Abstract
Background
Despite the promise shown by stem cells for restoration of cardiac function following myocardial infarction (MI), the poor survival of transplanted cells has been a major issue. Hypoxia inducible factor-1 (HIF-1) is a transcription factor that mediates adaptive responses to ischemia. Here we hypothesize that co-delivery of cardiac progenitor cells (CPCs) with a nonviral minicircle plasmid carrying HIF-1 (MC-HIF1) into the ischemic myocardium can improve the survival of transplanted CPCs.
Methods and Results
Following MI, CPCs were co-delivered intramyocardially into adult NOD/SCID mice with either saline, MC-GFP, or MC-HIF1 versus MC-HIF1 alone (N=10/group). Bioluminescence imaging (BLI) demonstrated better survival when CPCs were co-delivered with MC-HIF1. Importantly, echocardiography showed mice injected with CPCs + MC-HIF1 had the highest ejection fraction 6 weeks post-MI (57.1±2.6%) followed by MC-HIF1 alone (48.5±2.6%), with no significant protection for CPCs + MC-GFP (44.8±3.3%) compared to saline control (38.7±3.2%, P<0.05). In vitro mechanistic studies confirmed that cardiac endothelial cells (ECs) produced exosomes which were actively internalized by recipient CPCs. Exosomes purified from ECs overexpressing HIF-1 had higher contents of miR-126 and miR-210. These microRNAs activated pro-survival kinases and induced a glycolytic switch in recipient CPCs, giving them increased tolerance when subjected to in vitro hypoxic stress. Inhibiting both of these miRs blocked the protective effects of the exosomes.
Conclusions
In summary, HIF-1 can be used to modulate the host microenvironment for improving survival of transplanted cells. The exosomal transfer of miRs from host cells to transplanted cells represents a unique mechanism that can be potentially targeted for improving survival of transplanted cells.
Keywords: hypoxia inducible-factor 1, gene therapy, stem cells, exosomes, cross talk, microRNA
INTRODUCTION
Despite significant advances in treatment, coronary heart disease (CHD) is the leading cause of morbidity and mortality worldwide, accounting for the deaths of 3.8 million men and 3.4 million women annually according to the World Health Organization (WHO)1. Although current therapeutic interventions for CHD improve clinical outcomes and prolong life, they are palliative in nature as they fail to address the fundamental issue of the loss of myocardium. In light of this, stem cell-based therapies have gained increasing interest as a potential therapy for not only attenuating cardiac dysfunction, but also affording myocardial regeneration.
Unfortunately, progress in stem cell-based therapies has been hindered by the low percentages of transplanted cells that engraft and survive long-term (<1%). This is of particular concern as early stem cell engraftment has been shown to have a direct correlation to late cardiac functional recovery2. One possible reason for the poor survival of transplanted cells is the hostile ischemic and inflammatory environment into which the cells are introduced. Hypoxia inducible-factor 1 (HIF-1) is an oxygen-sensitive transcription factor, decreasing via proteolysis in response to normoxia and increasing due to stabilization under hypoxic conditions. Stabilization of HIF-1 has been shown to mediate activation of various adaptive responses under low levels of oxygen, including glucose metabolism, cell proliferation, neovascularization, inflammation, and cellular differentiation3. Previously, we have demonstrated the use of a novel vector expression system termed minicircles, which provided long-term transgene expression of HIF-1 (MC-HIF1) in vivo in the murine heart4. We have also shown that pro-survival microRNA (miR) cocktail involving miR-21, -24, and -221 can be used to improve the engraftment of transplanted cells and therapeutic efficiency for ischemic heart diseases5.
This follow-up study investigates our hypothesis that co-delivery of cardiac progenitor cells (CPCs) together with MC-HIF1 into the ischemic heart can improve the potency of CPCs for cardiac repair. We tested our hypothesis by determining the survival of CPCs following transplantation with or without MC-HIF1 and by monitoring cardiac function, infarct size, and vascularity. The effects of MC-HIF1 on the host microenvironment were investigated to identify molecules which could potentially mediate crosstalk between local transfected cells and transplanted CPCs. Finally, in vitro assays were performed to determine the molecular mechanisms that could give cultured CPCs increased resistance against ischemic stress.
METHODS
An extended methods section is available in the online-only Data Supplement.
Isolation and Maintenance of Sca1+ Cardiac Progenitor Cells (CPCs)
Heart tissue explants were isolated from transgenic L2G mice with an ubiquitin promoter constitutively driving firefly luciferase (Fluc) and green fluorescent protein (GFP). The minced heart pieces were enzymatically dissociated into a single cell suspension. Enrichment of Sca1+ cells was achieved by sorting using the Magnetic Cell Sorting (MACS) system (Miltenyi Biotec, Sunnyvale, CA). Whole primary cell suspension was incubated with PE-conjugated anti-Sca1 Miltenyi beads in PBS + 0.5% BSA, and then washed and isolated on a magnetic column to extract Sca1+ CPCs according to manufacturer’s instructions. To increase the purity of the Sca1+ cells, magnetic sorting was performed one more time. The Sca1+ cells were cultured on 1% gelatin-coated dishes in CPC media (DMEM/F12, 10% Embryonic Stem Cell-Grade FBS, PSG, Insulin-Transferring-Selenium, 1000 units/mL LIF, 40 ng/ml EGF, 20 ng/ml bFGF) and passaged no more than 4 times.
Murine Myocardial Infarction and Cell Delivery
All animal research protocols were approved by the Stanford Animal Research Committee. Ligation of the mid-left anterior descending artery (LAD) was performed in 8–10 weeks-old female NOD SCID mice (Jackson Laboratory, Bar Harbor, ME) under anesthesia (2% inhaled isoflurane) by a single experienced microsurgeon. Mice were randomized into 4 groups: (1) saline; (2) 1 × 106 CPCs with 20 μg MC-GFP; (3) 25 μg MC-HIF1 alone, and (4) 1 × 106 CPCs with 25 μg MC-HIF1 (N=10/group). The animals were injected in the peri-infarct zone with a total volume of 25 μL using a 31-gauge Hamilton syringe.
Preparation of Conditioned Medium and Exosomes
Conditioned medium (CM) collected from endothelial cells (ECs) transfected with MC-GFP or MC-HIF1 were named ECGFP-CM or ECHIF-CM, respectively. Cells and debris were removed by differential centrifugation at 300 g for 10 mins, 2, 000 g for 10 mins, and at 13, 000 g for 15 mins, followed by filtration (0.2 μM). The filtrated CM was then concentrated using an Ultracel-10K (Millipore, Billerica, MA) centrifugal device, to a protein concentration of ~0.1 mg/ml before being resuspended in a 1:9 ratio with CPC medium. Protein concentration was determined using a Micro BCA Assay Kit (Thermo Scientific, San Jose, CA). For isolation of exosomes, ECGFP-CM or ECHIF-CM were filtered (0.2 μM) and concentrated using Ultracel-100K (Millipore). Exosomes in CM were then precipitated using Invitrogen’s Total Exosome Isolation system according to manufacturer’s protocol overnight at 4°C, followed by centrifugation at 12, 000 g for 1 hour and resuspension in PBS.
Exosomal MicroRNA Array Profiling
Exosomal RNA from ECGFP-Exo versus ECHIF-Exo was quantitated using an UV-Vis spectrophotometer and quality was assessed using the Agilent 2000 Bioanalyzer. Based on the obtained concentration, 10 ng of input RNA from each group (N=4/group) was used as starting material for a qPCR-based microRNAs (miRs) array from System Biosciences performed according to manufacturer’s instructions.
Statistical Analysis
Data are expressed as mean ± standard error mean (SEM) and statistical analyses were performed using SigmaStat 3.5 (SPSS Inc., Chicago, IL). For a two-group comparison, a student t-test was applied if the pre-test for normality (Shapiro-Wilk normality test) was not rejected at 0.05 significance level; otherwise a Mann-Whitney test for nonparametric data was used. Multiple group comparisons were performed using ANOVA followed by Tukey’s post-test. Kruskal and Wallis test followed by Dunn test comparison of pairs was used to analyze data that did not show normal distribution. P values of <0.05 indicate statistical significance.
RESULTS
Co-Delivery of MC-HIF1 Improved Survival of Sca1+ CPCs
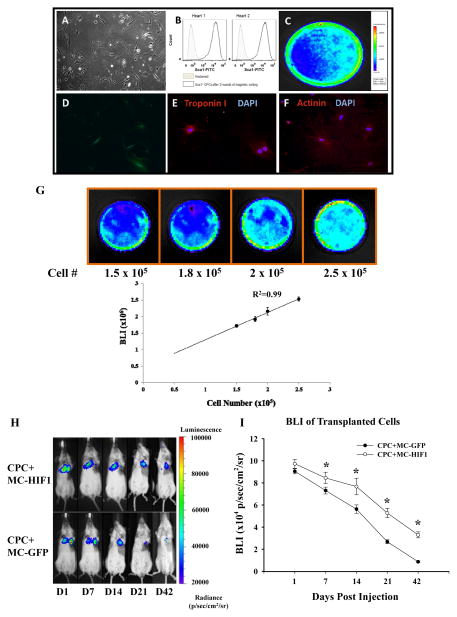
HIF-1 is known to mediate protective paracrine signaling in the ischemic heart6. Hence we evaluated whether co-delivery of stem cells together with MC-HIF1 could improve the survival of transplanted cells in the ischemic myocardium. Sca1+ CPCs isolated from transgenic L2G mice expressed both Fluc and GFP, and were amenable to lineage commitment including differentiation into cardiac lineage (Figure 1A–F). In vitro characterization demonstrated a robust linear correlation between bioluminescence signal intensity and cell numbers (R2=0.99), indicating the validity of this imaging method for assessing cell survival in vivo (Figure 1G). Next, we assessed the survival of these CPCs noninvasively by BLI when acutely co-delivered with MC-HIF1 or MC-GFP into mice subjected to MI. BLI showed CPCs co-delivered with MC-HIF1 was readily detectable as late as 42 days post-injection (3.3×104±2.7×103 p/sec/cm2/sr), in contrast to a barely detectable signal from CPCs co-delivered with MC-GFP (2.7×104±1.7×103 p/sec/cm2/sr; P<0.05) at day 21 and undetectable by day 42 (Figure 1H). These results were also confirmed by quantitative analysis of Fluc activity (Figure 1I) and immunofluorescence staining (Supplemental Figure 1A).
Figure 1.
Co-delivery of hypoxia inducible-factor 1 (HIF-1) driven by minicircle (MC) plasmid promotes survival of transplanted Sca1+ cardiac progenitor cells (CPCs) in the ischemic heart. (A) The morphology of isolated CPCs growing on gelatin coated dish. (B) Flow cytometric analysis of purified Sca1+ CPC population from two preparations. Typical purity of isolation is >95%. CPCs isolated from transgenic mice express robust (C) firefly luciferase (Fluc) and (D) GFP expression. After culturing in cardiac differentiation induction medium, differentiated CPCs stained positively with (E) cardiac Troponin I and (F) α-actinin demonstrating its amenability to lineage commitment. (G) CPCs were seeded into dishes with increasing cell numbers. Assessment of BLI signals showed a robust linear correlation (R2=0.99) between the cell number and Fluc expression, which is crucial for accurate tracking of cell survival by in vivo imaging. (H) Representative BLI of animals injected with CPCs intramyocardially together with either MC-GFP or MC-HIF1 following myocardial infarction (MI) at indicated time points. (I) Quantitative analysis of longitudinal BLI signal demonstrates that CPCs co-delivered with MC-HIF1 had better survival compared to CPCs co-delivered with MC-GFP over a period of 42 days. *P<0.05 (N=10/group).
Combined Cell & Gene Therapy Improves Cardiac Outcomes
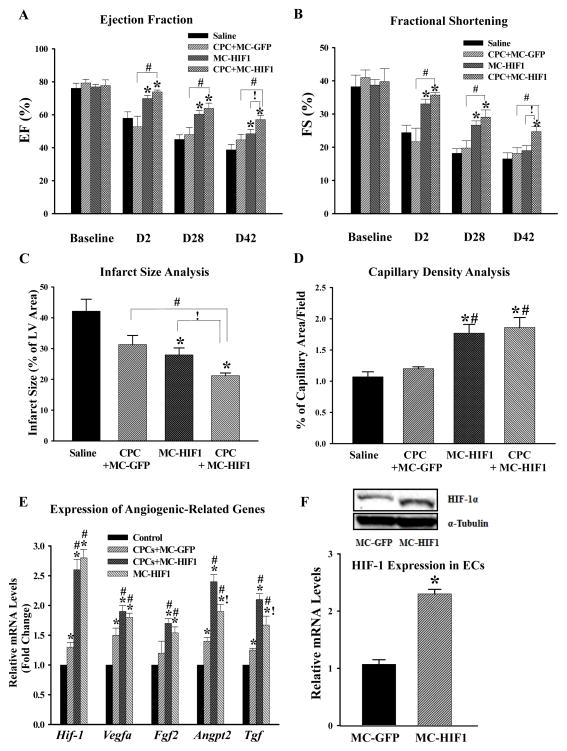
To determine whether enhanced survival of CPCs by co-delivery with MC-HIF1 could improve cardiac function, ten-week-old female NOD-SCID mice subjected to MI were divided into four groups and received either (i) saline control, (ii) CPCs + MC-GFP, (iii) MC-HIF1 alone, or (iv) CPCs + MC-HIF1 by intramyocardial injection into the peri-infarct area. Ejection fraction (EF) and fractional shortening (FS) prior to MI were comparable across all groups when measured by echocardiography (Figure 2A–B). In the saline control group, a significant decrease in EF and FS were observed suggesting compromised cardiac function. At all measured time points, the CPCs + MC-GFP group showed no significant improvement in EF and FS compared to saline control group. By contrast, mice receiving MC-HIF1 alone or CPCs + MC-HIF1 had significantly improved EF and FS as early as 2 days post-MI, and these changes were sustained through 6 weeks post-MI. Importantly, these changes were greater in CPCs + MC-HIF1 group compared to MC-HIF1 alone. These results suggest that co-delivery of CPCs together with MC-HIF1 provided superior therapeutic effects among all groups after MI.
Figure 2.
Combination of cell and gene therapy provides synergistic therapeutic effects following MI. Comparison of (A) ejection fraction and (B) fractional shortening among all 4 groups at indicated time points revealed that CPCs co-delivered with MC-HIF1 had superior therapeutic effects among all groups. *P<0.05 vs. saline group; #P<0.05 vs. CPCs + MC-GFP; !P<0.05 vs. MC-HIF1 (N=10/group). (C) Three days post-MI, mice from each group were sacrificed and hearts were collected for determination of infarct size by tetrazolium chloride staining. *P<0.05 vs. saline group; #P<0.05 vs. CPCs + MC-GFP; !P<0.05 vs. MC-HIF1 (N=6/group). (D) Vascular density in each group was determined by CD31 staining at 7 days post-MI. *P<0.05 vs. saline group; #P<0.05 vs. CPCs + MC-GFP (N=6/group). (E) Areas close to engrafted GFP+ CPCs were laser microdissected to assess levels of angiogenic gene activation. Samples were collected 5 days post-MI. qPCR showed that MC-HIF1 upregulates the expression of angiogenic genes, some of which were further augmented with the presence of CPCs. *P<0.05 vs. control (non-ischemic remote zone of MC-HIF1); #P<0.05 vs. CPCs + MC-GFP; !P<0.05 vs. CPCs + MC-HIF1 (N=5/group). (F) In a separate set of experiments, mice subjected to MI received either MC-GFP or MC-HIF1 only. Three days later, cardiac ECs were isolated from the peri-infarct region, ECs from MC-HIF1 group were found to have higher expression of HIF-1 at both protein (upper panel) and gene levels (lower panel) compared to ECs from MC-GFP group, indicating that cardiac ECs are receptive to MC-HIF1 transfection. *P<0.05 vs. MC-GFP (N=6/group).
As both the CPCs + MC-HIF1 and MC-HIF1 alone groups demonstrated preserved cardiac functions as early as day 2, we were interested to evaluate the infarct size. Six mice were randomly selected from each of the 4 groups to undergo infarct size analysis by tetrazolium chloride (TTC) staining at 3 days post-MI. While there was no discernible difference in the infarct size between saline–injected or CPCs + MC-GFP–injected mice, infarct size was significantly reduced in both CPCs + MC-HIF1–injected and MC-HIF1–injected MI mice, albeit to a lesser extent in the latter group (Figure 2C, Supplemental Figure 1B). Immunofluorescence staining with an endothelial marker (CD31) in the peri-infarct areas of hearts collected 7 days post-MI also revealed increased capillary density for CPCs + MC-HIF1 and MC-HIF1 alone groups compared to saline group. CPCs + MC-GFP group failed to show similar improvement compared to saline group (Figure 2D, Supplemental Figure 1C).
HIF-1 Modulates the Ischemic Milieu into a More Hospitable Environment
HIF-1 is a transcription factor that activates various adaptive responses against ischemic stress3. Therefore, we reasoned that sustained expression of HIF-1 would modulate the ischemic milieu by rendering it less hostile for transplanted CPCs. To test this hypothesis, myocardial tissue surrounding the transplanted Fluc+/GFP+ CPCs co-delivered with either MC-GFP or MC-HIF1 was laser-microdissected 5 days post-MI and qPCR for angiogenic-related genes (Hif-1α, Vegfa, Fgf2, Angpt2, and Tgf) was performed (Figure 2E). An additional group of MC-HIF1 delivery alone without cells was also included and the remote non-ischemic tissue of hearts from MC-HIF1 alone group was used as controls. A significant upregulation of several angiogenic genes was observed in the peri-transplant tissues of CPCs + MC-GFP group compared to controls, and this upregulation was significantly augmented in the CPCs + MC-HIF1 group (Figure 2E). Interestingly, although all these angiogenic genes were significantly upregulated in the MC-HIF1 alone group compared to control or CPCs + MC-GFP, expression of Angpt2 and Tgf were significantly lower compared to CPCs + MC-HIF1 group (Figure 2E). These results suggest that the presence of HIF-1 modifies the local microenvironment of transplanted CPCs at least through upregulation of angiogenic-related genes and the presence of CPCs helps to further augment the upregulation of selected genes such as Angpt2 and Tgf, hence conferring additional protection. To investigate whether host ECs could have taken up MC-HIF1, leading to a modified microenvironment, we next injected MC-GFP or MC-HIF into the peri-infarct zone of murine hearts (N=6/group). Three days later, hearts were explanted, digested into single cell suspensions and ECs were isolated using CD31 magnetic beads. ECs from MC-HIF1-injected hearts were found to have significantly elevated expression of HIF-1 both at gene and protein levels compared to MC-GFP (Figure 2F), indicating that ECs from host myocardium are receptive to injected MC-HIF1.
Molecular Crosstalk Between ECs and CPCs Involves Transfer of Exosomes
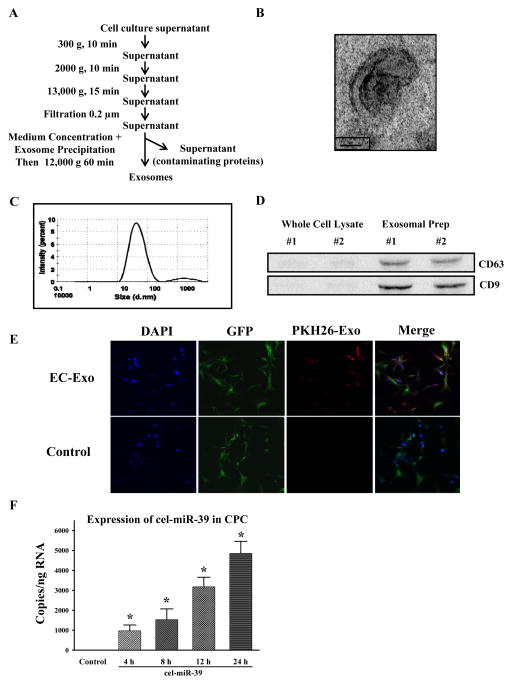
Since we had demonstrated that MC-HIF1 modifies the local ischemic milieu, we investigated the possibility that molecular crosstalk between host ECs and transplanted CPCs may lead to improved survival. Recently, it has been shown that cell-cell communication can be mediated by a class of extracellular vesicles termed exosomes7, 8. To study the potential involvement of exosomes in molecular crosstalk, we first determined whether cardiac ECs produce them. We isolated exosomes from the supernatants of cultured ECs through a series of microfiltration and centrifugation steps (Figure 3A). Exosome identity was assessed by electron microscopy which demonstrated a cup-shaped morphology, approximately 110 nm in size, as usually observed in exosomes (Figure 3B). Dynamic light scattering analysis further confirmed a size distribution consistent with exosomes vesicles in the range of 30–110 nm (Figure 3C). Comparison of cell lysate with exosomal preparations by immunoblotting revealed the enrichment of exosomal markers CD63 and CD9 confirming their purity (Figure 3D)9. We next determined the capacity of ECs transferring exosomes to recipient CPCs by pre-labeling isolated exosomes with the fluorescent dye PKH26 before adding them to the medium of CPCs. Typical labeling efficiency of exosomes is around 85% as determined by FACS (Supplemental Figure 2A). Following 6 hours of incubation, confocal imaging revealed the ability of CPCs to uptake the fluorescently labeled exosomes in a time-dependent manner (Figure 3E, Supplemental Figure 2B). To further confirm these findings, we transfected ECs with a miRNA that is naturally present only in Caenorhabditis elegans (cel-miR-39). Forty-eight hours later, exosomes from these cel-miR-39-transfected ECs were isolated and added to CPCs. Analysis of cel-miR-39 expression levels in CPCs demonstrated the presence of cel-miR-39, and that cel-miR-39 is transferred from ECs to CPCs in a temporal manner (Figure 3F).
Figure 3.
Purified exosomes produced by ECs are actively internalized by CPCs in vitro. (A) Purification scheme of exosomes from EC culture supernatant. (B) Cup-shaped morphology of purified ECs’ exosomes assessed by transmission electron microscopy. (C) Dynamic light scattering analysis of purified ECs’ exosomes demonstrating a physical size distribution of 10–110 nm. (D) Immunoblotting revealed an enrichment of exosomal markers CD63 and CD9 in purified exosomal fraction compared to whole EC lysates. Two separate preparations were shown. (E) CPCs were cultured in the presence of EC-derived PKH26-labeled exosomes or absence (control; same volume of PBS labeled similarly) for 6 hours. Exosomes were taken up CPCs as shown by confocal microscopy. (F) ECs were transfected with cel-miR-39 or left untransfected. CPCs were then treated with exosomes isolated from untransfected (control) or cel-miR-39 transfected ECs for the indicated times. *P<0.05 vs. control (N=4/group).
Overexpression of HIF-1 Alters the microRNA Contents of EC-derived Exosomes
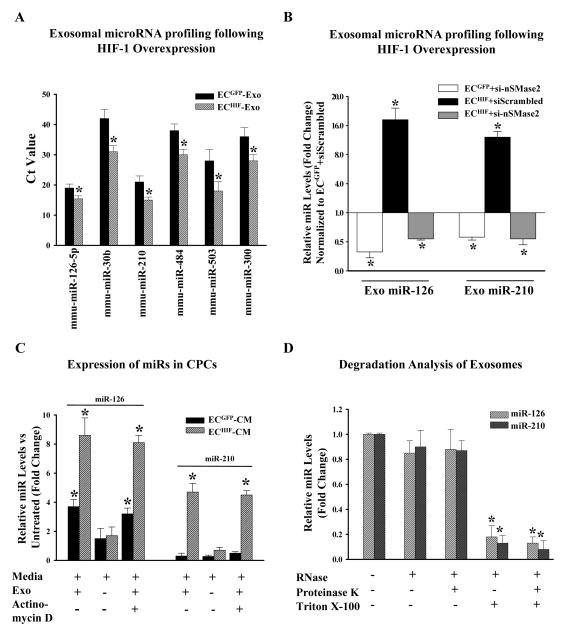
We next investigated the effects of MC-HIF1 transfection upon the EC-derived exosome composition. HIF-1 overexpression did not lead to a change in the amount of exosomes released from ECs (Supplemental Figure 2C). We next studied the repertoires of microRNAs (miRs) contained in exosomes secreted by both groups of cells (ECGFP-Exo vs. ECHIF-Exo) to determine potential miRs that might be regulated by overexpression of HIF-1. Using a qPCR array of 380 mouse miRs, profiling of RNA isolated from exosomes showed that overexpression of HIF-1 led to upregulation of several exosomal miRs (Figure 4A). We chose to focus on two specific miRs, namely the endothelial-specific miR-126; and also miR-210 which has been previously reported to be regulated by HIF-110 for subsequent experiments. We confirmed the involvement of HIF-1 in upregulating these miRs by obtaining similar results using a pharmacological activator of HIF-1, dimethyloxalylglycine (DMOG) (Supplemental Figure 2D). In addition, the requirement for exosome biogenesis in the increased expression of secreted miRs was demonstrated. Knockdown of sphingomyelinase (nSMase2), which has been shown to inhibit exosome generation11, reduced the presence of both exosomal miR-126 and miR-210 from ECGFP cells or ECHIF cells (Figure 4B).
Figure 4.
HIF-1 modulates the microRNA (miRs) repertoire in ECs-derived exosomes, which are transferable to CPCs. (A) ECs were transfected with either MC-GFP (control) or MC-HIF1. Following transfection, exosomes were purified from both groups and subjected to miRs profiling. Expression of selected exosomal miRs was increased following MC-HIF1 transfection. *P<0.05 vs ECGFP-Exo (N=4). (B) Based on the profiling results, miR-126 and miR-210 were chosen for detailed analysis. ECGFP and ECHIF were transfected with either scrambled siRNA or siRNA targeting nSMase2. Exosomes were then purified from each group, and the expression of miR-126 and miR-210 were determined by qPCR using Taqman probes, and expressed as relative fold-change normalized to ECGFP transfected with scrambled siRNA. *P<0.05 vs. ECGFP+siScrambled (N=6/group). (C) Recipient CPCs were treated with vehicle or actinomycin D (5 μg/ml). CPCs were then grown in normal growth medium or supplemented with either ECGFP-CM or ECHIF-CM in the absence or presence of exosomes (Exo) as indicated. After 24 hours, the expression of miR-126 and miR-210 in CPCs was determined by qPCR and expressed as fold-change normalized against CPCs grown in normal growth medium. *P<0.05 (N=6). (D) Exosomes were isolated from ECHIF and incubated with the indicated reagents for 45 mins at 37°C before isolation of RNA and measurement of expression levels of miR-126 and miR-210 by qPCR. *P<0.05 vs. untreated (N=4).
Following incubation with control ECGFP-CM, CPCs expressed significantly higher levels of miR-126 but levels of miR-210 remain unchanged, consistent with the fact that miR-126 is predominantly expressed in ECs (Figure 4C) rather than CPCs. In keeping with the profiling results, CPCs incubated with ECHIF-CM had a significantly higher expression of both miR-126 and miR-210 compared to CPCs supplemented with ECGFP-CM or untreated cells, indicating that HIF-1 leads to enriched expression of these miRs in target cells (Figure 4C). To directly investigate the requirement for exosomes in shuttling miRs, we depleted ECGFP-CM and ECHIF-CM of exosomes through ultracentrifugation. The depletion of exosomes considerably abrogated the expression of miR-126 and miR-210 in target CPCs (Figure 4C). The presence of a transcription inhibitor, actinomycin D, also did not affect levels of miR-126 and miR-210 in the treated CPCs, confirming the aforementioned effects as direct consequences of exosomal-mediated transfer (Figure 4C). Finally, degradation analysis of exosomes using a combination of ribonuclease (RNase), proteinase K (for degrading proteins), and Triton X-100 (disrupts phospholipid membranes) further demonstrated that the majority of endothelial-derived miR-126 and miR-210 are transmitted into CPCs through vesicle-protected RNA (Figure 4D), instead of forming soluble ribonucleoprotein complexes, as recently shown12–14.
Transferred miR-126 Modulates Biological Properties of CPCs
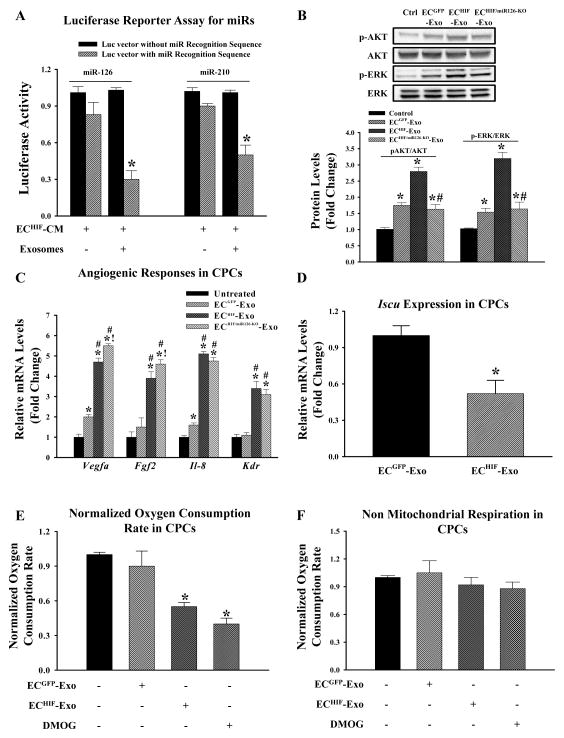
Next, to test the biological functionality of the transferred miR-126 and miR-210 in CPCs, cells were transfected with a 3′-UTR luciferase reporter vector harboring a binding site for either miR-126 or miR-210 respectively. We also included a second group with a control reporter vector without a miR recognition site. As shown in Figure 5A, when CPCs were cultured with ECHIF-CM, luciferase activity was decreased significantly in cells transfected with specific miR recognition sequence vector, but not the control vector or when exosomes were depleted from the CM through ultracentrifugation. Transfected CPCs in media alone displayed robust luciferase production (data not shown). These results show the transferred miRs were functional in the recipient CPCs. We then examined the biological impact of these transferred miRs that could potentially confer the demonstrated in vivo tolerance against ischemic stress in the recipient cells. We found that CPCs given ECGFP-Exo had increased phosphorylated levels of pro-survival kinases phospho-ERK (p-ERK) and phospho-AKT (p-AKT) and that these effects were further augmented when CPCs were given ECHIF-Exo, consistent with the established role of miR-126 in regulating these kinases (Figure 5B)15. Silencing of miR-126 blunted the phosphorylation of p-ERK and p-AKT, lending support to the importance of miR-126 in ECHIF-exosomes (Figure 5B). Furthermore, CPCs given ECHIF-Exo had elevated expression of several angiogenic-related genes such as Vegfa and Fgf2 compared to untreated or ECGFP-Exo cells (Figure 5C). These angiogenic factors have been previously shown to regulate p-ERK and p-AKT16. However, the upregulation of Vegfa is in contrary to the previously described role of miR-126 as a direct repressor of this gene. Hence, we subjected CPCs to culture conditions in which they were supplemented with exosomes derived from ECHIF co-transfected with antagomirs targeting miR-126 and we found that these cells had significantly higher expression of both Vegfa and Fgf2, but not Il-8 and Kdr compared to ECHIF transfected with scrambled antagomirs (we did not observe a difference between ECHIF vs. ECHIF-scrambled). These data suggest that while there were still partial repressive effects of miR-126, it was insufficient to lead to downregulation of Vegfa presumably due to the presence of other factors contained within ECHIF-Exo. We further confirmed the functionality of miR-126 in ECHIF-Exo through additional experiments by measuring the expression of Spred1, a validated miR-126 target gene in untreated CPCs, or CPCs given ECGFP-Exo, ECHIF-Exo, or ECHIF-miR126-KO-Exo. We found that exosomes derived from ECGFP led to partial suppression of Spred1 compared to untreated CPCs; these effects were further augmented when ECHIF-Exo were added and reversed when CPCs were treated with antagomirs targeting miR-126 (Supplemental Figure 2E).
Figure 5.
Transferred exosomal miRs regulate biological properties of recipient CPCs. (A) CPCs were transfected with a luciferase reporter vector containing either miR-126 or miR-210 recognition sequence or a control luciferase vector lacking the sequence. CPCs were then given ECHIF-CM in the presence or absence of exosomes as indicated. CPCs were then cultured for another 24 hours and luciferase activity was determined and expressed as fold-decrease of cells transfected with the luciferase reporter containing specific miR recognition sequence over the vector lacking the recognition sequence for each culture condition. *P<0.05 (N=4) vs. cells transfected with vector lacking miR recognition sequence. (B) Representative immunoblots and densitometry quantification of indicated proteins in CPCs grown in either normal conditions, or supplemented with ECGFP-Exo, ECHIF-Exo, or ECHIF/miR126-KO-exosomes. *P<0.05 vs. untreated CPCs; #P<0.05 vs. ECHIF (N=4). (C) Expression of angiogenic genes in CPCs grown in each culture condition as indicated was determined by qPCR. *P<0.05 vs. untreated; #P<0.05 vs. ECGFP-Exo; !P<0.05 vs. ECHIF-Exo (N=8). (D) Expression of Iscu, a validated miR-210 target gene, was determined in CPCs supplemented with either ECGFP-Exo or ECHIF-Exo. *P<0.05 (N=4) vs. CPCs supplemented with ECGFP-Exo. CPCs were cultured in conditions as specified for 24 hours. (E) Oxygen consumption and (F) non-mitochondrial respiration were then determined and expressed as fold-change compared to untreated control cells. Dimethyloxalylglycine (DMOG), a known HIF-1 activator was used as positive control for reduced oxygen consumption. *P<0.05 vs. untreated controls (N=6).
Transferred miR-210 Modulates Biological Properties of CPCs
Having shown that miR-126 affects CPCs, we postulated that transferred miR-210 would also have a biological relevance in CPCs. Iron-sulfur cluster scaffold homolog (Iscu) is a validated target of miR-210, which upon repression, reduces mitochondrial metabolism17. A significant reduction of Iscu was observed in CPCs treated with ECHIF-Exo indicative of repression through miR-210 (Figure 5D). To assess the metabolic profile of CPCs as a consequence of increased miR-210/repressed Iscu, we measured oxygen consumption rate (OCR) in these cells by SeaHorse XF bioenergetic system. CPCs treated with ECHIF-Exo displayed a significant reduction in basal oxygen consumption (normalized against cell numbers) compared to untreated CPCs or CPCs treated with ECGFP-Exo, showing that increased miR-210 leads to reduced mitochondrial metabolism in cells (Figure 5E). In contrast, non-mitochondrial respiration was similar among all groups suggesting that ECHIF-Exo act primarily in modulating mitochondrial respiration (Figure 5F). In addition, we noted an increase in intracellular lactate with CPCs treated with ECHIF-Exo, indicating increased glycolysis as usually seen along reduced mitochondrial metabolism (Supplemental Figure 2F).
Exosomes Reduce Cellular Damage of CPCs under Ischemic Conditions
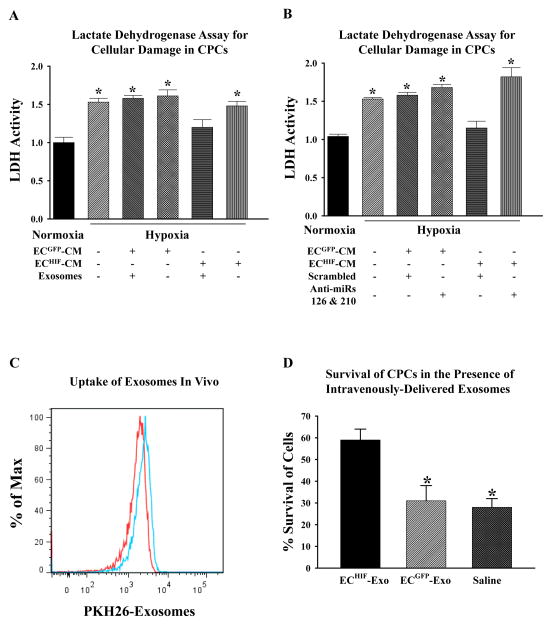
Given the known evidence for increased levels of pro-survival kinases, enhanced angiogenic response and reduced metabolic demand as adaptive beneficial responses18, 19, we hypothesized that these effects could collectively provide CPCs with increased tolerance to ischemic stress. Indeed, CPCs grown in ECHIF-CM had significantly reduced cellular damage as assessed by lactate dehydrogenase (LDH) release when subjected to in vitro hypoxia compared to untreated or cells grown in ECGFP-CM (Figure 6A). The protective effects of ECHIF-CM were dependent on the presence of exosomes as ultracentrifugation abrogated the aforementioned effects. Importantly, CM derived from ECs overexpressing HIF-1 that were additionally transfected with antagomirs against miR-126 and miR-210 failed to provide similar therapeutic effects when given to CPCs, indicating that these miRs are crucial for ECHIF-CM to afford CPCs with resistance against ischemic stress (Figure 6B). Finally, to determine whether ECHIF-Exo can directly protect CPCs following transplantation into the ischemic heart, we delivered CPCs into mice following MI concomitant with intravenous delivery of either saline, PKH26-labeled ECGFP-Exo, or PKH26-labeled ECHIF-Exo, respectively. Flow cytometry analysis of isolated CPCs from explanted hearts demonstrated the presence of PKH26 exosomes, confirming the uptake of these exosomes by CPCs in vivo (Figure 6C). Longitudinal BLI of mice showed that CPCs that received ECHIF-Exo had better survival in the group compared to saline or ECGFP-Exo group when measured 1 week post-injection (Figure 6D).
Figure 6.
Exosomes directly provide CPCs with increased tolerance against ischemic stress both in vitro and in vivo. (A) CPCs were grown in either normal conditions, or supplemented with ECGFP-CM or ECHIF-CM with or without the presence of exosomes as indicated, before being subjected to in vitro hypoxic stress. Lactate dehydrogenase (LDH) was then measured as an indicator of cellular damage and expressed as fold-change compared to untreated cells kept in normoxic conditions. *P<0.05 (N=6) vs. normoxic cells. (B) ECHIF were co-transfected with either scrambled antagomirs or antagomirs targeting miR-126 and miR-210. CPCs were then grown in conditioned medium from each group as indicated before being subjected to LDH assay to assess the importance of miR-126 and miR-210 in ECHIF-CM-mediated protection seen in (A). *P<0.05 vs. normoxic control (N=6). (C) Following MI, CPCs were intramyocardially injected into mice hearts, and a bolus of PKH26-labeled ECs-exosomes was delivered intravenously. After 12 hours, the animals were sacrificed and CPCs were re-isolated from the hearts. Samples were measured by flow cytometry for GFP+ and PKH26+ events. The histogram shows that PKH26-exosomes (blue lines) were detectable in ~10% of GFP+ CPCs (red lines) demonstrating that CPCs are capable of uptaking exosomes in vivo. (D) To determine whether ECHIF-Exo could confer increased tolerance to CPCs in vivo, cells were delivered intramyocardially post-MI into mice concomitantly with intravenous injection of either saline, ECGFP-Exo or ECHIF-Exo. BLI was then performed at day 1 and day 7 post-MI, and survival of CPCs was expressed as a % of signals intensity at day 7 compared to initial signals intensity of day 1. *P<0.05 vs. saline (N=6/group).
DISCUSSION
This study revealed a multifaceted mechanism by which delivery of HIF-1 via a MC plasmid platform enhances survival of co-transplanted Sca1+ CPCs. First, using a murine model of MI followed by co-delivery of CPCs with MC-GFP or MC-HIF, we observed that a combinatorial delivery with MC-HIF resulted in prolonged survival of transplanted CPCs, prevented cardiac remodeling, reduced infarct size, and enhanced vascularity. Second, HIF-1 modulates the local milieu of the ischemic heart at least partly by effects upon host cardiac ECs. Third, we found that these cardiac ECs generate and release exosomes in vitro, which are actively internalized by CPCs when present in the growth medium. Fourth, HIF-1 modifies the biological contents of these exosomes, resulting in a phenotypic change in recipient cells. Fifth, in vitro hypoxia assays revealed that exosomes derived from ECs overexpressing HIF-1 contributed to CPCs’ increased tolerance under hypoxic stress. These findings were validated in vivo since intravenous delivery of ECHIF-Exo significantly increased survival of transplanted CPCs in the ischemic heart as determined by BLI.
HIF-1 is a master transcriptional activator that mediates various physiological responses to hypoxia3. Here, we demonstrated that co-delivery of CPCs together with MC-HIF1 led to better survival of transplanted cells and was associated with preserved cardiac function. The synergistic effects of this combinatorial approach were evident early on, as documented by a smaller infarct size and increased vascularity when compared to other treatment groups. It is well known that stem/progenitor cells in adult organs reside in specialized niches that provide an ideal microenvironment for their maintenance20, 21. In this context, these cells are often found in close proximity to blood vessels, suggesting a role for these vessels in the regulation of stem cell self-renewal and differentiation. Following an ischemic event, ECs are highly susceptible to undergoing necrosis, potentially compromising the maintenance and expansion of resident stem cells. Using laser microdissection analysis, we observed that MC-HIF1 changes the gene expression landscape of the local milieu proximal to transplanted cells, including resident cardiac ECs.
Given that HIF-1 affects local cardiac ECs and improves survival of transplanted CPCs, it is highly plausible that potential beneficial crosstalk could occur between these two cell types. In recent years, exosomes have emerged as potential candidates for mediating cell-cell communication in various physiological or pathophysiological conditions through transfer of proteins, mRNAs and miRs7, 8. We confirmed that cardiac ECs promoted release and transfer of exosomes to CPCs in vitro, and demonstrated differences in miR profiling of ECGFP-Exo (control) versus ECHIF-Exo, most prominently miR-126 and miR-210. Our degradation analysis data also revealed that a significant amount of miR-126 and miR-210 is exosome-enclosed (unaffected by RNase treatment but susceptible to blockade of nSMase2), distinct from some previous studies indicating that miR-126 is transferred in a vesicle-free form or through apoptotic bodies13, 14. These data might reflect that additional mechanisms regulating the packaging of biological contents into exosomes are cell-type dependent. Recipient cell types might also differ in their ability to respond to exosomes, as previous studies have reported receptor specificity as a crucial factor for internalization of exosomes22.
Endothelial miR-126 has been shown to regulate multiple pathways, including the regulation of pro-survival kinases p-ERK and p-AKT. Our findings demonstrated that CPCs internalizing ECGFP-Exo had a transient activation of these kinases that were further increased by ECHIF-Exo. The transient phosphorylation of these kinases is known to be protective in the settings of ischemia by limiting both the apoptotic and necrotic components of cell death. Likewise, miR-210, a known HIF-1-regulated miR, was also shown to be preferentially upregulated in exosomes by HIF-1, switching recipient CPCs to a preferentially glycolytic state (reduced oxygen usage). This is consistent with previous studies documenting that one of the many targets of miR-210 is Iscu17, and repression of this gene leads to lower mitochondrial metabolism; both phenomena were observed in our study. This metabolic adaptation to oxygen conservation reflects a lower utilization of the mitochondrial electron transport chain (ETC), which reduced the generation of reactive oxygen species (ROS). Indirectly, this phenomenon could potentially function as a preemptive measure against ischemic stress, because the production of ROS is known to be greatly increased during ischemia and is highly detrimental to cellular health. In keeping with our hypothesis, we observed that CPCs in ECHIF-CM had less cellular damage when exposed to in vitro hypoxic stress. This increased tolerance is attributable to exosomes as we found that depletion of exosomes from the CM abrogated the protective effects. Additionally, the importance of both miR-126 and miR-210 mediating the protective effects of the ECHIF-CM was corroborated because such effects were abolished when the donor cardiac EC cells were transfected with antagomirs targeting both miRs.
In the present study, a full comparison of the abundance of exosomes in vitro and in vivo was not feasible as the concentrations and transfer efficiencies are probably different. Nevertheless, we have generated evidence to support a physiological role for exosomes in mediating increased tolerance against ischemic stress which helps to improve survival of transfected cells. Although we focused on the transfer of genetic material following HIF-1 overexpression from cardiac ECs to CPCs, we believe that reciprocal crosstalk could also occur as well with other resident cell types, including fibroblasts and host cardiomyocytes, and even the possibility of CPCs themselves taking up MC-HIF1 which warrants deeper investigation. Likewise, although our data indicated a physiological role for exosomal miR-126 and miR-210 in recipient cells that was reversed upon inhibition of both, this does not preclude the involvement of other miRs and proteins; further investigation is needed to show whether these proteins can improve survival of transplanted cells, as various studies have demonstrated the complexity of biological molecules enclosed within exosomes23. Finally, our results demonstrating the upregulation of Vegfa despite being a direct target of miR-126 reveals the intricate mechanisms mediated by exosomes.
Collectively, we show for the first time an intricate exosome-mediated crosstalk interface via HIF-1 between the vascular endothelium and transplanted stem cells. The improved tolerance against ischemic stress is afforded through activation of pro-survival kinases, increased angiogenic responses, and reduced metabolic demand, and involves, at least partially, miR-126 and miR-210 (Supplemental Figure 3). Importantly, these results suggest the combination of gene- and cell-based therapies should be explored in future clinical trials.
Supplementary Material
Acknowledgments
FUNDING SOURCES
We are grateful for the funding support by National Institutes of Health U01 HL099776, R01 EB009689, R01 HL093172, R01 HL095571, American Heart Association Established Investigator Award, and Fondation Leducq (JCW).
Footnotes
DISCLOSURES
None
References
- 1.Hausenloy DJ, Erik Botker H, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, Lecour S, van Laake LW, Madonna R, Ruiz-Meana M, Schulz R, Sluijter JP, Yellon DM, Ovize M. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2013;98:7–27. doi: 10.1093/cvr/cvt004. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Narsinh KH, Lan F, Wang L, Nguyen PK, Hu S, Lee A, Han L, Gong Y, Huang M, Nag D, Rosenberg J, Chouldechova A, Robbins RC, Wu JC. Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circ Cardiovasc Imaging. 2012;5:481–90. doi: 10.1161/CIRCIMAGING.111.969329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong SG, Hausenloy DJ. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol Ther. 2012;136:69–81. doi: 10.1016/j.pharmthera.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Huang M, Chen Z, Hu S, Jia F, Li Z, Hoyt G, Robbins RC, Kay MA, Wu JC. Novel minicircle vector for gene therapy in murine myocardial infarction. Circulation. 2009;120:S230–7. doi: 10.1161/CIRCULATIONAHA.108.841155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu S, Huang M, Nguyen PK, Gong Y, Li Z, Jia F, Lan F, Liu J, Nag D, Robbins RC, Wu JC. Novel microRNA prosurvival cocktail for improving engraftment and function of cardiac progenitor cell transplantation. Circulation. 2011;124:S27–34. doi: 10.1161/CIRCULATIONAHA.111.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang M, Nguyen P, Jia F, Hu S, Gong Y, de Almeida PE, Wang L, Nag D, Kay MA, Giaccia AJ, Robbins RC, Wu JC. Double knockdown of prolyl hydroxylase and factor-inhibiting hypoxia-inducible factor with nonviral minicircle gene therapy enhances stem cell mobilization and angiogenesis after myocardial infarction. Circulation. 2011;124:S46–54. doi: 10.1161/CIRCULATIONAHA.110.014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–8. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 9.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. Journal of proteomics. 2010;73:1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–8. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- 11.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 12.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen F, Yang X, Hoyer FF, Paul K, Heiermann N, Becher MU, Abu Hussein N, Kebschull M, Bedorf J, Franklin BS, Latz E, Nickenig G, Werner N. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1925–35. doi: 10.1161/ATVBAHA.112.253229. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Li YS, Nguyen P, Wang KC, Weiss A, Kuo YC, Chiu JJ, Shyy JY, Chien S. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ Res. 2013;113:40–51. doi: 10.1161/CIRCRESAHA.113.280883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JJ, Zhou SH. Mesenchymal stem cells overexpressing MiR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiol J. 2011;18:675–81. doi: 10.5603/cj.2011.0032. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–71. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–84. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, Harten SK, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–80. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 19.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–34. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 20.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–6. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 22.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380–5. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312–7. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.