Abstract
Background & Aims
Fibroblast growth factor 21 (FGF21) is a hepatic protein that plays a critical role in metabolism, stimulating fatty acid oxidation in liver and glucose uptake in fat. Systemic administration to obese rodents and diabetic monkeys leads to improved glucose homeostasis and weight loss. In rodents, FGF21 increases with fasting and consumption of a ketogenic diet (KD). In humans, FGF21 correlates with body mass index, but studies evaluating other parameters show inconsistent results. We examined FGF21 serum levels in lean and obese individuals and in response to dietary manipulation. We also evaluated FGF21 serum levels and liver mRNA expression in nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH).
Methods
Serum FGF21 was measured after an overnight fast in individuals with BMI ranging from normal to obese. Volunteers fasted for 16 or 72 hours and then ate a standard meal. Another group consumed KD for 12 days. Serum FGF21 and hepatic mRNA expression were measured in obese individuals with NAFLD or NASH.
Results
There was a positive correlation between BMI and FGF21. There was no change in FGF21 in response to a short fast or KD. A non-statistically significant fall in FGF21 levels was seen after a 72 hour fast. Hepatic FGF21 mRNA expression was significantly elevated in NAFLD, which correlated with a substantial increase in serum FGF21. In NASH, serum FGF21 but not liver mRNA was increased.
Conclusion
FGF21 correlates with BMI and may be a novel biomarker for NAFLD but is not nutritionally regulated in humans.
Keywords: FGF21, NAFLD, NASH, obesity
Introduction
Fibroblast growth factor 21 (FGF21) is a member of the FGF superfamily that is synthesized predominantly in the liver and released into the circulation. FGF21, together with FGF19 (FGF15 in mice) and FGF23, comprise an atypical subset of fibroblast growth factors that effectively function as hormones, exerting metabolic actions distant from the tissues from which they are secreted. FGF21 is highly homologous between rodents and humans (1). Recent studies indicate that in rodents, FGF21 regulates lipid oxidation in the liver (2, 3). In adipocytes, FGF21 has been shown to increase the expression of the glucose transporter GLUT-1, thereby stimulating glucose uptake in an insulin independent manner (4).
In mice, hepatic expression of FGF21 is induced by fasting and consumption of a ketogenic diet (KD) and is regulated by PPARα (2, 3, 5). Induction of expression is accompanied by an increase in circulating FGF21 levels (2). Mice lacking FGF21 respond inappropriately when fed KD, gaining rather than losing weight (6). Mice overexpressing FGF21 are more prone to ketosis and have increased lipolysis (3). In obese mice, administration of FGF21 reduces body weight and improves glycemia (7, 8).
Little is known about the role and regulation of FGF21 in humans. To date, published data are either cross-sectional or retrospective and report FGF21 levels from studies originally designed to measure other primary outcomes. A consistent finding in nearly all of these studies is that circulating FGF21 levels are increased in obesity, insulin resistance, impaired glucose tolerance, and hypertriglyceridemia (9,10,11) and also in individuals with biochemical evidence of liver injury (12). One group reported lower levels in a small group of women with anorexia nervosa compared to lean controls (13).
The effect of diet on FGF21 levels is also unclear. A few retrospective studies have examined the response of circulating FGF21 to dietary manipulation, including ketogenic diet and fasting, but the data as a whole are inconsistent (14, 15, 16). Galman et al. showed no increase in FGF21 levels among children with refractory epilepsy who consumed a KD for up to 16 months while Christodoulides et al. found that FGF21 levels decreased significantly among a small group of obese individuals with and without diabetes who consumed a KD for 3 months (14, 15). Another group found an increase in serum FGF21 levels in obese individuals who followed a very low calorie diet for 3 months (16).
In order to better understand the potential role of FGF21 in humans, we undertook a comprehensive prospective study and evaluated circulating levels in different nutritional states as well as in obesity, NAFLD, and NASH. We confirmed the positive correlation of FGF21 circulating levels with BMI. We found no significant change in FGF21 levels with a number of dietary interventions including short and long term fasting, refeeding, and 12 day consumption of KD. Although FGF21 levels fell in a small group of individuals fasted for 72 hours, the difference was not statistically significant. We found increased serum FGF21 levels in patients with either NAFLD or NASH and for the first time we report that FGF21 mRNA expression in human liver is significantly increased in NAFLD. As obesity is commonly associated with fatty liver, we propose that the increase seen in serum levels in obese humans reflects the presence of fatty liver and that FGF21 may be a biomarker of hepatic lipid accumulation in obesity.
Materials and Methods
Human subjects
Healthy adults ages 18–60, BMI 19–39 kg/m2 without significant medical illness were recruited by advertisement for participation. Individuals with diabetes, heart disease, liver disease, renal insufficiency, thyroid disease, gout or a history of kidney stones were excluded. Individuals with a history of alcohol abuse or marijuana use were also excluded. Women of childbearing age had a urine pregnancy test and were excluded if the test was positive. Subjects reported stable weight (+/− 3kg) for 6 months prior to participation. All subjects underwent a screening visit that included a medical history, physical exam and baseline laboratory tests. Patients gave written informed consent to all clinical investigations, which were performed in accordance with the principles embodied in the Declaration of Helsinki. The protocols were conducted in accordance with the ethical standards of the Committee on Clinical Investigation and were approved by the Beth Israel Deaconess Medical Center Institutional Review Board as well as the Institutional Review Board of Hospital Clínic de Barcelona. The studies were conducted from June, 2007 to August, 2008 at the Beth Israel Deaconess Medical Center, General Clinical Research Center, Boston, MA and in the Liver Unit at the Hospital Clinic, IDIBAPS, Barcelona, Spain. ClinicalTrials.gov identifier: NCT00476125.
Study Design
FGF21 and BMI
FGF21 levels were obtained after an overnight fast from 10 overweight or obese nondiabetic women (BMI 28–39 kg/m2) and compared to 20 healthy, lean volunteers (BMI 21–28 kg/m2).
Fasting studies
Study A: Following a screening visit, 20 healthy, nonobese subjects maintained their usual diet and exercise routine and kept detailed food records for a 3 day run-in period. Food records were analyzed for baseline carbohydrate intake and total daily caloric intake. Subjects then fasted overnight (16 hours total, typically starting at 4pm) and reported to the General Clinical Research Center (GCRC) the following morning for a 75 gram liquid glucose challenge. Blood samples were obtained through an indwelling intravenous catheter in the fasting state and 1, 2, and 5 hours after the oral glucose load. Glucose, insulin, and FGF21 levels were measured at all time points. Study B: Following a screening visit, 4 healthy, lean subjects reported to the GCRC at 8am for a standard 500 calorie breakfast. One hour after the meal, subjects underwent a subcutaneous fat biopsy began fasting. Subjects were typically fasted for 72 hours, except one individual who terminated the fast after 50 hours due to fatigue. During the fasting period, blood was drawn daily at 8am for measurement of standard chemistries, FGF21, insulin, and ketone levels. Urine ketones were also measured daily. Body weight was measured daily (data not shown). A second subcutaneous fat biopsy was done at the end of the fasting period, before the subject was fed a standard meal.
Ketogenic diet study
Following a screening visit, 11 healthy, nonobese subjects followed their usual diet and exercise routine and maintained detailed food records for a 3 day run-in period. Following the run-in period, subjects reported to the GCRC at 8am after an overnight fast. A blood sample was obtained and urine was tested for ketones. Resting energy expenditure was measured using indirect calorimetry. Subjects then received a standard test breakfast. After this breakfast was consumed, subjects carefully adhered to a ketogenic diet for 12 consecutive days. The diet was individually designed for each subject based on age and BMI and did not exceed 20g of carbohydrate per day. The diet was not designed to be hypocaloric and subjects were encouraged to eat ad libitum from the allowed foods. All meals and snacks were provided, and the food was typically eaten outside the GCRC. Subjects were required to eat only the food provided by investigators and maintain food logs while consuming KD. On days 4, 8, and 12, subjects reported to the GCRC for a blood test to measure FGF21, insulin, and β-hydroxybutyrate levels. After day 12 of the diet, subjects fasted overnight and reported the next morning to the GCRC for a single high carbohydrate refeed (100g carbohydrate breakfast). Resting energy expenditure was measured prior to the meal, and blood was drawn 1, 2, 4, and 6 hours postprandial through an indwelling catheter. Glucose, insulin, β-hydroxybutyrate, and FGF21 levels were measured at all time points.
FGF21 and nonalcoholic fatty liver disease
Serum and liver samples were obtained from patients with morbid obesity and biopsy-proven NAFLD or NASH undergoing laparoscopic bariatric surgery. Liver samples were also obtained from individuals with normal liver function undergoing cholycystectomy for cholelithiasis. A histologic diagnosis of NAFLD in the absence of other causes of liver disease (viral, alcoholic, metabolic) was established by a clinical pathologist. A diagnosis of NASH was established histologically by macrovesicular steatosis and lobular and portal inflammation in the absence of other causes of liver disease. In control subjects, histologic analysis revealed normal liver.
Clinical sample analyses
Serum insulin levels were measured with a human ELISA (Dako, Carpinteria, CA) and serum β-hydroxybutyrate levels were measured using a small-scale colorimetric assay (StanBio, Boerne, TX). FGF21 levels were measured using a commercially available human ELISA (BioVendor USA, Candler, NC). Samples for measurement of FGF-21 were collected in aprotinin pre-treated tubes, allowed to clot, spun for 15 minutes at 15,000g, and immediately frozen at −80° C. Samples were thawed at the time FGF21 levels were measured, within 12 months of sample collection.
Routine screening labs were performed to rule out occult disease. These included a, hematology panel (white blood cell count and differential, hemoglobin, hematocrit, platelet count), chemistry panel (sodium, potassium, chloride, bicarbonate, BUN, creatinine, glucose, calcium, magnesium, phosphate), lipid panel (total cholesterol, HDL, LDL, triglycerides), transaminases (ALT and AST), and uric acid levels were analyzed in the Beth Israel Deaconess Medical Center Clinical Laboratory according to standard procedures. Subjects had urine ketones measured at all study visits using commercially available ketostix.
Measurement of hepatic mRNA expression
RNA samples were obtained from frozen human liver tissue using RNeasy Mini Kit (Qiagen, Chatsworth, CA). 2 micrograms of total RNA was treated with DNase (Invitrogen, Carlsbad, CA) and cDNA was then synthesized with MMLV retrotranscriptase (Promega, Madison, WI) in the presence of random hexamer primers. PCRs were performed using BioRad iCycler Thermalcycler and all reactions were performed in triplicate. Primers used for FGF21 were: Forward AGTGGAGCGATCCATACAGG; Reverse ACTCCAGTCCTCTCCTGCAA Primers used for additional hepatic genes are as follows:
PPAR alpha: Forward ACACTGTGTATGGCTGAGAAGA
Reverse GACGGTCTCCACTGACGTG
CD36: Forward GGCCGATGCTGCTTCTACA
Reverse CCTCTGGATTGGTGACATTGAA
CPT1a: Forward GCCTCGTATGTGAGGCAAAA
Reverse TCATCAAGAAATGTCGCACG
Measurement of white adipose tissue mRNA expression
RNA samples were obtained from human subcutaneous white adipose tissue taken from lean volunteers one hour after a standard breakfast and after a prolonged fast (50–72 hours). RNA samples from subcutaneous and omental white adipose tissue from lean and obese women undergoing surgery were provided by Dr. Susan Fried through the Boston Obesity and Nutrition Research Center Adipocyte Biology Core. PCR was run for 40 cycles using the Applied Biosystems 7900HT Fast Real-Time PCR System, and all reactions were performed in duplicate. Primers used were hFGF21 F5: ACTCCAGTCCTCTCCTGCAA hFGF21 R5: GCACAGGAACCTGGATGTCT. The primers were validated using liver cDNA from mice overexpressing human FGF21.
Statistical analysis
Data are presented as mean ± standard deviation. Statistical analyses using paired t-tests were performed to test for differences in insulin and β-hydroxybutyrate levels. Correlations were calculated to determine the association between baseline carbohydrate intake and FGF21 levels. FGF21 levels before and after intervention were analyzed using a non-parametric Wilcoxon signed rank test. A Spearman’s rank correlation was used to examine changes across variables (eg FGF21 by baseline carbohydrate intake). A p-value of less than 0.05 is considered significant.
Results
FGF21 and BMI
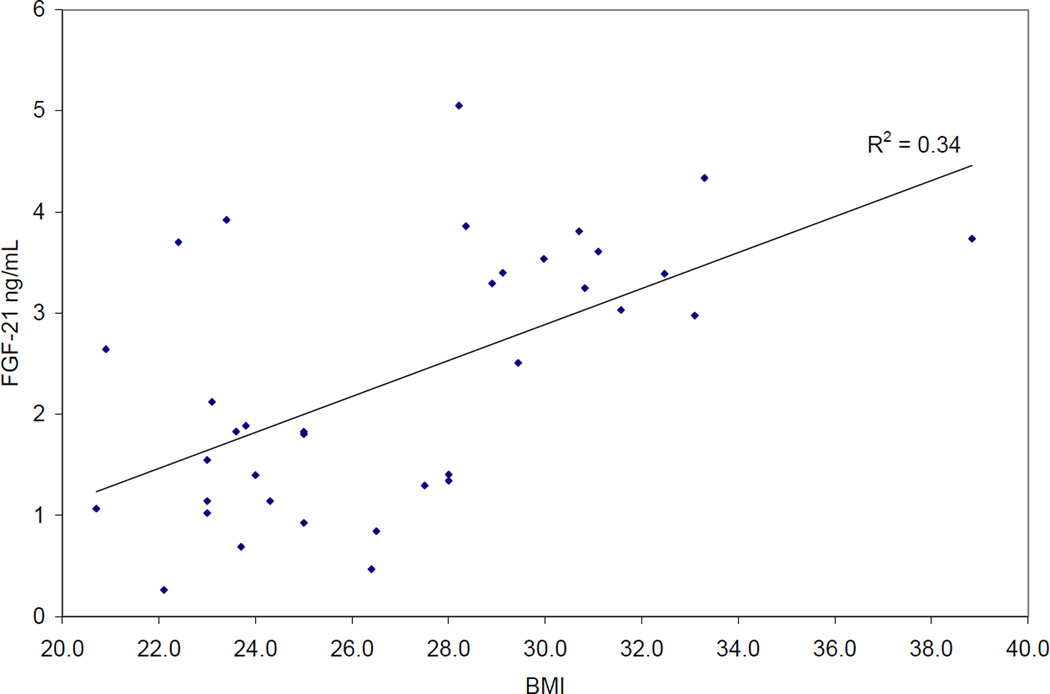
There was a positive correlation between FGF21 levels and BMI (r2 0.33, Figure 1).
Figure 1.
Relationship between BMI and serum FGF21 levels
Fasting studies
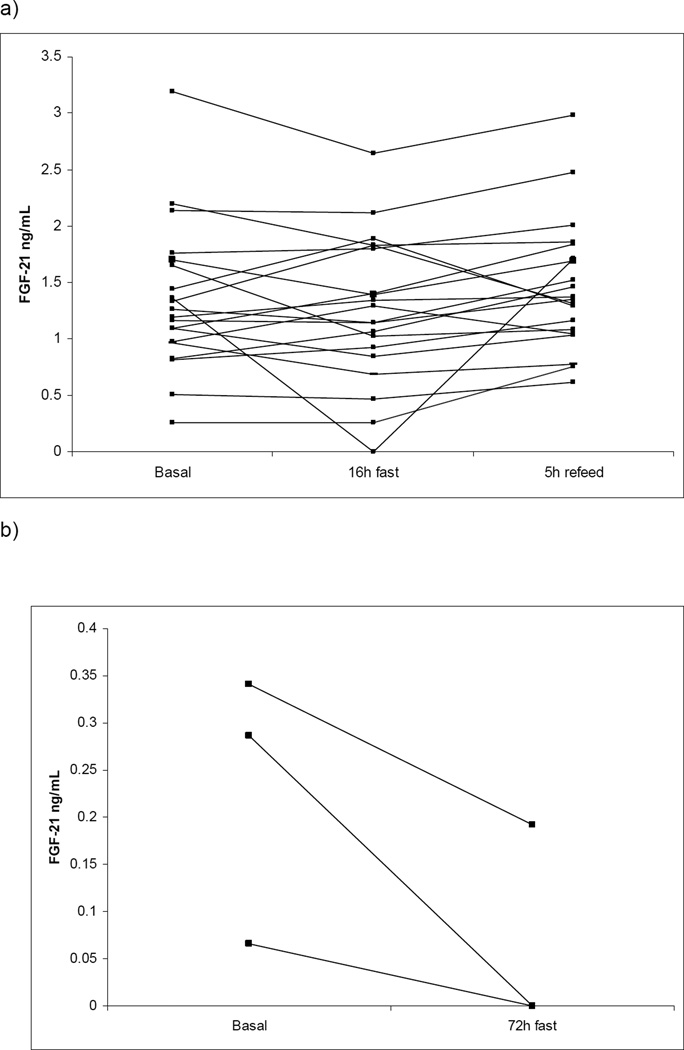
Baseline characteristics of participants in the 16 h fasting study are presented in Table 1. Subjects were 18–60 years of age with BMI ranging from 21–28 kg/m2. There was no correlation between carbohydrate intake and FGF21 levels in the ad lib fed state (r2 0.01). FGF21 levels did not increase significantly above baseline after a 16 hour fast or decrease following a 75 gram oral glucose challenge (Figure 2a). FGF21 levels decreased in all individuals after a 72 hour fast, but the difference did not reach statistical significance in this small cohort (Figure 2b).
Table 1.
Baseline characteristics of participants in fasting study (n = 20)
| Age (years) | 31 +/− 10 |
| Female | 13 |
| Weight (kg) | 67.8 +/− 8.6 |
| BMI (kg/m2) | 24.4 +/− 2.1 |
| Fasting glucose (mg/dL) | 78.4 +/− 5.9 |
| FGF21 (ng/mL) Range |
1.35 +/− 0.67 0.26–3.19 |
| Daily carbohydrate intake (g) | 247.2 +/− 79 |
Data are mean +/− s.d. FGF21 levels are in the fed state.
Figure 2.
Individual changes in serum FGF21 levels after a 16 hour overnight fast followed by a 75g oral glucose challenge (a) and in response to 72 hours of fasting (b). p=NS for all time points
Ketogenic diet study
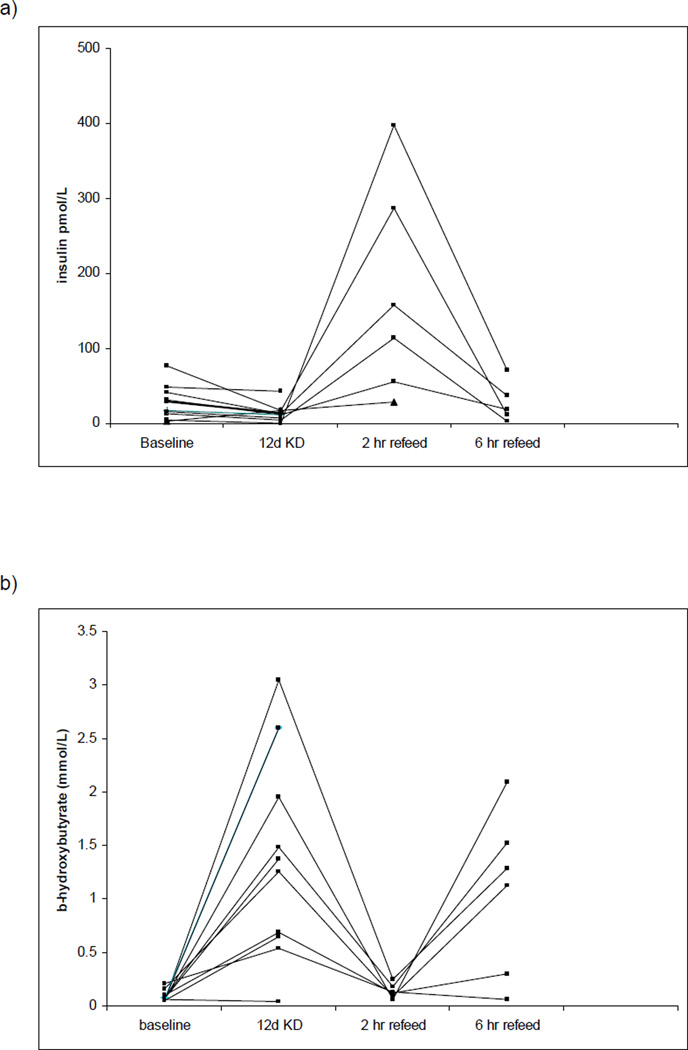
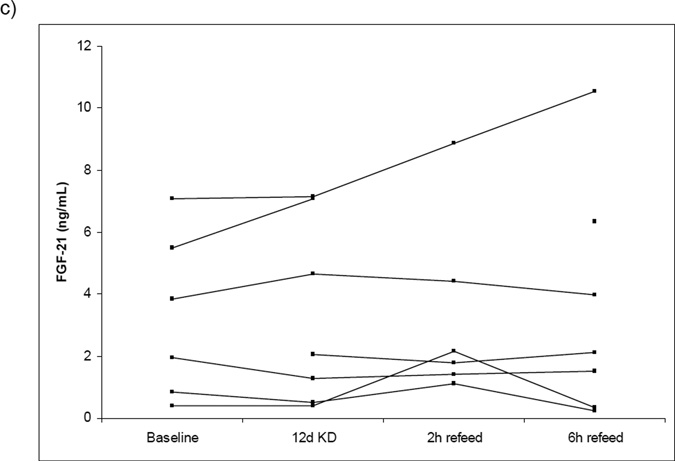
Baseline characteristics of participants in the KD study are presented in Table 2. Subjects were 21–40 years of age with BMI ranging from 19–27 kg/m2. In all subjects, consumption of KD for 12 days was associated with a decrease body weight and BMI (mean −2.3 kg). As expected, insulin levels decreased significantly (26.1 +/− 21.9 pmol/L to 10.9 +/− 5.4 pmol/L) and serum β - hydroxybutyrate levels increased significantly (0.09 +/− 0.05 mmol/L to 1.5 +/− 0.98 pmol/L). Insulin levels increased ~10-fold one hour following a breakfast containing 100g carbohydrate and decreased over the subsequent 6 hours (Figure 3a). Serum β-hydroxybutyrate butyrate levels decreased initially following the high carbohydrate refeed but after 4 hours returned to a level similar to the KD state (Figure 3b). FGF21 levels did not change significantly from baseline during KD feeding or following the high carbohydrate refeed (Figure 3c). Fasting glucose and HDL levels did not change during the ketogenic diet, but triglyceride levels decreased and LDL levels increased significantly. There was a reduction in the respiratory quotient but no change in resting energy expenditure during the ketogenic period.
Table 2.
Metabolic parameters of participants in ketogenic diet study
| Baseline | 12 days KD | p | |
|---|---|---|---|
| Age (years) | 24.9 +/− 5.4 | ||
| Male/Female | 1/10 | ||
| Weight (kg) | 64.3 +/− 10.5 | 62.0 +/− 10.2 | NS |
| BMI (kg/m2) | 23.2 +/− 2.9 | 22.3 +/− 2.6 | NS |
| FGF21 (ng/mL) Range |
3.3 +/− 1.1 0.4–7.1 |
3.3 +/− 1.2 0.4–7.1 |
NS |
| Insulin (pmol/L) | 26.1 +/− 21.9 | 10.9 +/− 5.4 | < 0.05 |
| βOH butyrate (mmol/L) | 0.09 +/− 0.05 | 1.5 +/− 0.98 | < 0.0001 |
| Fasting glucose (mg/dL) | 75.6 +/− 6.3 | 72 +/− 7.8 | NS |
| Total cholesterol (mg/dL) | 166 +/− 33.5 | 191.3 +/− 42.3 | NS |
| HDL (mg/dL) | 62.9 +/− 14.2 | 62.5 +/− 14.5 | NS |
| LDL (mg/dL) | 87.8 +/− 29 | 117 +/− 37.8 | < 0.02 |
| Triglycerides | 77.4 +/− 37.8 | 58.4 +/− 12.6 | NS |
| REE (kcal/kg) | 20.2 +/− 2.1 | 19.1 +/− 2.9 | NS |
| RQ | 0.87 +/− 0.04 | 0.78 +/− 0.04 | < 0.001 |
Data are mean +/− s.d. Baseline FGF21 levels are after an 8 hour overnight fast.
Figure 3.
Individual changes in serum insulin (a), ketones (b), and FGF21 levels (c) in response to 12 days of ketogenic diet (KD) followed by a single high carbohydrate reefed. p=NS baseline insulin to 12d KD, < 0.002 12d KD to 2h reefed. p < 0.001 baseline b-hydroxybutyrate to 12d KD
FGF21 and nonalcoholic fatty liver disease
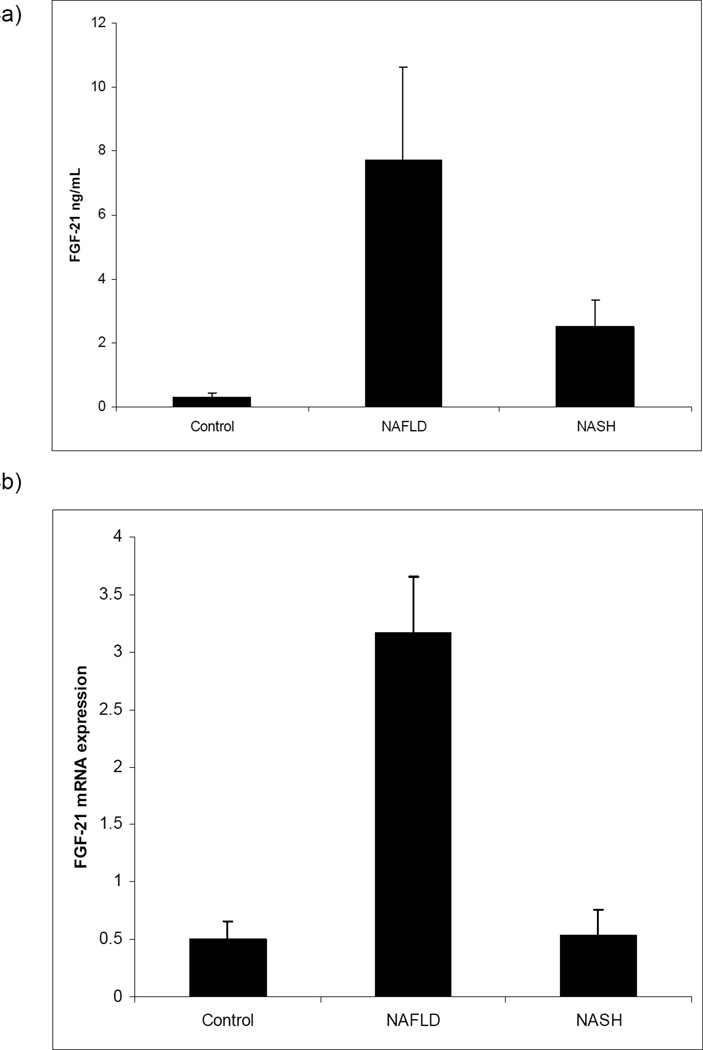
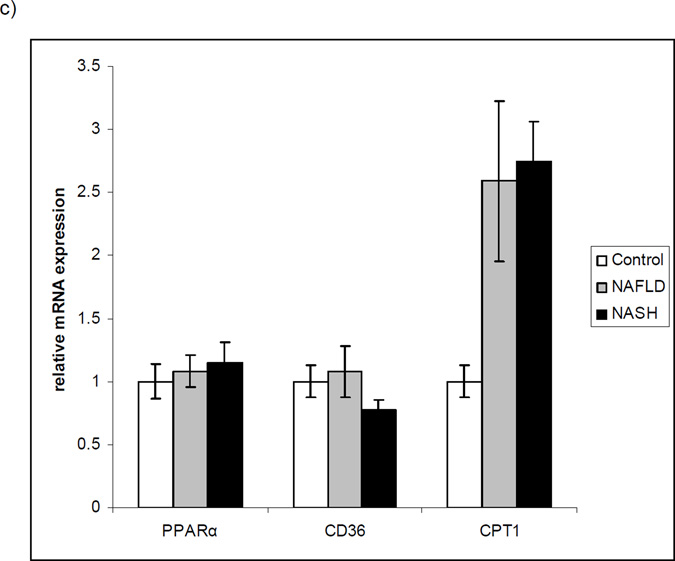
Baseline characteristics of the subjects with NAFLD and NASH are presented in Table 3. Serum FGF21 levels were significantly elevated in patients with NAFLD or NASH compared to controls (Figure 4a). Hepatic FGF21 expression was increased 6-fold among individuals with NAFLD but did not differ between controls and individuals with NASH (Figure 4b). To rule out a general effect of NAFLD on PPARα and its downstream targets, we examined expression of PPARα itself as well as 2 targets, CPT1 and CD36. Hepatic PPARα and CD36 expression did not change significantly in NAFLD, while CPT1 expression was increased (Figure 4c).
Table 3.
Metabolic parameters of patients with Steatosis, NASH, and controls
| Controls | Steatosis | NASH | p | |
|---|---|---|---|---|
| n | 6 | 6 | 9 | |
| M/F | 3/3 | 2/4 | 2/7 | |
| BMI (kg/m2) | 24.6 | 48.2 | 44.4 | |
| ALT/AST | 0.98 | 0.71 | 0.62 | |
| FGF21 (ng/mL) | 0.3 +/− 0.1 | 7.7 +/− 2.9 | 2.5 +/− 0.8 | < 0.02 steatosis vs control; < 0.03 NASH vs control |
| Hepatic FGF21 Relative expression | 0.5 +/− 0.2 | 3.2 +/− 0.5 | 0.5 +/− 0.2 | < 0.002 steatosis vs control |
Steatosis defined as histologic hepatic steatosis in the absence of other causes of liver disease. Controls had normal hepatic histology and liver function. NASH defined as steatohepatitis grade 1 based on histologic analysis revealing macrovesicular steatosis, lobular and portal inflammation in the absence of other causes of steatohepatitis.
Figure 4.
Serum (a) and hepatic (b) levels of FGF21 in controls and individuals with steatosis or NASH, and relative mRNA expression of PPARα, CPT1 and CD36 in controls and individuals with NAFLD or NASH (c). p < 0.02 NAFLD vs controls and < 0.03 NASH vs controls in serum. p < 0.002 NAFLD vs controls in hepatic expression. For CPT1, p < 0.03 NAFLD vs controls and 0.0003 NASH vs controls. p = NS for PPARα and CD36 across all groups
FGF21 expression in human fat
As mice are known to express FGF21 in fat and two groups have reported expression in human fat (10, 16), we also evaluated expression in this tissue. No amplification of FGF21 was seen in subcutaneous or visceral white adipose tissue after 30 amplification cycles, and only very weak amplification was seen in some samples at cycle 33 (data not shown). Robust expression of FGF21 was seen in the positive control samples, with amplification at cycle 17.
Discussion
Although a metabolic role for FGF21 in rodents is supported by a number of findings, the role of FGF21 in human physiology is unclear. In mice, circulating and hepatic FGF21 levels increase significantly with fasting and ingestion of KD and decrease following refeeding (2); in rats, hepatic FGF21 expression increases with fasting (17). Mice placed on a KD with subsequent adenovirus-mediated knockdown of FGF21 expression develop decreased ketosis, massive hepatosteatosis, and hyperlipidemia (2). FGF21 deficient mice (FGF21KO) placed on a KD also show an atypical response with weight gain, attenuated ketosis, and fail to show the expected increase in insulin sensitivity (6). As a pharmacologic agent, administration of FGF21 to obese mice and diabetic monkeys leads to weight loss and marked improvement in glucose and lipid parameters (7, 18, 19).
While it is known that FGF21 circulates in humans, its function and regulation are unclear. One consistent finding in human studies is a positive correlation between circulating FGF21 levels and obesity, type 2 diabetes, and features of the metabolic syndrome including insulin resistance, impaired glucose tolerance, and dyslipidemia (9, 10, 11, 12, 14, 20). This finding is in agreement with the increase in serum and liver expression of FGF21 seen in genetically obese mice (21, 22). However, some human studies report as much as 250-fold variability in serum FGF21 levels, which is unusual for a metabolic regulator (14, 15).
With respect to nutritional regulation, small retrospective studies in humans report inconsistent results. In order to better understand the physiology of FGF21 in humans, we prospectively examined serum levels in normal and overweight subjects in different nutritional states. Because fatty liver disease is often associated with obesity (23, 24, 25, 26), we hypothesized that the increased levels seen with rising BMI might reflect lipid accumulation in the liver. We therefore also evaluated serum FGF21 levels and hepatic mRNA expression in individuals with NAFLD or NASH. We found that FGF21 levels fell within a fairly narrow range in lean, healthy adults, which is consistent with FGF21 functioning as a metabolic regulator in humans. In agreement with several published studies, we found that FGF21 levels were positively correlated with BMI. With respect to nutritional regulation, we saw no change in circulating FGF21 levels when subjects were fasted for 16 hours and then given a 75 gram oral glucose challenge or fed a KD for 12 days followed by a single high carbohydrate meal. In contrast to mice, in which FGF21 increases significantly with prolonged fasting, we observed a consistent but not statistically significant fall in FGF21 levels after 72 hours of fasting in a small cohort of lean volunteers.
Interestingly, we found that FGF21 mRNA expression is regulated in human liver and is increased 6-fold in NAFLD. Similarly, serum FGF21 levels are also increased significantly in NAFLD. Among individuals with NASH, which represents a progression from steatosis to fibrosis, serum FGF21 levels were increased but not as elevated as in NAFLD, and hepatic mRNA expression was not increased above control samples. Lower hepatic FGF21 expression in NASH compared to NAFLD may reflect more advanced hepatic injury. It is unclear why serum levels remain elevated in NASH, but it may reflect differential clearance or a change in binding proteins. Our data on mRNA expression of CPT1 and CD36 revealed increased expression of only CPT1, confirming that there is not broad upregulation of PPARα targets in NAFLD or NASH.
To explore the potential contribution of fat-derived FGF21 in human serum, we looked at expression in subcutaneous and omental adipose tissue from men and women with BMI 21–55 kg/m2. We found no expression after 30 cycles of amplification and only a very weak signal at > 33 cycles in fewer than half of the samples. Similarly, we did not see any change in fat mRNA expression in subjects fasted for 72 hours. Although two groups have reported expression of FGF21 in human subcutaneous and visceral adipose tissue (10, 16), in neither case was absolute quantification provided. Our data suggest that if FGF21 is expressed in human adipose tissue, it is at very low levels and therefore is unlikely to contribute to the increased serum levels seen in obesity.
Our studies show that serum FGF21 levels correlate with BMI and that nutritional regulation of FGF21 is different in rodents and humans. We report for the first time that hepatic FGF21 expression is increased in NAFLD and appears to correlate with liver pathology. In humans, the major source of circulating FGF21 appears to be the liver, as we did not find FGF21 expression in either subcutaneous or omental human adipose tissue. The consistent increase in FGF21 seen in human obesity may reflect the presence of fatty liver disease, which raises the intriguing possibility that FGF21 could be a biomarker for NAFLD.
Acknowledgements
We acknowledge the assistance of Anna Moriarty and Marie LeClair. We thank Dr. Susan Fried for providing us with subcutaneous and visceral human fat samples. This study was supported in part by NIH Grant M01-RR01032, Beth Israel Deaconess Medical Center General Clinical Research Center, NIH grant AT-1576, SAF2005-00855, and HEPADIP-EULSHM-CT-205 (to M.L.M.-C), NIH grants 5R01DK069983-04 and NIH/5P01DK056106-10 (E.M.F.) and the Boston Obesity and Nutrition Research Center DK46200.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosures: There are no conflicts of interest to disclose for all authors.
Author contributions:
Dushay, study concept and design; data acquisition, analysis, and interpretation; drafting and critical revision of manuscript; statistical analysis; study supervision; Chui, data acquisition, analysis and interpretation, technical support, critical revision of manuscript; Gopalakrishnan: data acquisition and analysis, critical revision of manuscript, Varela-Rey: technical support, critical revision of manuscript; Crawley data acquisition and analysis, Fisher critical revision of manuscript, data analysis, Badman, data analysis and interpretation, critical revision of manuscript, technical support; Martinez-Chantar critical revision of manuscript, material support, obtained funding; Maratos-Flier study concept and design, data analysis and interpretation, drafting and critical revision of manuscript, obtained funding, study supervision.
REFERENCES
- 1.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochem Biophys Acta. 2000;1492(1):203–206. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 2.Badman M, Pissios P, Kennedy A, et al. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Kharitonenkov A, Shiyanova T, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy A, Pissios P, Otu H, et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292(6):E1724–E1739. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 6.Badman M, Koester A, Flier J, et al. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinol. 2009;150(11):1–10. doi: 10.1210/en.2009-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coskun T, Bina H, Schneider M, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrine. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 8.Berglund E, Candice Y, Bina H, et al. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinol. 2009;150(9):4084–4093. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Li L, Yang G, et al. Circulating FGF-21 levels in normal subjects and in newly diagnosed patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116(1):65–68. doi: 10.1055/s-2007-985148. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Yeung D, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 11.Chavez A, Molina-Carrion M, Abdul-Ghani M, et al. Circulating fibroblast growth factor 21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diab Care. 2009;32(8):1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Bao Y, Xu A, et al. Serum fibroblast growth factor 21 is associated with adverse lipid profiles and gamma-glutamyltransferase but not insulin sensitivity in Chinese subjects. J Clin Endocrin Metab. 2009;94:2151–2156. doi: 10.1210/jc.2008-2331. [DOI] [PubMed] [Google Scholar]
- 13.Dostalova I, Kavalkova P, Haluzikova D, et al. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab. 2008;93:3627–3632. doi: 10.1210/jc.2008-0746. [DOI] [PubMed] [Google Scholar]
- 14.Galman C, Lundasen T, Kharitonenkov A, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab. 2009;8(2):169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Christodoulides C, Dyson P, Sprecher D, et al. Circulating FGF21 is induced by PPAR agonists but not ketosis in man. J Clin Endocrin Metab. 2009;94:3594–3601. doi: 10.1210/jc.2009-0111. [DOI] [PubMed] [Google Scholar]
- 16.Mraz M, Bartlova M, Lacinova Z, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol. 2009;71(3):369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez J, Palou A, Pico C. Response to carbohydrate and fat refeeding in the expression of genes involved in nutrient partitioning and metabolism: striking effects on fibroblast growth factor-21 induction. Endocrinol. 2009;150:1–10. doi: 10.1210/en.2009-0466. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Stanislaus S, Chinookoswong N, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin resistant mouse models----Association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. 2009 Aug 25; doi: 10.1152/ajpendo.00348.2009. epub. [DOI] [PubMed] [Google Scholar]
- 19.Kharitonenkov A, Wroblewski VJ, Koester A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007 Feb;148(2):774–781. doi: 10.1210/en.2006-1168. Epub 2006 Oct 26. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Yang G, Ning H, et al. Plasma FGF-21 levels in type 2 diabetic patients with ketosis. Diabetes Res Clin Pract. 2008;82(2):209–213. doi: 10.1016/j.diabres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Badman M, Kennedy A, Adams A, et al. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independent of weight loss. Am J Physiol Endocrinol Metab. 2009;297:E1197–E1204. doi: 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundasen T, Hunt MC, Nilsson LM, et al. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun. 2007;360:437–440. doi: 10.1016/j.bbrc.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 23.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 24.Tran TT, Changsri C, Shackelton CR, et al. Living donor liver transplantation: histologic abnormalities found on liver biopsies of apparently healthy potential donors. J Gastroenterol Hepatol. 2006;21:381–383. doi: 10.1111/j.1440-1746.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- 25.Lazo M, Clark J. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liv Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 26.Neuschwander-Teri B, Caldwell S. Nonalcoholic steatohepatitis: summary of AASLD single topic conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]