Abstract
Objective
Obesity correlates with increased production of adipocyte-derived cytokines, which may contribute to a chronic subclinical inflammation seen in obese individuals. This study evaluated the ability of specific fatty acids to modulate production of the proinflammatory cytokine, tumor necrosis factor-α (TNF-α), and the anti-inflammatory cytokine, interleukin-10 (IL-10), in murine 3T3-L1 adipocytes. Effects on nuclear factor-κB (NF-κB), a key transcriptional activator of the inflammatory cascade, and suppressor of cytokine signaling 3 (SOCS-3), a negative regulator of cytokine signaling, were also determined.
Methods and Procedures
Adipocytes were incubated for 24 and 48 h with and without 50 or 500 μmol/l of palmitic acid, oleic acid, or docosahexaenoic acid, (DHA). Effects on gene expression and protein secretion of TNF-α and IL-10 were determined using real-time PCR and a murine multipex RIA kit. SOCS-3 expression was determined by northern blotting and NF-κB binding activity was assessed using a commercially available assay.
Results
Adipocytes treated for 24 h with palmitic acid exhibited a 70% increase in TNF-α production and up to a 75% decrease in IL-10 production, relative to untreated cells. In contrast, DHA treatment had no effect on TNF-α, but increased IL-10 production twofold. No effect of oleic acid was seen on either TNF-α or IL-10 production. Similar results were obtained during a 48-h incubation. Furthermore, NF-κB DNA-binding activity increased fourfold in response to palmitic acid and decreased 60% in response to DHA. Expression of SOCS-3 increased twofold in DHA-treated cells.
Discussion
In aggregate, these results suggest that dietary fatty acids act directly on adipocytes to modulate cytokine production. As circulating fatty acids levels are chronically elevated in obese individuals, this effect may account in part for obesity-associated inflammation.
Introduction
Obesity has become pandemic and is associated with a chronic state of low-grade inflammation, the etiology of which remains poorly understood. Over the past several years, in addition to its function as a repository for energy reserves, the role of the adipocyte has expanded to that of a dynamic endocrine organ that secretes a plethora of factors, including hormones and cytokines, which in turn regulate multiple biological processes (1–5). Several lines of evidence have shown that obesity triggers dysregulation of the endocrine function of adipose tissue (6,7). Adipose tissue comprises multiple cytokine-producing cell types, such as macrophages, preadipocytes, and adipocytes (8,9). Obesity promotes increased macrophage infiltration into adipose tissue to amplify production of inflammatory markers (10,11); furthermore, it correlates with increased secretion of several adipocyte-derived cytokines, which may contribute to a chronic state of low-grade inflammation. This inflammation, in turn, has been linked to the pathology of several metabolic and cardiovascular disorders, such as insulin resistance, type 2 diabetes, hyperlipidemia, and atherosclerosis (12–17). However, the capacity of the adipocyte itself for cytokine production and the mechanisms whereby the cellular inflammatory response of the adipocyte is initiated and maintained in an obese state, remain unclear.
Several studies of both rodent and human obesity indicate that expression of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) is markedly upregulated in adipose tissue (18–22) and null mutations in either the genes encoding TNF-α or its receptors ameliorate obesity-induced insulin resistance (23). It was recently reported that decreased signaling via the TNF-α inducible IKKβ/NF-κB pathway, a key signaling pathway in tissue inflammation, through the use of salicylate-based inhibitors or by decreased IKKβ expression improved insulin sensitivity in vivo (24,25).
Conversely, interleukin-10 (IL-10) is an anti-inflammatory cytokine secreted by white adipose tissue (26). IL-10 is also upregulated in an obese state, but acts to suppress macrophage function and inhibit the production of proinflammatory cytokines (27). It is generally accepted that IL-10 confers protection against an overwhelming inflammatory response. This theory is supported by genetic ablation studies showing that mice deficient for IL-10 have enterocolitis and impaired inflammatory responses (28–30).
Acute increases in plasma free fatty acids in humans trigger inflammatory and oxidative stress mechanisms that are implicated in the etiology of cardiovascular and metabolic anomalies, such as atherosclerosis and insulin resistance (31–33). We hypothesize that elevated circulating levels of free fatty acids in the obese state may have a direct role in regulating the cellular inflammatory response of the adipocyte. Furthermore, we hypothesize that specific classes of fatty acids may have differential effects on the production of adipocyte-derived cytokines. In this study, using fully differentiated murine 3T3-L1 adipocytes, we have evaluated the ability of a saturated fatty acid (palmitic) a monounsaturated fatty acid (oleic) and a polyunsaturated fatty acid (docosahexaenoic acid, DHA), to modulate production of TNF-α and IL-10 directly. We also begin to dissect the signal pathways mediating these potential effects, by focusing on associated changes in nuclear factor-κB (NF-κB) binding activity, and on associated changes in the suppressor of cytokine signaling 3 (SOCS-3), a negative regulator of cytokine signaling (34).
Methods And Procedures
Materials
Dulbecco’s modified Eagle’s medium (DMEM)-high glucose, fetal bovine serum, 3-isobutyl-1-methylxanthine, dexamethasone, fatty acid–free bovine serum albumin (BSA), and all fatty acids were acquired from Sigma (St. Louis, MO). Calf serum was obtained from HyClone (Logan, UT). Human insulin was from Boehringer Mannheim (Indianapolis, IN). Nylon membranes were purchased from Schleicher and Schuell (Needham, NH). A RadPrime DNA-labeling kit was purchased from GIBCO-BRL (Gaithersburg, MD) and [α-32P] dCTP was from PerkinElmer Life Sciences (Boston, MA).
3T3-L1 cell cultures
3T3-L1 preadipocytes were maintained in DMEM containing 10% calf serum in a 10% CO2, humidified environment at 37 °C. Cells were differentiated over an 8-day period with day 8 representing fully differentiated adipocytes. Differentiation of confluent preadipocyte cultures (day 0) was induced using DMEM containing 10% fetal bovine serum, 0.5 mmol/l 3-isobutyl-1-methylxanthine, 5 μg/ml insulin, and 0.4 μg/ml dexamethasone. On day 2, the media was replaced with DMEM containing 10% fetal bovine serum and 5 μg/ml insulin. Insulin was removed on day 4 by changing the media to DMEM containing 10% fetal bovine serum and cells were maintained thereafter in this medium.
Effects of free fatty acids on adipocyte-derived cytokines
To evaluate the ability of free fatty acids to modulate production of TNF-α and IL-10 by adipocytes directly, fully differentiated 3T3-L1 cells were incubated in serum-free medium (DMEM + 0.1% BSA) overnight, then incubated for 24 or 48 h in fresh DMEM containing 2% BSA in the absence and presence of 50 and 500 μmol/l each of palmitic acid, oleic acid, or DHA. These doses are well within the physiological range for free fatty acid concentrations reported for rodents and humans (35). Fatty acid–containing media was prepared by conjugation of each fatty acid to fatty acid–free BSA as described previously (36). Each fatty acid was dissolved in ethanol and diluted 1:100 in DMEM containing 2% (wt/vol) fatty acid–free BSA before being added to the cells. Control cells received vehicle. Total RNA was isolated from these cells and processed as described later. Cytokines secreted into the incubation medium were measured using a commercially available murine multiplex RIA kit (Linco Research, St. Charles, MO).
RNA isolation, real-time quantitative PCR, northern blotting, and NF-κB binding assays
Total RNA was isolated using the Ultraspec RNA Isolation System (Biotecx, Houston, TX) as described by the manufacturer. For real-time quantitative PCR (TaqMan), a 1 μg sample of RNA was used to synthesize cDNA using the Advantage One-Step RT-PCR kit (Clontech, Palo Alto, CA). Real-time PCR was performed using the ABI Prism 7700 sequence detection system according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Primers used were TNF-α (Fwd: 5′-CATCTTCTCAAAATTCGAGTGACAA-3′; Rev: 5′-TGGGAGTAGACAAGGTACAACCC-3′) and IL-10 (Fwd: 5′-GGTTGCCAAGCCTTATCGGA-3′; Rev: 5′-ACCTGCTCCACTGCCTTGCT-3′). Results were normalized against cyclophilin (Fwd: 5′-GGTGGAGAGCACCAAGACAGA-3′; Rev: 5′-GCCGGAGTCGACAATGATG-3′).
For northern blots, 10 μg samples of total RNA per lane were run in 1.2% formaldehyde-agarose denaturing gels and transferred to nylon membranes. Northern blotting was performed using a 0.8 kb [α-32P] dCTP-labeled mouse SOCS-3 cDNA kindly provided by Dr Jeffrey Flier, Beth Israel Deaconess Medical Center. Signals were visualized by autoradiography. RNA integrity and loading were verified by ethidium bromide staining of 28S ribosomal RNA. For NF-κB DNA-binding activity, nuclear extracts were prepared from 3T3-L1 adipocytes, and binding activity of the p65 subunit of NF-κB was measured using a commercially available BD TransFactor ELISA-based chemiluminescence assay (BD Biosciences Clontech, Mountain View, CA) as directed by the manufacturer.
Statistical analysis
Data are presented as means ± s.e. from three independent experiments. Statistical analyses were performed using StatView (Abacus Concepts, Berkley, CA). Differences between control and experimental values were determined by one-way ANOVA and Fisher’s test. A P value of <0.05 was deemed significant.
Results
Effects of palmitic acid, oleic acid, and DHA on TNF-α production by adipocytes
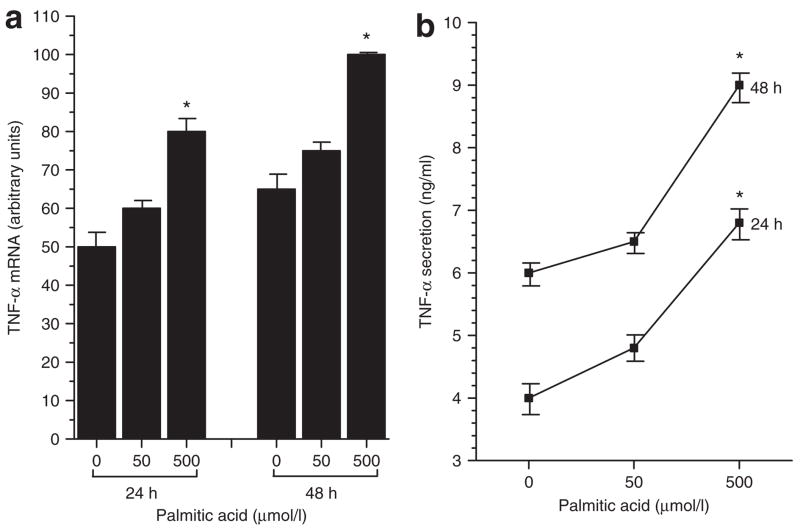
To begin characterizing the effects of specific dietary free fatty acids on the cellular inflammatory response of the adipocyte, fully differentiated 3T3-L1 cells were incubated for 24 and 48 h with 50 or 500 μmol/l of the saturated fatty acid, palmitic acid, and effects on synthesis and secretion of the proinflammatory cytokine TNF-α were determined. These doses are well within the physiological serum range for free fatty acid concentrations reported for both rodents and humans (35). As shown in Figure 1a,b, palmitic acid induced a concomitant increase in both TNF-α mRNA and TNF-α protein secretion at both doses and time points. Treatment with 500 μmol/l palmitic acid induced a 60% increase in TNF-α mRNA at 24 h (palmitic-treated 80 ± 3.2 vs. untreated 50 ± 3.6, P < 0.05, results expressed as arbitrary units) and a 54% increase in TNF-α mRNA at 48 h (palmitic-treated 100 ± 2.1 vs. untreated 65 ± 3.5, P < 0.05). Parallel increases in TNF-α protein secretion were observed. A 70% increase was seen at 24 h (palmitic-treated 6.8 ± 0.4 ng/ml vs. untreated 4.0 ± 0.3 ng/ml, P < 0.05) and a 50% increase at 48 h (palmitic-treated 9.0 ± 0.3 ng/ml vs. untreated 6.0 ± 0.2 ng/ml, P < 0.05). Cells incubated with the 50 μmol/l dose of each fatty acid showed an intermediate increase in TNF-α production that was not statistically significant (Figure 1a,b). Under the same incubation conditions, treatment with either a monounsaturated fatty acid (oleic) or a polyunsaturated fatty acid, (DHA), had no significant effect on TNF-α synthesis and secretion (data not shown).
Figure 1.
Effects of the saturated fatty acid, palmitic acid, on (a) gene expression and (b) protein secretion of the proinflammatory cytokine, tumor necrosis factor-α (TNF-α), by fully differentiated murine 3T3-L1 adipocytes. Cells were incubated in the absence (0) and presence of 50 or 500 μmol/l palmitic acid for 24 and 48 h. TNF-α mRNA was measured by real-time quantitative PCR and TNF-α protein secreted into the incubation medium was measured by immunoassay. Results are means ± s.e. for three independent experiments. *P < 0.05 vs. untreated (0).
Effects of palmitic acid, oleic acid, and DHA on IL-10 production by adipocytes
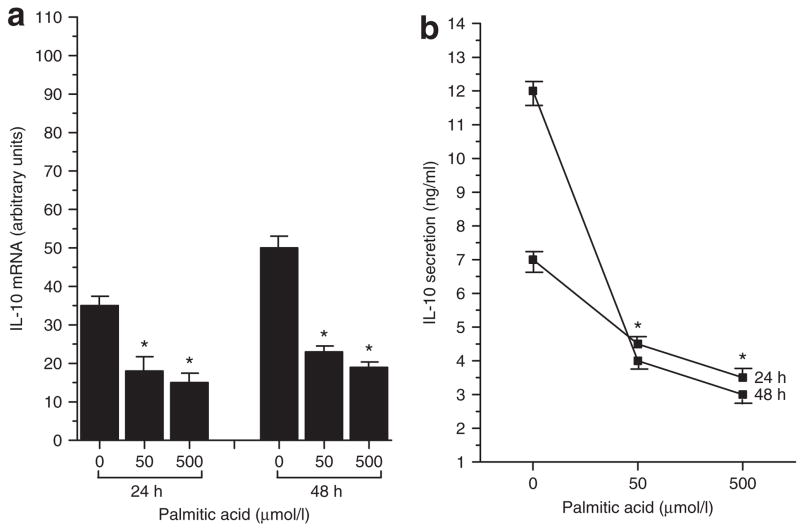
To further investigate the effects of dietary fatty acids on cytokine production by adipocytes, the effects of saturated, monounsaturated, and polyunsaturated fatty acids on synthesis and secretion of the anti-inflammatory cytokine, IL-10, were assessed. Cells were treated for 24 and 48 h with 50 or 500 μmol/l of palmitic acid, oleic acid, or DHA, and the effects on IL-10 production were determined. As shown in Figure 2a,b, the saturated fatty acid, palmitic acid, decreased both IL-10 mRNA and protein levels. Treatment with 50 μmol/l palmitic acid elicited a 49% decrease in IL-10 mRNA at 24 h (palmitic-treated 18 ± 3.5 vs. untreated 35 ± 2.6, P < 0.05) and a 54% decrease at 48 h (palmitic-treated 23 ± 2.1 vs. untreated 50 ± 3.1, P < 0.05). Similarly, 500 μmol/l palmitic acid induced a 57% decrease in IL-10 mRNA at 24 h (palmitic-treated 15 ± 1.8 vs. untreated 35 ± 2.6, P < 0.05) and a 62% decrease at 48 h (palmitic-treated 19 ± 1.5 vs. untreated 50 ± 3.1, P < 0.05). With regard to IL-10 protein secretion (Figure 2b), 50 μmol/l palmitic acid induced a 36% decrease at 24 h (palmitic-treated 4.5 ± 0.6 ng/ml vs. untreated 7 ± 1.1 ng/ml, P < 0.05) and a 67% decrease at 48 h (palmitic-treated 4 ± 0.5 ng/ml vs. untreated 12 ± 1.3 ng/ml, P < 0.05). The 500-μmol/l dose decreased IL-10 secretion by 50% at 24 h (palmitic-treated 3.5 ± 0.7 ng/ml vs. untreated 7 ± 1.1 ng/ml, P < 0.05) and 75% at 48 h (palmitic-treated 3 ± 0.5 ng/ml vs. untreated 12 ± 1.3 ng/ml, P < 0.05).
Figure 2.
Effects of the saturated fatty acid, palmitic acid, on (a) gene expression and (b) protein secretion of the anti-inflammatory cytokine, interleukin-10 (IL-10), by fully differentiated murine 3T3-L1 adipocytes. Cells were incubated in the absence (0) and presence of 50 or 500 μmol/l palmitic acid for 24 and 48 h. IL-10 mRNA was measured by real-time quantitative PCR and IL-10 protein secreted into the incubation medium was measured by immunoassay. Results are means ± s.e. for three independent experiments. *P < 0.05 vs. untreated (0).
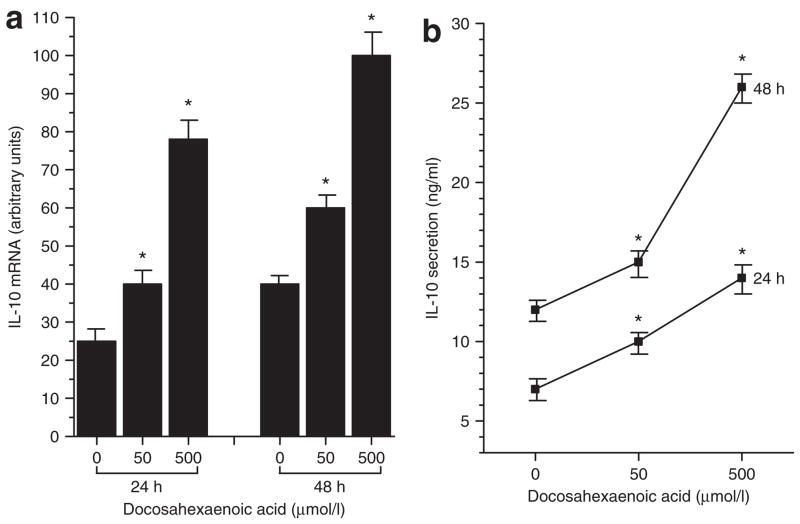
In contrast, the polyunsaturated fatty acid, DHA, at doses of 50 and 500 μmol/l increased expression of IL-10, as shown in Figure 3a. At the lower dose, IL-10 mRNA increased by 60% at 24 h (DHA-treated 40 ± 3.8 vs. untreated 25 ± 3.5, P < 0.05) and by 50% at 48 h (DHA-treated 60 ± 4.2 vs. untreated 40 ± 3.3, P < 0.05). Incubation with 500 μmol/l DHA stimulated a 3-fold increase in IL-10 mRNA at 24 h (DHA-treated 78 ± 5.1 vs. untreated 25 ± 3.5, P < 0.05) and a 2.5-fold increase at 48 h (DHA-treated 100 ± 5.2 vs. untreated 40 ± 3.3, P < 0.05). Concomitantly, as depicted in Figure 3b, cells treated with 50 μmol/l DHA showed a 43% increase in IL-10 protein secretion at 24 h (DHA-treated 10 ± 1.2 ng/ml vs. untreated 7 ± 1.3 ng/ml) and a 25% increase at 48 h (DHA-treated 15 ± 2.1 ng/ml vs. untreated 12 ± 1.4 ng/ml, P < 0.05), whereas 500 μmol/l DHA stimulated a twofold increase in IL-10 secretion at both 24 h (DHA-treated 14 ± 2.1 ng/ml vs. untreated 7 ± 1.3 ng/ml, P < 0.05) and 48 h (DHA-treated 26 ± 2.5 ng/ml vs. untreated 12 ± 1.4 ng/ml, P < 0.05). No significant effect of oleic acid was seen on IL-10 production (data not shown).
Figure 3.
Effects of the polyunsaturated fatty acid, docosahexaenoic acid (DHA), on (a) gene expression and (b) protein secretion of the anti-inflammatory cytokine, interleukin-10 (IL-10), by fully differentiated murine 3T3-L1 adipocytes. Cells were incubated in the absence (0) and presence of 50 or 500 μmol/l DHA for 24 and 48 h. IL-10 mRNA was measured by real-time quantitative PCR and IL-10 protein secreted into the incubation medium was measured by immunoassay. Results are means ± s.e. for three independent experiments. *P < 0.05 vs. untreated (0).
Effects of fatty acid–induced cytokine production on NF-κB binding activity in adipocytes
Initiation of the inflammatory cascade requires activation of a key transcriptional activator, NF-κB. To determine whether the changes in TNF-α and IL-10 production seen in response to specific fatty acids were accompanied by concomitant changes in NF-κB activity, nuclear extracts were prepared from cells treated for 24 h with 50 and 500 μmol/l palmitic acid, oleic acid, or DHA. Binding activity of the p65 subunit of NF-κB was determined using the commercially available BD TransFactor ELISA-based chemiluminescence assay. As shown in Figure 4a, consistent with its ability to stimulate the proinflammatory cytokine, TNF-α, 50 and 500 μmol/l palmitic acid treatment for 24 h elicited substantial increases in NF-κB binding activity. Treatment with 50 μmol/l palmitic acid led to a fourfold increase (palmitic-treated 2.1 ± 0.2 vs. untreated 0.5 ± 0.04, P < 0.05, results expressed as arbitrary units) while treatment with 500 μmol/l led to an approximately fivefold increase (palmitic-treated 2.4 ± 0.3 vs. untreated 0.5 ± 0.04, P < 0.05) in NF-κB binding activity. No significant effect of oleic acid treatment on NF-κB binding activity was observed (Figure 4b). Conversely, 50 and 500 μmol/l DHA treatment for 24 h, which stimulated increased secretion of the anti-inflammatory cytokine, IL-10, resulted in an ~60% decrease in NF-κB binding (Figure 4c; 50 μmol/l DHA-treated 0.2 ± 0.03 and 500 μmol/l DHA-treated 0.18 ± 0.03 vs. untreated 0.5 ± 0.04, P < 0.05).
Figure 4.
Nuclear factor-κB (NF-κB) DNA-binding activity in 3T3-L1 adipocytes treated for 24 h in the absence and presence of 50 and 500 μmol/l of (a) palmitic, (b) oleic, and (c) docosahexaenoic acid. Cellular nuclear extracts were prepared, and binding activity of the p65 subunit of NF-κB was measured using an ELISA-based assay. Results are means ± s.e. for three independent experiments. *P < 0.05 vs. untreated (0).
Effects of fatty acid–induced cytokine production on SOCS-3 expression in adipocytes
The inflammatory effects of cytokines may be partially regulated by induction of SOCS-3 (34). To determine whether the induction of TNF-α and IL-10 by palmitic acid and DHA, respectively, was accompanied by changes in SOCS-3 expression, northern blot analysis of RNA extracted from 3T3-L1 adipocytes treated for 24 h with palmitic acid, oleic acid, or DHA was performed using a 0.8 kb SOCS-3 cDNA. As depicted in Figure 5a, adipocytes exhibiting increased TNF-α production in response to palmitic acid treatment showed no significant change in SOCS-3 mRNA at the 24 h time point. Oleic acid treatment also had no effect on SOCS-3 expression (Figure 5b). However, 3T3-L1 adipocytes showing increased IL-10 secretion in response to 50 and 500 μmol/l DHA treatment for 24 h exhibited an approximately twofold increase in SOCS-3 mRNA (Figure 5c; 50 μmol/l DHA-treated 120 ± 3.2 and 500 μmol/l treated 140 ± 4.6 vs. untreated 60 ± 2.5, P < 0.05, results expressed as arbitrary units).
Figure 5.
Northern blot showing the effects of (a) palmitic acid, (b) oleic acid, and (c) docosahexaenoic acid, on suppressor of cytokine signaling 3 (SOCS-3) gene expression in 3T3-L1 adipocytes. Cells were incubated in the absence and presence of 50 and 500 μmol/l of each fatty acid for 24 h. Each lane was loaded with 10 μg of total RNA and blots were probed with a [α-32P] dCTP-labeled mouse SOCS-3 cDNA. Results are means ± s.e. for three independent experiments. *P < 0.05 vs. untreated (0).
Discussion
Ingested fats are typically stored as triglycerides in adipocytes and are released to provide energy in a fasting state; however, individuals with insulin-resistant states such as obesity or type 2 diabetes exhibit a chronic elevation in plasma free fatty acids and the normal suppression of plasma free fatty acids seen with food intake or in response to insulin is absent in these individuals (37–39). Elevated plasma free fatty acids have been linked to the pathology of insulin resistance, impaired endothelial function, and vascular inflammation (32,33,40,41). Several dietary fatty acids are capable of modulating the immune response via effects on lymphocyte proliferation, natural killer cell activity, phagocytosis, and cytokine production (42). However, studies related to the effects of dietary fatty acids on cytokine production have focused primarily on macrophages and have yielded conflicting results (42). Furthermore, although adipose tissue contains infiltrating macrophages and other cytokine-producing cell types, little is known about the effects of fatty acids on cytokine production specifically by adipocytes.
In this study, we show that the saturated fatty acid, palmitic acid, stimulated production of a proinflammatory cytokine and inhibited production of an anti-inflammatory cytokine, whereas the polyunsaturated fatty acid, DHA, stimulated production of the anti-inflammatory cytokine. Thus, our data suggest that chronically elevated plasma fatty acid levels in obese individuals may contribute at least in part to the initiation and/or maintenance of the cellular inflammatory response of adipocytes. To our knowledge, this is the first report of specific fatty acids acting directly on the adipocyte to modulate production of TNF-α and IL-10 in a differential manner. Our results further suggest that this effect of free fatty acids on cytokine production is transcriptionally mediated, as increases in mRNA levels of TNF-α and IL-10 were seen in conjunction with increased protein secretion of these cytokines. Similarly, decreased IL-10 protein secretion in response to palmitic acid treatment was accompanied by a concomitant decrease in IL-10 mRNA.
IL-10 is known to suppress macrophage function and to inhibit cytokine production (27). However, regulation of expression of IL-10 in adipocytes is not well understood. TNF-α and lipopolysaccharide are known to upregulate IL-10 in adipose tissue and circulating levels of IL-10 are upregulated in obese rodents and humans (9,26). However, obese individuals afflicted by the metabolic syndrome have significantly lower plasma IL-10 levels compared to their obese counterparts unafflicted by this syndrome (43). In general, reduced plasma IL-10 levels have been associated with hyperglycemia, dyslipidemia, and type 2 diabetes in both obese and nonobese individuals (43). Conversely, as noted earlier, increased TNF-α levels have been linked to the pathogenesis and sequelae of cardiovascular and metabolic disorders. Our findings show that in 3T3-L1 adipocytes, IL-10 production was decreased, whereas TNF-α levels were increased, in the presence of a saturated fatty acid. Furthermore, IL-10 levels were increased through exposure to a polyunsaturated fatty acid. We hypothesize that because saturated fatty acids are a major component of typical high-fat diets and are chronically elevated in an obese state, they may act directly on adipocytes to stimulate and inhibit the release of pro- and anti-inflammatory cytokines, respectively. This effect may potentially account in part for obesity-associated inflammation. On the other hand, polyunsaturated fatty acids, by stimulating the release of anti-inflammatory cytokines, may act to mitigate the inflammatory response. Consistent with this hypothesis, a very recently published study showed that palmitic acid–induced TNF-α expression in a macrophage cell line was completely inhibited by pretreatment with the polyunsaturated fatty acid, DHA (44).
Consonant with our hypothesis, adipocytes showing enhanced TNF-α production in response to treatment with palmitic acid also showed increased NF-κB binding activity. Interestingly, treatment with the lower dose of palmitic acid–induced only 30% of the maximal effect on TNF-α production seen with the higher dose, but nonetheless induced NF-κB binding activity to almost the same extent as the higher dose. These results suggest that palmitic acid–induced increases in NF-κB binding activity may also be mediated by TNF-α–independent signaling pathways.
Our finding on the ability of palmitic acid to increase NF-κB binding activity is in agreement with a previous report showing palmitate-induced activation of NF-κB in 3T3-L1 adipocytes (45). Furthermore, results from this earlier study indicated that palmitic acid had even more potent effects than the proinflammatory agonist, lipopolysaccharide, on the inflammatory response in 3T3-L1 adipocytes. However, the same study showed that palmitic acid increased expression but not secretion of TNF-α (45), whereas our results indicate both expression and secretion of TNF-α were increased in cells treated with palmitic acid. The basis for this disparity remains uncertain; however, it is possible that secreted TNF-α may not have been detected in the earlier study due to premature degradation.
DHA-treated cells, in which IL-10 production was increased, exhibited a marked decline in NF-κB activity. IL-10 has been shown to inhibit TNF-α–induced activation of NF-κB by inhibiting IKK activity in human monocytic cell lines (46,47). Our findings in the 3T3-L1 adipocyte cell line are consistent with the notion that the anti-inflammatory properties of IL-10 in adipocytes may be mediated, at least in part, via suppression of NF-κB activity.
The SOCS family, which comprises eight known members, has been shown to suppress cytokine signaling effectively (34). Expression of the SOCS proteins is increased by cytokine signaling through activation of signal transducers and activators of transcription as well as NF-κB mediated pathways (34). In this study, expression of a key member of this family, SOCS-3, was increased in 3T3-L1 adipocytes showing increased IL-10 synthesis and secretion in response to DHA. This finding is consistent with previous studies identifying SOCS-3 as an intracellular mediator of the anti-inflammatory actions of IL-10 (48), and with a previous study showing increased SOCS-3 protein, as well as increased TNF-α secretion, in response to treatment with a mixture of fatty acids (49). Furthermore, our observation of increased SOCS-3 mRNA a full 24 h after DHA treatment suggests that SOCS-3 may exert a sustained suppression of the inflammatory response. We did not observe increased expression of SOCS-3 in 3T3-L1 cells showing increased TNF-α production as a consequence of treatment with palmitic acid for 24 h. Previous studies have reported TNF-α to induce expression of SOCS-3 (50,51). However, in those studies, this effect was noted at much earlier time points than the 24-h time point used in our study. For example, one study in mice injected with TNF-α reported a marked induction of SOCS-3 within 1 h and a return to basal levels by 24 h (50).
A recently published study involving skeletal muscle cells has reported that palmitic acid increases both mRNA levels and protein secretion of another proinflammatory cytokine, IL-6, potentially via increased NF-κB activity, with associated decreases in Glut4 expression and glucose uptake in cultured skeletal muscle cells (52). In our study, we also assessed potential effects of palmitic acid (as well as oleic acid and DHA) on insulin sensitivity by measuring basal and insulin-stimulated glucose uptake in 3T3-L1 adipocytes after 24- and 48-h incubations, but saw no changes (data not shown). A similar lack of effect of unsaturated and saturated fatty acids on glucose transport in rat adipocytes has been reported (53). Another study has indicated that the primary effects of palmitic acid on 3T3-L1 adipocytes do not involve induction of insulin resistance in the adipocytes themselves (54). However, other studies have reported stimulatory effects of fatty acids on glucose uptake in rat adipocytes (55,56). These disparate results may be due to variations in cell culture models and incubation periods.
Very recently, it was reported that saturated fatty acids are naturally occurring ligands for Toll-like receptor 4 (44,57). Toll-like receptor 4 is an important mediator of the innate immune response and induces proinflammatory signaling pathways via activation of NF-κB (58). Toll-like receptor 4 is expressed on adipocytes, including 3T3-L1 cells (44,59), and a number of in vitro and in vivo studies have implicated a role for this receptor in mediating both high-fat diet–induced adipose tissue inflammation and high-fat diet–induced insulin resistance (44,60). This may be a possible pathway by which specific classes of dietary fatty acids differentially regulate cytokine production in 3T3-L1 adipocytes.
In summary, the results presented here show that dietary fatty acids are capable of directly acting on adipocytes to modulate production of TNF-α and IL-10. Furthermore, our findings underscore the inherent capacity of the adipocyte to fuel obesity-associated inflammation. Further studies will be required to more precisely define the relationship between fatty acid–induced changes in cytokine production and NFκB activation and to determine whether our observations in the 3T3-L1 cell line are mimicked in an in vivo model.
Acknowledgments
This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases grants RO1 DK-56113, DK53978 (E.M.-F.), and KO1 DK-063080 (R.L.B) and by an unrestricted educational grant from the Yamanouchi Foundation (E.M.-F.).
Footnotes
Disclosure
The authors declared no conflict of interest.
References
- 1.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22:1145–1158. doi: 10.1038/sj.ijo.0800770. [DOI] [PubMed] [Google Scholar]
- 2.Bradley RL, Cleveland KA, Cheatham B. The adipocyte as a secretory organ: mechanisms of vesicle transport and secretory pathways. Recent Prog Horm Res. 2001;56:329–358. doi: 10.1210/rp.56.1.329. [DOI] [PubMed] [Google Scholar]
- 3.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc. 2001;60:329–339. doi: 10.1079/pns200194. [DOI] [PubMed] [Google Scholar]
- 4.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 5.Gimeno RE, Klaman LD. Adipose tissue as an active endocrine organ: recent advances. Curr Opin Pharmacol. 2005;5:122–128. doi: 10.1016/j.coph.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Frayn KN. Obesity and metabolic disease: is adipose tissue the culprit? Proc Nutr Soc. 2005;64:7–13. doi: 10.1079/pns2004403. [DOI] [PubMed] [Google Scholar]
- 7.Staiger H, Haring HU. Adipocytokines: fat-derived humoral mediators of metabolic homeostasis. Exp Clin Endocrinol Diabetes. 2005;113:67–79. doi: 10.1055/s-2004-830555. [DOI] [PubMed] [Google Scholar]
- 8.Harkins JM, Moustaid-Moussa N, Chung YJ, et al. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–2677. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- 9.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 13.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 15.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 17.Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 18.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 20.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern PA, Saghizadeh M, Ong JM, et al. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mito N, Hosoda T, Kato C, Sato K. Change of cytokine balance in diet-induced obese mice. Metabolism. 2000;49:1295–1300. doi: 10.1053/meta.2000.9523. [DOI] [PubMed] [Google Scholar]
- 23.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 24.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 25.Hundal RS, Petersen KF, Mayerson AB, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juge-Aubry CE, Somm E, Pernin A, et al. Adipose tissue is a regulated source of interleukin-10. Cytokine. 2005;29:270–274. doi: 10.1016/j.cyto.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res. 2001;88:877–887. doi: 10.1161/hh0901.090440. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 29.Berg DJ, Leach MW, Kuhn R, et al. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory responses. J Exp Med. 1995;182:99–108. doi: 10.1084/jem.182.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg DJ, Kuhn R, Rajewsky K, et al. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (Lond) 2005;108:205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 32.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 33.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 35.Shimamura M, Matsuda M, Ando Y, et al. Leptin and insulin down-regulate angiopoietin-like protein 3, a plasma triglyceride-increasing factor. Biochem Biophys Res Commun. 2004;322:1080–1085. doi: 10.1016/j.bbrc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Chavez JA, Knotts TA, Wang LP, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 37.Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37:1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 39.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 40.Staiger H, Staiger K, Stefan N, et al. Palmitate-induced interleukin-6 expression in human coronary artery endothelial cells. Diabetes. 2004;53:3209–3216. doi: 10.2337/diabetes.53.12.3209. [DOI] [PubMed] [Google Scholar]
- 41.Shankar SS, Steinberg HO. FFAs: do they play a role in vascular disease in the insulin resistance syndrome? Curr Diab Rep. 2005;5:30–35. doi: 10.1007/s11892-005-0064-6. [DOI] [PubMed] [Google Scholar]
- 42.de Pablo MA, Alvarez de Cienfuegos G. Modulatory effects of dietary lipids on immune system functions. Immunol Cell Biol. 2000;78:31–39. doi: 10.1046/j.1440-1711.2000.00875.x. [DOI] [PubMed] [Google Scholar]
- 43.Esposito K, Pontillo A, Giugliano F, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab. 2003;88:1055–1058. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- 44.Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ajuwon KM, Spurlock ME. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J Nutr. 2005;135:1841–1846. doi: 10.1093/jn/135.8.1841. [DOI] [PubMed] [Google Scholar]
- 46.Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 47.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 48.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–573. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen MT, Satoh H, Favelyukis S, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 50.Emanuelli B, Peraldi P, Filloux C, et al. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944–47949. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- 51.Fasshauer M, Klein J, Lossner U, Paschke R. Isoproterenol is a positive regulator of the suppressor of cytokine signaling-3 gene expression in 3T3-L1 adipocytes. J Endocrinol. 2002;175:727–733. doi: 10.1677/joe.0.1750727. [DOI] [PubMed] [Google Scholar]
- 52.Jove M, Planavila A, Laguna JC, Vazquez-Carrera M. Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor kappaB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology. 2005;146:3087–3095. doi: 10.1210/en.2004-1560. [DOI] [PubMed] [Google Scholar]
- 53.Lundgren M, Eriksson JW. No in vitro effects of fatty acids on glucose uptake, lipolysis or insulin signaling in rat adipocytes. Horm Metab Res. 2004;36:203–209. doi: 10.1055/s-2004-814446. [DOI] [PubMed] [Google Scholar]
- 54.Usui I, Haruta T, Takata Y, et al. Differential effects of palmitate on glucose uptake in rat-1 fibroblasts and 3T3-L1 adipocytes. Horm Metab Res. 1999;31:546–552. doi: 10.1055/s-2007-978793. [DOI] [PubMed] [Google Scholar]
- 55.Hardy RW, Ladenson JH, Henriksen EJ, Holloszy JO, McDonald JM. Palmitate stimulates glucose transport in rat adipocytes by a mechanism involving translocation of the insulin sensitive glucose transporter (GLUT4) Biochem Biophys Res Commun. 1991;177:343–349. doi: 10.1016/0006-291x(91)91989-p. [DOI] [PubMed] [Google Scholar]
- 56.Hunnicutt JW, Hardy RW, Williford J, McDonald JM. Saturated fatty acid-induced insulin resistance in rat adipocytes. Diabetes. 1994;43:540–545. doi: 10.2337/diab.43.4.540. [DOI] [PubMed] [Google Scholar]
- 57.Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 58.Zuany-Amorim C, Hastewell J, Walker C. Toll-like receptors as potential therapeutic targets for multiple diseases. Nat Rev Drug Discov. 2002;1:797–807. doi: 10.1038/nrd914. [DOI] [PubMed] [Google Scholar]
- 59.Lin Y, Lee H, Berg AH, et al. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem. 2000;275:24255–24263. doi: 10.1074/jbc.M002137200. [DOI] [PubMed] [Google Scholar]
- 60.Suganami T, Mieda T, Itoh M, et al. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–49. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]