Abstract
Ingestion of very low-carbohydrate ketogenic diets (KD) is associated with weight loss, lowering of glucose and insulin levels and improved systemic insulin sensitivity. However, the beneficial effects of long-term feeding have been the subject of debate. We therefore studied the effects of lifelong consumption of this diet in mice. Complete metabolic analyses were performed after 8 and 80 weeks on the diet. In addition we performed a serum metabolomic analysis and examined hepatic gene expression. Lifelong consumption of KD had no effect on morbidity or mortality (KD vs. Chow, 676 vs. 630 days) despite hepatic steatosis and inflammation in KD mice. The KD fed mice lost weight initially as previously reported (Kennnedy et al., 2007) and remained lighter and had less fat mass; KD consuming mice had higher levels of energy expenditure, improved glucose homeostasis and higher circulating levels of β-hydroxybutyrate and triglycerides than chow-fed controls. Hepatic expression of the critical metabolic regulators including fibroblast growth factor 21 were also higher in KD-fed mice while expression levels of lipogenic enzymes such as stearoyl-CoA desaturase-1 was reduced. Metabolomic analysis revealed compensatory changes in amino acid metabolism, primarily involving down-regulation of catabolic processes, demonstrating that mice eating KD can shift amino acid metabolism to conserve amino acid levels. Long-term KD feeding caused profound and persistent metabolic changes, the majority of which are seen as health promoting, and had no adverse effects on survival in mice.
Keywords: Ketogenic diet, Liver, Ketogenesis, Hepatic steatosis, Free fatty acid metabolism, Metabolomics, Mass spectrometry
1. Introduction
Ketogenic diets are of interest because in humans, they have been used for weight loss [2,3], and are also used to treat refractory epilepsy, particularly in pediatric populations [4,5]. Studies of KD have been extended to mice in an attempt to identify potential mediators of their metabolic effects. Despite the high-fat content of the diet, mice fed KD fail to gain excess weight and develop a unique metabolic state characterized by increased energy expenditure, increased systemic insulin sensitivity, and a distinct hepatic pattern of gene expression. Consumption of KD is also effective in inducing weight loss in wild-type mice with diet-induced obesity, as well as improving glucose tolerance without weight loss in the ob/ob mouse [1,6,7]. Additional studies demonstrated that diabetic nephropathy can be reversed through the use of KD intervention in both Type 1 (Akita) and Type 2 (db/db) mouse models [8]. These results show promise for the therapeutic use of KD; yet there is controversy regarding the potential adverse long term consequences. One study suggests that chronic KD consumption leads to pathological hepatic steatosis as well as insulin resistance and glucose intolerance [9,10]. However, to date the longest duration of a study of mice eating KD has been 12 weeks [10]. Since human consumption of KD for weight loss or refractory epilepsy can continue for months or years, the long-term health and lifetime effects of this dietary state are of clinical importance.
To fully understand the long-term physiologic consequences of KD on systemic health, longevity, and metabolism, we compared both the short-term effects of 8 weeks of KD (STKD) versus consuming a standard mouse “chow” diet, (STCH) and long-term effects after 66–80 weeks on the diets (LTCH and LTKD for chow and KD respectively). We compared physiologic parameters, gene expression profiles, and serum metabolomic profiles of each of the four cohorts. We found that the previously reported increase in energy expenditure and insulin sensitivity [1] persisted for over a year. We also compared changes in amino acid metabolism in mice fed the diet for 8 weeks to changes in mice fed the diet for 80 weeks. In both STKD and LTKD cohorts we found differences in amino acid metabolism as assessed both by metabolomics and gene expression, which were consistent with decreased amino acid catabolism. We also found increases in markers of oxidative stress, and inflammation in the liver in both STKD and LTKD mice. As there was no premature mortality over duration of the study, which continued until the death of the last mouse in the longevity cohort, we found no adverse effects of KD on life span. Thus we demonstrate that ketogenic diets are well tolerated over the lifespan of the animal, that animals maintain improved glucose homeostasis, and that the mice develop sustained compensatory changes in amino acid metabolism which may allow them to compensate for decreases in dietary protein intake and de novo amino acid biosynthesis.
2. Materials and methods
2.1. Animals
Animals were obtained from The Jackson Laboratory (C57Bl6J, 000664) in Bar Harbor, ME and maintained at 24 °C on a 12:12-h light-dark cycle. Animals were allowed ad libitum access to food, except where stated otherwise. All procedures were in accordance with NIH Guidelines for the Care and Use of Animals and were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee (IACUC). C57BL/6J mice were split into two groups at 8 weeks of age and fed standard chow or KD ad libitum. Within dietary groups, subsets of mice were randomly selected for physiologic tests or terminal harvest for tissue analysis. Physiologic tests included nuclear magnetic resonance (qNMR), indirect calorimetry, glucose tolerance test (GTT), and insulin tolerance test (ITT). Mice selected for tissue analysis and harvested at 16 weeks for the short-term study and 88 ± 1 week for the long-term study.
2.2. Diet studies
LabDiet 5008 (Pharmaserv, Framingham, MA) consisting of 6.5% fat, 23.5% protein, and 56% carbohydrate (2.5% sucrose) wt/wt was used as standard chow. The ketogenic diet (KD) was obtained from Bio-Serv (F3666, Frenchtown, NJ) and consisted of 78.9% fat, 9.5% protein, and 0.76% carbohydrate (0% sucrose) wt/wt. This formulation has been demonstrated to induce ketosis in rodents [11] and used by us in previous studies [1]. The macronutrient-calorie percent composition from each diet was as follows: standard chow: 16.7% fat, 26.8% protein, and 56.4% carbohydrate (6.5% sucrose); KD: 95% fat, 0% carbohydrate (0% sucrose), and 5% protein.
2.3. Glucose and insulin tolerance tests
Glucose tolerance test: mice on standard chow (CH) and KD were fasted for 16 h before an intraperitoneal (ip) injection D-glucose (2 g/kg body weight; Sigma, St. Louis, MO) was administered 4 h after light onset. Tail blood glucose levels were measured using a OneTouch Ultra glucometer (Lifescan, Milpitas, CA). To assess insulin tolerance, 7 CH and 5 KD ad libitumfed-mice were injected ip, with insulin (0.75 U/kg) 8 h after onset of the light cycle (Lilly, Indianapolis, IN).
2.4. Indirect calorimetry
Mouse energy expenditure was measured by indirect calorimetry using the Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH). Mice fed chow for 60 weeks were housed singly with ad libitum access to food and water. Analysis was performed at 24 °C under a 12:12-h light-dark cycle (light period 0600–1800). Mice were acclimated in the metabolic chambers for 48 h before collecting measurements used for data analysis. Ambulatory activity, oxygen consumption, and carbon dioxide production were simultaneously determined.
2.5. Body composition analysis
Body composition, including lean and fat mass, was assessed using an EchoMRI 3-in-1 quantitative nuclear magnetic resonance (qNMR) system (Echo Medical Systems, Houston, TX). This test is performed in conscious mice that are immobilized for one minute.
2.6. Quantitative RT-PCR
Ribonucleic acid (RNA) was isolated from tissue flash-frozen in liquid nitrogen using an RNAeasy Lipid mini kit (Qiagen, Germantown, MD) according to manufacturer’s instructions. A DNase (Qiagen, Germantown, MD) step to digest the genomic DNA was included. Complementary DNA (cDNA) was made from isolated RNA using oligo(dt) and random hexamer primers and reverse transcriptase (QuantiTech RT Kit; Qiagen, Germantown, MD). Quantitative PCR was performed using the 7800HT (Applied Biosystems, Foster City, CA) thermal cycler and SYBR Green master mix (Applied Biosystems, Foster City, CA). Relative mRNA abundance was calculated and normalized to levels of the housekeeping gene 36B4. Primers are included in Supplementary Table 1.
2.7. Survival analysis
C57BL/6J mice were split into two groups at 8 weeks of age and allowed ad libitum access to either standard chow or KD. The Kaplan–Meier survival curve was created with the log-rank test equal to the Mantel–Haenszel test. All statistical analyses were performed with GraphPad Prism v6.04 for Windows (GraphPad Software, San Diego, California, USA).
2.8. Serum and liver assays
Serum FGF21 concentrations were measured using Quantikine mouse FGF21 enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN), nonesterified free fatty acids (NEFAs) (NEFA C colorimetric assay; Wake Chemicals, Richmond, VA), triglycerides (Triglyceride Colorimetric Assay; Stanbio Laboratory, Boerne, TX), β-hydroxybutyrate (β-hydroxybutyrate colorimetric assay, Stanbio Laboratory), cholesterol (Cholesterol Liquicolor, colorimetric assay; Stanbio Laboratory), insulin (ultra-sensitive mouse insulin ELISA; Crystal Chem, Chicago, IL), and alanine aminotransferase (ALT Colorimetric Assay, Pointe Scientific, Canton, MI). For tissue cholesterol and triglyceride assays, a Folch extraction was first performed on 100 mg of frozen tissue [12] before colorimetric analysis (Cholesterol Liquicolor, colorimetric assay; Stanbio Laboratory), (Triglyceride Colorimetric assay; Stanbio Laboratory, Boerne, TX).
2.9. Targeted mass spectrometry and metabolomics
Serum (100 μL) was collected and isolated from mice 16 weeks (STCH and STKD) and 88 weeks (LTCH and LTKD) of age. Serum samples were extracted at dry ice temperatures using a 1:4 ratio of serum:methanol resulting in a total volume of 80% methanol. Samples were centrifuged and resulting supernatant was dried under vacuum. Samples were resuspended using 20 μL high-performance liquid chromatography (HPLC) grade water for mass spectrometry and 10 μL was injected and analyzed using a 5500 QTRAP triple quadrupole mass spectrometer (AB/Sciex) coupled to a Prominence UFLC HPLC system (Shimadzu) via selected reaction monitoring (SRM) of a total of 249 endogenous water soluble metabolites as previously described [13]. All mass spectrometry data were collected and processed as previous reported [13]. Data are presented as mean ± SEM. Comparisons between two groups were performed using an unpaired t test.
2.10. Statistics
Data are presented as mean ± SEM. Comparisons between two groups were performed using an unpaired t test, while for 4-group comparisons a two-way ANOVA utilized where appropriate, the effects of duration and diet were queried and post-hoc comparisons were determined by Sidak’s post-hoc test for individual time point analysis.
3. Results
3.1. KD-fed mice remain leaner and lighter than WT controls
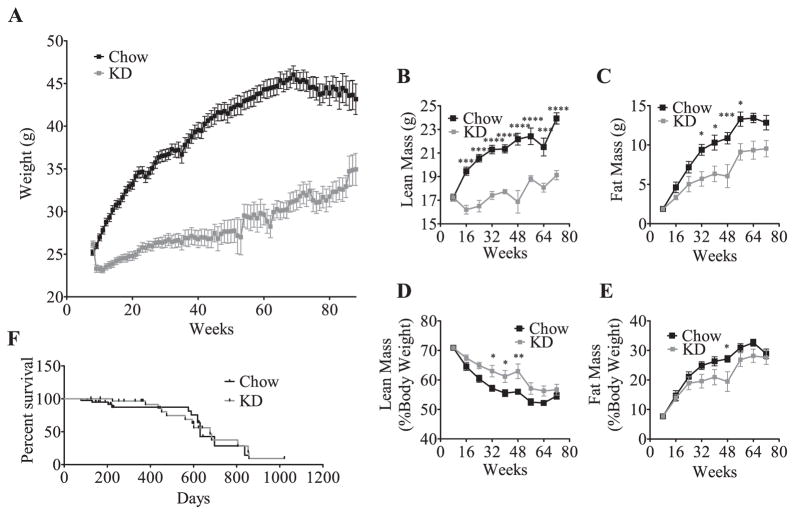
To evaluate the effects of chronic feeding of KD we generated a cohort of mice that were fed KD for 8 weeks (designated short term or STKD) as a baseline group for comparison. In this cohort we reproduced our previous reported findings [1]. Mice fed KD initially lose weight dramatically; however, after 18 weeks their weight returns to the baseline and the animals gradually accumulate mass. Over a 40-week period, chow-fed mice gained 16 g (1.6 g/month), while the KD-fed mice gained only 1.2 g over the same period (0.12 g/month) (Fig. 1A). Body composition analysis revealed that the weight differential results from both a lower lean mass (Fig. 1B) and fat mass in KD compared to chow fed animals (Fig. 1C). As a percent of total mass, the lean mass of the KD-fed mice is greater (Fig. 1D) than that of chow-fed controls (Fig. 1E), indicating that relative lean mass was preserved. Indeed, tissue weights after 80-weeks on KD revealed that the relative mass of the liver, brown fat and epididymal fat was the same in KD and chow fed animals (Supplemental Fig. 1). Together, these findings show that long-term administration of KD maintains a lean phenotype.
Fig. 1.
Ketogenic diet fed mice remain lighter and leaner than chow fed controls and have similar life expectancy. KD fed mice initially lose weight (A) and after a few months return to baseline, but remain lighter than chow-fed controls (effect of diet P < 0.0001). The values represent group means (±SEM, initial group size CH, n = 48; KD, n = 39). Significance in weight curve was determined with two-way ANOVA. Both lean mass (B) and fat (C) mass are reduced in KD fed mice compared to chow fed counterparts as measured by MRI, but when corrected for body mass (D & E), KD-fed mice have a greater percentage of lean mass (effect of diet P < 0.0001) and less fat mass (effect of diet P = 0.0002). The values represent group means ± SEM, CH, n = 6–13 per time point, KD, n = 8–11 per time point. Significance in body composition was determined with two-way ANOVA with Sidak’s post-hoc test for individual time point analysis, significance is designated by asterisks with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Survival analysis in animals fed chow or KD demonstrating no significant alteration in morbidity or mortality in KD fed mice compared to CH fed mice (F). Comparison of survival curves was conducted with a log-rank (Mantel–Cox) test, P = 0.9092. Starting survival study contained CH n = 40, KD n = 31.
Given the dramatic difference in nutrient availability of the two diets, we postulated that chronic consumption of KD could influence the morbidity or mortality. We therefore conducted a survival study and found that lifelong consumption of KD had no influence upon survival including no major morbidity when compared to chow (Fig. 1F). Despite profound systemic metabolic and physiologic changes in mice, including increases in metabolic rate and altered glucose homeostasis, KD was not shown to alter survival curves, although median survival was slightly longer on KD (676 days compared to 630 days for chow).
3.2. LTKD-fed mice demonstrated increased energy expenditure and loss of a diurnal rhythm in RER
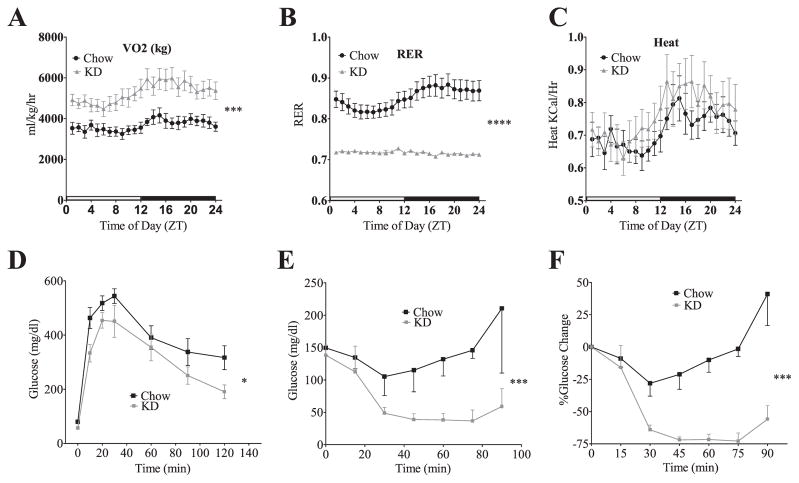
We have previously shown that short-term consumption of KD is associated with increased energy expenditure. To assess the persistence of this effect, we performed indirect calorimetry at 60 weeks. We found that mice fed KD exhibited greater oxygen consumption (VO2) than chow-fed cohorts (Fig. 2A) (p = 0.003, Repeat Measures ANOVA). Furthermore, in short term KD fed animals there was a loss of diurnal pattern of the respiratory exchange ratio (RER), which indicated continuous use of fatty acids as an energy substrate (Fig. 2B). Although the KD-fed mice were smaller, they generated the same heat as larger mice on chow (Fig. 2C). Combined, these results demonstrate that long-term consumption of KD sustains increases in energy expenditure.
Fig. 2.
Mice fed KD for 60 weeks have sustained changes in metabolic rate and glucose homeostasis. KD-fed mice have a higher metabolic rate of VO2 consumption (A). The cyclic nature of the respiratory exchange rate (RER) is lost in the KD-fed mice (B) due to constant utilization of lipid as a fuel source. An indirect measure of heat (C) trends higher in the KD mice. The values for VO2 consumption, RER and heat all represent group means (±SEM, group size CH, n = 8; KD, n = 7). Mice fed KD for 60 weeks maintain glucose tolerance as measured by an IP glucose tolerance test (D). The values represent group means (±SEM, group size CH, n = 8; KD, n = 7). Mice fed KD for 60 weeks remain insulin-sensitive as shown by an ITT either by glucose level (E) or %change (F). The values represent group means (±SEM, group size CH, n = 7; KD, n = 5). Two-way Repeat Measures ANOVA for all datasets in this figure, significance is designated by asterisks with *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3.3. LTKD-feeding improved glucose tolerance and enhanced insulin sensitivity
As seen in STKD, LTKD-fed animals maintained glucose tolerance and exhibited improved insulin sensitivity, based on several parameters. Glucose tolerance testing (GTT) demonstrated that KD-fed mice were glucose tolerant (Fig. 2D) (p < 0.05 RM ANOVA). Additionally, LTKD fed mice had dramatically lower baseline insulin levels than LTCH fed mice (LTCH = 4.01 ng/ml, LTKD = 0.41 ng/ml, Student’s t-test p < 0.01). Mice on LTKD also showed a more robust glucose lowering response to exogenous insulin. Upon insulin administration, the lowest glucose level achieved in chow fed mice was 105 mg/dl compared to 37 mg/dl in LTKD. Furthermore, hypoglycemia persisted for a longer period. While glucose levels returned to the baseline by 75 min in chow fed animals, glucose levels were still low at 90 min in LTKD animals. Thus, LTKD mice demonstrated enhanced insulin sensitivity as determined by an insulin tolerance test (ITT) (Fig. 2E & F).
3.4. Metabolic profile
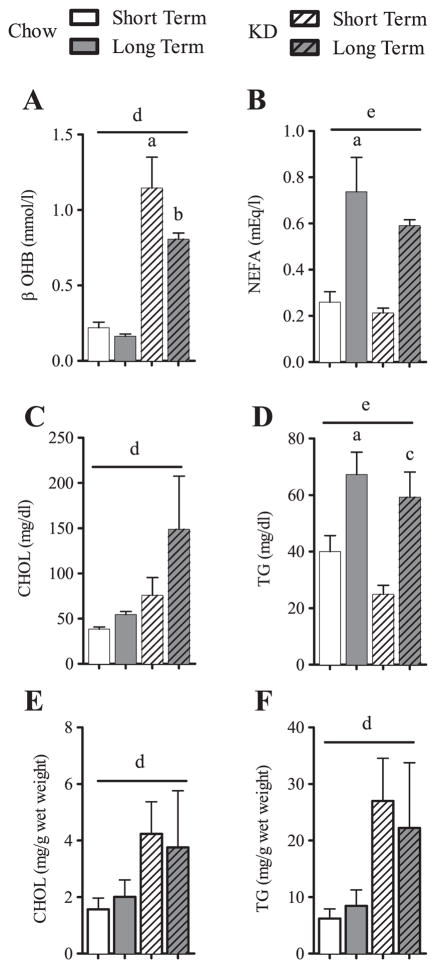
KD feeding led to a five-fold increase in β-hydroxybutyrate levels, an effect noted at 8 weeks, which persisted at 22 months demonstrating a significant effect of KD (Two-way ANOVA, P < 0.0001) (Fig. 3A). Circulating levels of nonessential fatty acid (NEFA) levels were the same in STCH and STKD fed animals. Interestingly, NEFAs rose in both the LTCH and LTKD fed animals, demonstrating a significant effect of age (Two-way ANOVA, P = 0.0005) (Fig. 3B). Serum levels of cholesterol were higher in mice fed KD (Two-way ANOVA, P = 0.0087) (Fig. 3C). Triglyceride levels were higher in LTCH mice and LTKD mice, demonstrating an age or “duration” effect (Two-way ANOVA, P = 0.0002) (Fig. 3D). Analysis of hepatic cholesterol and triglycerides demonstrated increased concentrations of these lipids in all KD-fed mice (Two-way ANOVA, effect of diet P = 0.0378 CHOL and P = 0.0075 TG) (Fig. 3E and F). Together, these results show that hepatic lipids increase upon KD feeding; however, long-term KD feeding has no additive effects upon the increased levels seen short-term.
Fig. 3.
Serum nutrient profiles and liver lipids for short or long-term feeding of chow and KD. Data is shown as means ± SEM and is shown for serum (A–D) or for liver (E–F). Two-way ANOVA with Sidak’s post-hoc test for individual time point analysis, significance (P < 0.05) is designated by letters: asignificantly different from STCH; bsignificantly different from LTCH; csignificantly different from STKD; dsignificant effect of diet; and esignificant effect of age. n = 4–9 per group.
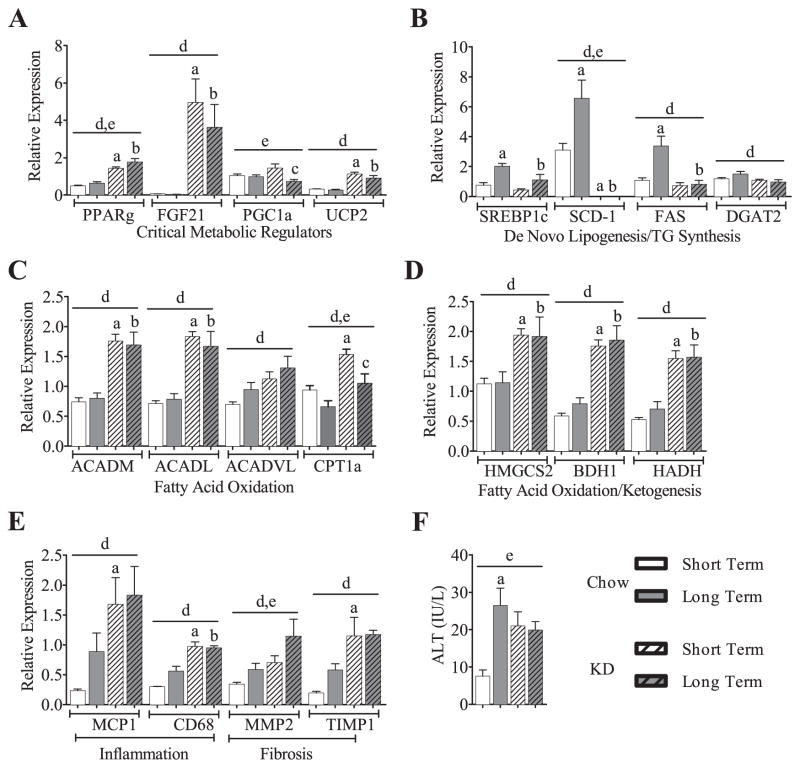
3.5. KD-fed mice have altered expression of critical hepatic regulators of metabolism, genes involved in fatty acid oxidation, ketogenesis and fatty acid transport
Several reports note that KD feeding leads to altered expression of genes involved in energy balance [1,6,10,14]. Consistent with these studies, we found that the expression of uncoupling protein 2 (UCP2), fibroblast growth factor 21 (FGF21), and peroxisome proliferator-activated receptor gamma (PPARγ) were all elevated under STKD feeding. These changes persisted with LTKD feeding (Fig. 4A). Decreased expression of gene encoding rate-limiting enzymes of de novo lipogenesis and triglyceride synthesis was observed (Fig. 4B), while increases in fatty acid oxidation and ketogenesis (Fig. 4C & D) gene expression were all largely attributable to KD alone. Given previous findings of hepatic steatosis in STKD mice [1,10], we examined markers of inflammation and fibrosis. We found that KD consumption caused increases in the expression of chemokines/cytokines or genes related to pro-inflammatory pathways (Fig. 4E) and these too were principally ascribed to diet. The serum marker of liver injury, alanine transaminase (ALT), was also elevated in all KD fed mice, and in the LTCH fed mice (P < 0.01) (Fig. 4F). Interestingly, the effects of age and feeding on ALT expression were not cumulative; there were no exaggerated effects of LTKD feeding compared to LTCH feeding. Together, these findings suggest that many of the effects previously observed during STKD feeding persist in LTKD.
Fig. 4.
Hepatic gene expression: during short or long-term feeding of KD, critical metabolic regulators remain elevated (A) and de novo lipogenic/triglyceride synthesis-genes remain depressed (B). With a few exceptions, expression in the liver of genes involved in fatty acid oxidation and ketogenesis elevate upon KD feeding and remain elevated long-term (C & D). KD feeding and/or aging increases gene expression markers (E) and the serum marker aminotransferase (F) of hepatic inflammation and fibrosis, but the increase is not cumulative. PPARγ, peroxisome proliferator-activated receptor gamma; FGF21, fibroblast growth factor 21; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; UCP2, uncoupling protein 2, SCD-1, stearoyl-CoA desaturase-1; FAS, fatty acid synthase; DGAT2, diglyceride acyltransferase; SREBP1c, sterol regulatory element-binding transcription factor 1c; ACADM, acyl-Coenzyme A dehydrogenase [medium chain]; ACADL, acyl-Coenzyme A dehydrogenase [long chain]; ACADVL, acyl-Coenzyme A dehydrogenase [very long chain]; Hmgcs2, 3-hydroxy-3-methylgutaryl-CoA synthase 2 (mitochondrial); BDH1, 3-hydroxybutyrate dehydrogenase (type 1); HADH, hydroxyacyl-CoA dehydrogenase; MCP1, monocyte chemotactic protein-1; Cd68, cluster of differentiation 68; Mmp2, matrix metalloproteinase-2; TIMP1, tissue inhibitor of metalloproteinase 1. Graphs are shown means ± SEM. Two-way ANOVA utilized on all data sets, effects of duration and diet queried, post-hoc comparisons determined by Sidak’s post-hoc test for individual time point analysis, significance (P < 0.05) is designated by letters: asignificantly different from STCH; bsignificantly different from LTCH; csignificantly different from STKD; dsignificant effect of diet; and esignificant effect of age. n = 4–9 per group.
3.6. Metabolic profiling identifies a long-term compensatory amino acid metabolism in response to KD
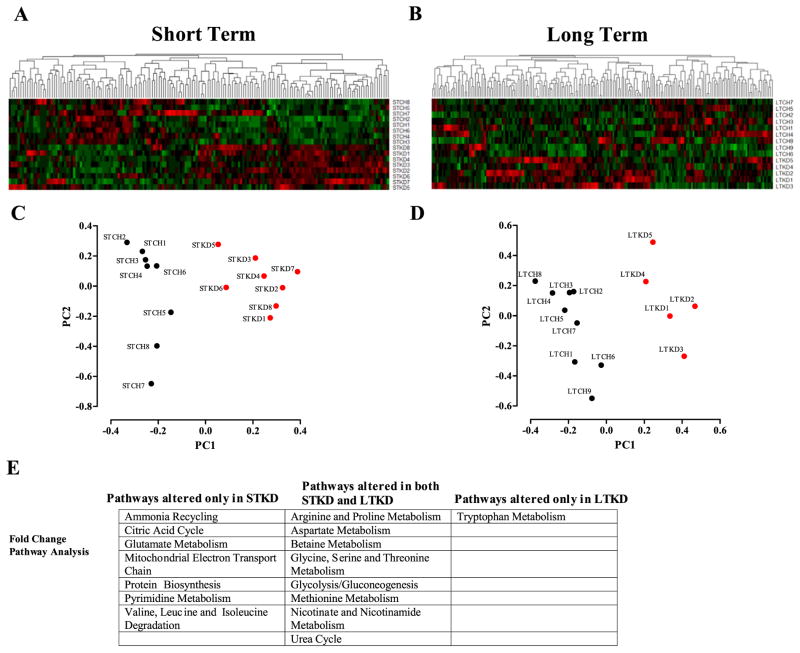
The differences in systemic metabolism suggested that KD-fed mice would have a distinct metabolic profile. To study the associated changes in the metabolome, we used a targeted-mass-spectrometry based metabolomics platform to profile polar metabolites present in the serum of our four cohorts. We carried out several unbiased forms of classification to identify metabolic signatures that distinguished chow-versus KD-fed mice. We detected 217 and 239 metabolites in the short term and long term cohorts respectively (Supplemental Files 1 and 2). Of these more than 125 metabolites were used for analysis which identified metabolic signatures unique to KD-fed mice compared to chow-fed mice, shown here in an unsupervised hierarchical clustering of serum metabolite profiles (Fig. 5A). This clustering revealed a distinct pattern of metabolites differentially (either elevated or decreased) in the sera of KD-fed mice relative to chow-fed mice (Fig. 5A and Supplemental Fig. 2A, B for short term and Supplemental Fig. 3A, B for long term). The tree structure obtained from clustering each durational cohort exhibits two distinct subclusters corresponding to the two different diets (Supplemental Fig. 4A, B). We then performed a principal component analysis (PCA) to determine the specific metabolites characteristic of these diet-dependent signatures (Fig. 5C, D). The analysis revealed that the first principal component was of sufficient magnitude to distinguish KD and chow-fed animals. To further identify the specific pathways involved in response to KD, we used the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to group the most significant metabolites changing between the four cohorts (Fig. 5E). The pathway analysis revealed overlap in pathways that altered by both short and long term KD.
Fig. 5.
Heatmaps depicting hierarchical clustering of CH vs. KD metabolite composition. Unsupervised hierarchical clustering of serum metabolite profiles of STCH vs. STKD (A). Hierarchical clustering of LTCH vs. LTKD serum metabolite composition (B) (gradient of red color for metabolites for relative positive correlation and green colors for metabolites negatively correlated). Projection of individual samples onto the first two principal components segregate into their respective groups of short-term chow vs. KD (C) or long-term chow vs. KD (D). Table depicting a KEGG pathway analysis using metabolite fold change values for STCH vs. STKD and LTCH vs. LTKD (E). n = 5–9 per group.
KEGG analysis summarized discriminant metabolites between the four cohorts. By comparing both ST cohorts, we found that short-term effects of KD led to alterations in the metabolic signatures associated with pathways for glutamate metabolism, protein biosynthesis, mitochondrial electron transport chain, the ammonia cycle and the tricarboxylic acid cycle. By comparing both KD cohorts, we found that the KD over time led to alterations in metabolic signatures associated with pathways for arginine and proline metabolism, glycine, serine and threonine metabolism, glycolysis/gluconeogenesis, aspartate metabolism, nicotinate and nicotinamide metabolism, the urea cycle and methionine metabolism. Interestingly, although circulating methionine levels were not significantly changed, the intermediate metabolite homocysteine was increased in both STKD and LTKD.
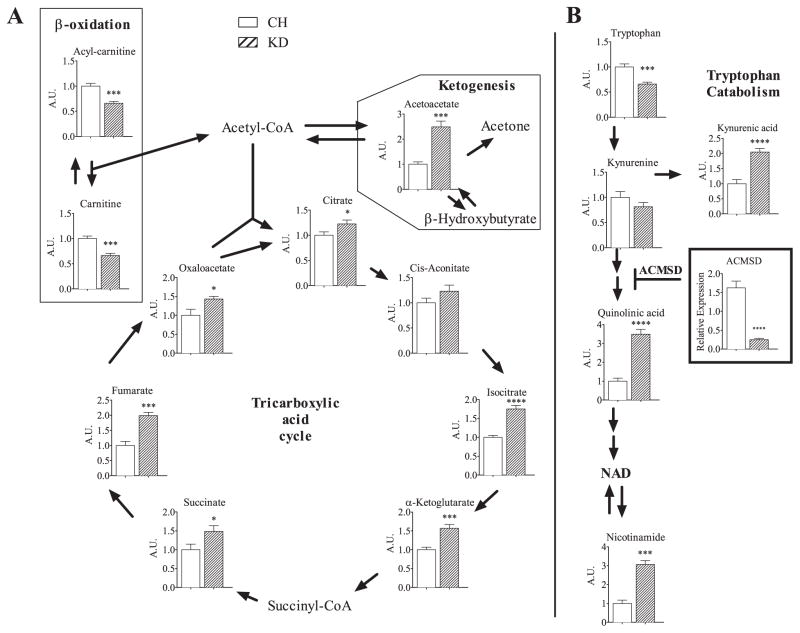
Examples of some pathway-wide metabolic changes used in the pathway analysis are depicted in Fig. 6, demonstrating that critical metabolic networks are susceptible to diet-induced changes under KD. These changes include increases in critical intermediates of the tricarboxylic acid cycle (TCA cycle), β-oxidation, and ketogenesis. Metabolomic analysis revealed a large increase in the ketone body acetoacetate in both short-term and long-term fed KD groups, compared to their respective chow-fed controls (Fig. 6A). These data, combined with the direct serum measurement of β-hydroxybutyrate (Fig. 3A), are consistent with the extreme state of ketosis in the KD-fed mice. Gene expression analysis revealed increased hepatic expression of genes involved in β-oxidation (Fig. 4A, B). Serum concentrations of β-oxidation intermediates carnitine and acylcarnitine were paradoxically decreased in STKD mice, indicating a possible dissociation between hepatic gene expression and serum content of oxidative markers (Fig. 6A).
Fig. 6.
Pathway depiction of metabolites altered with STCH or STKD feeding. Critical metabolic intermediates in BCAA metabolism, the citric acid cycle, ketogenesis, β-oxidation, and tryptophan catabolism pathways are shown. Chow values are normalized to “1”. The graph depicting Aminocarboxymuconate Semialdehyde Decarboxylase (ACMSD) denotes gene expression. Data is shown as means ± SEM. Asterisks designate significance by Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. n = 8 per group.
KD alters the normal TCA cycle as STKD fed mice up-regulated isocitrate, α-ketoglutarate, succinate, fumarate, oxaloacetate, and citrate compared to the chow fed controls (Fig. 6A). Another pathway that changed when comparing chow and KD involved tryptophan metabolism including reduced circulating tryptophan and increased downstream metabolites kynurenic acid, quinolinic acid and nicotinamide along with decreased gene expression of the enzyme Aminocarboxymuconate Semialdehyde Decarboxylase (ACMSD) (Fig. 6B). Analysis of the TCA cycle and tryptophan metabolism in LTKD fed mice revealed a similar pattern of expression (Supplemental Fig. 5A, B).
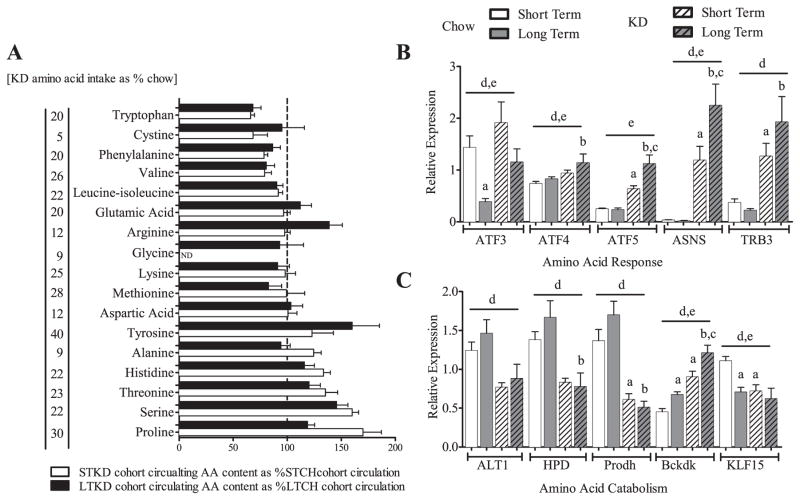
To further investigate the metabolic adaptations to the different amino acid (AA) contents of the diets, we evaluated both the amount of AA available in the two diets and the concentration of circulating AA in the serum. In Fig. 7A, we plotted the quantity of AA found in the KD as a percentage found in the chow, juxtaposed with the amount of AA found in the serum of LTKD as a percentage of that found in the serum of LTCH cohort. Two observations are notable: 1) the AA content in the KD is extremely low and 2) there is a striking maintenance of circulating AA metabolites in KD-mouse serum. We postulate that AA starvation triggers the amino acid response (AAR), a reaction observed during calorie and protein restriction, and that this down-regulation of AA catabolic genes likely offsets the low concentration of protein substrate in the diet [15]. We examined hepatic gene expression involved in the AAR and found that activating transcription factors 3, 4, and 5 (ATF3-5), asparagine synthetase (ASNS), and tribbles homolog 3 (TRB3), all markers of AAR [16–19], were increased in KD-fed mice (Fig. 7B). We also examined hepatic AA catabolism genes (Fig. 7C). We found reduced expression of krüppel-like factor 15 (KLF15), a gene associated with amino acid catabolism and a known regulator of alanine aminotransferase (ALT1), involved in pyruvate metabolism, 4-hydroxyphenylpyruvate dioxygenase (HPD) involved in tyrosine metabolism, and proline dehydrogenase 1 (Prodh), involved in proline degradation. There was also an increase in branched chain ketoacid dehydrogenase kinase (Bckdk) which catalyzes the phosphorylation and inactivation of the branched-chain alpha-ketoacid dehydrogenase complex, the key regulatory enzyme of the valine, leucine and isoleucine catabolic pathways. Proteomic analysis revealed altered expression in the KD fed mice of all five of these key mediators of AA.
Fig. 7.
Low AA availability leads to a hepatic amino acid response and down-regulation of hepatic amino acid catabolism. Mice eating KD consumed 5–30%/daily of the AA of the mice on chow [levels in brackets], yet despite this low AA availability circulating levels of AA are largely maintained (A). Hepatic gene expression: Increases in amino acid response gene markers in KD-fed mice. ATF3, ATF4, ATF5, activating transcription factor 3,4,5, ASNS, asparagine synthetase (glutamine-hydrolyzing), TRB3, tribbles homolog 3 (B). Hepatic amino acid catabolism genes are decreased in KD samples suggesting protection of amino acid content. ALT1, glutamic-pyruvate transaminase (alanine aminotransferase); HPD, 4-hydroxyphenylpyruvate dioxygenase; Prodh, proline dehydrogenase (oxidase) 1; Bckdk, branched chain ketoacid dehydrogenase kinase; KLF15, Krüppel-like factor 15 (C). Data is shown as means ± SEM. Two-way ANOVA utilized on hepatic gene expression, effects of duration and diet queried, post-hoc comparisons determined by Sidak’s post-hoc test for individual time point analysis, significance (P < 0.05) is designated by letters: asignificantly different from STCH; bsignificantly different from LTCH; csignificantly different from STKD; dsignificant effect of diet; and esignificant effect of age. ND = none detected. Gene expression: n = 5–9 per group.
4. Discussion
In mice, consumption of a ketogenic diet results in a unique physiologic state as metabolism shifts from a glucose-based “economy” to one that is fatty acid-based wherein oxidation of FFA results in the generation of ketones as the primary systemic fuel source. To understand the long-term consequences of consumption of this very high-fat, low-carbohydrate, low-protein diet, we assessed longevity and evaluated the metabolic state of animals after 80 weeks on the diet. Although KD is associated with fatty liver and inflammation [10], long-term consumption of the diet appears to have no adverse health consequences in mice as evidenced by normal life expectancy without observed changes in morbidity and mortality. Animals eating KD maintain a relative low body weight compared to chow-fed, although weight gain accelerates at around 40 weeks of age. KD-fed animals had improved glucose homeostasis as assessed by glucose tolerance and systemic insulin sensitivity at 60 weeks.
To better understand the metabolic adaptions to KD consumption that permit normal long-term survival, we first performed unsupervised clustering analysis on serum metabolites. This revealed a distinct metabolic signature in mice fed KD in comparison to mice fed standard chow with major differences in pathways involving amino acid metabolism and specific metabolites associated with energetic processes. For example, levels of substrates of the TCA cycle are generally higher in mice consuming KD, reflecting differences in metabolic flux in these essential pathways. The KD that we use in our studies is a low protein diet and supplies relatively low amounts of essential amino acids including methionine and choline [20]. However, the levels of circulating amino acids in the serum of KD-fed animals were largely maintained at levels similar to that seen in chow-fed mice; although valine and tryptophan concentrations were depressed on the diet. There is a complex relationship between glucose homeostasis and circulating levels of a subset of amino acids. A report in humans demonstrated significant associations linking increased concentrations of the branched chain amino acids (BCAAs) leucine, isoleucine, valine and the aromatic amino acids phenylalanine (Phe) and tyrosine (Tyr) with a predisposition to diabetes [21]. Other work showed an association between insulin resistance and metabolites including BCAAs, Phe, Tyr and products of amino acid catabolism [22,23]. Indeed, providing a Leu-depleted diet to mice resulted in increased insulin sensitivity [24]. Interestingly, FGF21 production is stimulated upon Leu-deprivation [25]. However, the Leu effect may be complex as doubling dietary Leu intake via Leu-containing water improved glucose homeostasis including insulin sensitivity in a mouse model [26]. Circulating levels of branched chain amino acids per se may not be critical for insulin sensitivity, as our KD mice were insulin sensitive and yet maintained relatively normal circulating levels of BCAA. Rather, reduced amino acid catabolism might affect insulin sensitivity through influences on insulin-sensitizing pathways. Indeed, we found that KD-fed mice demonstrated decreased hepatic expression of genes responsible for amino acid catabolism including BCAA catabolism. For example, we found altered expression of Bckdk (Fig. 7), a kinase that catalyzes the phosphorylation and inactivation of the branched-chain alpha-ketoacid dehydrogenase complex and is a key regulator of the BCAA catabolic pathways.
With respect to specific amino acids, we noticed that KD changes several metabolites of the tryptophan-NAD pathway. KD feeding decreased tryptophan levels while downstream metabolites quinolinic acid and nicotinamide are increased. This metabolite pattern suggests a shift at the branch point of tryptophan metabolism towards NAD synthesis as opposed to complete oxidation. The enzyme ACMSD plays a key role in this pathway as increased expression reduces quinolinic acid levels. We found that ACMSD is suppressed in KD resulting in increased quinolinic acid levels and increased nicotinamide. This is consistent with what is known about PPARα activation by KD consumption [27] as well as the fact that PPARα activation suppresses the enzyme ACMSD, which causes the flux of tryptophan metabolites towards NAD synthesis [28–30].
Although the compensatory responses to low protein content in KD preserved circulating amino acid levels, we nevertheless expected that the amino acid response (AAR) would be activated in the liver. The AAR is a conserved canonical stress-response pathway that is enacted as a cytoprotective response to nutrient limitation [16,17,31,32]. AA restriction activates the serine/threonine protein kinase General control nonrepressed 2 (GCN2) and switches on the AAR initiating a cascade of events including the phosphorylation of Eukaryotic translation initiation factor 2A (eIF2a), a common factor in multiple cellular stress-response pathways that are called the integrated stress response. The phosphorylation of eIF2a increases the translation of a subset of mRNAs including the transcription factor activating transcription factor 4 (ATF4). ATF4 activates target genes including pseudokinase tribbles homolog 3 (TRB3) and ATF3, which respond to AA deficiencies [33–36]. We found expected alterations many genes in this pathway including ATF3, ATF4, ATF5, asparagine synthetase (glutamine-hydrolyzing) (ASNS), and TRB3. Interestingly, ATF4 regulates FGF21 and this may in part explain FGF21’s induction upon ketogenic diet feeding [25,37]. We also found diminished expression of the transcript KLF15, which is associated with reduction of amino acid catabolism pathways as it regulates catabolic processes through genes encoding the transcripts for ALT1, HPD, and Prodh [38,39].
In humans KD has clinical utility as a therapy for refractory epilepsy [4,5] and is also used for weight loss [3]. KD has been modeled in mice in attempts to understand the physiologic states that mediate both seizure suppression and weight loss. Previous feeding studies were limited to 3–4 months. Furthermore there are concerns about long term consumption of KD because of increased fatty liver and inflammation. In this first life-long KD feeding study, we found that the diet was well tolerated, as mice had normal life expectancy and median life span. Mice consuming KD remained lighter and had a higher relative lean body mass. The KD cohorts remained more responsive to insulin and glucose tolerant at advanced ages of 60 weeks. The low carb content of the diet, especially the absence of sugars may also contribute to the improved glucose and decreased insulin. Gene expression and metabolomic analysis revealed sustained changes in critical metabolic regulators including compensatory responses in amino acid metabolism, possibly due to chronic activation of the amino acid starvation response. All of these metabolic adaptations likely contribute to the fact that the diet is well-tolerated long-term. Diet studies in mice, including longevity studies, are held in conditions of standard laboratory housing that typically lack access to exercise and enrichment. Such enhancements may alter the degree of stress in mice and could change morbidity/mortality and should be evaluated in less stressful environments in the future as these might also impart an increase in inflammatory and fibrosis markers.
Understanding the ketotic state is of signiifcance consequence with wide implications for the patients who depend on this diet for various therapies. In addition, a recent report highlighted the important role of ketogenesis as a critical regulator in hepatic metabolism to prevent diet-induced fatty liver disease and hyperglycemia [40]. Further inquiry into the metabolic flux brought on by ketogenesis is important and should reveal valuable contributions to the understanding of metabolism.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbadis.2015.07.009.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by the NIH Grant DK028082 (to E.M.F.), DK083694 (to P.P.) and an Institutional Research Training Grant NRSA 5T32DK007516 (to N.D.). JWL is supported by an NIH pathway to independence award CA168997.
Footnotes
Transparency document
The Transparency document associated with this article can be found, in the online version.
Disclosure summary: The authors have nothing to disclose.
References
- 1.Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292:E1724–E1739. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 2.Paoli A, Grimaldi K, D’Agostino D, Cenci L, Moro T, Bianco A, Palma A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J Int Soc Sports Nutr. 2011;9:34. doi: 10.1186/1550-2783-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volek J, Sharman M, Gómez A, Judelson DA, Rubin M, Watson G, Sokmen B, Silvestre R, French D, Kraemer W. Comparison of energy-restricted very low-carbohydrate and low-fat diets on weight loss and body composition in overweight men and women. Nutr Metab. 2004;1:13. doi: 10.1186/1743-7075-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 5.Keene DL. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediatr Neurol. 2006;35:1–5. doi: 10.1016/j.pediatrneurol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Badman M, Kennedy A, Adams A, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab. 2009;297:204. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okuda T, Morita N. A very low carbohydrate ketogenic diet prevents the progression of hepatic steatosis caused by hyperglycemia in a juvenile obese mouse model. Nutr Diabetes. 2012;2 doi: 10.1038/nutd.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, Mobbs CV. Reversal of diabetic nephropathy by a ketogenic diet. PLoS One. 2010;6 doi: 10.1371/journal.pone.0018604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jornayvaz F, Jurczak M, Lee H-Y, Birkenfeld A, Frederick D, Zhang D, Zhang X-M, Samuel V, Shulman G. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab. 2010;299:15. doi: 10.1152/ajpendo.00361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, Ezenwajiaku N, Cotter DG, Brunt EM, Crawford PA. Hepatic steatosis, inflammation and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011;300:67. doi: 10.1152/ajpgi.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bough K, Eagles D. A ketogenic diet increases the resistance to pentylenetetrazole-induced seizures in the rat. Epilepsia. 1999;40:138–143. doi: 10.1111/j.1528-1157.1999.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 12.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 13.Locasale JW, Melman T, Song S, Yang X, Swanson KD, Cantley LC, Wong ET, Asara JM. Metabolomics of human cerebrospinal fluid identifies signatures of malignant glioma. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.014688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endo-crinology. 2009;150:4931–4940. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilberg MS, Balasubramanian M, Fu L, Shan J. The transcription factor network associated with the amino acid response in mammalian cells. Adv Nutr (Bethesda) 2012;3:295–306. doi: 10.3945/an.112.001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilberg M, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20:436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carraro V, Maurin A-C, Lambert-Langlais S, Averous J, Chaveroux C, Parry L, Jousse C, Ord D, Ord T, Fafournoux P, Bruhat A. Amino acid availability controls TRB3 transcription in liver through the GCN2/eIF2α/ATF4 pathway. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Y-X, Chen H, Thiaville M, Kilberg M. Activation of the ATF3 gene through a coordinated amino acid-sensing response programme that controls transcriptional regulation of responsive genes following amino acid limitation. Biochem J. 2007;401:299–307. doi: 10.1042/BJ20061261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan J, Lopez M-C, Baker H, Kilberg M. Expression profiling after activation of the amino acid deprivation response in HepG2 human hepatoma cells. Physiol Genomics. 2010;41:315–327. doi: 10.1152/physiolgenomics.00217.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pissios P, Hong S, Kennedy AR, Prasad D, Liu F-FF, Maratos-Flier E. Methionine and choline regulate the metabolic phenotype of a ketogenic diet. Mol Metab. 2012;2:306–313. doi: 10.1016/j.molmet.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Larson M, Vasan R, Cheng S, Rhee E, McCabe E, Lewis G, Fox C, Jacques P, Fernandez C, O’Donnell C, Carr S, Mootha V, Florez J, Souza A, Melander O, Clish C, Gerszten R. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newgard C, An J, Bain J, Muehlbauer M, Stevens R, Lien L, Haqq A, Shah S, Arlotto M, Slentz C, Rochon J, Gallup D, Ilkayeva O, Wenner B, Yancy W, Eisenson H, Musante G, Surwit R, Millington D, Butler M, Svetkey L. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huffman K, Shah S, Stevens R, Bain J, Muehlbauer M, Slentz C, Tanner C, Kuchibhatla M, Houmard J, Newgard C, Kraus W. Relationships between circulating metabolic intermediates, insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J, Liu Y, Guo F. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Sousa-Coelho AL, Relat J, Hondares E, Pérez-Martí A, Ribas F, Villarroya F, Marrero PF, Haro D. FGF21 mediates the lipid metabolism response to amino acid starvation. J Lipid Res. 2013;54:1786–1797. doi: 10.1194/jlr.M033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Guo K, LeBlanc R, Loh D, Schwartz G, Yu Y-H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 27.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Delaney J, Hodson MP, Thakkar H, Connor SC, Sweatman BC, Kenny SP, McGill PJ, Holder JC, Hutton KA, Haselden JN, Waterfield CJ. Tryptophan-NAD+ pathway metabolites as putative biomarkers and predictors of peroxisome proliferation. Arch Toxicol. 2005;79:208–223. doi: 10.1007/s00204-004-0625-5. [DOI] [PubMed] [Google Scholar]
- 29.Shin M, Kim I, Inoue Y, Kimura S, Gonzalez FJ. Regulation of mouse hepatic alpha-amino-beta-carboxymuconate-epsilon-semialdehyde decarboxylase, a key enzyme in the tryptophan-nicotinamide adenine dinucleotide pathway, by hepatocyte nuclear factor 4alpha and peroxisome proliferator-activated receptor alpha. Mol Pharmacol. 2006;70:1281–1290. doi: 10.1124/mol.106.026294. [DOI] [PubMed] [Google Scholar]
- 30.Zhen Y, Krausz KW, Chen C, Idle JR, Gonzalez FJ. Metabolomic and genetic analysis of biomarkers for peroxisome proliferator-activated receptor alpha expression and activation. Mol Endocrinol. 2007;21:2136–2151. doi: 10.1210/me.2007-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Guo F. Effects of activating transcription factor 4 deficiency on carbohydrate and lipid metabolism in mammals. IUBMB Life. 2012;64:226–230. doi: 10.1002/iub.605. [DOI] [PubMed] [Google Scholar]
- 32.Guo F, Cavener D. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–114. doi: 10.1016/j.cmet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ord D, Ord T. Characterization of human NIPK (TRB3, SKIP3) gene activation in stressful conditions. Biochem Biophys Res Commun. 2005;330:210–218. doi: 10.1016/j.bbrc.2005.02.149. [DOI] [PubMed] [Google Scholar]
- 35.Jousse C, Deval C, Maurin A-C, Parry L, Chérasse Y, Chaveroux C, Lefloch R, Lenormand P, Bruhat A, Fafournoux P. TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J Biol Chem. 2007;282:15851–15861. doi: 10.1074/jbc.M611723200. [DOI] [PubMed] [Google Scholar]
- 36.Schwarzer R, Dames S, Tondera D, Klippel A, Kaufmann J. TRB3 is a PI 3-kinase dependent indicator for nutrient starvation. Cell Signal. 2006;18:899–909. doi: 10.1016/j.cellsig.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 37.De Sousa-Coelho AL, Marrero PF, Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem J. 2012;443:165–171. doi: 10.1042/BJ20111748. [DOI] [PubMed] [Google Scholar]
- 38.Gray S, Wang B, Orihuela Y, Hong E-GG, Fisch S, Haldar S, Cline GW, Kim JK, Peroni OD, Kahn BB, Jain MK. Regulation of gluconeogenesis by Krüppel-like factor 15. Cell Metab. 2007;5:305–312. doi: 10.1016/j.cmet.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sasse SK, Mailloux CM, Barczak AJ, Wang Q, Altonsy MO, Jain MK, Haldar SM, Gerber AN. The glucocorticoid receptor and KLF15 regulate gene expression dynamics and integrate signals through feed-forward circuitry. Mol Cell Biol. 2013;33:2104–2115. doi: 10.1128/MCB.01474-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cotter DG, Ercal B, Huang X, Leid JM, d’Avignon DA, Graham MJ, Dietzen DJ, Brunt EM, Patti GJ, Crawford PA. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest. 2014;124:5175–5190. doi: 10.1172/JCI76388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.