receiving microbial transplantation with NZW/LacJ cecal contents (Figure 5C,D). Moreover, recipient mice following microbial transplantation with C57BL/6J cecal contents on the choline supplemented diet showed ~10-fold increase in plasma TMAO level and accompanying enhanced platelet responsiveness to submaximal ADP dose during platelet aggregometry studies compared with chow-fed controls (p<0.0001; Figure 5C). Similarly, recipients of the C57BL/6J cecal microbes maintained on the choline supplemented diet also showed significant shortening of time to blood flow cessation during in vivo thrombosis assay (p<0.0001; Figure 5D). In contrast, a blunted (relative to C57BL/6J) rise in TMAO in the recipients receiving NZW/LacJ cecal microbes and placed on choline supplemented diet was observed, with a correspondingly reduced effect of choline diet on both platelet responsiveness and in vivo thrombosis potential (Figure 5C,D).
Figure 5. Thrombosis potential is a transmissible trait.
(A) Scheme illustrating cecal microbial transplant study design. Germ-free C57BL/6J mice (recipients) had cecal microbes introduced by gastric gavage from conventionally reared C57BL/6J (high TMA/TMAO producer) and NZW/LacJ (low TMA/TMAO producer) donors. Recipients (with and without microbe transplants) were placed on sterile chemically defined chow (0.08% choline) vs. choline supplemented (1.0% total) diets. (B) Cecal choline TMA lyase enzyme activity was quantified in donor mice (left panel), and recipient germ-free mice without (middle) or following (right) microbial transplantation and maintenance on the indicated diets within individual gnotobiotic isolators. Platelet function was assessed following microbial transplantation in the indicated numbers of recipient mice both by (C) ex vivo monitoring of aggregation response to ADP (1 µM), and (D) in vivo measuring of time to occlusive thrombus formation using the FeCl3-induced carotid artery injury model. (B,C and D) Plasma TMAO levels (mean ±SEM) were also determined at completion of all studies. For significance, a Kruskal Wallis test was used for multiple group comparison and a Mann Whitney test was used for two group comparison. See also Figure S6.
Transplanted cecal microbial taxa associate with TMAO levels and thrombosis potential
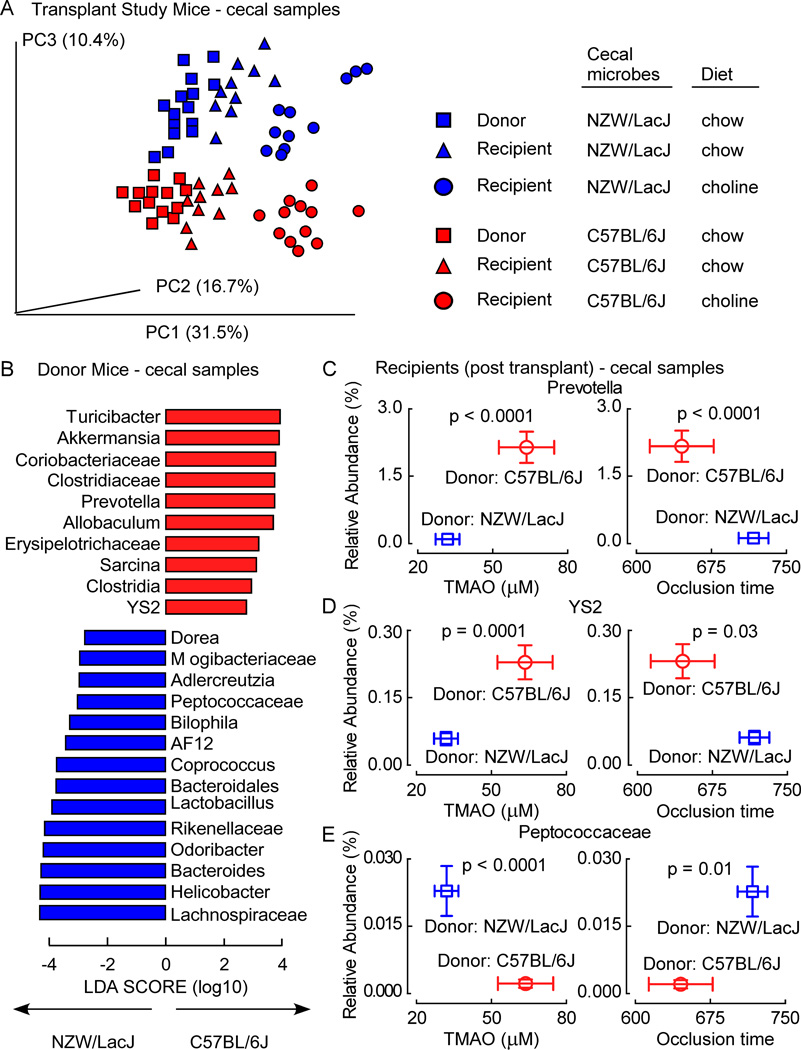
Cecal microbial composition in donors (on chow diet) and germ-free recipients post transplantation on the distinct diets (chow versus choline supplemented) was examined as before by sequencing microbial DNA encoding 16S ribosomal RNA and performing Principal Coordinates Analyses. Clear differences in community structure were visualized between cecal microbes recovered from the donors (Figure 6A). Further, following transplantation, recipients of C57BL/6J cecal microbes showed cecal microbial composition similar to the C57BL/6J donors, and recipients of NZW/LacJ cecal microbes show cecal microbial composition similar to the NZW/LacJ donors (Figure 6A). In addition, exposure to the high choline diet induced a shift in community composition within each recipient group, but the differences between (and similarities with their respective donor) the microbial community compositions in the respective recipients remained visible (Figure 6A).
Figure 6. Characterization of donor-characteristic cecal microbiota associated with TMAO and in vivo thrombosis potential in recipients following microbial transplantation.
Intestinal microbial community composition was assessed by (A) Principle coordinates analysis for donor and recipient mouse strains on the indicated diets. (B) Linear Discriminant Analysis (LDA) Effect Size (LEfSe) analyses were performed to identify taxa most characteristic (increased abundance) in NZW/LacJ and C57BL/6J donors. (C) Illustration of three taxa identified in recipient cecal microbes whose proportions are significantly associated with both plasma TMAO levels and occlusion time when grouped by dietary treatment (blue squares, recipients of NZW/LacJ donor cecal microbes; red circles, recipients of C57BL/6J microbes). Values in both x and y directions are plotted as mean±SE. Significance was determined using unpaired Student’s t test. See also Figure S7 and Table S5.
In further studies we examined whether the proportions of any cecal taxa in recipient mice from the above microbial transplantation study were significantly correlated with either plasma TMAO levels or in vivo thrombosis potential (occlusion time) at time of sacrifice (Figure S7). Of note, the proportions of several taxa previously identified in analyses of mice on chow versus choline supplemented diets as being associated with TMAO levels and in vivo thrombosis potential (Figures 4E, Figure S4, Table S2) were again observed to be significantly associated with TMAO levels and occlusion time. Specifically, proportions of three taxa in recipient cecal microbes were identified when grouped by dietary treatment as again being significantly associated with both plasma TMAO levels and occlusion time [Allobaculum with TMAO, p<0.0001, and with occlusion time, p=0.01 (Figure S7, panel B); Candidatus Arthromitus with TMAO, p<0.0001, and with occlusion time, p=0.007 (Figure S7, panel C); and Lachnospiraceae with TMAO, p<0.0001, and with occlusion time, p=0.01 (Figure S7, panel D)].
LEfSe analyses was next performed to identify cecal microbial taxa that account for the greatest differences between C57BL/6J and NZW/LacJ donor mice (Figure 6B), and the relationships of these donor characteristic taxa with both plasma TMAO and in vivo thrombosis potential examined in recipients following cecal microbial transplantation. Interestingly, of the ten taxa identified as characteristic for C57BL/6J donors (Figure 6B, taxa depicted in red), examination of these in recipient cecal microbes revealed that the proportions of seven taxa either trended or were significantly positively associated with TMAO and negatively with time to cessation of flow (i.e. associated with a more “pro-thrombotic” phenotype). Genus Prevotella and order YS2 from the Cyanobacteria phylum are examples of C57BL/6J donor characteristic taxa identified by the LEfSe analyses that in recipients were positively associated (after FDR adjustment) with TMAO and negatively with time to occlusive clot formation (Figure 6C, D). In similar analyses, of the fourteen taxa identified as being characteristic of the NZW/LacJ donors in LEfSe analyses (Figure 6B, taxa depicted in blue), when examined in recipient cecal microbes, the proportions of eight taxa either trended or were significantly negatively associated with TMAO levels and positively associated with time to cessation of flow (i.e. less thrombotic phenotype). The genus Peptococcaceae is an example of a taxa identified as being characteristic of the NZW/LacJ donors and which was observed to be both significantly negatively associated with TMAO levels and positively associated with in vivo time to occlusive clot formation within recipients (Figure 6E).
DISCUSSION
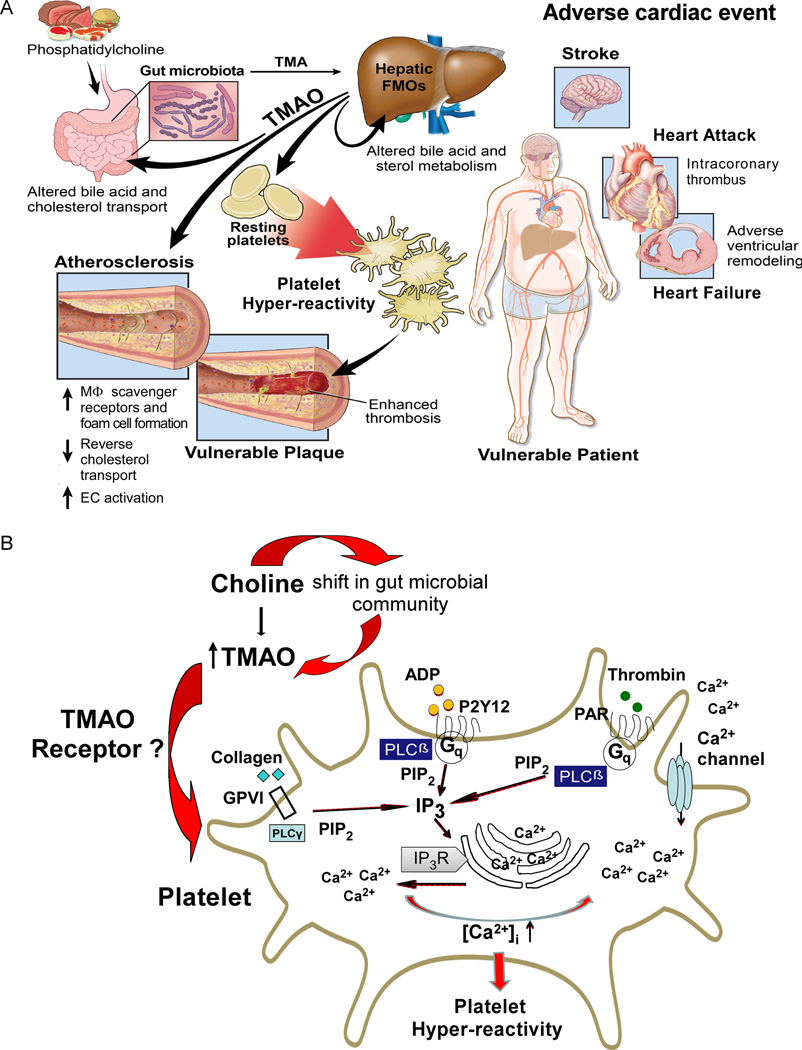
The present studies reveal the interesting finding that gut microbes modulate thrombosis potential in vivo. They also expand upon our understanding of the relationship between TMAO and CVD risk. The TMAO meta-organismal pathway has been shown to possess numerous clinical and mechanistic links (Figure 7A) with both atherosclerotic plaque development and whole body cholesterol and sterol metabolism (Wang et al., 2011; Koeth et al., 2013; Koeth et al., 2014; Gregory et al., 2015; Miao et al., 2015; Shih et al., 2015; Warrier et al., 2015). Moreover, circulating levels of TMAO have been shown in multiple distinct clinical studies and cohorts to be associated with CVD risks (Wang et al., 2011; Tang et al., 2013; Lever et al., 2014; Wang et al., 2014a; Tang et al., 2015 ; Trøseid et al., 2015), and targetted suppression of microbial TMA/TMAO production has recently been shown to inhibit diet induced atherosclerosis (Wang et al, 2015). The present studies expand upon these findings, and show that gut microbes, via generation of TMAO, can directly modulate platelet hyperresponsiveness and clot formation rate in vivo. A scheme illustrating the overall involvement of gut microbes and the TMAO meta-organismal pathway in platelet hyperresponsiveness and in vivo thrombosis potential, a critical determinant of patient vulnerability for adverse cardiac event risk, is illustrated in Figure 7A. Also shown are known effects of the gut microbial TMAO pathway on CVD, including the recent demonstration of adverse ventricular remodeling and heart failure risk (Organ et al., 2015), and arterial endothelial cell activation in vivo (Seldin et al., 2016). The influence of gut microbes on thrombosis risk via TMAO production requires the presence of an appropriate dietary input capable of producing TMA (e.g. foods rich in choline or phosphatidylcholine), the precursor for TMAO generation. Further, an obligatory role of gut microbes in choline dietenhanced thrombosis was confirmed both in studies using germ-free mice and in conventional mouse studies in the presence versus absence of oral poorly absorbed broad spectrum antibiotics. Cecal microbial transplantation studies confirm that both choline diet-enhanced platelet responsiveness and in vivo thrombosis potential are transmissible traits. Finally, results of large scale human clinical studies with over 4000 subjects underscore the potential clinical significance of the pathway, since elevated TMAO levels are independently associated with incident risk for thrombotic events (myocardial infarction or stroke) in subjects, even following adjustments for multiple risk factors, medication use and CVD status.
Figure 7. Summary schemes illustrating gut-microbial involvement in development of platelet hyperresponsiveness and athero-thrombotic heart disease.
(A) Global schema illustrating metaorganismal pathway linking dietary sources of choline abundant in a Western diet, gut microbiota and host hepatic FMOs, resultant TMAO production, and subsequent development of hyperresponsive platelet phenotype and enhanced thrombotic event risk. Also shown are reported pro-atherosclerotic effects of TMAO and the potential involvement of TMAO in development of vulnerable plaque. EC, endothelial cell; FMOs, flavin monooxygenases; M[phage], macrophage; TMA, trimethylamine; TMAO trimethylamine N-oxide. (B) Chronic exposure to a diet rich in choline leads to a shift in the gut microbial composition and function, with consequent enhancement in host TMAO plasma levels. Platelet exposure to high levels of TMAO enhances sub-maximal stimulus (thrombin, ADP, collagen) evoked release of intracellular calcium stores, and platelet hyperresponsiveness. ADP, adenosine diphosphate; GPVI, glycoprotein VI; G<q>, G protein q; IP<3>, inositol 1,4,5-triphosphate; IP<3>R, Inositol 1,4,5-triphosphate receptor; P2Y12, purinergic receptor P2Y12; PAR, protease activated receptor; PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate.
The mechanisms through which dietary choline influences platelet responsiveness and function, impacting overall in vivo thrombosis risk, are illustrated in Figure 7B. First, a diet rich in choline alters microbial composition and function. Specifically, with choline supplementation, total cecal microbial choline TMA lyase activity was shown to increase, with parallel increases in both plasma TMAO levels and proportions of specific taxa associated with TMAO. The present studies also show that the effect of TMAO on platelet hyperresponsiveness is not stimulus specific; rather, multiple distinct agonists including ADP, thrombin, collagen and arachidonic acid all showed TMAO-dependent enhancement in Ca2+ release from platelet intracellular stores and activation in the setting of submaximal agonist stimulation (Figure 7B). In addition, studies examining the effect of TMAO on IP3 generation in platelets, a second messenger signaling molecule produced by various phospholipase C isoforms that triggers intracellular Ca2+ release leading to platelet activation, similarly showed TMAO-dependent enhancement. These observations collectively suggest the site of TMAO action in platelets is at a molecular level at or proximal to this step. Importantly, the effect of TMAO on platelet function was also dose-dependent across the physiological range of TMAO concentrations observed in both humans and in animal models. Finally, the effect of TMAO on platelet reactivity and in vivo thrombosis was observed to be both rapid and reversible.
Despite the numerous findings and mechanistic insights revealed regarding the impact of TMAO on platelet function, the “chemical sensor” for TMAO within platelets remains unknown. Indeed, a receptor for TMAO in eukaryotic cells has not yet been reported. In contrast, TMA is a well-known ligand for an olfactory G-protein coupled receptor, trace amine-associated receptor 5 (TAAR5), which is specific for TMA and dimethylamine, but reportedly does not recognize either TMAO or numerous alternative methylamines (Wallrabenstein et al., 2013). Since orally ingested TMAO can in part be converted back to TMA by gut microbes, one might speculate that TMA, and thus TAAR5, might participate in the observed platelet phenotype. However, results of platelet aggregometry studies with direct addition of chemically pure TMAO, as well as studies employing germ-free mice and dietary TMAO supplementation, both strongly indicate that it is TMAO itself, and not TMA, that mediates the observed platelet hyperresponsiveness ex vivo, and the pro-thrombotic phenotype in vivo. Whether there is a dedicated receptor on the platelets for TMAO, or TMAO instead acts as an allosteric modulator of existing signaling pathways is unknown. TMAO is known to function as a small molecule chaperone mimetic, influencing protein conformation and function. Studies with isotope labeled TMAO reveal it can enter cells (data not shown), but its site of action in modulating platelet function is at present unknown. Biophysically, TMAO is rather unique and possesses both hydrophobic and hydrophilic properties and the known ability to alter protein confirmation and stability (Brown et al., 1997; Singh et al., 2007). Discovery of the molecular receptor(s) that senses TMAO within cells like platelets is an area of intense interest.
In summary, the present work reveals the clinically relevant discovery that gut microbes, via generation of TMAO, participate in modulating platelet function and generation of a prothrombotic phenotype in vivo. The results thus suggest new potential therapeutic targets and refinement of nutritional interventions for the prevention of adverse cardiovascular event risk. It is remarkable that numerous large scale epidemiological studies have noted a relationship between a Western diet, which is enriched in known nutrient TMAO precursors (e.g. choline, phosphatidylcholine and carnitine), and enhanced risk for myocardial infarction, stroke and death (Iqbal et al., 2008; Rohrmann et al., 2013). It also is relevant that anti-platelet agents are a cornerstone of current pharmacological interventions for the treatment and prevention of acute complications of CVD. These interventions are, however, hampered by their potential for bleeding as a complication (Jennings, 2009). The present studies suggest that targeting the gut microbial TMAO pathway as a treatment strategy, whether it be via dietary manipulation, alteration in microbial community with a probiotic or prebiotic, or direct pharmacological inhibition of microbial enzymes involved in TMA production, has the potential to temper platelet hyperresponsiveness associated with elevated TMAO. Moreover, such pharmacological interventions would, in contrast to existing anti-platelet agents, be expected to reduce platelet hyperresponsiveness to normal range, and not induce impairment in overall platelet function. Thus, one could speculate that targeting this microbial pathway would foster a beneficial “anti-platelet” effect, attenuating pro-thrombotic tendencies, but without enhanced bleeding complications as a consequence. Studies into this possibility represent an attractive future area of investigation. Further examination of the clinical utility of TMAO levels as an indication of subjects who may benefit from anti-platelet prophylaxis therapy with low dose aspirin or other platelet directed antithrombotic drugs is also an area of considerable interest.
EXPERIMENTAL PROCEDURES
More complete experimental procedures are provided in Supplemental Experimental Procedures.
General procedures and reagents
Reagents were purchased from Sigma unless otherwise stated. Protein concentration was determined by bicinchoninic acid assay (BCA) assay (Bio-Rad). Hepatic FMO activity was quantified as described previously (Bennett et al., 2013). Mouse plasma total cholesterol and triglycerides were measured using Abbott ARCHITECT platform model ci8200 (Abbott Diagnostics).
Ethical considerations
All animal model studies were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic. All study protocols and informed consent for human subjects were approved by the Cleveland Clinic Institutional Review Board. Informed consents were obtained from all subjects.
Mass spectrometry quantification of plasma and dietary analytes
Stable isotope dilution liquid chromatography with on-line tandem mass spectrometry (LC/MS/MS) was used for quantification of plasma TMA and TMAO, as well as the content of free ant total choline of all chemically defined diets, as previously described (Wang et al., 2011; Wang et al., 2014b).
Human studies
The association between TMAO levels and incident thrombotic event risk was examined in the Genebank Study (Tang et al., 2013). Platelets from consenting healthy volunteers were drawn into citrate tubes. Donors were prescreened and confirmed to have fasting TMAO <2.5 µM the day of blood draw. Platelet-rich plasma, isolated human platelets and aggregometry studies were performed as described in Supplemental Experimental Procedures. Thrombosis studies in whole blood at physiological levels of shear stress were performed using the Cellix Microfluidics System (Cellix Ltd.,) with computer image assisted quantification of adherent platelets as described in Supplemental Experimental Procedures. Inositol phosphate levels were quantified using the IP-One ELISA kit (Cisbio Bioassays) according to the manufacturer's instruction.
In vivo carotid artery thrombosis models
Two in vivo thrombosis models were used. Mice were typically placed for 6 weeks on the indicated chemically defined diets (± oral broad-spectrum antibiotics provided in the drinking water, where indicated) as previously described (Wang et al., 2011). For most studies, mice were subjected to common carotid artery injury by application of 10% FeCl3 for 1 min (Chen et al., 2008). In alternative studies, a photochemical carotid artery injury model was used as described in Supplemental Experimental Procedures. In some experiments, plasma TMAO was acutely elevated by intraperitoneal injection (100 µl) of sterile stock solution (290 mM final in sterile water, pH adjusted to 7.4 using 0.1N HCl) or sterile normal saline, and the carotid artery thrombosis model was performed two hours later.
Intracellular Ca2+ measurements
Ratiometric fluorescence measurements were conducted with 8×108 washed human platelets re-suspended in Hank’s buffered salt solution supplemented with bovine serum albumin and glucose and after incubation with Fura 2-AM (1 µM) and removal of unabsorbed FURA 2-AM. Changes in [Ca2+]i were monitored by measuring Fura 2-AM fluorescence using 340/380 nm dual–wavelength excitation and an emission of 510 nm, as described in Supplemental Experimental Procedures.
Germ-free mouse studies
Germ-free C57BL/6J female mice bred and housed at the National Gnotobiotic Rodent Resource Center, University of North Carolina (UNC) were used in various studies. Mice were exposed to 3 weeks of the indicated chemically defined sterile diets as described in Supplemental Experimental Procedures. Conventionalization of germ-free mice and microbial transplantation studies were performed as described in Supplemental Experimental Procedures.
Microbiota studies
Cecal microbial community composition was assessed by sequencing 16S rRNA gene amplicons (Gregory et al., 2015), and metagenomic analyses were performed as described in Supplemental Experimental Procedures, following shotgun sequencing of cecal microbes. Metagenomic data are deposited in MG-RAST-Argonne National Laboratory (http://metagenomics.anl.gov/) and can be accessed through project name: Platelet function and TMAO.
Statistical analysis
Detailed statistical methods are described in Supplemental Experimental Procedures. All studies were repeated at least 3 independent times with the number of biological replicates (animal numbers) for a given experiment shown. Kaplan-Meier survival plots and Cox proportional hazards analysis were used to determine Hazard ratio (HR) and 95% confidence intervals (95%CI) for thrombotic event (MI and stroke) risk stratified according to TMAO quartiles. Adjustments were made for individual traditional risk factors including age, sex, SBP, LDL, HDL, triglycerides, smoking, diabetes, estimated creatinine clearance, medications, and CVD status. All data were analyzed using R 3.1.0 (Vienna, Austria) and JMP (SAS Institute). For all statistical tests p < 0.05 was considered significant.
Supplementary Material
Highlights.
Elevated TMAO levels predict incident risk for thrombotic events in human subjects
TMAO enhances submaximal stimulus-dependent platelet activation
Dietary choline, gut microbes, and TMAO are linked to thrombotic potential in vivo
Microbial transplantation shows that thrombosis potential is a transmissible trait
Acknowledgments
We thank Daniel I. Simon and Yunmei Wang (Case Western Reserve University) for technical assistance on the photochemical thrombosis model. We thank Sorel Fitz-Gibbon (UCLA) for assistance with metagenomic sequencing data. This research was supported by grants from the NIH and the Office of Dietary Supplements (R01HL103866, P20HL113452, HL28481, HL30568, R01DK106000, R01 HL122283, P40 OD010995 and P30 DK034987). GeneBank was supported by NIH grants P01HL076491, P01HL098055, R01HL103931 and UL1TR 000439. W.Z. and Z.W. were supported in part by AHA Scientist Development Grants. E.O was supported by FP7 grant no 330381. SLH was partially supported by the Leonard Krieger endowment. Mass spectrometry instruments used were supported in part by AB SCIEX.
Disclosures
Drs. Wang and Hazen are named as co-inventors on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics or therapeutics. Drs. Hazen and Wang report having the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics from Cleveland Heart Lab, Inc. Dr. Hazen also reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Siemens, Esperion, and Frantz Biomarkers, LLC. Dr. Hazen reports having been paid as a consultant for Esperion and P&G, and receiving research funds from Astra Zeneca, P&G, Pfizer Inc., Roche Diagnostics and Takeda.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures and five tables.
AUTHOR CONTRIBUTIONS:
W.Z. participated in the design, performance and analyses of most studies and the drafting of the manuscript. J.C.G. participated in the design and performance of microbial transplantation studies and analyses, human platelet related studies and the drafting of the manuscript. J.A.B. assisted in all mouse studies, and N.G. helped with both microfluidic device studies and human platelet related studies. Z.W. and F.X. performed all mass spectrometry analyses. L.L. and Y.W. assisted with statistical analyses. R.B.S. supervised some of the germ-free mice experiments. E.O., M.M., and A.J.L. performed gut microbial composition analyses. T.M.M., R.L.S., W.H.W.T., J.A.D, and J.M.B. assisted with experimental design input and thoughtful discussions. S.L.H. conceived of the project idea, and participated in the design of experiments, data analyses and the drafting of the manuscript. All authors critically reviewed and edited the manuscript.
REFERENCES
- Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CR, Hong-Brown LQ, Welch WJ. Correcting temperature-sensitive protein folding defects. J Clin Invest. 1997;99(6):1432–1444. doi: 10.1172/JCI119302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ Res. 2008;102(12):1512–1519. doi: 10.1161/CIRCRESAHA.108.172064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad of Sci. 2012;109:21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G, Vieira-Silva S, Raes J. Microbiology Meets Big Data: The Case of Gut Microbiota-Derived Trimethylamine. Annual Review of Microbiology. 2015;69:305–321. doi: 10.1146/annurev-micro-091014-104422. [DOI] [PubMed] [Google Scholar]
- Frossard M, Fuchs I, Leitner JM, Hsieh K, Vlcek M, Losert H, Domanovits H, Schreiber W, Laggner AN, Jilma B. Platelet function predicts myocardial damage in patients with acute myocardial infarction. Circulation. 2004;110(11):1392–1397. doi: 10.1161/01.CIR.0000141575.92958.9C. [DOI] [PubMed] [Google Scholar]
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, et al. Transmission of Atherosclerosis Susceptibility with Gut Microbial Transplantation. J Biol Chem. 2015;290(9):5647–5660. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.who.int/mediacentre/factsheets/fs317/en/, World Health Organization Fact Sheet N°317, CVDs (CVDs).
- Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, Chifamba J, Al-Hinai A, Keltai M, Yusuf S, et al. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation. 2008;118(19):1929–1937. doi: 10.1161/CIRCULATIONAHA.107.738716. [DOI] [PubMed] [Google Scholar]
- Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102(2):248–257. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- Koch R. Investigations into the Etiology of Traumatic Infective Diseases. The New Sydenham Society; 1880. [Google Scholar]
- Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, et al. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20(5):799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, McEntyre CJ, Elmslie JL, Atkinson W, Molyneux SL, et al. Betaine and Trimethylamine-N-Oxide as Predictors of Cardiovascular Outcomes Show Different Patterns in Diabetes Mellitus: An Observational Study. PLoS One. 2014;9(12):e114969. doi: 10.1371/journal.pone.0114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Ling AV, Manthena PV, Gearing ME, Graham MJ, Crooke RM, Croce KJ, Esquejo RM, Clish CB Morbid Obesity Study Group. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WHW, Wu Y, Hazen SL, Lefer DJ. Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circulation: Heart Failure. 2015 doi: 10.1161/CIRCHEARTFAILURE.115.002314. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13(9):1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink TJ, Sage SO. Calcium signaling in human platelets. Annu Rev Physiol. 1990;52:431–449. doi: 10.1146/annurev.ph.52.030190.002243. [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, Nailler L, Boutron-Ruault MC, Clavel-Chapelon F, Krogh V, et al. Meat consumption and mortality--results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013;11:63. doi: 10.1186/1741-7015-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. 2015;6(2):e02481-14. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-kappaB. Journal of the American Heart Association. 2016;5:e002767. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56(1):22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh LR, Chen X, Kozich V, Kruger WD. Chemical chaperone rescue of mutant human cystathionine β-synthase Mol Genet Metab. Mol Genet Metab. 2007;91(4):335–342. doi: 10.1016/j.ymgme.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: Refining the gut hypothesis. J Am Coll Cardiol. 2014;64(18):1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WW, Wang Z, Kennedy DJ, Wu Y, Buffa J, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut Microbiota-Dependent Trimethylamine N-oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ Res. 2015;116(3):448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, Stone GW, Curzen N, Geisler T, Ten Berg J, et al. Working Group on On-Treatment Platelet Reactivity. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62(24):2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- Trøseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjørndal B, Halvorsen B, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277(6):717–726. doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Wallrabenstein I, Kuklan J, Weber L, Zborala S, Werner M, Altmüller J, Becker C, Schmidt A, Hatt H, Hummel T, et al. Human Trace Amine-Associated Receptor TAAR5 Can Be Activated by Trimethylamine. PLoS One. 2013;8(2):e54950. doi: 10.1371/journal.pone.0054950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes CVD. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014a;35(14):904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014b;455:35–40. doi: 10.1016/j.ab.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015;S2211-1247(14):01065-1. doi: 10.1016/j.celrep.2014.12.036. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Li W, Silverstein RL. Advanced glycation end products induce a prothrombotic phenotype in mice via interaction with platelet CD36. Blood. 2012;119(25):6136–6144. doi: 10.1182/blood-2011-10-387506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.