Abstract
Inferior vena cava (IVC) filters play an important role in preventing pulmonary embolism in patients with deep venous thrombosis. When preparing for IVC filter placement, there are several important anatomic and technical considerations. The IVC has complex embryologic origins, and normal variants are relatively common which may necessitate a change in technique or approach. When performing the procedure, the choice in imaging modality for deployment, location of deployment, and route of access must be considered. The pediatric and pregnant populations present unique situations that require special consideration and close examination of indications and contraindications.
Keywords: IVC anatomy, IVC filter, interventional radiology
Objectives: Upon completion of this article, the reader will be able to identify common anatomic variations of the inferior vena cava and how to approach the placement of a filter in populations that require special considerations.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Inferior Vena Cava Anatomic Variants
In 96 to 97% of individuals, the inferior vena cava (IVC) is a single, right-sided venous structure formed at the confluence of the iliac veins and draining to the inferior right atrium.1 2 The hepatic IVC is formed from the vitelline veins,2 3 the normal infrarenal IVC is formed from the right supracardinal vein, and the left supracardinal vein regresses to form the hemiazygos veins. Finally, the suprarenal IVC is formed from the subcardinal veins.1 2 4 Most anomalies of the IVC are a result of aberrations with normal embryological processes. Variations in typical anatomy of the IVC require different approaches to planning placement of an IVC filter. As these abnormalities develop during embryogenesis, there is appropriate opportunity for the body to develop sufficient collaterals. As a result, these IVC anomalies tend to be asymptomatic, and are of greatest value in planning interventions appropriately.5
Mega Vena Cava
A typical infrarenal IVC measures approximately 23 mm.1 A mega vena cava, though not embryologically derived as most anatomic variants, is diagnosed when the IVC measures greater than 28 mm. This anomaly occurs in less than 1% of the population.6 7 Most commercially available IVC filters are specifically indicated for use in IVCs that measure 28 mm or less; however, the Bird's Nest filter (Cook Inc, Bloomington, IN) is designed for use in IVCs up to 40 mm.1 8 An alternative approach to the patient with a mega vena cava is to deploy IVC filters in the iliac veins bilaterally.6
Retroaortic/Circumaortic Left Renal Vein
The left renal vein is normally derived from the anterior subcardinal veins. It courses anteriorly across the midline anteriorly, between the aorta and superior mesenteric artery.1 If this vein regresses, the supracardinal vein which is anatomically located behind the aorta maintains venous outflow of the left kidney.5 9 A retroaortic left renal vein is seen in approximately 3% of people (Fig. 1).1 9 A circumaortic left renal vein occurs due to persistence of both the retroaortic and normal preaortic components; this anomaly has a prevalence of 7% (Fig. 2).1 Circumaortic left renal veins are classified by the course and quantity of the veins: type I occurs with a single renal vein bifurcating into a pre- and retroaortic branches, type II has two separate renal veins, and type III has an anastomosis of the pre- and retroaortic veins or multiple pre- or retroaortic veins.4 Generally, the retroaortic renal vein or the retroaortic component of a circumaortic renal vein drain into the IVC more caudally than the typical renal veins.7
Fig. 1.
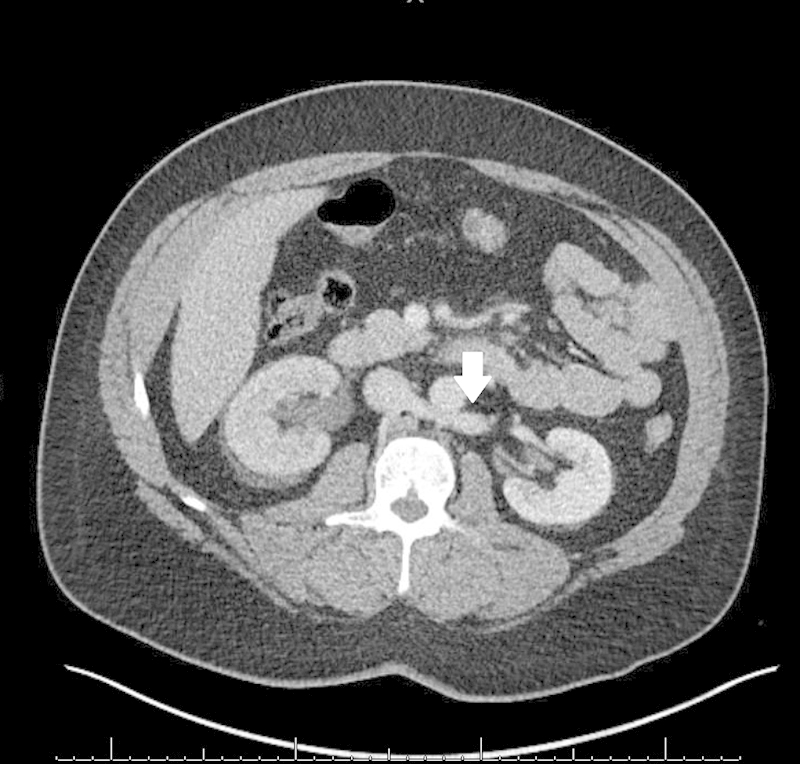
Axial contrast-enhanced CT showing left renal vein passing dorsal to the aorta (arrow).
Fig. 2.
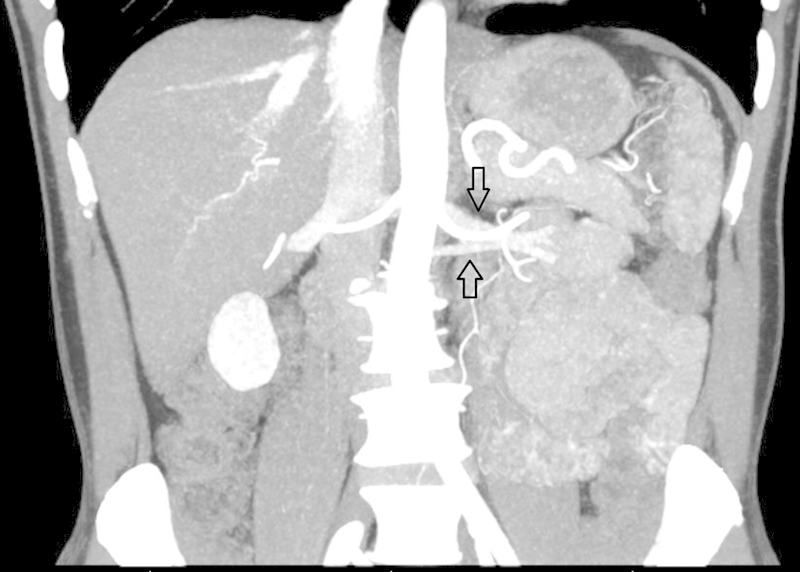
Coronal contrast-enhanced CT showing left renal veins (arrows) diverging caudally and cranially, the most common configuration for circumaortic left renal veins.
Left Inferior Vena Cava
A left-sided IVC occurs when the left supracardinal vein persists and the right supracardinal vein regresses.2 5 9 This anomaly occurs in approximately 0.5% of the population.1 2 5 9 There are two common configurations of the left-sided IVC. Most commonly, the IVC crosses the midline via the left renal vein to form a normal right-sided suprarenal IVC (Fig. 3)1 2; alternatively, the IVC may drain into the left-sided hemiazygos vein, which crosses the midline and drains into the azygos veins. The azygos veins then drain into the superior vena cava (SVC) or, rarely, the left brachiocephalic vein.4 9 The intrahepatic IVC is not necessarily affected in this anomaly, as it arises from the subcardinal veins as opposed to the supracardinal veins.1 As a result, the liver typically drains normally in this anomaly into the right atrium. Clinically, a left-sided IVC may be more difficult to access by a transjugular approach.5 If there is a normal configuration of the suprarenal IVC, a left IVC may cause confusion if not identified on pre-procedural imaging.2
Fig. 3.
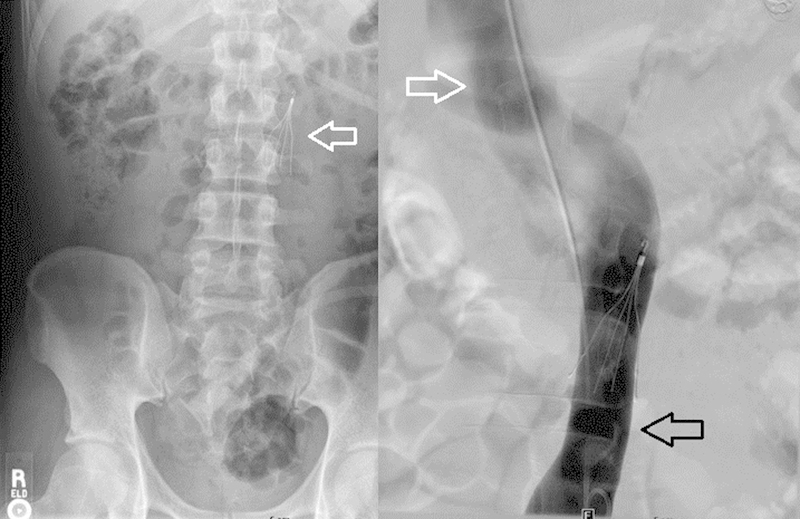
Abdominal radiograph demonstrating left-sided filter deployment (arrow) and venogram demonstrating left-sided inferior vena cava (IVC) (black arrow) crossing midline to form a normal right-sided suprarenal IVC (white arrows).
Double Inferior Vena Cava
A duplicated IVC occurs when the supracardinal veins persist bilaterally.2 4 9 This configuration is estimated to be present in 0.2 to 3% of the general population.1 4 5 9 With this anomaly, the iliac veins typically drain into the ipsilateral IVC.1 The IVCs vary greatly in size and may be symmetric, but a dominant IVC typically occurs, usually on the right side.4 5 This anomaly has multiple associations with cardiovascular and urinary system malformation.4
A double IVC has multiple common variants in the configuration of the venous system. The most common is that the separate IVCs may simply unite via the left-sided IVC draining to the left renal vein, and joining the right IVC via a normal suprarenal IVC (Figs. 4 and 5).1 2 4 5 It is possible for the right renal vein to be associated with the left IVC and to cross posterior to the aorta, as well as for the left IVC to be continuous with the hemiazygos veins.9 The hemiazygos veins may drain into the azygos veins, the coronary vein of the heart through a left-sided SVC, or the left brachiocephalic vein.9 Owing to the relative rarity of this anomaly, the approach to filter placement is debatable. The most often described approach is placement of IVC filters in the bilateral infrarenal IVCs3 7; however, there are reports of placing an IVC filter in the common suprarenal IVC.5 7 If one of the IVCs is small and communicates with the main IVC inferiorly and superiorly, embolizing the nondominant IVC and placing a filter in the remaining patent IVC may also be a reasonable approach.5 7 Patients with duplicated IVCs have higher incidences of recurrent pulmonary emboli compared with those with normal anatomy.4
Fig. 4.
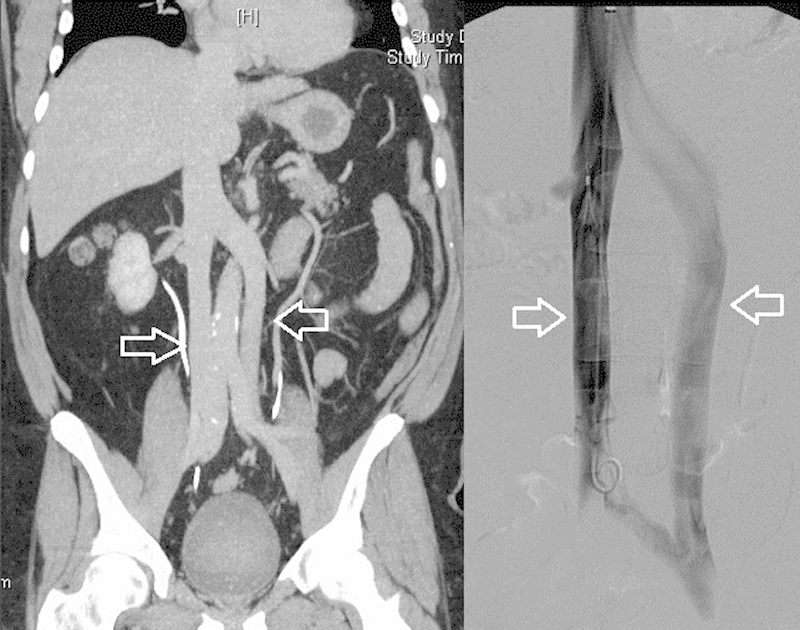
Coronal CT and venogram demonstrating duplicated infrarenal inferior vena cava (arrows) which join at the levels of the iliac veins and the renal veins.
Fig. 5.
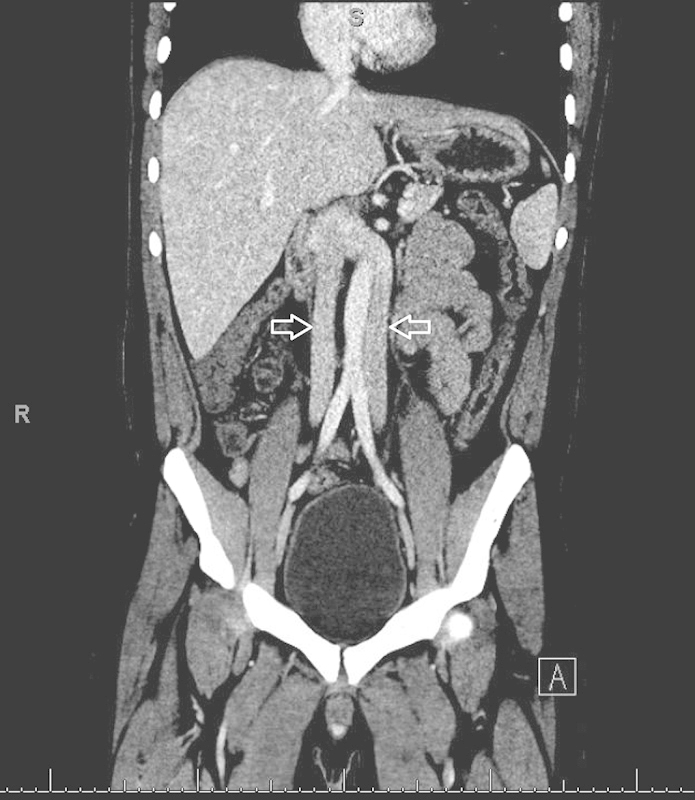
Coronal contrast-enhanced CT demonstrating bilateral inferior vena cava (IVCs) (arrows) which join at the level of the renal veins to form a single right-sided suprarenal IVC.
Interrupted Inferior Vena Cava
The subcardinal veins are responsible for the appropriate formation of the suprarenal IVC. If these regress or do not appropriately develop, the IVC does not follow its typical course through the liver. To compensate, the infrahepatic IVC becomes continuous with the azygos vein, which drains into the SVC.4 9 The prevalence of this anomaly is estimated to be approximately 0.6%,2 9 and this specific anomaly is associated with situs and cardiovascular anomalies.5 9 If there is a situs abnormality with a persistent left SVC, then the azygos may drain into the hemiazygos, to the left SVC, and into the coronary sinus.2
Technical Considerations in Inferior Vena Cava Filter Placement
Cross-sectional imaging, although not required, can be of great utility in planning prior to IVC intervention. The imaging should not only be used to evaluate the IVC but also the venous access site. If prior imaging is not available, complete cavography should be performed. Close and careful examination of cross-sectional imaging must be performed, as left-sided IVCs can be misinterpreted as lymphadenopathy.2 3 4 5
Approach and Deployment
The choice of approach for placement of a filter has been well studied. The right internal jugular vein is a common choice for venous access because of its ease, comfort, and relatively straight course to the IVC. However, the femoral veins are also commonly used. In cases where access to those vessels is not feasible, brachial vein access has also been described.6 8 In general, right-sided vessels are believed to have the lowest rate of tilt during deployment when compared with left-sided vessels, with the internal jugular vein being superior to the femoral vein.6 Tilt is clinically significant when greater than 15 degrees, which results in decreased filter efficacy and increased risk of caval rupture.6 10 When using intravascular ultrasound, however, a femoral approach is generally preferred.7
Most commonly, a filter is deployed in an infrarenal position to minimize the risk of filtered occlusive thrombus occluding the renal vein outflow.8 However, due to anatomic variation, pregnancy, presence of infrarenal IVC thrombus, or for other reasons, it may be necessary to deploy a filter in the suprarenal IVC.8 11 The suprarenal IVC tends to be of larger diameter than the infrarenal IVC due to inflow from the renal veins. Although suprarenal filter placement is generally safe and similarly effective to infrarenal placement, careful consideration is needed for patients who have significant renal impairment.6 8 That said, if filter placement is indicated and infrarenal placement is not feasible, the evidence of renal dysfunction from suprarenal IVC filter placement derived from high-level evidence is very sparse at best.11
Imaging in Placement
Multiple imaging modalities may be used for guidance during deployment of a filter device. Such options include the traditional use of fluoroscopy in an angiography suit; however, bedside placement of a filter can be performed using either duplex ultrasound or intravascular ultrasound (IVUS).
Real-time fluoroscopy remains the most common imaging modality used for IVC filter deployment.8 Cavography from the left iliac vein can be performed to determine if there is either a duplicated or left-sided IVC.2 Alternatively, contrast reflux into the left iliac from right-sided access can also confirm conventional anatomy.1 Carbon dioxide and iodinated contrast material have both been demonstrated to be equally safe and effective when used in the IVC,7 although the risk of nephrotoxicity and anaphylaxis is eliminated by using carbon dioxide.
Conventional transcutaneous ultrasound and IVUS are both safe and effective imaging techniques for bedside IVC filter placement.8 12 IVUS may be better utilized in patients with large abdominal girths or in other circumstances (e.g., ileus) when transcutaneous ultrasound cannot evaluate the IVC.7 8 12 One limitation to using IVUS for IVC filter placement is that it requires either two separate venous access sites or withdrawal of the IVUS probe prior to placement of the device,12 eliminating real-time imaging during deployment. The primary vascular anatomic landmarks with IVUS are the confluence of the iliac veins, the renal and hepatic veins, and the cavoatrial junction.1 For patients with nontraditional IVC anatomy, this may be a less desirable approach. As with other imaging techniques, operator experience is highly variable and can have a significant effect on filter placement. One report recommends utilizing fluoroscopy while learning the use of IVUS.12 Because this technique requires an additional site of venous access and imaging expertise, fluoroscopy may be more appropriate in the general population. However, bedside placement presents a useful alternative for intensive care patients who may not be able to be safely transported to the interventional radiology suite.
Special Populations
IVC filters were originally developed with the adult population in mind. However, deep venous thrombosis (DVT) is recognized more frequently in the pediatric population than in previous decades. Although IVC filters are used in children, recommendations for their use are based largely on adult trials; recommendations in the pediatric literature are limited to retrospective studies and case reports.13 14 Current guidelines vary based on specific medical societies: the American College of Chest Physicians recommends filter use in patients weighing at least 10 kg and with known acute DVT and contraindication to anticoagulation, the American Heart Association includes use in recurrent DVT, and the Society of Interventional Radiology includes a recommendation for prophylactic use in high-risk populations.14 The unique concern for patient (and IVC) growth and for greater expected survival in these patients should give the clinician pause when considering IVC filter placement over mechanical, pharmacologic, or other alternative therapies. A single-center retrospective study of IVC filters in children found there were no complications related to deployment, indicating that the procedure is, at the very least, safe in this population. However, in this study, 10% of patients failed to have their filters retrieved,13 which may have significant long-term implications.
Another population that requires special attention is pregnant patients. In the United Kingdom, venous thromboembolic (VTE) events have been cited as the most common primary cause of maternal mortality, with a relative risk of death in patients with VTE of approximately six times greater than in the normal population.6 15 Vitamin K antagonists cannot be utilized during pregnancy because of the teratogenicity risk, which leaves low molecular weight heparin as the only currently viable pharmaceutical anticoagulation option.6 15 Traditionally, suprarenal IVC filter placement is performed in gravid patients due to concern for filter deformation or malpositioning, secondary to the mass effect from the enlarging uterus. A secondary benefit to suprarenal placement is a reduction in the radiation dose to the fetus, which can further be reduced by utilizing shielding and conservative use of fluoroscopy.7 16 A relative indication for placement of an IVC filter in pregnant patients is when the DVT is upstream from the uterus, due to concern for a thrombotic event in the perinatal period with shrinking of the uterus.16 For women with known prior high-risk pregnancies pursuing another pregnancy, prophylactic IVC filter placement may be performed prior to conception.15 However, no prospective trials are available to offer strong evidence-based guidelines for IVC filter utilization in this population.15
Conclusion
Many specific anatomical and technical considerations must be taken into account when placing IVC filters. Many of these anatomical considerations are clinically silent, and the interventional radiologist placing filters must be acutely aware of the possibility of such anomalies existing. A priori knowledge of these anomalies is vital.
References
- 1.Kaufman J A, Lee M J. Philadelphia, PA: Elsevier Saunders; 2014. Vascular and interventional radiology. 2nd edition; pp. 287–292. [Google Scholar]
- 2.Smillie R P, Shetty M, Boyer A C, Madrazo B, Jafri S Z. Imaging evaluation of the inferior vena cava. Radiographics. 2015;35(2):578–592. doi: 10.1148/rg.352140136. [DOI] [PubMed] [Google Scholar]
- 3.Awais M, Rehman A, Baloch N U-A, Salam B. Multiplanar imaging of inferior vena cava variants. Abdom Imaging. 2015;40(1):159–166. doi: 10.1007/s00261-014-0187-9. [DOI] [PubMed] [Google Scholar]
- 4.Spentzouris G, Zandian A, Cesmebasi A. et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and considerations for clinicians. Clin Anat. 2014;27(8):1234–1243. doi: 10.1002/ca.22445. [DOI] [PubMed] [Google Scholar]
- 5.Kandpal H, Sharma R, Gamangatti S, Srivastava D N, Vashisht S. Imaging the inferior vena cava: a road less traveled. Radiographics. 2008;28(3):669–689. doi: 10.1148/rg.283075101. [DOI] [PubMed] [Google Scholar]
- 6.Harvey J J, Hopkins J, McCafferty I J, Jones R G. Inferior vena cava filters: what radiologists need to know. Clin Radiol. 2013;68(7):721–732. doi: 10.1016/j.crad.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Kandarpa K, Machan L. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2011. Handbook of Interventional Radiologic Procedures. [Google Scholar]
- 8.Weinberg I, Kaufman J, Jaff M R. Inferior vena cava filters. JACC Cardiovasc Interv. 2013;6(6):539–547. doi: 10.1016/j.jcin.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Petik B Inferior vena cava anomalies and variations: imaging and rare clinical findings Insights Imaging 2015; Available at: http://link.springer.com/10.1007/s13244-015-0431-z. Accessed March 24, 2016 [DOI] [PMC free article] [PubMed]
- 10.Knott E M, Beacham B, Fry W R. New technique to prevent tilt during inferior vena cava filter placement. J Vasc Surg. 2012;55(3):869–871. doi: 10.1016/j.jvs.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Kalva S P, Chlapoutaki C, Wicky S, Greenfield A J, Waltman A C, Athanasoulis C A. Suprarenal inferior vena cava filters: a 20-year single-center experience. J Vasc Interv Radiol. 2008;19(7):1041–1047. doi: 10.1016/j.jvir.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 12.Hodgkiss-Harlow K, Back M R, Brumberg R. et al. Technical factors affecting the accuracy of bedside IVC filter placement using intravascular ultrasound. Vasc Endovascular Surg. 2012;46(4):293–299. doi: 10.1177/1538574411434495. [DOI] [PubMed] [Google Scholar]
- 13.Rottenstreich A, Revel-Vilk S, Bloom A I, Kalish Y. Inferior vena cava (IVC) filters in children: A 10-year single center experience. Pediatr Blood Cancer. 2015;62(11):1974–1978. doi: 10.1002/pbc.25641. [DOI] [PubMed] [Google Scholar]
- 14.Blevins E M, Glanz K, Huang Y-SV, Raffini L, Shinohara R T, Witmer C. A multicenter cohort study of inferior vena cava filter use in children. Pediatr Blood Cancer. 2015;62(12):2089–2093. doi: 10.1002/pbc.25662. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Ettles D F, Robinson G J, Lindow S W. Inferior vena cava filter use in pregnancy: preliminary experience. BJOG. 2008;115(6):785–788. doi: 10.1111/j.1471-0528.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 16.Kiguchi M M, Dillavou E D. IVC filters: challenges and future directions. Adv Vasc Med. 2014;201:6. [Google Scholar]


