Abstract
Many inferior vena cava (IVC) filter types, along with their specific risks and complications, are not recognized. The purpose of this study was to evaluate the various FDA-approved IVC filter types to determine device-specific risks, as a way to help identify patients who may benefit from ongoing follow-up versus prompt filter retrieval. An evidence-based electronic search (FDA Premarket Notification, MEDLINE, FDA MAUDE) was performed to identify all IVC filter types and device-specific complications from 1980 to 2014. Twenty-three IVC filter types (14 retrievable, 9 permanent) were identified. The devices were categorized as follows: conical (n = 14), conical with umbrella (n = 1), conical with cylindrical element (n = 2), biconical with cylindrical element (n = 2), helical (n = 1), spiral (n = 1), and complex (n = 1). Purely conical filters were associated with the highest reported risks of penetration (90–100%). Filters with cylindrical or umbrella elements were associated with the highest reported risk of IVC thrombosis (30–50%). Conical Bard filters were associated with the highest reported risks of fracture (40%). The various FDA-approved IVC filter types were evaluated for device-specific complications based on best current evidence. This information can be used to guide and optimize clinical management in patients with indwelling IVC filters.
Keywords: IVC filters, nonthrombotic complications, interventional radiology
CME Objective: Upon completion of this article, the reader should be able to distinguish the various retrievable and permanent IVC filter designs, and explain the most common complications associated with the various designs.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
The use of inferior vena cava (IVC) filters has dramatically increased over the past three decades in the United States, and the number of filter insertions doubled between 1998 and 2008.1 2 By 2012, an estimated 259,000 IVC filters were placed in the United States alone,3 coinciding with a growing number of Food and Drug Administration (FDA)-approved devices. Consequently, the increasing variety of filters along with rising overall use has resulted in increased complications from indwelling IVC filters; this prompted the FDA to issue a safety alert urging all physicians caring for patients with indwelling filters to consider removing the filter as soon as protection from pulmonary embolism (PE) is no longer needed.4 Despite this recommendation, many devices are not adequately followed for removal, and the large number of filter types now encountered on routine imaging has made proper device identification difficult. The purpose of this study was to evaluate the various FDA-approved IVC filter designs to determine device-specific risks, as a way of helping to identify patients who may benefit from ongoing follow-up or prompt filter retrieval.
Materials and Methods
Identification of IVC Filter Types
The FDA Premarket Notification Database5 was searched electronically to identify all IVC filters receiving 510(k) clearance (product code DTK—filter, intravascular, cardiovascular) between 1980 and 2014.
Classification of Filter Complications
IVC filter complications were classified according to the Society of Interventional Radiology (SIR) guidelines6 as follows:
Fracture: Breakage or separation of any filter component due to structural failure.6 The fractured components can remain in situ or undergo distal embolization (Fig. 1).
Fig. 1.
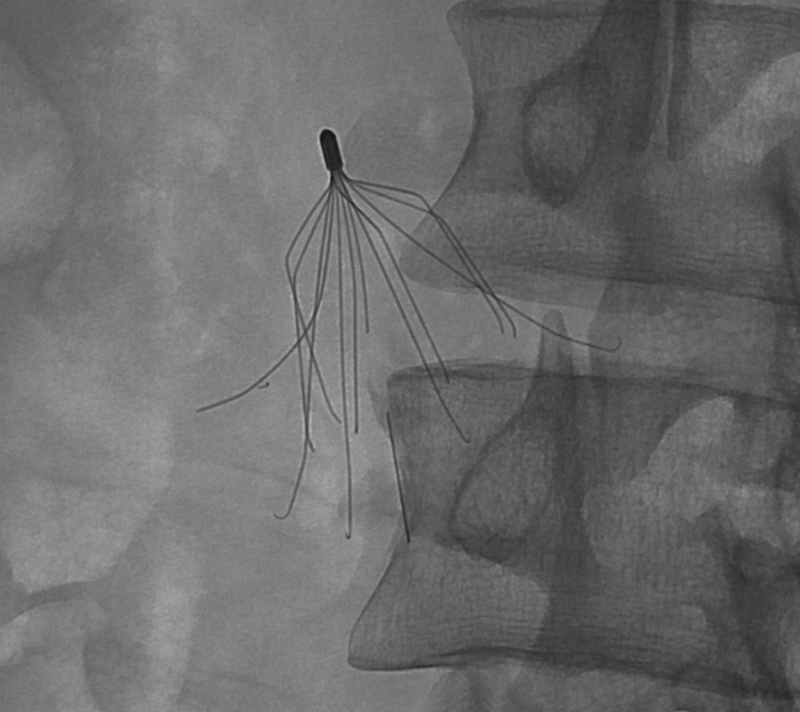
Fluoroscopic image showing a G2 filter with multiple fractured components.
Insertional problems: Malfunctions in filter deployment including tilting of the filter more than 15 degrees from the IVC axis, incomplete opening, and prolapse of filter components6 (Fig. 2).
Fig. 2.
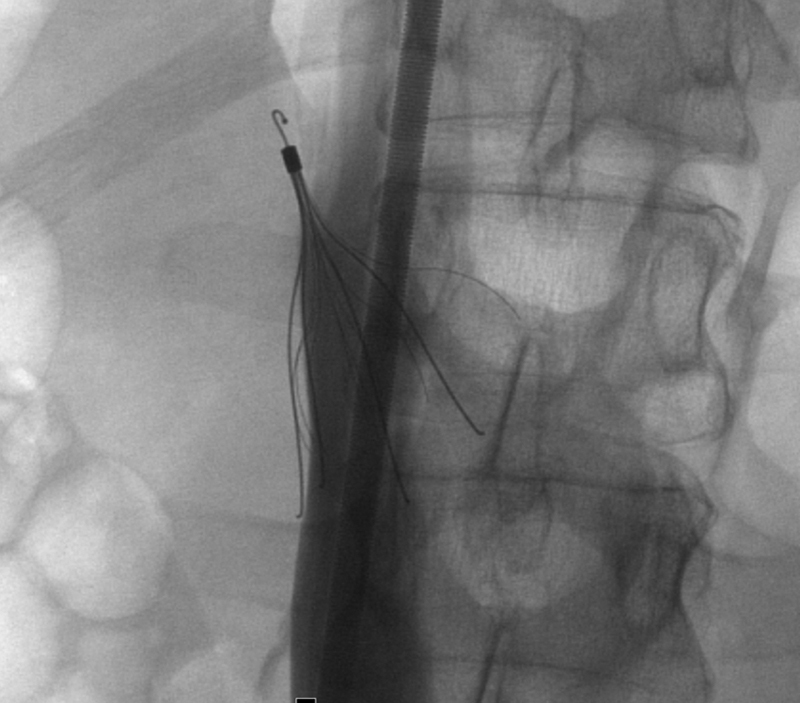
Fluoroscopic images showing a severely tilted and tip embedded Celect filter.
IVC perforation: Visualization of one or more filter components extending greater than 3 mm beyond the caval wall or into an adjacent structure6 such as the duodenum, aorta, psoas muscle, kidney, or vertebral body. Grading schemes defining the degree of perforation have been described in the literature7 (Fig. 3).
Fig. 3.
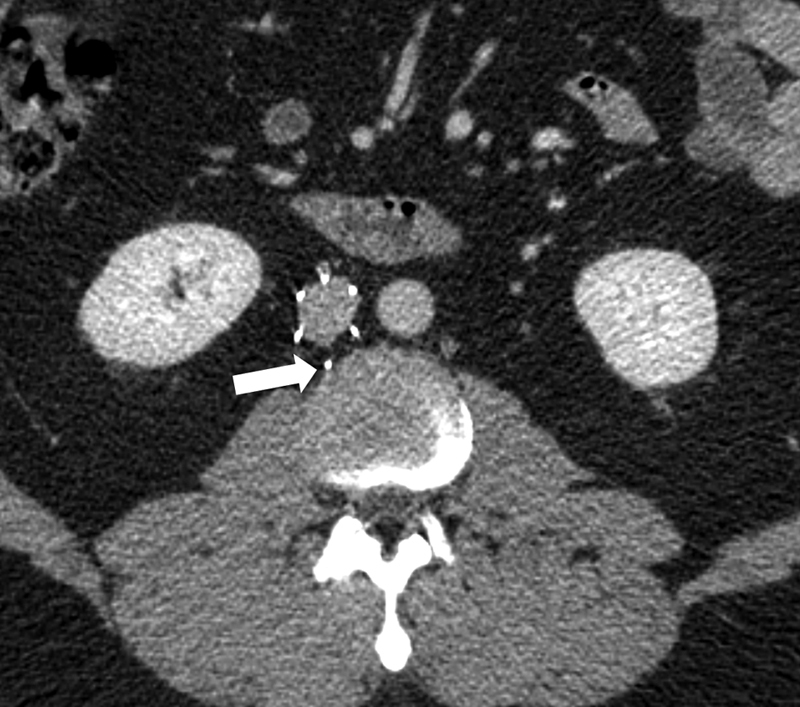
Axial CT image showing a 12F Stainless Steel Greenfield filter in place complicated by component perforation (arrow).
Migration: Movement of an IVC filter greater than 2 cm along the IVC beyond the initial placement position.6 Filter migration may result in filter embolization into the right atrium, right ventricle, or pulmonary arteries (Fig. 4).
Fig. 4.
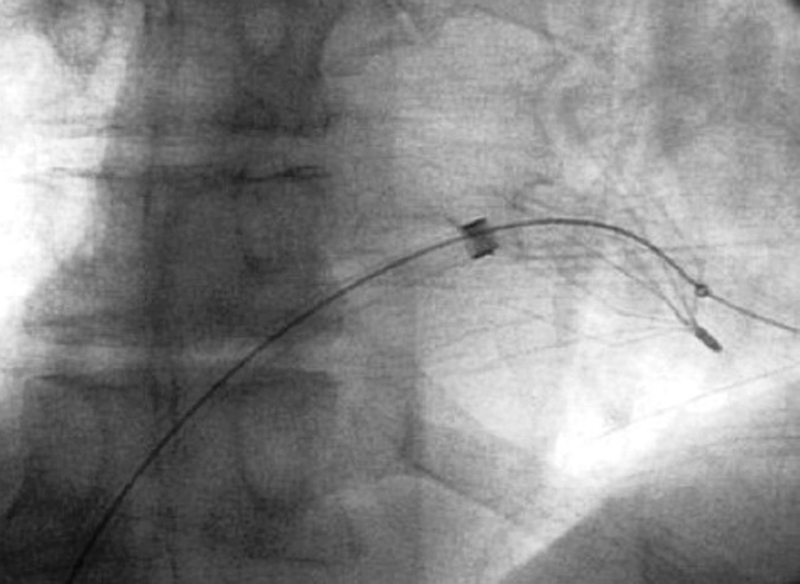
Fluoroscopic image demonstrating an IVC filter that has migrated into the right ventricle.
IVC occlusion: Acute or chronic thrombotic occlusion of the IVC following filter placement6 (Fig. 5).
Fig. 5.
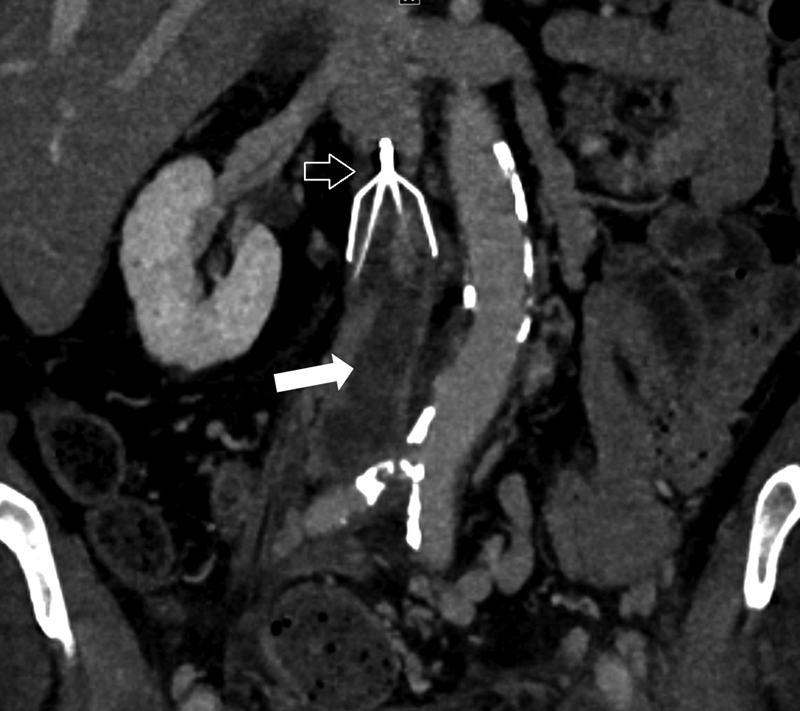
Coronal CT image showing a Bard filter (open arrow) in place with acute thrombotic occlusion of the IVC (solid arrow).
Evidence-Based Search of Filter Complications
An electronic MEDLINE search was performed using the following index search terms: “IVC filter” OR “inferior vena cava filter” OR “ALN filter” OR “Bard Eclipse” OR “Bard G2” OR “Bard G2X” OR “Bard Recovery” OR “Bard Denali” OR “Bard Meridian” OR “Simon Nitinol” OR “Vena Tech LGM” OR “Vena Tech LP” OR “Greenfield filter” OR “Bird's Nest filter” OR “Celect filter” OR ” Günther Tulip” OR “Optease” OR “Trapease” OR “Safeflo” OR “Option filter” OR “Crux vena cava filter.” The results were filtered for English language, clinical trial study type, human species, and date range from 1980 to 2014 (Fig. 6). All potentially relevant articles were collected for analysis. The references within these articles were reviewed to obtain additional relevant articles for analysis. A data extraction form was used to record the following information: filter type, retrieval rate, complications, and frequency of complications per filter type. Two reviewers verified the accuracy of all data prior to analysis. The FDA Manufacturer and User Facility Device Experience (MAUDE) database8 was queried electronically (1992–2014) to identify additional adverse events associated with IVC filter use (product class—filter, intravascular, cardiovascular).
Fig. 6.
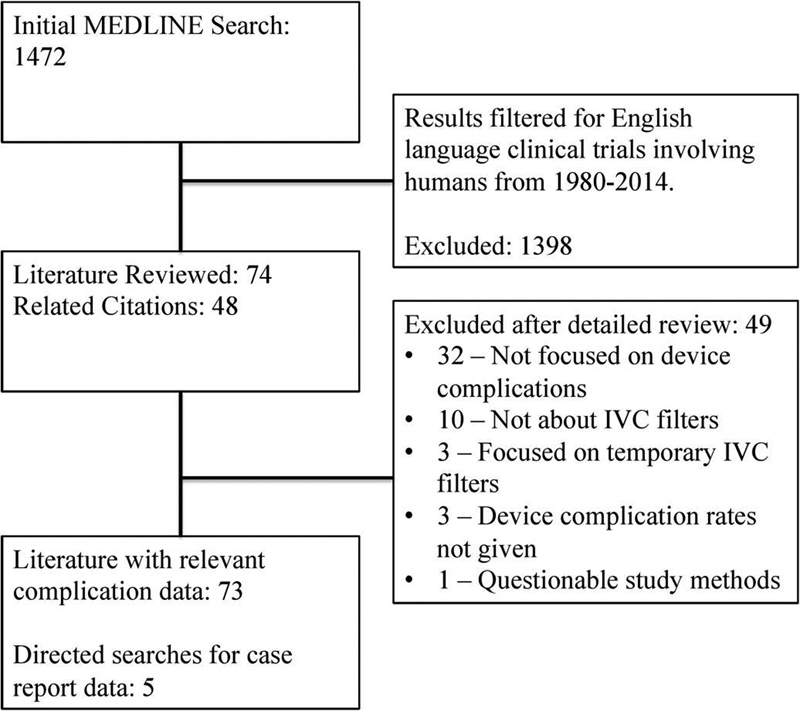
Literature screening flowchart.
Results
Twenty-four IVC filters were identified. From this group, the Edwards Mobin-Uddin device was excluded as it was removed from the market in 1986.9 From the remaining group, nine filters cleared for permanent use, and 14 filters cleared for retrievable or permanent use, were identified (Table 1). The device distribution based on geometry was as follows: conical (n = 15), conical with umbrella (n = 1), conical with cylindrical element (n = 2), biconical with cylindrical element (n = 2), helical (n = 1), spiral with umbrella (n = 1), and complex (n = 1) (Fig. 7).
Table 1. IVC Filters in the United States (1980–2014).
| Permanent filters | Retrievable filtersa |
|---|---|
| • 24F stainless steel Greenfield (Boston Scientific, Natick, MA)b
• 12F stainless steel Greenfield (Boston Scientific) • Titanium Greenfield (Boston Scientific) • Vena Tech LGM (B. Braun Medical, Bethlehem, PA)b • Vena Tech LP (B. Braun Medical) • Trapease (Cordis Endovascular, Warren, NJ) • Bird's Nest (Cook, Bloomington, IN) • Simon Nitinol (Bard Peripheral Vascular, Tempe, AZ) • SafeFlo (Rafael Medical Technologies, Dover, DE)b |
• ALN (ALN International, Miami, FL) • Recovery (Bard Peripheral Vascular)b • G2 (Bard Peripheral Vascular)b • G2X (Bard Peripheral Vascular)b • Eclipse (Bard Peripheral Vascular)b • Meridian (Bard Peripheral Vascular)b • Denali (Bard Peripheral Vascular) • Günther Tulip (Cook) • Celect (Cook)b • Celect Platinum (Cook) • Optease (Cordis Endovascular, Warren, NJ) • Option (Argon, Plano, TX)b • Option Elite (Argon, Plano, TX) • Crux (Volcano, San Diego, CA) |
All retrievable filters are also approved for permanent use.
No longer manufactured but may still be encountered from prior implantation.
Fig. 7.
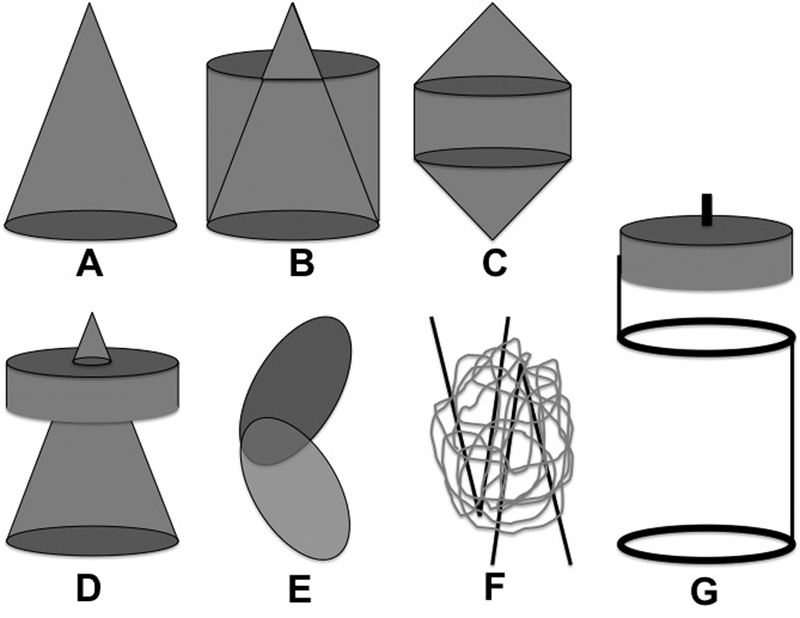
IVC filter geometries: (A) conical, (B) conical with cylindrical element, (C) biconical with cylindrical element, (D) conical with umbrella, (E) helical, (F) complex, (G) spiral with umbrella.
Reported device-specific complications were identified among all filter types, and the highest reported complications for each device are summarized in Table 2. The risk of complications was found to vary widely depending on the specific IVC filter type.
Table 2. Highest reported radiographically identifiable complications for each filter type.
| Device (year FDA cleared) | Fracture | IVC perforation | Migration | IVC occlusion |
|---|---|---|---|---|
| ALN (2008) | Case reports (0%)54 55 56 |
3.4% (3.4%)54 |
3% (1.4–3%)57 58 |
Case reports (0%)54 58 59 |
| Recovery (2003/2005a) | 39.5% at 65.7 mo 25% (5.5–25%)10 11 12 13 14 |
100% (27–100%)12 14 |
10% (0–10%)10 12 20 21 |
Case reports (0%)14 |
| G2 (2005/2008a) G2X (2008) Eclipse (2008) Meridian (2011) |
38% at 60 mo 12% (1.2–12%)11 13 15 16 23 60 |
44% (18–44%)15 23 60 61 |
25% (12–25%)15 20 23 |
2.2% (0–2.2%)22 60 |
| Denali (2013) | Case reports62 | 2.5% (2.5%)63 |
Limited data | Limited data |
| Simon Nitinol (1990) | 16% (10–16%)17 18 |
95% (25–95%)17 18 |
5% (0–5%)18 47 |
50% (3.5–50%)17 47 48 49 |
| LGM/Vena Tech LGM (1989) | Case reports64 | Case reports (0%)44 65 | 18.4% (6–18.4%)33 44 45 66 |
65% at 9 y (3.7%/y)45 |
| Vena Tech LP (2001) | Limited data | Case reports (0%)67 | Case reports (0%)67 | Limited data |
| 24F SS Greenfield (1973) | Case reports68 | 15% (2–15%)69 70 |
2% (2%)69 |
5% (2–5%)28 29 71 72 |
| 12F SS Greenfield (1995) | 0.3% (0.3%)73 |
1% (1%)73 |
2.6% (2.6%)73 |
12% (5–12%)31 74 |
| Titanium Greenfield (1989) | 3.8% (3.8%)74 |
50% (13–50% prior to hook modification, 1% with MH design)40 41 |
15% (7.5–15%)33 42 |
20% (3.5–20%)32 75 |
| Günther Tulip (2000/2003a) | 0.3% (0.3%)36 |
78% (22–78%)7 35 36 37 |
12.5% (2.4–12.5%)26 36 43 |
4.1% (2.4–4.1%)36 43 |
| Celect (2007/2008a) Celect Platinum (2012) |
5.6% (4.3–5.6%)39 76 |
93% (22–93%)7 35 38 39 |
4.3% (0–4.3%)38 76 77 |
2.5% (2.5%)38 |
| Bird's Nest (1989) | 4% (3–4%)41 |
85% (85%)34 |
1.1% (1.1%)78 |
4.7% (2.9–4.7%)34 78 |
| Optease (2002/2004a) Trapease (2000) |
50% (0–50%)19 79 80 81 82 83 84 |
Case Reports (0%)79 83 84 | 0.9% (0–0.9%)79 80 81 83 84 |
29% (0.8–29%)46 79 83 84 85 86 |
| Option (2009) | Limited data | 10% (2.9–10%)37 87 |
2% (2%)87 |
4% (4%)87 |
| Crux (2012) | Limited data (0%)88 | Limited data | Limited data (0%)88 |
Limited data (7.2% nonocclusive IVC thrombus)88 |
Abbreviations: FDA, Federal Drug Administration; IVC, inferior vena cava.
Note: For the SafeFlo filter (2009), no significant clinical data are available, and the device is no longer manufactured.
Subsequent year when filter was cleared for retrieval indication.
Fracture
Early conical Bard Peripheral Vascular (Tempe, AZ) filters were associated with the highest reported rates of fracture. The fracture rate for the original Bard Recovery device was 5.5 to 25% with an estimated incidence of 39.5% at 65.7 months.10 11 12 13 14 The fracture rate for the Bard G2 devices (G2, G2X, Eclipse, Meridian) was initially 1.2 to 12%, but the highest reported rate was later found to be 38% at 60 months.11 13 15 16 High fracture rates were also reported for the Simon Nitinol filter (Bard) (10–16%)17 18 and the Optease/Trapease (Cordis, Miami Lakes, FL) (up to 50%).19
Insertional Issues
Filter tilting greater than 15 degrees during insertion were reported among the following conical filters: Bard Recovery (2.3–15%),20 21 Bard G2/G2X/Eclipse (14–18%),15 22 23 Cook (Bloomington, IN) Günther Tulip (11.5–24%),24 25 26 27 24F Greenfield (Boston Scientific, Marlborough, MA) (7–12%),28 29 12F Stainless Steel Greenfield (Boston Scientific) (9.9–55%),30 31 and Titanium Greenfield (Boston Scientific) (8.3–41%).30 32 33 In addition, wire prolapse up to 70% was reported for the Cook Bird's Nest filter.34
Inferior Vena Cava Perforation
Purely conical filters were associated with the highest reported rates of IVC perforation and were reported as follows: Bard Recovery (27–100%),12 14 Bard G2/G2X/Eclipse (26–44%),15 23 Bard Simon Nitinol (25–95%),17 18 Cook Günther Tulip (22–78%),7 35 36 37 Cook Celect (22–93%),7 35 38 39 and Titanium Greenfield (prior to hook modification) (13–50%).40 41 In addition, IVC strut perforation up to 85% was reported for the Cook Bird's Nest filter.34
Migration
Migration rates greater or equal to 10% were reported among the following devices: Bard Recovery (0–10%),10 12 20 Bard G2 (12–25%),15 20 Titanium Greenfield (7.5–15%),33 42 Cook Günther Tulip (2.4–12.5%),26 36 43 and Vena Tech LGM (6–18.4%).33 44 45
Inferior Vena Cava Occlusion
Filters with a cylindrical component (Vena Tech LGM, Trapease/Optease) or umbrella element (Simon-Nitinol) were associated with high rates of caval thrombosis. The highest reported rate of IVC occlusion for the Trapease/Optease filters was 28.6%.46 The rates of chronic IVC occlusion with the Simon Nitinol filter range from 3.5 to 50%,17 47 48 49 and for the VenaTech LGM, the IVC occlusion rate is as high as 65% at 9 years.45
Discussion
Over the past few decades, IVC filter use has risen in the United States,1 2 which has led to increased recognition of a wide range of potential filter-related complications. These complications include fracture, IVC perforation, component embolization, device migration, and IVC occlusion. In response to rising complication rates, the current FDA Safety Alert on IVC filters recommends filter removal when protection from PE is no longer needed. More recently, the FDA released an additional Safety Communication stating that the risk-to-benefit ratio begins to favor IVC filter removal within 29 to 54 days after implantation, if the risk of PE has passed.50 51
The systematic review by Angel et al52 concluded that filter complications are a serious concern associated with long-term filter use, but the study did not address device-specific risks, and there was no analysis specifically of complications from permanent IVC filters. A large variety of retrievable and permanent IVC filters are commonly encountered on routine imaging studies, but the myriad number of filter types and their associated complications prevents interpretation of such radiographic findings. Filter-related complications may therefore go unrecognized or underappreciated as potential causes of morbidity in patients, including those presenting with intractable abdominal pain from filter penetration.53
The goal of this study was to evaluate the various FDA-approved IVC filter designs to determine device-specific risks, and to help identify patients who may benefit from ongoing follow-up versus prompt filter retrieval. First, we identified the 23 filter types currently encountered in the United States. Next, the complications associated with each filter type were identified. Although we initially searched the FDA MAUDE database, we soon realized these data were based on voluntary reporting and there was gross underreporting of complications. Therefore, we chose to use evidence-based methods to identify the highest reported complication rates in the literature for each filter type (Table 2).
These data revealed a high risk of fracture among Bard and Cordis (Miami Lakes, FL) IVC filters, including a fracture incidence of 39.5% at 65.7 months with the Bard Recovery device,10 11 12 13 14 a 38% risk of fracture at 60 months among the Bard G2 type filters,11 13 15 16 and a 50% risk of fracture with Cordis Optease/Trapease devices.19 A high risk of IVC perforation was reported with the Bard Recovery and Cook Celect filters, with penetration rates exceeding 90% for both.7 12 For permanent filter types, a high risk of IVC occlusion was reported among the Simon Nitinol and Vena Tech LGM,9 filters with occlusion rates of 50 and 65% (at 9 years),45 49 respectively. Overall, as these complications appear to be related to filter geometry, one should always assess for IVC perforation, when a conical device is identified, and IVC occlusion, when a cylindrical or umbrella filter component is identified.
This study is limited by the quality of available data on IVC filters, and some filter types in this study were limited published data. In addition, many studies had limited long-term follow-up; therefore, it is possible that the true risk of complications for these filter types could be even higher than currently reported, as complications tend to increase after longer dwell times. Nevertheless, mitigation against these effects was attempted by identifying the highest complication rates reported so far in the literature. Future studies should involve methods to provide constant updating of filter complication rates as new data emerge in larger cohorts.
References
- 1.Duszak R Jr, Parker L, Levin D C, Rao V M. Placement and removal of inferior vena cava filters: national trends in the Medicare population. J Am Coll Radiol. 2011;8(7):483–489. doi: 10.1016/j.jacr.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Stein P D, Kayali F, Olson R E. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164(14):1541–1545. doi: 10.1001/archinte.164.14.1541. [DOI] [PubMed] [Google Scholar]
- 3.Smouse B J. Is market growth of vena cava filters justified? Endovasc Today. 2010:74–77. [Google Scholar]
- 4.Food and Drug Administration Removing Retrievable Inferior Vena Cava Filters: Initial Communication Published August 9, 2010. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221676.htm. Accessed July 30, 2015
- 5.Food and Drug Administration 510(K) Premarket Notification Database Published 2014. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm. Accessed January 1, 2015
- 6.Caplin D M Nikolic B Kalva S P Ganguli S Saad W E Zuckerman D A; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for the performance of inferior vena cava filter placement for the prevention of pulmonary embolism J Vasc Interv Radiol 201122111499–1506. [DOI] [PubMed] [Google Scholar]
- 7.Durack J C, Westphalen A C, Kekulawela S. et al. Perforation of the IVC: rule rather than exception after longer indwelling times for the Günther Tulip and Celect retrievable filters. Cardiovasc Intervent Radiol. 2012;35(2):299–308. doi: 10.1007/s00270-011-0151-9. [DOI] [PubMed] [Google Scholar]
- 8.Food and Drug Administration MAUDE - Manufacturer and User Facility Device Experience Database Published 2014. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. Accessed January 1, 2015
- 9.Bakal C W, Silberzweig J E. New York, NY: Thieme; 2011. Vascular and Interventional Radiology: Principles and Practice. [Google Scholar]
- 10.Tam M D, Spain J, Lieber M, Geisinger M, Sands M J, Wang W. Fracture and distant migration of the Bard Recovery filter: a retrospective review of 363 implantations for potentially life-threatening complications. J Vasc Interv Radiol. 2012;23(2):199–2050. doi: 10.1016/j.jvir.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson W, Nicholson W J, Tolerico P. et al. Prevalence of fracture and fragment embolization of Bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med. 2010;170(20):1827–1831. doi: 10.1001/archinternmed.2010.316. [DOI] [PubMed] [Google Scholar]
- 12.Hull J E, Robertson S W. Bard Recovery filter: evaluation and management of vena cava limb perforation, fracture, and migration. J Vasc Interv Radiol. 2009;20(1):52–60. doi: 10.1016/j.jvir.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Vijay K, Hughes J A, Burdette A S. et al. Fractured Bard Recovery, G2, and G2 express inferior vena cava filters: incidence, clinical consequences, and outcomes of removal attempts. J Vasc Interv Radiol. 2012;23(2):188–194. doi: 10.1016/j.jvir.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Kalva S P, Athanasoulis C A, Fan C M. et al. “Recovery” vena cava filter: experience in 96 patients. Cardiovasc Intervent Radiol. 2006;29(4):559–564. doi: 10.1007/s00270-005-0271-1. [DOI] [PubMed] [Google Scholar]
- 15.Binkert C A, Drooz A T, Caridi J G. et al. Technical success and safety of retrieval of the G2 filter in a prospective, multicenter study. J Vasc Interv Radiol. 2009;20(11):1449–1453. doi: 10.1016/j.jvir.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 16.An T, Moon E, Bullen J. et al. Prevalence and clinical consequences of fracture and fragment migration of the Bard G2 filter: imaging and clinical follow-up in 684 implantations. J Vasc Interv Radiol. 2014;25(6):941–948. doi: 10.1016/j.jvir.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Poletti P A, Becker C D, Prina L. et al. Long-term results of the Simon nitinol inferior vena cava filter. Eur Radiol. 1998;8(2):289–294. doi: 10.1007/s003300050382. [DOI] [PubMed] [Google Scholar]
- 18.McCowan T C, Ferris E J, Carver D K, Molpus W M. Complications of the nitinol vena caval filter. J Vasc Interv Radiol. 1992;3(2):401–408. doi: 10.1016/s1051-0443(92)72053-3. [DOI] [PubMed] [Google Scholar]
- 19.Sano M, Unno N, Yamamoto N, Tanaka H, Konno H. Frequent fracture of TrapEase inferior vena cava filters: a long-term follow-up assessment. Arch Intern Med. 2012;172(2):189–191. doi: 10.1001/archinternmed.2011.548. [DOI] [PubMed] [Google Scholar]
- 20.Cantwell C P, Pennypacker J, Singh H, Scorza L B, Waybill P N, Lynch F C. Comparison of the recovery and G2 filter as retrievable inferior vena cava filters. J Vasc Interv Radiol. 2009;20(9):1193–1199. doi: 10.1016/j.jvir.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 21.Binkert C A Sasadeusz K Stavropoulos S W Retrievability of the recovery vena cava filter after dwell times longer than 180 days J Vasc Interv Radiol 200617(2, Pt 1):299–302. [DOI] [PubMed] [Google Scholar]
- 22.Charles H W, Black M, Kovacs S. et al. G2 inferior vena cava filter: retrievability and safety. J Vasc Interv Radiol. 2009;20(8):1046–1051. doi: 10.1016/j.jvir.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Lynch F C, Kekulawela S. Removal of the G2 filter: differences between implantation times greater and less than 180 days. J Vasc Interv Radiol. 2009;20(9):1200–1209. doi: 10.1016/j.jvir.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 24.Marquess J S, Burke C T, Beecham A H. et al. Factors associated with failed retrieval of the Günther Tulip inferior vena cava filter. J Vasc Interv Radiol. 2008;19(9):1321–1327. doi: 10.1016/j.jvir.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Smouse H B Rosenthal D Thuong V H et al. Long-term retrieval success rate profile for the Günther Tulip vena cava filter J Vasc Interv Radiol 2009207871–877., quiz 878 [DOI] [PubMed] [Google Scholar]
- 26.Wicky S, Doenz F, Meuwly J-Y, Portier F, Schnyder P, Denys A. Clinical experience with retrievable Günther Tulip vena cava filters. J Endovasc Ther. 2003;10(5):994–1000. doi: 10.1177/152660280301000524. [DOI] [PubMed] [Google Scholar]
- 27.Xiao L, Huang D S, Shen J, Tong J J. Introducer curving technique for the prevention of tilting of transfemoral Günther Tulip inferior vena cava filter. Korean J Radiol. 2012;13(4):483–491. doi: 10.3348/kjr.2012.13.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenfield L J, Peyton R, Crute S, Barnes R. Greenfield vena caval filter experience: late results in 156 patients. Arch Surg. 1981;116(11):1451–1456. doi: 10.1001/archsurg.1981.01380230065010. [DOI] [PubMed] [Google Scholar]
- 29.Messmer J M, Greenfield L J. Greenfield caval filters: long-term radiographic follow-up study. Radiology. 1985;156(3):613–618. doi: 10.1148/radiology.156.3.4023218. [DOI] [PubMed] [Google Scholar]
- 30.Kinney T B, Rose S C, Weingarten K E, Valji K, Oglevie S B, Roberts A C. IVC filter tilt and asymmetry: comparison of the over-the-wire stainless-steel and titanium Greenfield IVC filters. J Vasc Interv Radiol. 1997;8(6):1029–1037. doi: 10.1016/s1051-0443(97)70706-1. [DOI] [PubMed] [Google Scholar]
- 31.Johnson S P, Raiken D P, Grebe P J, Diffin D C, Leyendecker J R. Single institution prospective evaluation of the over-the-wire Greenfield vena caval filter. J Vasc Interv Radiol. 1998;9(5):766–773. doi: 10.1016/s1051-0443(98)70389-6. [DOI] [PubMed] [Google Scholar]
- 32.Sweeney T J, Van Aman M E. Deployment problems with the titanium Greenfield filter. J Vasc Interv Radiol. 1993;4(5):691–694. doi: 10.1016/s1051-0443(93)71950-8. [DOI] [PubMed] [Google Scholar]
- 33.Wittenberg G, Kueppers V, Tschammler A, Scheppach W, Kenn W, Hahn D. Long-term results of vena cava filters: experiences with the LGM and the Titanium Greenfield devices. Cardiovasc Intervent Radiol. 1998;21(3):225–229. doi: 10.1007/s002709900249. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson A A, Ettles D F, Paddon A J, Dyet J F. Long-term follow-up of the bird's nest IVC filter. Clin Radiol. 1999;54(11):759–764. doi: 10.1016/s0009-9260(99)91180-7. [DOI] [PubMed] [Google Scholar]
- 35.McLoney E D, Krishnasamy V P, Castle J C, Yang X, Guy G. Complications of Celect, Günther tulip, and Greenfield inferior vena cava filters on CT follow-up: a single-institution experience. J Vasc Interv Radiol. 2013;24(11):1723–1729. doi: 10.1016/j.jvir.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Hoffer E K, Mueller R J, Luciano M R, Lee N N, Michaels A T, Gemery J M. Safety and efficacy of the Gunther Tulip retrievable vena cava filter: midterm outcomes. Cardiovasc Intervent Radiol. 2013;36(4):998–1005. doi: 10.1007/s00270-012-0517-7. [DOI] [PubMed] [Google Scholar]
- 37.Olorunsola O G, Kohi M P, Fidelman N. et al. Caval penetration by retrievable inferior vena cava filters: a retrospective comparison of Option and Günther Tulip filters. J Vasc Interv Radiol. 2013;24(4):566–571. doi: 10.1016/j.jvir.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Zhou D, Spain J, Moon E, Mclennan G, Sands M J, Wang W. Retrospective review of 120 Celect inferior vena cava filter retrievals: experience at a single institution. J Vasc Interv Radiol. 2012;23(12):1557–1563. doi: 10.1016/j.jvir.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Sangwaiya M J, Marentis T C, Walker T G, Stecker M, Wicky S T, Kalva S P. Safety and effectiveness of the celect inferior vena cava filter: preliminary results. J Vasc Interv Radiol. 2009;20(9):1188–1192. doi: 10.1016/j.jvir.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 40.Greenfield L J, Cho K J, Proctor M. et al. Results of a multicenter study of the modified hook-titanium Greenfield filter. J Vasc Surg. 1991;14(3):253–257. doi: 10.1067/mva.1991.29913. [DOI] [PubMed] [Google Scholar]
- 41.Ferris E J, McCowan T C, Carver D K, McFarland D R. Percutaneous inferior vena caval filters: follow-up of seven designs in 320 patients. Radiology. 1993;188(3):851–856. doi: 10.1148/radiology.188.3.8351361. [DOI] [PubMed] [Google Scholar]
- 42.Greenfield L J, Cho K J, Pais S O, Van Aman M. Preliminary clinical experience with the titanium Greenfield vena caval filter. Arch Surg. 1989;124(6):657–659. doi: 10.1001/archsurg.1989.01410060019002. [DOI] [PubMed] [Google Scholar]
- 43.Hoppe H, Nutting C W, Smouse H R. et al. Günther Tulip filter retrievability multicenter study including CT follow-up: final report. J Vasc Interv Radiol. 2006;17(6):1017–1023. doi: 10.1097/01.rvi.90000223689.49091.76. [DOI] [PubMed] [Google Scholar]
- 44.Millward S F, Peterson R A, Moher D. et al. LGM (Vena Tech) vena caval filter: experience at a single institution. J Vasc Interv Radiol. 1994;5(2):351–356. doi: 10.1016/s1051-0443(94)71501-3. [DOI] [PubMed] [Google Scholar]
- 45.Crochet D P Brunel P Trogrlic S Grossetëte R Auget J-L Dary C Long-term follow-up of Vena Tech-LGM filter: predictors and frequency of caval occlusion J Vasc Interv Radiol 199910(2, Pt 1):137–142. [DOI] [PubMed] [Google Scholar]
- 46.Corriere M A, Sauve K J, Ayerdi J. et al. Vena cava filters and inferior vena cava thrombosis. J Vasc Surg. 2007;45(4):789–794. doi: 10.1016/j.jvs.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 47.Simon M, Athanasoulis C A, Kim D. et al. Simon nitinol inferior vena cava filter: initial clinical experience. Work in progress. Radiology. 1989;172(1):99–103. doi: 10.1148/radiology.172.1.2662259. [DOI] [PubMed] [Google Scholar]
- 48.Kim D, Edelman R R, Margolin C J. et al. The Simon nitinol filter: evaluation by MR and ultrasound. Angiology. 1992;43(7):541–548. doi: 10.1177/000331979204300701. [DOI] [PubMed] [Google Scholar]
- 49.Grassi C J, Matsumoto A H, Teitelbaum G P. Vena caval occlusion after Simon nitinol filter placement: identification with MR imaging in patients with malignancy. J Vasc Interv Radiol. 1992;3(3):535–539. doi: 10.1016/s1051-0443(92)72008-9. [DOI] [PubMed] [Google Scholar]
- 50.Food and Drug Administration Removing Retrievable Inferior Vena Cava Filters: FDA Safety Communication Published 2014. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm396377.htm. Accessed January 1, 2015
- 51.Morales J P, Li X, Irony T Z, Ibrahim N G, Moynahan M, Cavanaugh K J Jr. Decision analysis of retrievable inferior vena cava filters in patients without pulmonary embolism. J Vasc Surg Venous Lymphat Disord. 2013;1(4):376–384. doi: 10.1016/j.jvsv.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Angel L F, Tapson V, Galgon R E, Restrepo M I, Kaufman J. Systematic review of the use of retrievable inferior vena cava filters. J Vasc Interv Radiol. 2011;22(11):1522–1.53E6. doi: 10.1016/j.jvir.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 53.Meyer A, Schönleben F, Heinz M, Lang W. Perforated inferior vena cava filters as the cause of unclear abdominal pain. Ann Vasc Surg. 2013;27(3):3.54E11–3.54E14. doi: 10.1016/j.avsg.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Pellerin O, di Primio M, Sanchez O, Meyer G, Sapoval M. Successful retrieval of 29 ALN inferior vena cava filters at a mean of 25.6 months after placement. J Vasc Interv Radiol. 2013;24(2):284–288. doi: 10.1016/j.jvir.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Pellerin O, Barral F G, Lions C, Novelli L, Beregi J P, Sapoval M. Early and late retrieval of the ALN removable vena cava filter: results from a multicenter study. Cardiovasc Intervent Radiol. 2008;31(5):889–896. doi: 10.1007/s00270-008-9357-x. [DOI] [PubMed] [Google Scholar]
- 56.Kuo W T Robertson S W Odegaard J I Hofmann L V Complex retrieval of fractured, embedded, and penetrating inferior vena cava filters: a prospective study with histologic and electron microscopic analysis J Vasc Interv Radiol 2013245622–6300., quiz 631 [DOI] [PubMed] [Google Scholar]
- 57.Mismetti P, Rivron-Guillot K, Quenet S. et al. A prospective long-term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism. Chest. 2007;131(1):223–229. doi: 10.1378/chest.06-0631. [DOI] [PubMed] [Google Scholar]
- 58.Imberti D, Bianchi M, Farina A, Siragusa S, Silingardi M, Ageno W. Clinical experience with retrievable vena cava filters: results of a prospective observational multicenter study. J Thromb Haemost. 2005;3(7):1370–1375. doi: 10.1111/j.1538-7836.2005.01448.x. [DOI] [PubMed] [Google Scholar]
- 59.Caronno R, Piffaretti G, Tozzi M. et al. Mid-term experience with the ALN retrievable inferior vena cava filter. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2006;32(5):596–9. doi: 10.1016/j.ejvs.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Zhu X, Tam M DBS, Bartholomew J, Newman J S, Sands M J, Wang W. Retrievability and device-related complications of the G2 filter: a retrospective study of 139 filter retrievals. J Vasc Interv Radiol. 2011;22(6):806–812. doi: 10.1016/j.jvir.2011.01.430. [DOI] [PubMed] [Google Scholar]
- 61.Oliva V L, Perreault P, Giroux M-F, Bouchard L, Therasse E, Soulez G. Recovery G2 inferior vena cava filter: technical success and safety of retrieval. J Vasc Interv Radiol. 2008;19(6):884–889. doi: 10.1016/j.jvir.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 62.Kuo W T Robertson S W Bard Denali IVC filter fracture and embolization resulting in cardiac tamponade: a device failure analysis. Journal of vascular and interventional radiology J Vasc Interv Radiol 2014; In press [DOI] [PubMed] [Google Scholar]
- 63.Stavropoulos S W Sing R F Elmasri F et al. The DENALI Trial: an interim analysis of a prospective, multicenter study of the Denali retrievable inferior vena cava filter J Vasc Interv Radiol 201425101497–1505., 1505.e1 [DOI] [PubMed] [Google Scholar]
- 64.Awh M H, Taylor F C, Lu C T. Spontaneous fracture of a Vena-Tech inferior vena caval filter. AJR Am J Roentgenol. 1991;157(1):177–178. doi: 10.2214/ajr.157.1.2048515. [DOI] [PubMed] [Google Scholar]
- 65.Ricco J-B Dubreuil F Reynaud P et al. The LGM Vena-Tech caval filter: results of a multicenter study Ann Vasc Surg 19959(Suppl):S89–S100. [DOI] [PubMed] [Google Scholar]
- 66.Murphy T P, Dorfman G S, Yedlicka J W. et al. LGM vena cava filter: objective evaluation of early results. J Vasc Interv Radiol. 1991;2(1):107–115. doi: 10.1016/s1051-0443(91)72482-2. [DOI] [PubMed] [Google Scholar]
- 67.Le Blanche A F, Benazzouz A, Reynaud P. et al. The VenaTech LP permanent caval filter: effectiveness and safety in the prevention of pulmonary embolism–a European multicenter study. Journal of vascular and interventional radiology. J Vasc Interv Radiol. 2008;19(4):509–515. doi: 10.1016/j.jvir.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 68.Taheri S A, Kulaylat M N, Johnson E, Hoover E. A complication of the Greenfield filter: fracture and distal migration of two struts—a case report. J Vasc Surg. 1992;16(1):96–99. [PubMed] [Google Scholar]
- 69.Carabasi R A III, Moritz M J, Jarrell B E. Complications encountered with the use of the Greenfield filter. Am J Surg. 1987;154(2):163–168. doi: 10.1016/0002-9610(87)90171-1. [DOI] [PubMed] [Google Scholar]
- 70.Wingerd M, Bernhard V M, Maddison F, Towne J B. Comparison of caval filters in the management of venous thromboembolism. Arch Surg. 1978;113(11):1264–1271. doi: 10.1001/archsurg.1978.01370230054006. [DOI] [PubMed] [Google Scholar]
- 71.Greenfield L J, Michna B A. Twelve-year clinical experience with the Greenfield vena caval filter. Surgery. 1988;104(4):706–712. [PubMed] [Google Scholar]
- 72.Greenfield L J, Proctor M C. Twenty-year clinical experience with the Greenfield filter. Cardiovasc Surg. 1995;3(2):199–205. doi: 10.1016/0967-2109(95)90895-c. [DOI] [PubMed] [Google Scholar]
- 73.Greenfield L J, Proctor M C. The percutaneous greenfield filter: outcomes and practice patterns. J Vasc Surg. 2000;32(5):888–893. doi: 10.1067/mva.2000.110346. [DOI] [PubMed] [Google Scholar]
- 74.Cho K J, Greenfield L J, Proctor M C. et al. Evaluation of a new percutaneous stainless steel Greenfield filter. J Vasc Interv Radiol. 1997;8(2):181–187. doi: 10.1016/s1051-0443(97)70536-0. [DOI] [PubMed] [Google Scholar]
- 75.Greenfield L J Proctor M C Cho K J et al. Extended evaluation of the titanium Greenfield vena caval filter J Vasc Surg 1994203458–464., discussion 464–465 [DOI] [PubMed] [Google Scholar]
- 76.Wang W, Zhou D, Obuchowski N, Spain J, An T, Moon E. Fracture and migration of Celect inferior vena cava filters: a retrospective review of 741 consecutive implantations. J Vasc Interv Radiol. 2013;24(11):1719–1722. doi: 10.1016/j.jvir.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 77.Lyon S M, Riojas G E, Uberoi R. et al. Short- and long-term retrievability of the Celect vena cava filter: results from a multi-institutional registry. J Vasc Interv Radiol. 2009;20(11):1441–1448. doi: 10.1016/j.jvir.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 78.Roehm J O Jr, Johnsrude I S, Barth M H, Gianturco C. The bird's nest inferior vena cava filter: progress report. Radiology. 1988;168(3):745–749. doi: 10.1148/radiology.168.3.3043548. [DOI] [PubMed] [Google Scholar]
- 79.Ziegler J W, Dietrich G J, Cohen S A, Sterling K, Duncan J, Samotowka M. PROOF trial: protection from pulmonary embolism with the OptEase filter. J Vasc Interv Radiol. 2008;19(8):1165–1170. doi: 10.1016/j.jvir.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 80.Oliva V L, Szatmari F, Giroux M-F, Flemming B K, Cohen S A, Soulez G. The Jonas study: evaluation of the retrievability of the Cordis OptEase inferior vena cava filter. J Vasc Interv Radiol. 2005;16(11):1439–1445, quiz 1445. doi: 10.1097/01.RVI.0000171699.57957.C7. [DOI] [PubMed] [Google Scholar]
- 81.Rosenthal D, Swischuk J L, Cohen S A, Wellons E D. OptEase retrievable inferior vena cava filter: initial multicenter experience. Vascular. 2005;13(5):286–289. doi: 10.1258/rsmvasc.13.5.286. [DOI] [PubMed] [Google Scholar]
- 82.Kalva S P, Wicky S, Waltman A C, Athanasoulis C A. TrapEase vena cava filter: experience in 751 patients. J Endovasc Ther. 2006;13(3):365–372. doi: 10.1583/05-1741.1. [DOI] [PubMed] [Google Scholar]
- 83.Liu W C, Do Y S, Choo S W. et al. The mid-term efficacy and safety of a permanent nitinol IVC filter(TrapEase) Korean J Radiol. 2005;6(2):110–116. doi: 10.3348/kjr.2005.6.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rousseau H, Perreault P, Otal P. et al. The 6-F nitinol TrapEase inferior vena cava filter: results of a prospective multicenter trial. J Vasc Interv Radiol. 2001;12(3):299–304. doi: 10.1016/s1051-0443(07)61907-1. [DOI] [PubMed] [Google Scholar]
- 85.Kalva S P, Marentis T C, Yeddula K, Somarouthu B, Wicky S, Stecker M S. Long-term safety and effectiveness of the “OptEase” vena cava filter. Cardiovasc Intervent Radiol. 2011;34(2):331–337. doi: 10.1007/s00270-011-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Usoh F, Hingorani A, Ascher E. et al. Prospective randomized study comparing the clinical outcomes between inferior vena cava Greenfield and TrapEase filters. J Vasc Surg. 2010;52(2):394–399. doi: 10.1016/j.jvs.2010.02.280. [DOI] [PubMed] [Google Scholar]
- 87.Johnson M S, Nemcek A A Jr, Benenati J F. et al. The safety and effectiveness of the retrievable option inferior vena cava filter: a United States prospective multicenter clinical study. J Vasc Interv Radiol. 2010;21(8):1173–1184. doi: 10.1016/j.jvir.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Smouse H B Mendes R Bosiers M Van Ha T G Crabtree T Investigators R; RETRIEVE Investigators. The RETRIEVE trial: safety and effectiveness of the retrievable crux vena cava filter J Vasc Interv Radiol 2013245609–621. [DOI] [PubMed] [Google Scholar]


