Abstract
Objectives To determine the utility of three-dimensional (3D) printed models in individualized petroclival tumor resection planning by measuring the fidelity of printed anatomical structures and comparing tumor exposure afforded by different approaches.
Design Case series and review of the literature.
Setting Tertiary care center.
Participants Three patients with petroclival lesions.
Main Outcome Measures Subjective opinion of access by neuro-otologists and neurosurgeons as well as surface area of tumor exposure.
Results Surgeons found the 3D models of each patient's skull and tumor useful for preoperative planning. Limitations of individual surgical approaches not identified through preoperative imaging were apparent after 3D models were evaluated. Significant variability in exposure was noted between models for similar or identical approaches. A notable drawback is that our printing process did not replicate mastoid air cells.
Conclusions We found that 3D modeling is useful for individualized preoperative planning for approaching petroclival tumors. Our printing techniques did produce authentic replicas of the tumors in relation to bony structures.
Keywords: petroclival, 3D printer, individualized surgical planning, surgical education
Introduction
Petroclival tumors are therapeutic dilemmas given their central location and potential involvement of the internal carotid artery and cranial nerves. Once considered to be inoperable due to unacceptable morbidity and mortality rates,1 2 advances in preoperative imaging, microsurgery, and perioperative care have progressively made safe resection feasible. Given the potential for growth and tendency to cause cranial nerve deficits, petroclival tumors such as meningiomas, chondrosarcomas, and chordomas necessitate individualization through consideration of tumor and patient factors for optimal treatment.
Depending on a tumor's size, extent of middle and posterior fossa involvement, and cranial nerve deficits, options for surgical approaches to the petroclival region range from orbitozygomatic to posterior fossa approaches with or without petrosectomy. The choice of approach is typically based on imaging; patient factors such as age, hearing status, and related deficits; and comorbidities.
Most anatomical research on skull base approaches consists of cadaveric studies. These studies require a surgeon to generalize objective exposure data from various approaches as well as degrees of freedom available at critical anatomical sites. Cadaveric studies are limited in that static nonpathologic models must be applied to dynamic pathologic states. In addition, several clinical studies have described the outcomes and relative merits of various skull base approaches to the petroclival region.3 4 5 6 7 8 9 10
Printed three-dimensional (3D) models are currently used to facilitate maxillofacial, orbital, and head and neck reconstruction after trauma or exonerative surgery, in addition to various uses in other medical specialties. Our goal was to determine the helpfulness of these models in preoperatively planning surgical approaches for unique individual patients with representation of their pathology by comparing the models' accuracy with actual surgical exposure as well as measuring the surface area of tumor exposure afforded through the various approaches in practice.
Methods
Patient Specifics
Patient 1 was a 60-year-old woman with multiple intracranial meningiomas who underwent a preceding frontal craniotomy for removal of a frontal subfrontal meningioma (Fig. 1). She demonstrated growth of both subfrontal residual and a separate petroclival meningioma with decreased hearing, facial numbness, and intermittent sharp facial pain. There was brainstem compression from the latter tumor, and the basilar artery was displaced to the contralateral side. The anterior skull base tumor was resected first, and the petrous apex tumor was resected 6 months later. Surface area measurements suggest that the retrosigmoid approach offered three times more tumor surface area than the middle fossa approach. However, as noted in our discussion of limitations, the surface area measurement did not take into account the obstruction caused by the brainstem. We opted for a extended middle fossa approach with a drill-out of the Kawase triangle. Near-total removal of the meningioma was performed.
Fig. 1.
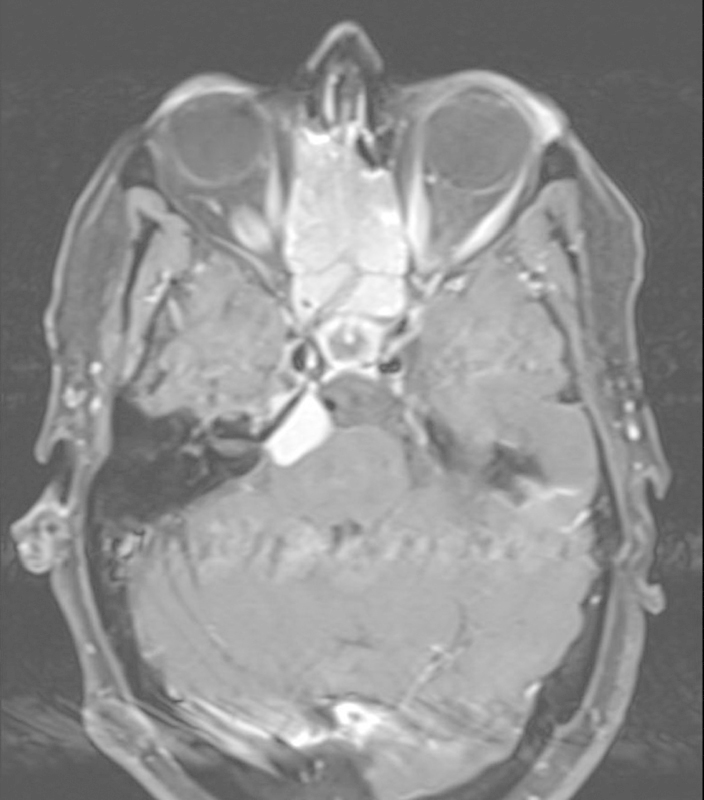
Patient 1 had multiple meningiomas. Her endonasal and subfrontal component was removed first followed by her petroclival component via a Kawase approach.
Patient 2 was a 54-year-old man with a history of diplopia, abducens nerve palsy, and partial oculomotor nerve palsy (Fig. 2A, B). He was found to have a large petroclival meningioma with invasion of the cavernous sinus. We performed a left combined petrosal approach, retrolabyrinthine as well as middle fossa. Subtotal removal of petroclival meningioma was performed after it was found to lie intimately between nerves VII and V. Nerve VII stimulated well during the procedure and also had normal function afterward.
Fig. 2a.
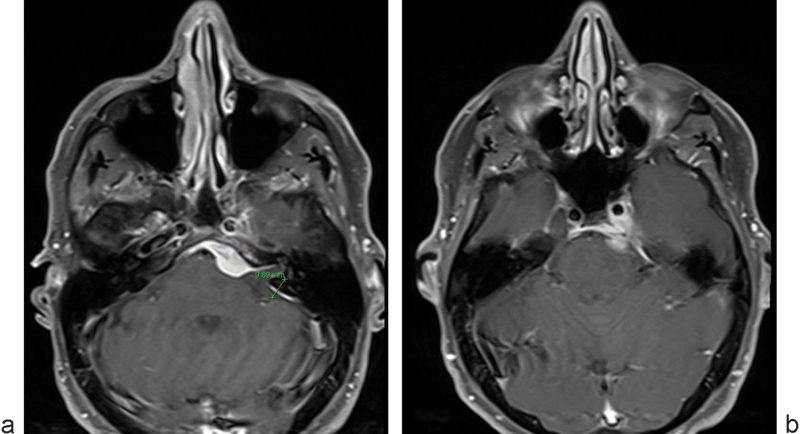
and b Patient 2 had diploplia and was noted to have a left petroclival meningioma with cavernous sinus involvement. His CPA component was removed through a combined retrolabyrinthine and middle fossa petrosal approach.
Patient 3 was a 44-year-old woman who presented with headaches and intermittent diplopia due to abducens nerve palsy (Fig. 3). She was diagnosed radiographically with a petrous apex chondrosarcoma appearing to arise from the petrooccipital synchondrosis and reaching the jugular foramen. We opted to perform a middle fossa approach and achieved removal of the entire petrous apex mass as well as debulking the inside of the cavernous sinus component. We debulked it inferiorly to the level of the internal auditory canal and were unable to access the jugular foramen component from this middle fossa approach.
Fig. 3.
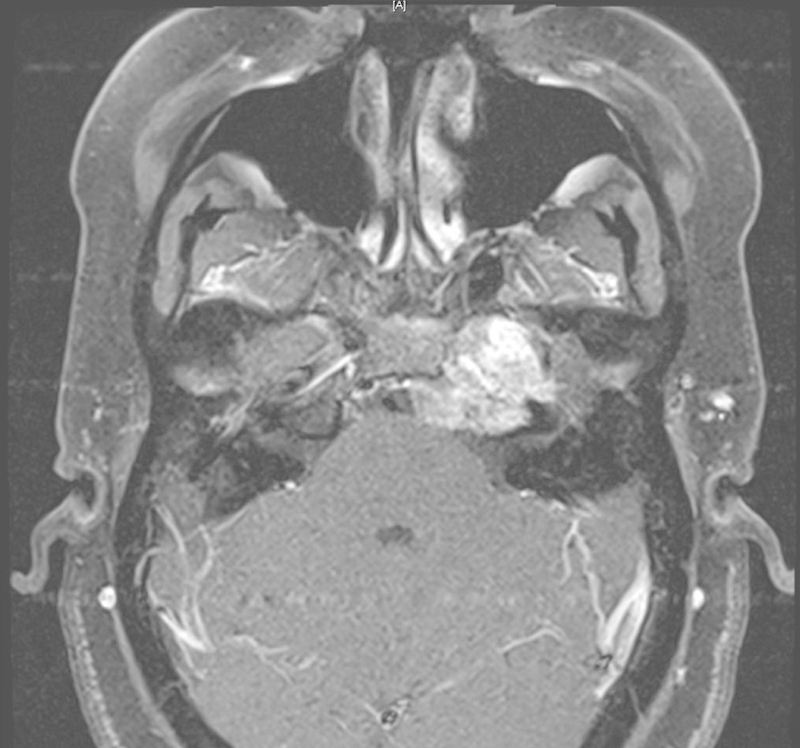
Patient 3 had a left petrous apex chondrosarcoma with headaches and diploplia that we debulked down to the level of the jugular foramen via a Kawase approach.
We created 3D models of skulls with tumors using a fusion of high-resolution skull base or temporal bone computed tomography (CT) and brain magnetic resonance imaging (MRI) scans in three patients with petroclival tumors. Brainlab (Brainlab iPlan v.3.0.5, Feldkirchen, Germany) image navigation software was used to perform fusion of imaging and object creation. Tumor and bone were fused into one object and exported as stereolithography (STL) files. Catalyst EX v.4.4 (Dimension, Stratasys, Eden Prairie, Minnesota, United States) was used to format the STL files for printing from our Stratasys uPrint SE Plus 3D printer, which printed the skulls in a uniform color of production-grade thermoplastic material by a fused deposition modeling printing method. The printer is able to print to a resolution of 0.254 mm and requires a minimum wall thickness of 0.914 mm.
After printing the tumor and skull model, we used bright acrylic paint to highlight the tumor surface. Middle fossa and retrosigmoid approaches were then performed by the senior author, with photodocumentation of each tumor exposure. We attempted to perform a transmastoid approach on the skulls as well.
Brainlab software was used to register each 3D model to the skull surface rendering in Brainlab at eight preselected registration points. Next the navigation pointer was used to create points on the surface of the tumor that were saved on the Brainlab system. The points were selectively placed to divide the visible tumor surface area for each approach into a series of boxes. The outlined distance between points was measured in millimeters using Brainlab to create a two-dimensional area. We then used the application sketchandcalc (www.sketchandcalc.com) to redraw the corresponding measurements and calculate the area of each box. The sum of these areas represents an approximation of the total exposed surface area.
Results
Surface area measurements for patient 1 was 52 mm2 afforded by the middle fossa approach and 148 mm2 by the retrosigmoid approach. For patient 2, it was 103 mm2 by the middle fossa approach and 188 mm2 by the retrosigmoid approach. Patient 2's skull can be visualized in Figs. 4 5 6 7. For patient 3, it was 378 mm2 by the middle fossa approach and 75 mm2 by the retrosigmoid approach. Patient 3's skull can be seen in Fig. 6. Table 1 summarizes these results.
Fig. 4.
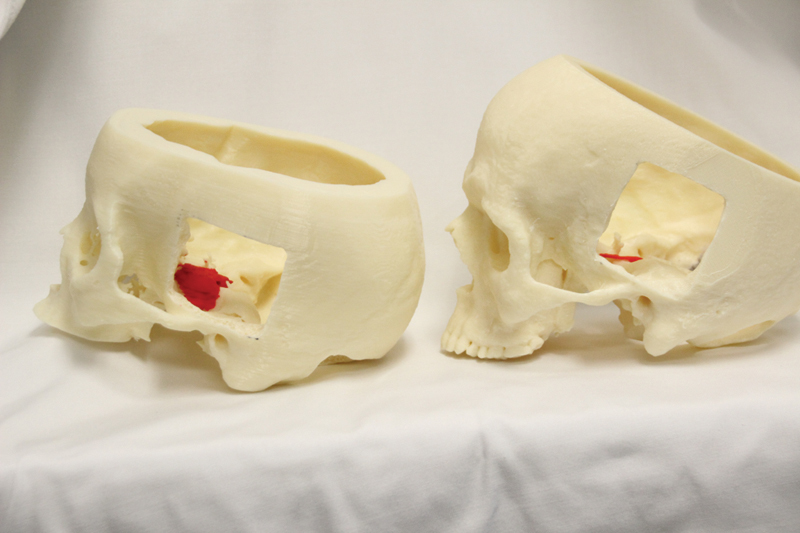
Photograph of three-dimensional printed skulls and tumors with middle fossa approach visible. Left: patient 3; right: patient 2.
Fig. 5.

Photograph of three-dimensional printed skull and tumor retrosigmoid approach: patient 2.
Fig. 6.
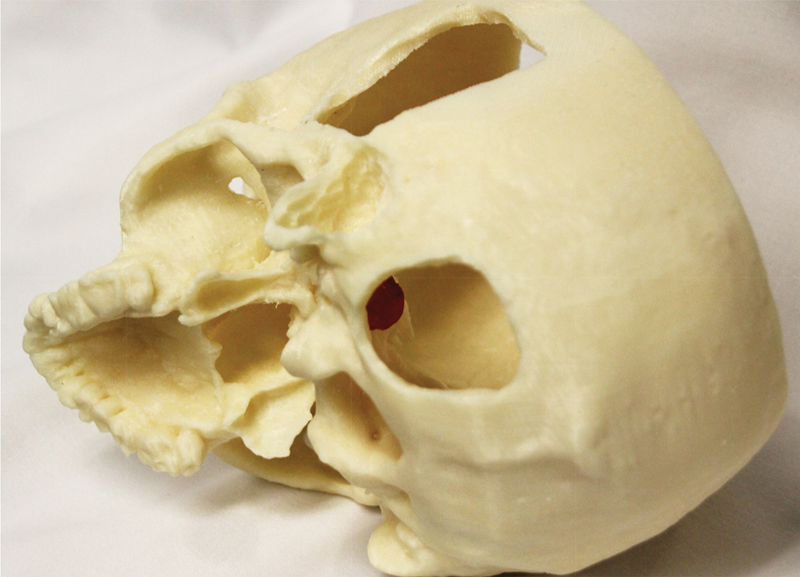
Photograph of three-dimensional printed skull and tumor middle fossa transmastoid and retrosigmoid approaches: patient 2.
Fig. 7.

Photograph of three-dimensional printed skull and tumor petroclival region from the supratentorial view: patient 2.
Table 1. Surface areas of petroclival tumors accessible from two skull base approaches.
| Skull base approach | Middle fossa approach | Retrosigmoid approach |
|---|---|---|
| Patient 1 | 52 mm2 | 148 mm2 |
| Patient 2 | 103 mm2 | 188 mm2 |
| Patient 3 | 378 mm2 | 75 mm2 |
We were unable to perform a transmastoid approach because the printer could not recreate mastoid air cells, middle ear, and other bony landmarks within the temporal bone.
Discussion
Study Findings
We were able to create 3D printed skulls with tumors that were anatomically accurate to within 0.254 mm. The 3D printer and software available to us was able to print a single-color single-texture model from the fusion of CT and MRI scans. We were able to measure the surface area exposed by the middle fossa and retrosigmoid skull base approaches successfully using the skulls and navigation equipment. These skulls serve as tangible models for surgeons, trainees, and patients during surgical planning, education, and discussion.
Limitations of the study include the minimum wall thickness of 0.914 required by our printer that made it impossible for us to recreate the eggshell quality of the mastoid air cells. Another limitation was that we were only able to print the skull and the tumor. To print and differentiate surrounding vessels, nerves, and brain parenchyma, we would have required magnetic resonance angiography and magnetic resonance venography data to input into our STL file. A more advanced printer would have also been required so we could assign different colors and textures to the various anatomical structural categories. These structures would have provided information about the degree of obstruction that nerves or parenchyma would have caused for each approach. Prints of this quality would provide a means of calculating volume of resection, which may be a more useful measure to compare approaches.
Cadaveric Studies
Several cadaveric studies have quantified and compared the working surface area of the ventral brainstem and petroclival area that various approaches afford the surgeon.11 12 13 14 Of these studies, two performed calculations to determine the degree of operative freedom a surgeon may have at specific delicate anatomical sites.13 14 Another calculated the mean exposure of the parasellar region and clivus.15 In 2007 Safavi-Abbasi et al used an inflated balloon catheter to mimic tumor mass effect and quantified the amount of shift of various structures as well as the improved exposure of the petroclival region by tumor compression of the brainstem.16 Our 3D printed skulls provided high-fidelity replicas of individuals' unique pathologic anatomy to the surgeon, a technique that has not yet been described in the literature.
Three-Dimensional Printers in Medicine
Three-dimensional printers have a growing number of applications in various medical specialties. In a recent project, otolaryngology residents drilled cadaveric temporal bones and their corresponding 3D printed models; they concluded the models were realistic representations of the cadaver temporal bones.17 Other specialties have used 3D printers for individualized surgical planning including cardiology18 and orthopedics.19 Cardiovascular regenerative researchers have printed scaffolds20 and molds21 for growing autologous heart valves. Two recent articles highlighted the surgical educational merits of 3D printed models for neurosurgical trainees.22 23 A 2007 article investigated the use of 3D printers for planning orthognathic surgeries and showed the models to be valuable for preoperative planning and practice.24 Our models and results describe the utility of 3D printers for operative planning for skull base tumors in anatomical locations that are difficult to access.
The printer and software used in this study cost approximately $50,000 USD. The cost of thermoplastic “ink” for each skull was approximately $150 to $200. Top-of-the-line printers that would be able to print multiple colors and textures range from $350,000 to $600,000.
Goals of Surgical Resection for Petroclival Meningiomas: Trending Away from the Transpetrosal Approach
Surgical excision of petroclival meningiomas has trended toward less use of aggressive surgical approaches to minimize surgical morbidity.10 25 26 That is, the transpetrosal approaches are used less frequently given the risk of (or inherent) damage to the vestibulocochlear and facial nerves.9 It is important to note that the goal of near-total resection, as compared with gross total resection, has been shown to reduce surgical morbidity significantly without resulting in a great difference in tumor recurrence rates.26 The retrosigmoid approach provides equivalent working area and operative angles as does the combined transpetrosal approach.14
Stereotactic radiosurgery is also a viable primary treatment option and has been found to have low rates of progression-free survival in this setting. It also can be used successfully as an adjuvant treatment to surgical resection.27 Stereotactic radiosurgery in the setting of complex petroclival anatomy also demonstrates a risk of cranial nerve injury. Clival- or petrous-based tumor locations were predictive of an increased risk of new or worsening neurologic deficit following Gamma Knife surgery.28 In general, Gamma Knife surgery offers an acceptable rate of tumor control for posterior fossa meningiomas with a low incidence of neurologic deficits for primary treatment28 as well as adjuvant treatment after surgery.29 One large study of 121 patients undergoing Gamma Knife surgery for skull base meningiomas showed a very low incidence of treatment-related cranial nerve dysfunction (1.7%).30
Conclusions
Three-dimensional printers have a growing role in health care and are becoming more readily available. The 3D models we created are useful for a new surgeon or for an experienced surgeon planning a difficult case. The models can help surgical trainees visualize and explore pathologic states, as well as simulate skull base approaches. The models are also useful as tangible representations for discussion with patients to improve their understanding of their pathology.
The amount of information provided by 3D printed skulls is proportional to the amount of realistic anatomy that the printers can recreate. As 3D printer technology continues to prove useful in various medical fields, both surgeons and patients stand to benefit.
References
- 1.Campbell E, Whitfield R D. Posterior fossa meningiomas. J Neurosurg. 1948;5(2):131–153. doi: 10.3171/jns.1948.5.2.0131. [DOI] [PubMed] [Google Scholar]
- 2.Yasargil M G, Mortara R W, Curcic M. Vienna, Austria: Springer; 1980. Meningiomas of basal posterior cranial fossa; pp. 1–115. [Google Scholar]
- 3.Xu F, Karampelas I, Megerian C A, Selman W R, Bambakidis N C. Petroclival meningiomas: an update on surgical approaches, decision making, and treatment results. Neurosurg Focus. 2013;35(6):E11. doi: 10.3171/2013.9.FOCUS13319. [DOI] [PubMed] [Google Scholar]
- 4.Al-Mefty O, Fox J L, Smith R R. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22(3):510–517. doi: 10.1227/00006123-198803000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Kawase T Shiobara R Toya S Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients Neurosurgery 1991286869–875.; discussion 875–876 [PubMed] [Google Scholar]
- 6.Seifert V Raabe A Zimmermann M Conservative (labyrinth-preserving) transpetrosal approach to the clivus and petroclival region—indications, complications, results and lessons learned Acta Neurochir (Wien) 20031458631–642.; discussion 642 [DOI] [PubMed] [Google Scholar]
- 7.Brandt M G, Poirier J, Hughes B, Lownie S P, Parnes L S. The transcrusal approach: a 10-year experience at one Canadian center. Neurosurgery. 2010;66(5):1017–1022. doi: 10.1227/01.neu.0000368102.22612.47. [DOI] [PubMed] [Google Scholar]
- 8.Xiao X Zhang L Wu Z et al. Surgical resection of large and giant petroclival meningiomas via a modified anterior transpetrous approach Neurosurg Rev 2013364587–593.; discussion 593–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bambakidis N C, Kakarla U K, Kim L J. et al. Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurgery. 2008;62(6) 03:1182–1191. doi: 10.1227/01.neu.0000333784.04435.65. [DOI] [PubMed] [Google Scholar]
- 10.Nanda A, Javalkar V, Banerjee A D. Petroclival meningiomas: study on outcomes, complications and recurrence rates. J Neurosurg. 2011;114(5):1268–1277. doi: 10.3171/2010.11.JNS10326. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M, Ambekar S, Guthikonda B, Nanda A. A comparison between the Kawase and extended retrosigmoid approaches (retrosigmoid transtentorial and retrosigmoid intradural suprameatal approaches) for accessing the petroclival tumors. A cadaveric study. J Neurol Surg B Skull Base. 2014;75(3):171–176. doi: 10.1055/s-0033-1359305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambekar S, Amene C, Sonig A, Guthikonda B, Nanda A. Quantitative comparison of retrosigmoid intradural suprameatal approach and retrosigmoid transtentorial approach: implications for tumors in the petroclival region. J Neurol Surg B Skull Base. 2013;74(5):300–304. doi: 10.1055/s-0033-1348025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang S W Wu A Gore P et al. Quantitative comparison of Kawase's approach versus the retrosigmoid approach: implications for tumors involving both middle and posterior fossae Neurosurgery 200964(3, Suppl):ons44–ons51.; discussion ons51–ons52 [DOI] [PubMed] [Google Scholar]
- 14.Siwanuwatn R, Deshmukh P, Figueiredo E G, Crawford N R, Spetzler R F, Preul M C. Quantitative analysis of the working area and angle of attack for the retrosigmoid, combined petrosal, and transcochlear approaches to the petroclival region. J Neurosurg. 2006;104(1):137–142. doi: 10.3171/jns.2006.104.1.137. [DOI] [PubMed] [Google Scholar]
- 15.Little A S Jittapiromsak P Crawford N R et al. Quantitative analysis of exposure of staged orbitozygomatic and retrosigmoid craniotomies for lesions of the clivus with supratentorial extension Neurosurgery 200862502ONS318–ONS323.; discussion ONS323–ONS324 [DOI] [PubMed] [Google Scholar]
- 16.Safavi-Abbasi S, Zabramski J M, Deshmukh P. et al. Moving toward the petroclival region: a model for quantitative and anatomical analysis of tumor shift. J Neurosurg. 2007;107(4):797–804. doi: 10.3171/JNS-07/10/0797. [DOI] [PubMed] [Google Scholar]
- 17.Unger B J, Kraut J, Rhodes C, Hochman J. Design and validation of 3D printed complex bone models with internal anatomic fidelity for surgical training and rehearsal. Stud Health Technol Inform. 2014;196:439–445. [PubMed] [Google Scholar]
- 18.Jacobs S, Grunert R, Mohr F W, Falk V. 3D-Imaging of cardiac structures using 3D heart models for planning in heart surgery: a preliminary study. Interact Cardiovasc Thorac Surg. 2008;7(1):6–9. doi: 10.1510/icvts.2007.156588. [DOI] [PubMed] [Google Scholar]
- 19.Niikura T, Sugimoto M, Lee S Y. et al. Tactile surgical navigation system for complex acetabular fracture surgery. Orthopedics. 2014;37(4):237–242. doi: 10.3928/01477447-20140401-05. [DOI] [PubMed] [Google Scholar]
- 20.Lueders C, Jastram B, Hetzer R, Schwandt H. Rapid manufacturing techniques for the tissue engineering of human heart valves. Eur J Cardiothorac Surg. 2014;46(4):593–601. doi: 10.1093/ejcts/ezt510. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama Y, Takewa Y, Sumikura H. et al. In-body tissue-engineered aortic valve (Biovalve type VII) architecture based on 3D printer molding. J Biomed Mater Res B Appl Biomater. 2015;103(1):1–11. doi: 10.1002/jbm.b.33186. [DOI] [PubMed] [Google Scholar]
- 22.Waran V, Narayanan V, Karuppiah R. et al. Injecting realism in surgical training--initial simulation experience with custom 3D models. J Surg Educ. 2014;71(2):193–197. doi: 10.1016/j.jsurg.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Waran V, Narayanan V, Karuppiah R, Owen S L, Aziz T. Utility of multimaterial 3D printers in creating models with pathological entities to enhance the training experience of neurosurgeons. J Neurosurg. 2014;120(2):489–492. doi: 10.3171/2013.11.JNS131066. [DOI] [PubMed] [Google Scholar]
- 24.Mavili M E, Canter H I, Saglam-Aydinatay B, Kamaci S, Kocadereli I. Use of three-dimensional medical modeling methods for precise planning of orthognathic surgery. J Craniofac Surg. 2007;18(4):740–747. doi: 10.1097/scs.0b013e318069014f. [DOI] [PubMed] [Google Scholar]
- 25.Jung H W Yoo H Paek S H Choi K S Long-term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases Neurosurgery 2000463567–574.; discussion 574–575 [DOI] [PubMed] [Google Scholar]
- 26.Little K M Friedman A H Sampson J H Wanibuchi M Fukushima T Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients Neurosurgery 2005563546–559.; discussion 546–559 [DOI] [PubMed] [Google Scholar]
- 27.Subach B R Lunsford L D Kondziolka D Maitz A H Flickinger J C Management of petroclival meningiomas by stereotactic radiosurgery Neurosurgery 1998423437–443.; discussion 443–445 [DOI] [PubMed] [Google Scholar]
- 28.Starke R M, Nguyen J H, Rainey J. et al. Gamma Knife surgery of meningiomas located in the posterior fossa: factors predictive of outcome and remission. J Neurosurg. 2011;114(5):1399–1409. doi: 10.3171/2010.11.JNS101193. [DOI] [PubMed] [Google Scholar]
- 29.Zachenhofer I Wolfsberger S Aichholzer M et al. Gamma-knife radiosurgery for cranial base meningiomas: experience of tumor control, clinical course, and morbidity in a follow-up of more than 8 years Neurosurgery 200658128–36.; discussion 28–36 [DOI] [PubMed] [Google Scholar]
- 30.Eustacchio S, Trummer M, Fuchs I, Schröttner O, Sutter B, Pendl G. Preservation of cranial nerve function following Gamma Knife radiosurgery for benign skull base meningiomas: experience in 121 patients with follow-up of 5 to 9.8 years. Acta Neurochir Suppl (Wien) 2002;84:71–76. doi: 10.1007/978-3-7091-6117-3_8. [DOI] [PubMed] [Google Scholar]


