Abstract
Objective To explore the use of the endoscopic endonasal transclival approach (EEA) for clipping anterior inferior cerebellar artery (AICA), posterior inferior cerebellar artery (PICA), and vertebral artery (VA) aneurysms.
Design Anatomical study.
Participants Fifteen adult cadavers.
Main Outcome Measures Length of artery exposed and distance from the nasal ala to the arteries.
Results The length of the right and left VA exposed were 1.7 ± 0.6 cm and 1.6 ± 0.6 cm, respectively. The distance to the right VA was 11.1 ± 0.9 cm and to the left was 11.1 ± 0.8 cm. Right and left AICA were exposed for an average length of 1.1 ± 0.3 cm and 0.8 ± 0.3 cm, respectively. The distance to the right AICA was 10.3 ± 0.8 cm and to the left was 10.3 ± 0.8 cm. The right PICA was exposed for a length of 0.5 ± 0.2 cm at a distance of 10.9 ± 0.5 cm. The left PICA was exposed for a length of 0.5 ± 0.2 cm at a distance of 11.1 ± 0.9 cm.
Conclusion The EEA can provide direct access to AICA, PICA, and VA, making it a potential alternative to the traditional approaches for the clipping of aneurysms arising from those arteries.
Keywords: aneurysms, endoscopic endonasal, surgical clipping, transclival
Introduction
The endoscopic endonasal transclival approach (EEA) is a minimally invasive approach that has been adopted by neurosurgeons in recent years for the resection of pituitary tumors, skull base tumors, and other pathologies arising from the anterior skull base. EEA provides direct access to the ventral skull base while obviating the need for brain retraction. In addition, it involves minimal manipulation of the neurovasculature and thus carries a lower risk of potential cranial nerve injury often incurred during lateral approaches to these arteries when compared with traditional craniotomies. EEA has been extended beyond the standard transsphenoidal approach to include the transcribriform, transclival, and transodontoid approach, allowing for the expansion of its application to other pathologies.1 Most recently, the use of EEA has been expanded to surgical clipping of cerebral aneurysms in both cadaveric and clinical studies.2 3 4 5 6
Approximately 35% of cerebral aneurysms involve the posterior cerebral circulation. When they rupture they carry a significantly higher mortality rate than ruptured aneurysms of the anterior cerebral circulation.7 The treatment of these aneurysms is also associated with much higher morbidity and mortality rates when compared with the anterior circulation.8 The location of these aneurysms make them very challenging to treat, particularly those arising from the anterior inferior cerebellar artery (AICA), posterior inferior cerebellar artery (PICA), and vertebral artery (VA). AICA is located in close proximity to cranial nerves VI to VIII and the pons; PICA is located in close proximity to cranial nerves IX to XII and the medulla oblongata. The VA lies on top of the medullary-pontine junction, pons, and spinal cord and is located in close relation to the origin of cranial nerves VI and XII. AICA and PICA aneurysms are rare, accounting for ∼ 1.3% and 3%, respectively, of cerebral aneurysms, and most of these aneurysms arise proximally near the origin of these arteries, often requiring surgical exposure of the ventral brainstem necessitating a more invasive lateral approach.9 10 Endovascular treatment of these aneurysms is challenging as well because the parent vessel cannot be easily separated from the aneurysm, resulting in the potential risk of sacrificing the parent vessel, leading to potential AICA or PICA territory infarcts.11 Aneurysms of the vertebral artery make up < 5% of cerebral aneurysms, and most of them are located near the origin of PICA.12 13 The purpose of this study is to explore the feasibility of using the EEA specifically the transclival approach for the surgical clipping of aneurysms arising from AICA, PICA, and VA by assessing the visualization afforded by this approach as well as the maneuverability of the surgical instruments.
Materials and Methods
Materials
Fifteen fresh adult cadaver heads injected with dyed latex were used for the dissections. A rigid 0-degree endoscope (Stryker, Kalamazoo, Michigan, United States), attached to a light source and a high-definition camera (Stryker) was used for the visualization and obtaining images.
Surgical Dissection
The EEA was performed as outlined by Cavallo et al.1 The middle turbinate is first fractured and removed on one side. The posterior portion of the nasal septum is then removed to allow a binarial approach. Part of the sphenoid floor and vomer is drilled out, using the pterygoid canals as the lateral boundary.6 This exposes the superior third of the clivus and creates a wide surgical corridor. The rhinopharyngeal mucosa is removed to expose the inferior part of the clivus and C1. The entire clivus is drilled out, with the auditory tubes marking the lateral limits in the inferior third, the paraclival internal carotid arteries marking the lateral limits in the superior and middle thirds, the sella floor marking the superior limit, and C1 marking the inferior limit.6 The dura mater is removed with Kerrison forceps, exposing the basilar artery, posterior cerebral arteries, superior cerebellar arteries, VA, AICA, and PICA.
Measurements
The lengths of the exposed basilar artery, VA, AICA, and PICA were measured. The distance from the bottom of the nares to those arteries was also measured.
Clipping
AICA, PICA, and VA were clipped using extended vascular clip applicators (Aesculap AG, Tuttlingen, Germany).
Results
The EEA allowed visualization of both left and right VA and AICA in all 15 of the specimens (Figs. 1 and 2). The right PICA was exposed in 11 of the specimens (73%); the left PICA was exposed in 12 (80%) (Fig. 3). Table 1 summarizes the length of the artery exposed; Table 2 summarizes the distance from the bottom of the nares to the arteries.
Fig. 1.
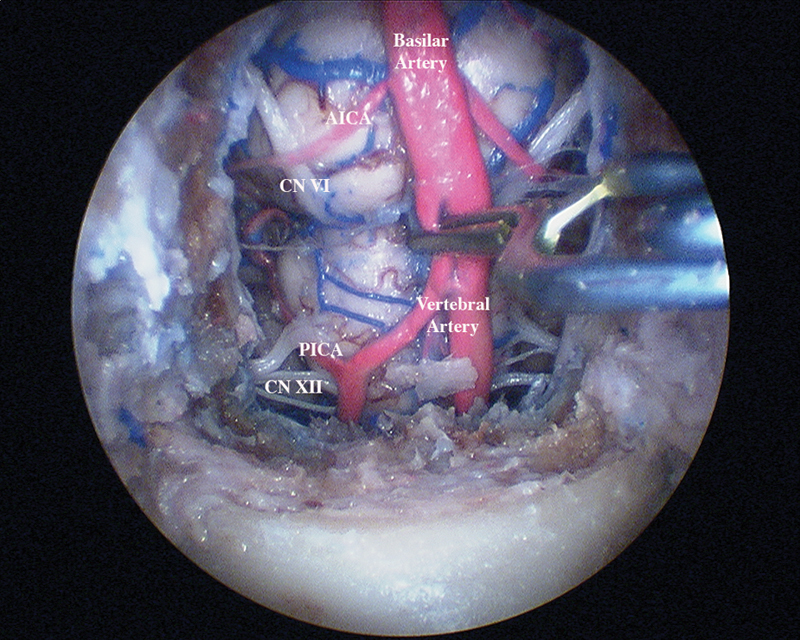
Endoscopic view with a clip placed on the proximal right vertebral artery.
Fig. 2.
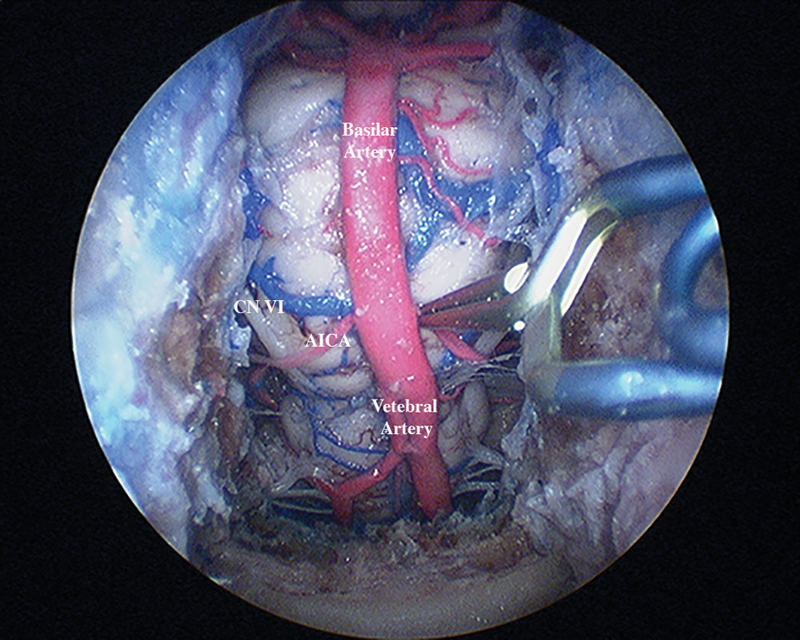
Endoscopic view with a clip placed on the proximal left anterior inferior cerebellar artery.
Fig. 3.
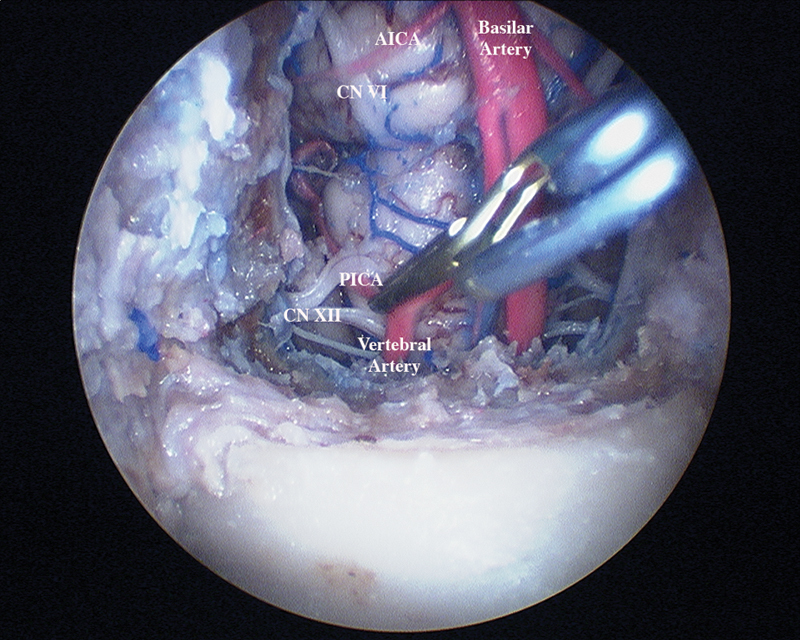
Endoscopic view with a clip placed on the proximal right posterior interior cerebellar artery.
Table 1. Length of the artery exposed.
| Average length ± SD, cm (range) | |
|---|---|
| Basilar artery | 2.6 ± 0.3 (2.0–3.2) |
| Right VA | 1.7 ± 0.6 (0.8–2.8) |
| Left VA | 1.6 ± 0.6 (0.8–2.5)a |
| Right AICA | 1.1 ± 0.3 (0.6–1.7)a |
| Left AICA | 0.8 ± 0.3 (0.4–1.5)a |
| Right PICA | 0.5 ± 0.2 (0.2–1.4)a |
| Left PICA | 0.5 ± 0.2 (0.1–1.0)a |
Abbreviations: AICA, anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery; SD, standard deviation; VA, vertebral artery.
Indicates samples where there were outliers, defined as > 2 ± SDs. The mean and standard deviations do not include the outliers.
Table 2. Distance from bottom of the nares to arterial landmarks.
| Average distance from nares ± SD, cm (range) | |
|---|---|
| VBJ | 10.3 ± 0.7 (9.5–11.5) |
| Lowest level of the right VA | 11.1 ± 0.9 (10.0–13.0) |
| Lowest level of the left VA | 11.1 ± 0.8 (10.0–12.8) |
| Origin of right AICA | 10.3 ± 0.8 (9.3–11.8) |
| Origin of left AICA | 10.3 ± 0.8 (9.5–11.8) |
| Origin of right PICA | 10.9 ± 0.5 (10.0–12.0)a |
| Origin of left PICA | 11.1 ± 0.9 (9.5–12.5) |
Abbreviations: AICA, anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery; SD, standard deviation; VA, vertebral artery; VBJ, vertebrobasilar junction.
Indicates samples where there were outliers, defined as > 2 ± SDs. The mean and SDs do not include the outliers.
Discussion
AICA, PICA, and VA aneurysms can be treated with either surgical clipping or endovascular treatment. Traditionally, a variety of surgical approaches including, but not limited to, retrosigmoid, lateral suboccipital, and far lateral are used for clipping these aneurysms.9 14 15 16 17 For the treatment of PICA and VA aneurysms, these approaches have a success rate, as defined by having a favorable outcome, > 85%.15 18 These data are not available for AICA aneurysms. Although these procedures are very successful, they are highly invasive and require navigating through important neurovascular structures, leading to high morbidity rates. The most common morbidities are lower cranial nerve palsies, with incidence ranging from 10% to 56%.9 18 19 Other complications include cerebrospinal fluid (CSF) leakage, meningitis, and hydrocephalus.20 Although endovascular treatment has lower morbidity rates than clipping, it has higher failure rates, an increased probability of recurrence, and lower rates of achieving complete occlusion.11 In addition, coiling is not feasible in all situations, so the parent artery often has to be embolized and sacrificed, risking strokes. In the International Subarachnoid Aneurysm Trial, the authors were able to obtain complete occlusion in only 66% of cases with endovascular treatment, compared with the 82% they achieved with surgical clipping for ruptured cerebral aneurysms.13 In a 2010 report by Suh et al, good clinical outcomes were achieved in only seven of nine AICA aneurysm cases (78%).21 Chalouhi et al performed endovascular treatment on PICA aneurysms in 76 patients and reported failure in 6.6% of cases, recurrence in 22.9% of cases, infarction due to PICA occlusion in 36.4% of cases, and only 63.4% achievement of complete occlusion of proximal and distal PICA aneurysms.11 Pandey and colleagues were able to achieve complete occlusion in 82.9% of their 41 PICA/VA aneurysm cases, but they concluded that endovascular coiling is more effective as a short-term treatment because of the high rate of recurrence that requires close monitoring.22
Because the EEA carries a lower risk of lower cranial nerve palsies compared with the retrosigmoid, lateral suboccipital, far lateral approaches, and so on, it is an appealing alternative approach for surgical clipping of certain cerebral aneurysms. Several articles have demonstrated the use of EEA for this purpose. Kassam et al published a report in 2006 about using the extended EEA for clipping a large vertebral aneurysm in conjunction with endovascular techniques.5 In 2011, Germanwala et al used solely EEA to clip a ruptured ophthalmic artery aneurysm and an unruptured paraclinoid aneurysm.4 In a 2012 report, Drazin et al clipped a ruptured basilar aneurysm and an associated artery feeding into an arteriovenous malformation using the extended EEA.2 Clipping of aneurysms arising from anterior communicating artery complexes have been demonstrated using the transtuberculum-transplanum approach in cadaveric studies by Di Somma and Lai.23 24 The only report to date of using EEA for clipping PICA aneurysms is from 2011 in a case report by Enseñat et al.3 The authors were able to clip a saccular VA-PICA aneurysm successfully, demonstrating that EEA provides sufficient exposure of the proximal PICA and provides enough space to maneuver and place distal and proximal surgical clips. No report has been published on the use of EEA for clipping AICA aneurysms to date.
Although EEA remains a promising alternative for the treatment of different pathologies, its use is confined to pathologies located toward the midline. Furthermore, the exposure afforded by EEA is limited compared with traditional approaches, and endoscopy, which has a steep learning curve, is relatively new to neurosurgery. Most importantly, there is more limited vascular control with EEA because of the smaller surgical corridor. This makes it especially challenging to restore hemostasis if there is bleeding, particularly from the venous plexus. Complications resulting from EEA include CSF leakage, meningitis, intracranial abscesses, neurologic deficits, and vascular injury. The high CSF leakage rates are of concern for many, especially because of the increased risk of infection. One retrospective study noted a rate of 15.8% of 800 cases.25 However, with the adoption of nasoseptal flaps and secondary flaps for reconstruction, the incidence has decreased to as low as 3%.26 27
In this article, we have demonstrated the use of the EEA to reach AICA, PICA, and VA. Removing the sellar floor and the entire clivus allows us to get a visual from the basilar apex superiorly extending down to the VA inferiorly. Visualization of AICA and VA was possible in 100% of the specimens. There was more anatomical variation with PICA with regard to its origin. Some originated a few centimeters from the vertebrobasilar junction and were easily located; others originated far too inferiorly to be seen with the transclival approach. As a result, we were successful in reaching the right PICA in 73% of the 15 specimens and the left PICA in 80% of the specimens. The exposure of these arteries was more than adequate, and the superior view afforded by this approach facilitated placement of the clips.
With the endonasal approach, the nasal structures were a slight hindrance because they limit the maneuverability of the instruments; however, the fine movements necessary for drilling, removing the dura, and clipping the arteries were still possible. In addition, the binarial approach allowed greater flexibility with the movements. Although the surgical corridor was adequate, several technical barriers were encountered. The operative field was deeper than some instruments could reach, which can limit the ability to position the clips. Depth perception is sacrificed with the two-dimensional perspective of the endoscope, making it a challenge to gauge the three-dimensional spatial relationships. Lastly, the standard clips used for aneurysms are too large, with the ends sticking out beyond the dura. This can be problematic for dural closures that require a tight seal to prevent CSF leakage. Mini aneurysm clips would be better suited for this purpose; specially designed clips for endonasal surgery would be ideal.2 6 It should be noted that not all aneurysms are amenable to clipping via EEA, and this approach should only be attempted by experienced skull base teams. In addition to location of the aneurysm, the location of the neck of the aneurysm should be taken into consideration as well as what determines the orientation of the clip.28
There have been many advances made in the field of endoscopic endonasal surgery in technique and technology, but it still lacks the surgical tools designed specifically for this approach. These tools will allow us to overcome some of the technical barriers and further advance the field.
Conclusion
The EEA allows for direct access to AICA, PICA, and VA and provides an incomparable view of these arteries. The surgical corridor created by this approach is wide enough to allow sufficient maneuverability of the surgical tools and placement of the clips. Although standard treatments should always be considered first for the treatment of aneurysms arising from AICA, PICA, and VA, the EEA should be considered by experienced skull-based teams when the standard treatments have failed or are high risk, but the possibility of CSF leak and infection must be taken into account.
References
- 1.Cavallo L M, Messina A, Cappabianca P. et al. Endoscopic endonasal surgery of the midline skull base: anatomical study and clinical considerations. Neurosurg Focus. 2005;19(1):E2. [PubMed] [Google Scholar]
- 2.Drazin D, Zhuang L, Schievink W I, Mamelak A N. Expanded endonasal approach for the clipping of a ruptured basilar aneurysm and feeding artery to a cerebellar arteriovenous malformation. J Clin Neurosci. 2012;19(1):144–148. doi: 10.1016/j.jocn.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Enseñat J Alobid I de Notaris M et al. Endoscopic endonasal clipping of a ruptured vertebral-posterior inferior cerebellar artery aneurysm: technical case report Neurosurgery 201169(1, Suppl Operative):E121–E127.; discussion E127–E128 [DOI] [PubMed] [Google Scholar]
- 4.Germanwala A V Zanation A M Endoscopic endonasal approach for clipping of ruptured and unruptured paraclinoid cerebral aneurysms: case report Neurosurgery 201168(1, Suppl Operative):234–239.; discussion 240 [DOI] [PubMed] [Google Scholar]
- 5.Kassam A B Mintz A H Gardner P A Horowitz M B Carrau R L Snyderman C H The expanded endonasal approach for an endoscopic transnasal clipping and aneurysmorrhaphy of a large vertebral artery aneurysm: technical case report Neurosurgery 200659101E162–E165.; discussion E162–E165 [DOI] [PubMed] [Google Scholar]
- 6.Lai L T, Morgan M K, Chin D CW. et al. A cadaveric study of the endoscopic endonasal transclival approach to the basilar artery. J Clin Neurosci. 2013;20(4):587–592. doi: 10.1016/j.jocn.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Schievink W I, Wijdicks E FM, Piepgras D G, Chu C P, O'Fallon W M, Whisnant J P. The poor prognosis of ruptured intracranial aneurysms of the posterior circulation. J Neurosurg. 1995;82(5):791–795. doi: 10.3171/jns.1995.82.5.0791. [DOI] [PubMed] [Google Scholar]
- 8.Ogilvy C S Hoh B L Singer R J Putman C M Clinical and radiographic outcome in the management of posterior circulation aneurysms by use of direct surgical or endovascular techniques Neurosurgery 200251114–21.; discussion 21–22 [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez L F, Alexander M J, McDougall C G, Spetzler R F. Anteroinferior cerebellar artery aneurysms: surgical approaches and outcomes—a review of 34 cases. Neurosurgery. 2004;55(5):1025–1035. doi: 10.1227/01.neu.0000141083.00866.82. [DOI] [PubMed] [Google Scholar]
- 10.Hudgins R J, Day A L, Quisling R G, Rhoton A L Jr, Sypert G W, Garcia-Bengochea F. Aneurysms of the posterior inferior cerebellar artery. A clinical and anatomical analysis. J Neurosurg. 1983;58(3):381–387. doi: 10.3171/jns.1983.58.3.0381. [DOI] [PubMed] [Google Scholar]
- 11.Chalouhi N, Jabbour P, Starke R M. et al. Endovascular treatment of proximal and distal posterior inferior cerebellar artery aneurysms. J Neurosurg. 2013;118(5):991–999. doi: 10.3171/2012.12.JNS121240. [DOI] [PubMed] [Google Scholar]
- 12.Drake C G. The treatment of aneurysms of the posterior circulation. Clin Neurosurg. 1979;26:96–144. doi: 10.1093/neurosurgery/26.cn_suppl_1.96. [DOI] [PubMed] [Google Scholar]
- 13.Molyneux A, Kerr R, Stratton I. et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomized trial. . Lancet. 2002;360(9342):1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 14.Bambakidis N C, Manjila S, Dashti S, Tarr R, Megerian C A. Management of anterior inferior cerebellar artery aneurysms: an illustrative case and review of literature. Neurosurg Focus. 2009;26(5):E6. doi: 10.3171/2009.1.FOCUS0915. [DOI] [PubMed] [Google Scholar]
- 15.D'Ambrosio A L Kreiter K T Bush C A et al. Far lateral suboccipital approach for the treatment of proximal posteroinferior cerebellar artery aneurysms: surgical results and long-term outcome Neurosurgery 200455139–50.; discussion 50–54 [PubMed] [Google Scholar]
- 16.Heros R C. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64(4):559–562. doi: 10.3171/jns.1986.64.4.0559. [DOI] [PubMed] [Google Scholar]
- 17.Krayenbühl N, Guerrero C, Krisht A F. Technical strategies to approach aneurysms of the vertebral and posterior inferior cerebellar arteries. Neurosurg Focus. 2005;19(2):E4. doi: 10.3171/foc.2005.19.2.5. [DOI] [PubMed] [Google Scholar]
- 18.Al-khayat H Al-Khayat H Beshay J Manner D White J Vertebral artery-posteroinferior cerebellar artery aneurysms: clinical and lower cranial nerve outcomes in 52 patients Neurosurgery 20055612–10.; discussion 11 [PubMed] [Google Scholar]
- 19.Andoh T Shirakami S Nakashima T et al. Clinical analysis of a series of vertebral aneurysm cases Neurosurgery 1992316987–993.; discussion 993 [DOI] [PubMed] [Google Scholar]
- 20.Horowitz M, Kopitnik T, Landreneau F. et al. Posteroinferior cerebellar artery aneurysms: surgical results for 38 patients. Neurosurgery. 1998;43(5):1026–1032. doi: 10.1097/00006123-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Suh S H, Kim D J, Kim D I. et al. Management of anterior inferior cerebellar artery aneurysms: endovascular treatment and clinical outcome. AJNR Am J Neuroradiol. 2011;32(1):159–164. doi: 10.3174/ajnr.A2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pandey A S Koebbe C Rosenwasser R H Veznedaroglu E Endovascular coil embolization of ruptured and unruptured posterior circulation aneurysms: review of a 10-year experience Neurosurgery 2007604626–636.; discussion 636–637 [DOI] [PubMed] [Google Scholar]
- 23.Di Somma A, de Notaris M, Stagno V. et al. Extended endoscopic endonasal approaches for cerebral aneurysms: anatomical, virtual reality and morphometric study. Biomed Res Int. 2014;2014:703792. doi: 10.1155/2014/703792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai L T, Morgan M K, Dalgorf D. et al. Cadaveric study of the endoscopic endonasal transtubercular approach to the anterior communicating artery complex. J Clin Neurosci. 2014;21(5):827–832. doi: 10.1016/j.jocn.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Kassam A B, Prevedello D M, Carrau R L. et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg. 2011;114(6):1544–1568. doi: 10.3171/2010.10.JNS09406. [DOI] [PubMed] [Google Scholar]
- 26.Hadad G, Bassagasteguy L, Carrau R L. et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 27.Patel M R, Taylor R J, Hackman T G. et al. Beyond the nasoseptal flap: outcomes and pearls with secondary flaps in endoscopic endonasal skull base reconstruction. Laryngoscope. 2014;124(4):846–852. doi: 10.1002/lary.24319. [DOI] [PubMed] [Google Scholar]
- 28.Heiferman D M, Somasundaram A, Alvarado A J, Zanation A M, Pittman A L, Germanwala A V. The endonasal approach for treatment of cerebral aneurysms: a critical review of the literature. Clin Neurol Neurosurg. 2015;134:91–97. doi: 10.1016/j.clineuro.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


