Abstract
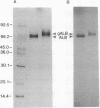
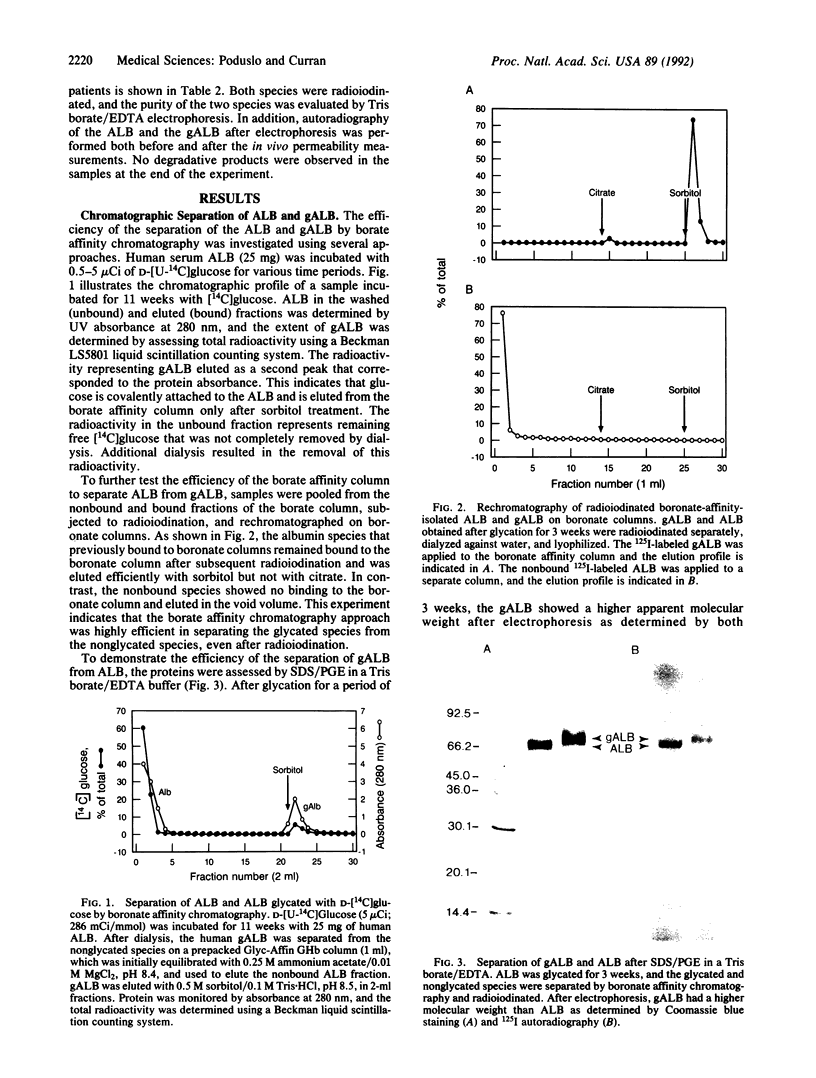
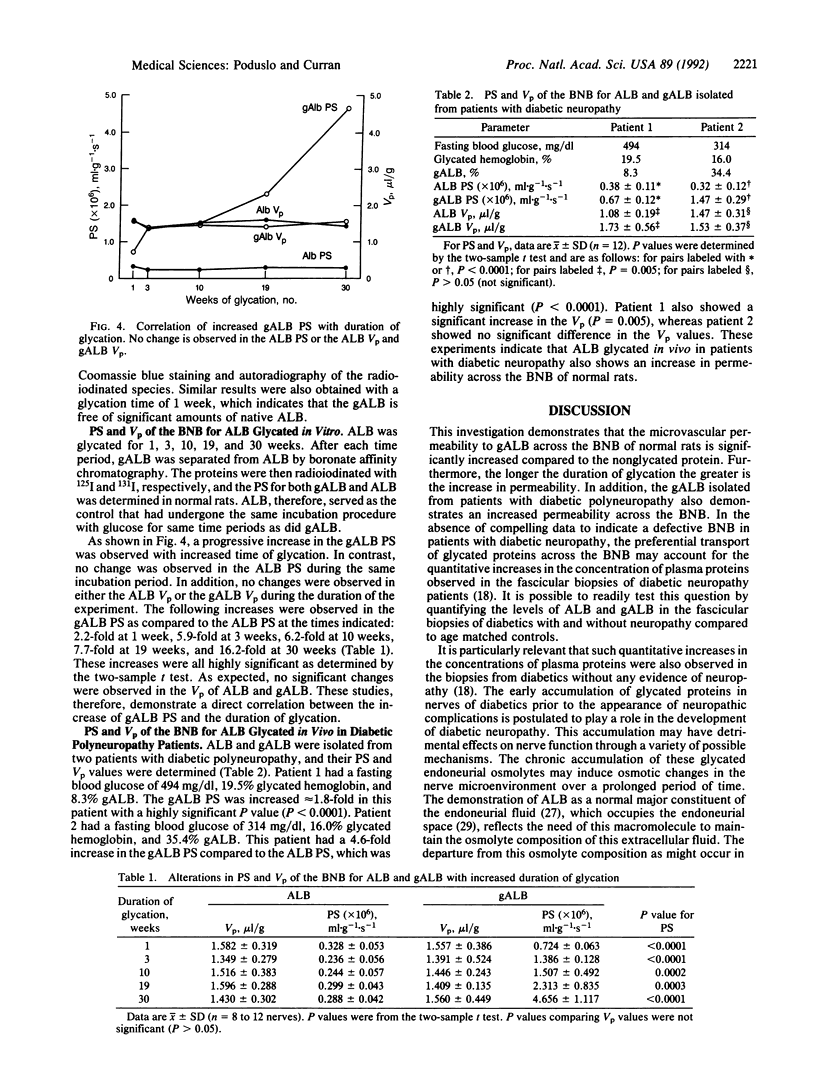
The blood-nerve transfer of human plasma albumin glycated with D-glucose was investigated by measuring the permeability coefficient-surface area product (PS) of the blood-nerve barrier to radioiodinated albumin in normal adult rat sciatic nerve. Human albumin (ALB) from normal individuals, freshly isolated by CM-Affi-Gel Blue affinity chromatography, was glycated in vitro for 1, 3, 10, 19, and 30 weeks. Glycated ALB (gALB) was separated from the nonglycated form by boronate-affinity chromatography. The efficiency of this separation was assessed by chromatography of ALB glycated with [14C]glucose and by rechromatography of isolated ALB and gALB after radioiodination. The gALB was also shown to have a higher molecular weight and be completely separated from ALB after SDS/pore gradient electrophoresis in a Tris borate/EDTA buffer. After 1 week of glycation, the gALB PS was 2.2-fold greater than the ALB PS (0.724 +/- 0.063 x 10(-6) vs. 0.328 +/- 0.053 x 10(-6) ml.g-1.s-1; mean +/- SD; P less than 0.0001) and it increased with the time of glycation reaching a maximum value of 16.2-fold greater at 30 weeks (4.656 +/- 1.117 x 10(-6) vs. 0.288 +/- 0.042 x 10(-6) ml.g-1.s-1; mean +/- SD; P less than 0.0001). No change was observed in the residual endoneurial plasma volume. In addition, the PS of gALB isolated from patients with diabetic polyneuropathy was significantly increased (P less than 0.0001) compared to the PS for ALB isolated from the same patients. It is hypothesized that the increased permeability of gALB and presumably other glycated serum components across the blood-nerve barrier, as well as the observed quantitative increase in ALB, IgG, and IgM in sural nerve biopsies from patients with diabetic polyneuropathy contribute to the development of diabetic polyneuropathy over a prolonged period of time by mechanisms that might involve osmotic changes in the nerve microenvironment, direct toxic effects of glycated macromolecules on cells within the endoneurium, or nerve damage by classical immunological mechanisms due to trapping of glycated immunoglobulins within nerve.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee M., Cerami A. The biochemistry of the complications of diabetes mellitus. Annu Rev Biochem. 1981;50:385–432. doi: 10.1146/annurev.bi.50.070181.002125. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Pongor S., Cerami A. Covalent attachment of soluble proteins by nonenzymatically glycosylated collagen. Role in the in situ formation of immune complexes. J Exp Med. 1983 Nov 1;158(5):1739–1744. doi: 10.1084/jem.158.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Trapped immunoglobulins on peripheral nerve myelin from patients with diabetes mellitus. Diabetes. 1986 Sep;35(9):999–1003. doi: 10.2337/diab.35.9.999. [DOI] [PubMed] [Google Scholar]
- Day J. F., Thornburg R. W., Thorpe S. R., Baynes J. W. Nonenzymatic glucosylation of rat albumin. Studies in vitro and in vivo. J Biol Chem. 1979 Oct 10;254(19):9394–9400. [PubMed] [Google Scholar]
- Garlick R. L., Mazer J. S., Higgins P. J., Bunn H. F. Characterization of glycosylated hemoglobins. Relevance to monitoring of diabetic control and analysis of other proteins. J Clin Invest. 1983 May;71(5):1062–1072. doi: 10.1172/JCI110856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick R. L., Mazer J. S. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem. 1983 May 25;258(10):6142–6146. [PubMed] [Google Scholar]
- Gonen B., Baenziger J., Schonfeld G., Jacobson D., Farrar P. Nonenzymatic glycosylation of low density lipoproteins in vitro. Effects on cell-interactive properties. Diabetes. 1981 Oct;30(10):875–878. doi: 10.2337/diab.30.10.875. [DOI] [PubMed] [Google Scholar]
- Kennedy L., Baynes J. W. Non-enzymatic glycosylation and the chronic complications of diabetes: an overview. Diabetologia. 1984 Feb;26(2):93–98. doi: 10.1007/BF00281113. [DOI] [PubMed] [Google Scholar]
- Mamo J. C., Szeto L., Steiner G. Glycation of very low density lipoprotein from rat plasma impairs its catabolism. Diabetologia. 1990 Jun;33(6):339–345. doi: 10.1007/BF00404637. [DOI] [PubMed] [Google Scholar]
- Mata M., Staple J., Fink D. J. The distribution of serum albumin in rat peripheral nerve. J Neuropathol Exp Neurol. 1987 Jul;46(4):485–494. doi: 10.1097/00005072-198707000-00007. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Gravallese E., Bunn H. F. Nonenzymatic glycosylation of erythrocyte membrane proteins. Relevance to diabetes. J Clin Invest. 1980 Apr;65(4):896–901. doi: 10.1172/JCI109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier V. M., Stevens V. J., Cerami A. Nonenzymatic glycosylation, sulfhydryl oxidation, and aggregation of lens proteins in experimental sugar cataracts. J Exp Med. 1979 Nov 1;150(5):1098–1107. doi: 10.1084/jem.150.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi T., Poduslo J. F., Curran G. L., Dyck P. J. Quantitative method for detection of blood-nerve barrier alterations in experimental animal models of neuropathy. Exp Neurol. 1985 Nov;90(2):365–372. doi: 10.1016/0014-4886(85)90025-1. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Curran G. L., Dyck P. J. Increase in albumin, IgG, and IgM blood-nerve barrier indices in human diabetic neuropathy. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4879–4883. doi: 10.1073/pnas.85.13.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo J. F. Glycoprotein molecular-weight estimation using sodium dodecyl sulfate-pore gradient electrophoresis: comparison of tris-glycine and tris-borate-EDTA buffer systems. Anal Biochem. 1981 Jun;114(1):131–139. doi: 10.1016/0003-2697(81)90463-2. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Low P. A., Nickander K. K., Dyck P. J. Mammalian endoneurial fluid: collection and protein analysis from normal and crushed nerves. Brain Res. 1985 Apr 15;332(1):91–102. doi: 10.1016/0006-8993(85)90392-0. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Low P. A., Windebank A. J., Dyck P. J., Berg C. T., Schmelzer J. D. Altered blood-nerve barrier in experimental lead neuropathy assessed by changes in endoneurial albumin concentration. J Neurosci. 1982 Oct;2(10):1507–1514. doi: 10.1523/JNEUROSCI.02-10-01507.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Effects of age and diabetes mellitus on the solubility and nonenzymatic glucosylation of human skin collagen. J Clin Invest. 1981 Jun;67(6):1630–1635. doi: 10.1172/JCI110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklai N., Garlick R. L., Bunn H. F. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem. 1984 Mar 25;259(6):3812–3817. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tarsio J. F., Reger L. A., Furcht L. T. Decreased interaction of fibronectin, type IV collagen, and heparin due to nonenzymatic glycation. Implications for diabetes mellitus. Biochemistry. 1987 Feb 24;26(4):1014–1020. doi: 10.1021/bi00378a006. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Nonenzymatic glycosylation of peripheral nerve protein in diabetes mellitus. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5190–5192. doi: 10.1073/pnas.78.8.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Valinsky J., Brownlee M., Cerami C., Nishimoto S., Cerami A. Advanced glycosylation endproducts on erythrocyte cell surface induce receptor-mediated phagocytosis by macrophages. A model for turnover of aging cells. J Exp Med. 1987 Aug 1;166(2):539–549. doi: 10.1084/jem.166.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasuriya A., Curran G. L., Poduslo J. F. Blood-nerve transfer of albumin and its implications for the endoneurial microenvironment. Brain Res. 1989 Aug 7;494(1):114–121. doi: 10.1016/0006-8993(89)90149-2. [DOI] [PubMed] [Google Scholar]
- Weerasuriya A., Curran G. L., Poduslo J. F. Developmental changes in blood-nerve transfer of albumin and endoneurial albumin content in rat sciatic nerve. Brain Res. 1990 Jun 25;521(1-2):40–46. doi: 10.1016/0006-8993(90)91522-i. [DOI] [PubMed] [Google Scholar]
- Weerasuriya A., Curran G. L., Poduslo J. F. Physiological changes in the sciatic nerve endoneurium of lead-intoxicated rats: a model of endoneurial homeostasis. Brain Res. 1990 May 28;517(1-2):1–6. doi: 10.1016/0006-8993(90)91000-7. [DOI] [PubMed] [Google Scholar]
- Williams S. K., Devenny J. J., Bitensky M. W. Micropinocytic ingestion of glycosylated albumin by isolated microvessels: possible role in pathogenesis of diabetic microangiopathy. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2393–2397. doi: 10.1073/pnas.78.4.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L., Mahoney E. M., Branks M. J., Fisher M., Elam R., Steinberg D. Nonenzymatic glucosylation of low-density lipoprotein alters its biologic activity. Diabetes. 1982 Apr;31(4 Pt 1):283–291. doi: 10.2337/diab.31.4.283. [DOI] [PubMed] [Google Scholar]
- Yatscoff R. W., Mehta A., Gerrard J. M., Thliveris J. Glycation of platelet protein in diabetes mellitus: lack of correlation with platelet function. Clin Biochem. 1987 Oct;20(5):359–363. doi: 10.1016/s0009-9120(87)80087-5. [DOI] [PubMed] [Google Scholar]