Abstract
Objectives Postoperative hearing preservation rates for patients with large vestibular schwannomas range from 0 to 43%. The clinical and radiographic factors predicting hearing preservation in smaller vestibular schwannomas are well described; however, their importance in larger tumors is unclear. We investigated factors predicting hearing preservation in large vestibular schwannomas.
Design Retrospective review.
Setting Quaternary care academic center.
Participants A total of 85 patients with unilateral vestibular schwannomas > 3 cm underwent retrosigmoid resections.
Main Outcomes Measures Preoperative and postoperative serviceable hearing rates.
Methods Clinical and radiographic data including preoperative and postoperative audiograms, preoperative symptoms, magnetic resonance imaging features, and postoperative facial weakness were analyzed.
Results Hearing was preserved in 41% of patients (17 of 42) with preoperative serviceable hearing. Hypertension and diabetes increased the likelihood of preoperative hearing loss. Preoperative tinnitus predicted a lower likelihood of hearing preservation. No radiographic factors predicted hearing preservation; however, larger tumor size, smaller fourth ventricular width, and the presence of a cerebrospinal fluid cleft surrounding the tumor predicted postoperative facial weakness.
Conclusion Systemic comorbidities may influence hearing loss preoperatively in patients with large vestibular schwannomas. The absence of tinnitus may reflect hearing reserve and propensity for hearing preservation. Preoperative radiographic features did not predict hearing preservation despite some associations with postoperative facial weakness.
Keywords: hearing preservation, microsurgery, vestibular schwannoma
Introduction
Vestibular schwannomas are benign intracranial neoplasms of the eighth cranial nerve that present with hearing loss, tinnitus, and ataxia. Management options include observation, surgical resection, and radiosurgery or radiotherapy. Radiosurgery and fractionated or unfractionated radiotherapy are generally reserved for tumors < 3 cm due to the risk of radiation edema with increasing tumor size. Although advancements in microsurgical techniques and intraoperative neurophysiologic monitoring have dramatically improved outcomes following surgical resection,1 hearing preservation rates for tumors > 3 cm reported in the literature are relatively low ranging from 0% to 43%.2 3 4 5 6 7 8 9 10 11 12 13
A variety of studies have investigated the clinical and radiographic factors predicting hearing preservation in vestibular schwannoma surgery for tumors < 3 cm. The impact of these factors on surgery for large vestibular schwannomas remains unclear. We retrospectively reviewed our series of large vestibular schwannomas to investigate the effect of patient factors, preoperative hearing, radiographic parameters, and surgical factors on the rates of hearing preservation following vestibular schwannoma surgery. Patients with severe preoperative hearing loss were included to investigate factors that predict preoperative hearing loss in large vestibular schwannomas.
Methods
Patients who underwent resection of a vestibular schwannoma using a hearing-preserving approach (retrosigmoid craniotomy) between January 1, 2002, and December 1, 2013, at the Vancouver General Hospital institution were identified. Inclusion criteria were (1) unilateral tumors, (2) maximal extrameatal diameter > 3 cm, (3) preoperative magnetic resonance imaging (MRI) available, and (4) documented preoperative and postoperative audiograms in patients with serviceable hearing on the tumor side. Patients were excluded if they had bilateral tumors and neurofibromatosis type II or if they had undergone previous surgery or radiation therapy. This study was approved by the local institutional research ethics board.
In all cases, surgery was performed by a team consisting of the same neurosurgeon (R.A.) and neuro-otologist (B.D.W.). Intraoperative electrophysiologic monitoring was performed by a neurophysiologist (C.D.). Details on the surgical technique are described elsewhere.9 Brainstem auditory evoked responses were monitored if patients had meaningful waveforms on the day of surgery.
The primary outcome was postoperative hearing status based on the American Academy of Otolaryngology-Head and Neck Surgery Foundation. Serviceable hearing was defined as a speech discrimination score (SDS) ≤50% or a pure tone average (PTA) < 50 dB. Patients were categorized into preoperative serviceable versus nonserviceable hearing. Those with preoperative serviceable hearing were categorized into postoperative hearing preserved versus lost. Secondary outcomes were House-Brackmann grading of immediate facial nerve function in the postoperative period (day 1 or 2) and delayed grading at 6 weeks to 6 months of follow-up. Facial weakness was defined as House-Brackmann grade ≥3.
The clinical factors investigated were patient demographics (age and gender), comorbidities (diabetes, hypertension, active smoking, and alcohol consumption), and clinical presentation (hearing loss, tinnitus, ataxia, facial weakness, facial numbness, facial pain, and hydrocephalus). The radiographic factors investigated were maximal extrameatal tumor diameter (anteroposterior, transverse, and vertical), extrameatal tumor volume (ABC/2 equation),14 intrameatal length and width, percentage of internal auditory canal (IAC) filling according to Mohr et al,5 the presence of a tumor cyst, tumor heterogeneity on T1 with gadolinium imaging, the presence of a cerebrospinal fluid (CSF) cleft around 50% of the tumor or within the IAC on fast imaging employing steady-state acquisition sequences, narrowest width of the ipsilateral middle cerebellar peduncle (MCP), narrowest width of the fourth ventricle, brainstem edema and cerebellar edema on T2 or fluid-attenuated inversion recovery images, and hydrocephalus.
Statistical Analysis
All data were analyzed with SPSS v.19.0 (IBM, Inc.., Armonk, New York, United States). Categorical predictors were gender, diabetes, hypertension, smoking, alcohol consumption, hearing loss, ataxia, facial weakness, facial numbness, facial pain, hydrocephalus, cystic or solid, tumor heterogeneity, CSF cleft in IAC, CSF cleft surrounding tumor, brainstem edema, cerebellar edema, and hydrocephalus. Categorical variables were analyzed with the Pearson chi-square statistic. Continuous variables were age, maximal extrameatal diameter, intrameatal length and width, MCP width, and fourth ventricle width. Continuous variables were analyzed with univariate analysis of variance. The p values < 0.05 were considered significant.
Results
Eighty-five patients met inclusion criteria and were reviewed. The mean age was 48.9 years; 43 patients (50.6%) were male. Mean maximal tumor diameter was 36.3 mm; mean extrameatal volume was 18.7 cm3. Table 1 illustrates demographic characteristics and quantitative tumor parameters.
Table 1. Demographic characteristics and quantitative tumor parameters.
| Mean (SD) | Range | |
|---|---|---|
| Age, y | 48.9 (12.2) | 24–75 |
| Maximum tumor dimension, mm | 36.3 (5.7) | 30–58 |
| Extrameatal volume, cm3 | 18.7 (9.6) | 6.7–52.2 |
| IAC length, mm | 9.4 (4.2) | 1.9–25.5 |
| IAC width, mm | 8.5 (3.1) | 2.0–18.8 |
| Percentage filling of IAC, % | 71.5 (27.3) | 13–100 |
Abbreviations: IAC, internal auditory canal; SD, standard deviation.
Hearing Outcomes
Forty-two of 85 patients had serviceable hearing preoperatively (50.5%). Following surgery, hearing was preserved in 17 of 42 patients (41.5%). Table 2 illustrates the preoperative and postoperative SDS and PTA in patients with hearing preservation and loss.
Table 2. Preoperative and postoperative audiometry in patients with postoperative hearing preservation and hearing loss.
| Preoperative | Postoperative | |||
|---|---|---|---|---|
| Hearing lost | Hearing preserved | Hearing lost | Hearing preserved | |
| SDS % (mean, SD) | 83 (15) | 84 (24) | NA | 88 (14) |
| PTA dB (mean, SD) | 32.9 (16.2) | 28.7 (12.5) | 69.8 (12.0) | 40.1 (15.6) |
Abbreviations: NA, not applicable; PTA, pure tone average; SD, standard deviation; SDS, speech discrimination score.
Older age was associated with worse preoperative hearing (F = 16.288; p < 0.001). Preoperative hearing was significantly lower in patients with hypertension and diabetes (hypertension: chi-square = 6.664, p = 0.010; diabetes: chi-square = 4.950, p = 0.026) (Table 3). Radiographically, larger length and width of the tumor in the IAC were associated with worse preoperative hearing (length: F = 10.849, p < 0.001; width: F = 11.590, p < 0.001) as was a higher percentage of IAC filling (F = 5.354, p = 0.023). The presence of a CSF cleft in the IAC was associated with improved preoperative hearing (chi-square = 7.749, p = 0.005).
Table 3. Clinical and radiographic factors associated with preoperative hearing.
| Preoperative hearing present | Preoperative hearing absent | |
|---|---|---|
| Clinical factors | ||
| Age, y, mean (SD) | 43.8 (10.9)a | 53.6 (11.4) |
| Gender, N (% male) | 19 (46.3) | 24 (54.5) |
| Hypertension, N (%) | 5 (12.2)a | 16 (36.4) |
| Diabetes mellitus, N (%) | 0 (0)a | 5 (11.4) |
| Active smoker, N (%) | 2 (4.9) | 3 (6.8) |
| Frequent alcohol consumption, N (%) | 13 (31.7) | 10 (22.7) |
| Radiographic factors | ||
| Maximum extrameatal tumor diameter, mean, mm (SD) | 36.0 (5.2) | 36.5 (6.1) |
| Extrameatal tumor volume, cm3 (SD) | 17.2 (7.9) | 20.0 (10.9) |
| Intrameatal length, mm (SD) | 7.9 (3.4)a | 10.7 (4.3) |
| Intrameatal width, mm (SD) | 7.4 (2.2)a | 9.4 (3.2) |
| Percentage of IAC filling, % (SD) | 65.0 (27.5)a | 78.6 (26.0) |
| Tumor cyst present, N (%) | 27 (67.5) | 25 (56.8) |
| Tumor heterogeneity, N (%) | 28 (71.8) | 29 (65.9) |
| CSF cleft within IAC, N (%) | 32 (80.0)a | 19 (50.0) |
| CSF cleft surrounding tumor, N (%) | 15 (40.5) | 15 (42.9) |
| Narrowest width of ipsilateral MCP, mm (SD) | 7.2 (2.9) | 7.2 (2.3) |
| Narrowest width of fourth ventricle, mm (SD) | 8.7 (3.3) | 9.6 (4.0) |
| Brainstem edema present, N (%) | 5 (12.2) | 5 (11.9) |
| Cerebellar edema present, N (%) | 18 (43.9) | 17 (40.5) |
| Hydrocephalus present, N (%) | 3 (7.3) | 9 (20.9) |
Abbreviations: CSF, cerebrospinal fluid; IAC, internal auditory canal; MCP, middle cerebellar peduncle; SD, standard deviation.
p < 0.05.
The absence of tinnitus preoperatively was associated with a higher likelihood of preserved hearing postoperatively (Fig. 1; chi-square = 3.939, p = 0.047). Preoperative headaches were associated with a lower likelihood of postoperative hearing preservation (chi-square = 8.912, p = 0.003). The proportion of patients with gross total resections was not significantly different between patients with preserved versus lost hearing (gross total hearing lost: 83.9%; hearing preserved: 70.6%; near-total hearing lost: 16.1%; hearing preserved: 17.6%; subtotal: hearing lost: 0%, hearing preserved: 11.8%, p > 0.05). No radiographic factors were predictive of hearing preservation.
Fig. 1.
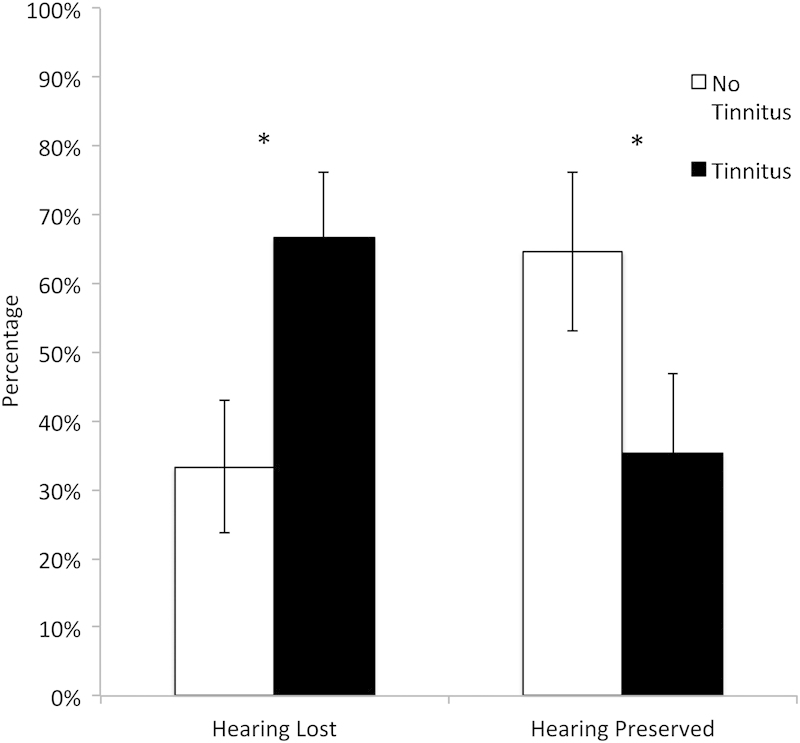
Percentage of patients with postoperative hearing preservation versus hearing loss in the presence and absence of preoperative tinnitus.
Facial Nerve Function
Overall, 65 of 84 patients (77.4%) had preserved facial nerve function in the early postoperative period (House-Brackmann 1–2). An additional eight patients recovered facial nerve function by 6-week follow-up for a total facial nerve preservation rate of 85.9% (73 of 84 patients). Active smoking was associated with a higher rate of immediate postoperative facial weakness (F = 4.244, p = 0.039) but this effect was not sustained in delayed follow-up (p > 0.05) (Table 4). Radiographic features associated with worse immediate facial nerve function were larger maximal tumor diameter (F = 7.855, p = 0.006), larger extrameatal volume (F = 7.345, p = 0.008), smaller fourth ventricle width (F = 4.539, p = 0.036), and the presence of a CSF cleft surrounding the tumor (chi-square = 4.872, p = 0.027). The presence of a CSF cleft surrounding the tumor also predicted worse delayed facial nerve function (chi-square = 7.021, p = 0.008).
Table 4. Clinical and radiographic factors associated with immediate postoperative facial nerve function.
| House-Brackmann 1–2 | House-Brackmann 3–6 | |
|---|---|---|
| Clinical factors | ||
| Age, mean (SD) | 48.8 (11.5) | 49.6 (11.7) |
| Gender, N, (% male) | 34 (52.3) | 9 (47.4) |
| Hypertension, N (%) | 48 (73.8) | 15 (78.9) |
| Diabetes mellitus, N (%) | 3 (4.6) | 2 (10.5) |
| Active smoker, N (%) | 2 (3.1)a | 3 (15.8) |
| Frequent alcohol consumption, N (%) | 20 (30.8) | 3 (15.8) |
| Radiographic factors | ||
| Maximum extrameatal tumor diameter, mean, mm (SD) | 35.3 (4.9)a | 39.2 (6.9) |
| Extrameatal tumor volume, cm3 (SD) | 17.0 (7.7)a | 23.3 (12.6) |
| Intrameatal length, mm (SD) | 9.3 (3.9) | 9.4 (5.2) |
| Intrameatal width, mm (SD) | 8.4 (3.0) | 8.3 (2.7) |
| Percentage of IAC filling, % (SD) | 72.6 (26.9) | 69.1 (29.8) |
| Tumor cyst present, N (%) | 22 (34.4) | 10 (52.6) |
| Tumor heterogeneity, N (%) | 45 (70.3) | 12 (66.7) |
| CSF cleft within IAC, N (%) | 41 (67.2) | 10 (58.8) |
| CSF cleft surrounding tumor, N (%) | 20 (35.1)a | 10 (66.7) |
| Narrowest width of ipsilateral MCP, mm (SD) | 7.5 (2.5) | 6.4 (2.8) |
| Narrowest width of 4th ventricle, mm (SD) | 9.7 (3.8)a | 7.7 (2.7) |
| Brainstem edema present, N (%) | 7 (11.1) | 2 (10.5) |
| Cerebellar edema present, N (%) | 25 (39.7) | 9 (47.4) |
| Hydrocephalus present, N (%) | 6 (9.4) | 5 (26.3) |
Abbreviations: CSF, cerebrospinal fluid; IAC, internal auditory canal; MCP, middle cerebellar peduncle; SD, standard deviation.
p < 0.05.
Discussion
Vestibular schwannomas are benign tumors that can be managed with observation, radiation, or surgery. Larger vestibular schwannomas are only amenable to surgical resection. The goal of surgery is to achieve a maximal resection while preserving neurologic structures, mainly facial nerve function and hearing if possible. With modern-day microsurgical techniques and intraoperative monitoring, facial function preservation has improved dramatically for larger tumors.1 In contrast, hearing preservation rates in large tumors remain relatively low.2 3 4 5 6 7 8 9 10 11 12 13 In this study we investigated the clinical and radiographic factors that affect hearing preservation rates in large vestibular schwannomas. We report an overall hearing preservation rate of 41.5% in patients with vestibular schwannomas > 3 cm who had serviceable hearing preoperatively.
Preoperative hearing was affected by age in addition to comorbid hypertension and diabetes. Radiographically, larger tumor size within the IAC increased the likelihood of preoperative deafness, a finding noted in several other studies.5 15 16 17 To our knowledge, this is the first study to report the impact of hypertension and diabetes on preoperative hearing. Diabetes is a leading cause of peripheral neuropathy, and hypertension increases the risk of developing peripheral neuropathy.18 This finding may be relevant for counseling patients who are being observed with asymptomatic or minimally symptomatic small tumors. The presence of diabetes or hypertension appears to increase the chances of developing preoperative hearing loss; patients with vestibular schwannomas who are being observed with serial imaging should be counseled on the apparent impact of these comorbidities on their hearing.
A variety of studies have investigated factors predicting hearing preservation in vestibular schwannoma surgery for tumors < 3 cm. These factors can be categorized as patient factors, preoperative hearing, radiographic parameters, and surgical factors. Relevant patient factors include the presence of neurofibromatosis or bilateral tumors. Patients with neurofibromatosis have a higher incidence of cochlear and facial nerve schwannomas that may limit the ability to preserve hearing.3 19 Patients with bilateral tumors are less likely to have preserved hearing postoperatively.20 Of the radiographic factors, the most robust variable is tumor size; larger tumors have lower rates of hearing preservation.16 The degree of IAC has been shown to predict postoperative hearing preservation.5 17 21 Comparison of the intralabyrinthine fluid intensity signal on T1 spin-echo and gradient-echo MRI is a prognostic factors.17 22 Lastly, length of tumor contact with the vestibulocochlear nerve has been shown to predict postoperative hearing preservation.23
Several groups have reported on tumor factors that influence hearing preservation. The most widely accepted factor is the surgical approach; a systematic review concluded the middle fossa approach is best for hearing preservation in small tumors, and the retrosigmoid approach is best for facial nerve preservation.24 Hearing is sacrificed in the translabyrinthine approach. Preoperative facial and trigeminal nerve dysfunction has been associated with lower rates of hearing preservation.25 The presence of adhesions between the tumor and the vestibulocochlear nerve is a negative prognostic factor.26 27 In addition, tumors originating from the superior vestibular nerve have higher rates of postoperative hearing preservation compared with tumors originating from the inferior vestibular nerve.2 28 29
The only factors predicting hearing preservation in large tumors identified in this study were the presence of preoperative tinnitus and headaches. Tinnitus is the subjective experience of ringing in the ear or head. Baguley et al30 investigated the clinical characteristics of tinnitus in patients with vestibular schwannoma and proposed the likely mechanism is brainstem compression. Tinnitus was associated with contralateral auditory brainstem response abnormalities that were independent of tumor size. Another proposed mechanism for tinnitus is deafferentation of cochlear inner hair cells.31 Tinnitus may be a useful clinical marker of brainstem compression, cochlear nerve damage, and hearing reserve. Patients with tinnitus have a lower chance of hearing preservation using a retrosigmoid approach for large vestibular schwannomas.
Postoperative facial weakness was predicted by larger extrameatal tumor size and dimensions and by a smaller width of the fourth ventricle, consistent with findings from previous studies.32 33 Postoperative facial nerve function depends on extracanalicular tumor size and dimensions, whereas auditory nerve function appears to be affected by intracanalicular size and dimensions. Interestingly, smoking was associated with early postoperative facial weakness; however, this effect was not sustained in follow-up. It nonetheless highlights the importance of vascular risk factors and comorbidities in nerve recovery following surgery.
Anatomical or radiologic factors did not appear to be as significant for hearing preservation as can be demonstrated in facial nerve preservation. This is likely because the total number of patients with intact hearing preoperatively is still relatively small in this series, whereas the whole cohort is available for facial function evaluation. Previous studies have shown that an estimate of the length of tumor contact with the cochlear nerve and a larger proportion of tumor anterior to the IAC predicted hearing preservation.9 23 We were unable to replicate these findings potentially because of the small numbers in the series.
An important limitation of this study is the retrospective nature of the data reviewed. Despite all the attempts to obtain a complete data set, some data were missing. Nonetheless, the study investigated a large group of patients with larger tumors. The patients investigated in this study had audiograms performed in many different audiology centers that used variable stimulus intensities for determining the speech discrimination score. Previous studies of hearing preservation in vestibular schwannomas using variable methodologies for defining hearing function complicates comparison with other studies. We used a clinically pragmatic method for determining hearing function by defining hearing as serviceable or nonserviceable based on the American Academy of Otolaryngology-Head and Neck Surgery Foundation.
Conclusion
Hearing preservation in larger vestibular schwannomas is possible and worthwhile attempting. Systemic comorbidities may influence hearing loss preoperatively in patients with large vestibular schwannomas. Tinnitus may be an indicator of hearing reserve and potential for hearing preservation. The total number of patients available for attempted hearing preservation in larger tumors is relatively small, and those who are able to enjoy preserved hearing postoperatively are few even in a moderate-volume institution. This poses a challenge in making generalizable comments about the population of patients with large tumors that have intact preoperative hearing. In this study, preoperative radiographic features were not found to predict hearing preservation despite some features being associated with postoperative facial weakness.
References
- 1.Koerbel A, Gharabaghi A, Safavi-Abbasi S, Tatagiba M, Samii M. Evolution of vestibular schwannoma surgery: the long journey to current success. Neurosurg Focus. 2005;18(4):e10. doi: 10.3171/foc.2005.18.4.11. [DOI] [PubMed] [Google Scholar]
- 2.Cohen N L, Lewis W S, Ransohoff J. Hearing preservation in cerebellopontine angle tumor surgery: the NYU experience 1974–1991. Am J Otol. 1993;14(5):423–433. doi: 10.1097/00129492-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Fischer G, Fischer C, Rémond J. Hearing preservation in acoustic neurinoma surgery. J Neurosurg. 1992;76(6):910–917. doi: 10.3171/jns.1992.76.6.0910. [DOI] [PubMed] [Google Scholar]
- 4.Kemink J L, LaRouere M J, Kileny P R, Telian S A, Hoff J T. Hearing preservation following suboccipital removal of acoustic neuromas. Laryngoscope. 1990;100(6):597–602. doi: 10.1288/00005537-199006000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Mohr G, Sade B, Dufour J J, Rappaport J M. Preservation of hearing in patients undergoing microsurgery for vestibular schwannoma: degree of meatal filling. J Neurosurg. 2005;102(1):1–5. doi: 10.3171/jns.2005.102.1.0001. [DOI] [PubMed] [Google Scholar]
- 6.Gormley W B Sekhar L N Wright D C Kamerer D Schessel D Acoustic neuromas: results of current surgical management Neurosurgery 199741150–58.; discussion 58–60 [DOI] [PubMed] [Google Scholar]
- 7.Sugita K, Kobayashi S. Technical and instrumental improvements in the surgical treatment of acoustic neurinomas. J Neurosurg. 1982;57(6):747–752. doi: 10.3171/jns.1982.57.6.0747. [DOI] [PubMed] [Google Scholar]
- 8.Post K D, Eisenberg M B, Catalano P J. Hearing preservation in vestibular schwannoma surgery: what factors influence outcome? J Neurosurg. 1995;83(2):191–196. doi: 10.3171/jns.1995.83.2.0191. [DOI] [PubMed] [Google Scholar]
- 9.Di Maio S Malebranche A D Westerberg B Akagami R Hearing preservation after microsurgical resection of large vestibular schwannomas Neurosurgery 2011683632–640.; discussion 640 [DOI] [PubMed] [Google Scholar]
- 10.Yokoh A, Kobayashi S, Tanaka Y, Gibo H, Sugita K. Preservation of cochlear nerve function in acoustic neurinoma surgery. Acta Neurochir (Wien) 1993;123(1–2):8–13. doi: 10.1007/BF01476279. [DOI] [PubMed] [Google Scholar]
- 11.Umezu H, Aiba T. Preservation of hearing after surgery for acoustic schwannomas: correlation between cochlear nerve function and operative findings. J Neurosurg. 1994;80(5):844–848. doi: 10.3171/jns.1994.80.5.0844. [DOI] [PubMed] [Google Scholar]
- 12.Iwai Y Yamanaka K Ishiguro T Surgery combined with radiosurgery of large acoustic neuromas Surg Neurol 2003594283–289.; discussion 289–291 [DOI] [PubMed] [Google Scholar]
- 13.Samii M Matthies C Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections Neurosurgery 1997402248–260.; discussion 260–262 [DOI] [PubMed] [Google Scholar]
- 14.Yu Y L, Lee M S, Juan C J, Hueng D Y. Calculating the tumor volume of acoustic neuromas: comparison of ABC/2 formula with planimetry method. Clin Neurol Neurosurg. 2013;115(8):1371–1374. doi: 10.1016/j.clineuro.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Khrais T, Sanna M. Hearing preservation surgery in vestibular schwannoma. J Laryngol Otol. 2006;120(5):366–370. doi: 10.1017/S002221510600332X. [DOI] [PubMed] [Google Scholar]
- 16.Kari E, Friedman R A. Hearing preservation: microsurgery. Curr Opin Otolaryngol Head Neck Surg. 2012;20(5):358–366. doi: 10.1097/MOO.0b013e3283579673. [DOI] [PubMed] [Google Scholar]
- 17.Somers T, Casselman J, de Ceulaer G, Govaerts P, Offeciers E. Prognostic value of magnetic resonance imaging findings in hearing preservation surgery for vestibular schwannoma. Otol Neurotol. 2001;22(1):87–94. doi: 10.1097/00129492-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Tesfaye S, Chaturvedi N, Eaton S E. et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 19.Friedman R A, Goddard J C, Wilkinson E P. et al. Hearing preservation with the middle cranial fossa approach for neurofibromatosis type 2. Otol Neurotol. 2011;32(9):1530–1537. doi: 10.1097/MAO.0b013e3182355855. [DOI] [PubMed] [Google Scholar]
- 20.Sanna M, Zini C, Mazzoni A. et al. Hearing preservation in acoustic neuroma surgery. Middle fossa versus suboccipital approach. Am J Otol. 1987;8(6):500–506. [PubMed] [Google Scholar]
- 21.Tringali S, Ferber-Viart C, Fuchsmann C, Buiret G, Zaouche S, Dubreuil C. Hearing preservation in retrosigmoid approach of small vestibular schwannomas: prognostic value of the degree of internal auditory canal filling. Otol Neurotol. 2010;31(9):1469–1472. [PubMed] [Google Scholar]
- 22.Goddard J C, Schwartz M S, Friedman R A. Fundal fluid as a predictor of hearing preservation in the middle cranial fossa approach for vestibular schwannoma. Otol Neurotol. 2010;31(7):1128–1134. doi: 10.1097/MAO.0b013e3181e8fc3f. [DOI] [PubMed] [Google Scholar]
- 23.Yong R L, Westerberg B D, Dong C, Akagami R. Length of tumor-cochlear nerve contact and hearing outcome after surgery for vestibular schwannoma. J Neurosurg. 2008;108(1):105–110. doi: 10.3171/JNS/2008/108/01/0105. [DOI] [PubMed] [Google Scholar]
- 24.Ansari S F, Terry C, Cohen-Gadol A A. Surgery for vestibular schwannomas: a systematic review of complications by approach. Neurosurg Focus. 2012;33(3):E14. doi: 10.3171/2012.6.FOCUS12163. [DOI] [PubMed] [Google Scholar]
- 25.Nadol J B Jr, Chiong C M, Ojemann R G. et al. Preservation of hearing and facial nerve function in resection of acoustic neuroma. Laryngoscope. 1992;102(10):1153–1158. doi: 10.1288/00005537-199210000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Moriyama T, Fukushima T, Asaoka K, Roche P H, Barrs D M, McElveen J T Jr. Hearing preservation in acoustic neuroma surgery: importance of adhesion between the cochlear nerve and the tumor. J Neurosurg. 2002;97(2):337–340. doi: 10.3171/jns.2002.97.2.0337. [DOI] [PubMed] [Google Scholar]
- 27.Nonaka Y Fukushima T Watanabe K et al. Contemporary surgical management of vestibular schwannomas: analysis of complications and lessons learned over the past decade Neurosurgery 201372(2, Suppl Operative):ons103–ons115.; discussion ons115 [DOI] [PubMed] [Google Scholar]
- 28.Brackmann D E, Owens R M, Friedman R A. et al. Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otol. 2000;21(3):417–424. doi: 10.1016/s0196-0709(00)80054-x. [DOI] [PubMed] [Google Scholar]
- 29.Rachinger J, Rampp S, Prell J, Scheller C, Alfieri A, Strauss C. Tumor origin and hearing preservation in vestibular schwannoma surgery. J Neurosurg. 2011;115(5):900–905. doi: 10.3171/2011.7.JNS102092. [DOI] [PubMed] [Google Scholar]
- 30.Baguley D M, Humphriss R L, Axon P R, Moffat D A. The clinical characteristics of tinnitus in patients with vestibular schwannoma. Skull Base. 2006;16(2):49–58. doi: 10.1055/s-2005-926216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knipper M, Van Dijk P, Nunes I, Rüttiger L, Zimmermann U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 2013;111:17–33. doi: 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Lalwani A K, Butt F Y, Jackler R K, Pitts L H, Yingling C D. Facial nerve outcome after acoustic neuroma surgery: a study from the era of cranial nerve monitoring. Otolaryngol Head Neck Surg. 1994;111(5):561–570. doi: 10.1177/019459989411100505. [DOI] [PubMed] [Google Scholar]
- 33.Sampath P, Holliday M J, Brem H, Niparko J K, Long D M. Facial nerve injury in acoustic neuroma (vestibular schwannoma) surgery: etiology and prevention. J Neurosurg. 1997;87(1):60–66. doi: 10.3171/jns.1997.87.1.0060. [DOI] [PubMed] [Google Scholar]


