Abstract
Background
Weight loss following gastrectomy for patients with gastric cancer has not been well characterized. We assessed the impact of patient and procedure-specific variables on postoperative weight loss following gastrectomy for cancer.
Methods
A prospectively maintained gastric cancer database identified patients undergoing gastrectomy for cancer. Clinical and pathologic characteristics, baseline body mass index (BMI), and postoperative weights were extracted. Change in weight was analyzed by percent change in weight and absolute change in BMI. Random coefficients models were used to test whether the rate of change in weight over time differed by factors of interest.
Results
Of 376 consecutive patients who underwent resection for gastric adenocarcinoma, 55 % were male, median age 66 years, and mean preoperative BMI 27.1 (range 16.2–45.6). Total gastrectomy was associated with more weight loss than subtotal gastrectomy at 1 year (15 vs. 6 %, early stage; 17 vs. 7 %, late stage). Maximum weight change was observed at 6–12 months after operation and remained stable or improved at 2 years. For early- and late-stage patients, median percent weight loss at 1 year was greater for BMI ≥ 30 versus BMI < 30 (14 vs. 8 %, early stage; 15 vs. 9 %, late stage).
Conclusions
The extent of weight loss after gastrectomy for gastric cancer is dependent on preoperative BMI and extent of gastric resection. Maximum weight change is expected by 12 months after operation and will stabilize or improve over time.
Gastric adenocarcinoma has a worldwide incidence of approximately 952,000 cases, approximately 22,220 of which occur in the United States, where malignancy is the most common indication for gastrectomy.1,2 Following upper gastrointestinal track surgery, the benefits of early oral feeding are well established, whereas supplemental nutrition often is provided to assuage concerns about weight loss, malnutrition, and poor quality of life.3–5
Our current understanding of post-gastrectomy weight loss is predominantly informed by resection for peptic ulcer disease. In Johnson’s 1958 cohort, for example, fewer than 10 % of patients had a resection for benign or malignant tumors of the stomach or esophagus.6 Contemporary studies of Asian gastric cancer patients have focused on the impact of postoperative weight loss related to the delivery of adjuvant therapy, and disease recurrence and survival.7,8 Despite these reports, our ability to identify patients at risk of excess postoperative weight loss and to predict weight loss for individual patients following gastrectomy is limited.
The purpose of this study was to describe patterns of weight loss following gastrectomy for gastric cancer and identify patient and treatment-specific factors associated with the duration or degree of weight loss. We examined patients with early-stage gastric cancer treated with gastrectomy as monotherapy to assess the impact of operation on weight loss. Because preoperative body mass index (BMI) has previously been correlated with postoperative complications, we also investigate the influence of BMI on postoperative weight loss.9 Additionally, we reviewed late-stage patients who underwent multimodality therapy to examine the impact of treatment related factors on postoperative weight loss.
METHODS
Following institutional review board approval, all patients undergoing curative-intent gastrectomy for gastric adenocarcinoma between July 2007 and July 2013 were identified from a prospectively maintained, institutional gastric cancer database. Patients were excluded if they did not have postoperative weight recorded, for palliative or R2 resections, proximal gastrectomy and esophagogastrectomy, death within 90 days or disease recurrence within 12 months of operation, and concurrent cancer. Preoperative height and weight, recorded within 10 days of operation, and postoperative weight were extracted from the electronic medical record. Change in weight was analyzed by percent change in weight (kilograms) and absolute change in BMI.
All patients underwent gastrectomy at a single institution and received standardized postoperative care. All patients received either a D1 or D2 lymphadenectomy. Extent of gastrectomy was based on tumor location and reconstruction according to the amount of residual stomach. Roux-en-Y reconstruction (RY) was utilized for total gastrectomy and subtotal gastrectomies where more than 50 % of the stomach was removed; Billroth II reconstruction (BII) when less than 50 % of the stomach was removed. Patients were classified as having early-stage (postoperative T1/T2, N0, M0) or late-stage (postoperative T3/T4, N0, M0 or any T, N1–3, M0–1) tumors based on final pathology according to the 7th edition American Joint Committee on Cancer (AJCC) staging system.10 All patients receive postoperative dietary counseling; dietary supplementation is not provided as part of routine clinical care.
Statistical Analysis
All analyses were performed separately for early- and late-stage patients. In the early-stage group, multivariate random coefficient models with robust standard errors and unstructured covariance matrix were used to predict average percent weight loss from surgery, and least square means were used to predict percent weight loss for the covariates of interest (age, sex, preoperative BMI, type of operation, major postoperative complication, neoadjuvant chemotherapy).11 Visual examination of plots of the data indicated a quadratic relationship with time, thus the multivariate models included a quadratic term between time and covariates of interest, which is supported by smaller Akaike information criterion for the quadratic random coefficient models compared with the linear models. All models were adjusted for age, sex, and BMI. In addition to multivariate regression methods, summary statistics were used to describe the change in weight from baseline to 1 month (3–6 weeks), 3 months (10–14 weeks), 6 months (5–7 months), 12 months (10–14), and 24 months (22–26) after operation.
For late-stage patients, multimodality therapy is frequently employed and is subject to variability in time to initiation of postoperative treatment, dose modification, and duration of therapy. Due to these sources of variation, we did not employ modeling and instead present the observed postoperative weights for all late-stage patients. All analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC). P values were based on two-tailed statistical analysis, and p < 0.05 indicated statistical significance.
RESULTS
Of the 376 patients included in the analysis, 55 % (n = 207) were male, the median age was 65.7 years (Table 1), and median follow-up was 25.8 months. Mean preoperative weight was 74.3 kg (range 42.3–163), and mean preoperative BMI was 27.1 (16.2–45.6). The majority of patients (n = 273, 73 %) were normal or overweight (BMI 18.5–30). Six patients (2 %) had BMI < 18.5, and 97 patients (26 %) were obese (BMI ≥ 30).
TABLE 1.
Clinical and pathologic characteristics of patients with gastric cancer
| Characteristics | Total (n = 376) | Early stage* (n = 155) | Late stage** (n = 221) |
|---|---|---|---|
| Age at operation, median (IQR) | 65.7 (54.7–74.2) | 67.5 (54.4–75.3) | 65.3 (54.9–73.6) |
| Sex, number (%) | |||
| Female | 169 (45) | 66 (43) | 103 (47) |
| Male | 207 (55) | 89 (57) | 118 (53) |
| Preoperative weight (kg), mean | 74.3 | 76.6 | 72.7 |
| Preoperative BMI, mean | 27.1 | 27.4 | 26.9 |
| BMI < 30 kg/m2, number (%) | 279 (74) | 111 (72) | 168 (76) |
| BMI ≥ 30 kg/m2, number (%) | 97 (26) | 44 (28) | 53 (24) |
| Race/ethnicity, number (%) | |||
| White, non-Hispanic | 243 (65) | 106 (68) | 137 (62) |
| Black, non-Hispanic | 30 (8) | 12 (8) | 18 (8) |
| Hispanic | 32 (9) | 11 (7) | 21 (10) |
| Asian | 60 (16) | 22 (14) | 38 (17) |
| Other/unknown | 11 (3) | 4 (3) | 7 (3) |
| Postoperative pathologic stage, number (%) | |||
| 0 | 16 (4) | 16 (10) | 0 (0) |
| I | 153 (41) | 139 (90) | 14 (6) |
| II | 121 (32) | 0 (0) | 121 (55) |
| III | 82 (22) | 0 (0) | 82 (37) |
| IV | 4 (1) | 0 (0) | 4 (2) |
| Type of operation, number (%) | |||
| Subtotal/BII | 142 (38) | 60 (39) | 82 (37) |
| Subtotal/RY | 92 (24) | 40 (26) | 52 (24) |
| Total/RY | 142 (38) | 55 (35) | 87 (39) |
| Major complication (≥Grade 3), number (%) | 54 (14) | 20 (13) | 34 (15) |
| Neoadjuvant chemotherapy, number (%) | 150 (40) | 42 (27) | 108 (49) |
| Adjuvant chemotherapy, number (%) | 132 (35) | 23 (15) | 109 (49) |
| Postoperative feeding tube, number (%) | 20 (5) | 6 (4) | 14 (6) |
| Median follow-up, months (IQR) | 25.8 (13.4–42.1) | 26.4 (13–45.1) | 25 (13.9–40.1) |
Early stage = T1/T2, N0
Late stage = T3/T4, N0 and Any T, N1–3;
BMI body mass index, BII Billroth-II, RY Roux-en-Y
Tumors were most frequently located in the distal stomach (n = 187, 50 %) or body (n = 106, 28 %). Seven patients (2 %) had Siewert III gastroesophageal junction adenocarcinoma, eight patients (2 %) had diffusely infiltrating tumors, and four patients (1 %) had tumors in the remnant stomach after previous partial gastrectomy. Lauren’s type was recorded as diffuse (n = 122, 32 %), intestinal (n = 168, 45 %), mixed (n = 79, 21 %) or unknown (n = 7, 2 %). Neoadjuvant chemotherapy was administered to 40 % of our cohort (n = 150), whereas neoadjuvant radiation therapy was rare (n = 2, 0.5 %). Patients underwent either subtotal gastrectomy with BII gastrojejunostomy (subtotal/BII, n = 142), subtotal gastrectomy with RY gastrojejunostomy (subtotal/RY, n = 92), or total gastrectomy with RY esophagojejunostomy (total/RY, n = 142). Overall, 23 % (85/376) of all patients received a minimally invasive gastrectomy, and there was no difference in weight loss between patients who underwent an open gastrectomy and those who underwent a minimally invasive gastrectomy.
The distribution of postoperative pathologic stage was: stage 0 (n = 16, 4 %, all of whom received neoadjuvant chemotherapy with complete pathological response), stage I (n = 153, 41 %), stage II (n = 121, 32 %), stage III (n = 82, 22 %), and stage IV (n = 4, 1 %). Adjuvant chemotherapy was administered to 35 % (n = 132) of patients; 7 % (n = 25) received adjuvant radiation therapy. Major complications (grade 3 and 4) were recorded in 54 patients (14 %). A minority of patients had enteral feeding tubes placed (n = 20, 5 %), always in the context of postoperative complications.12
Early-Stage Disease
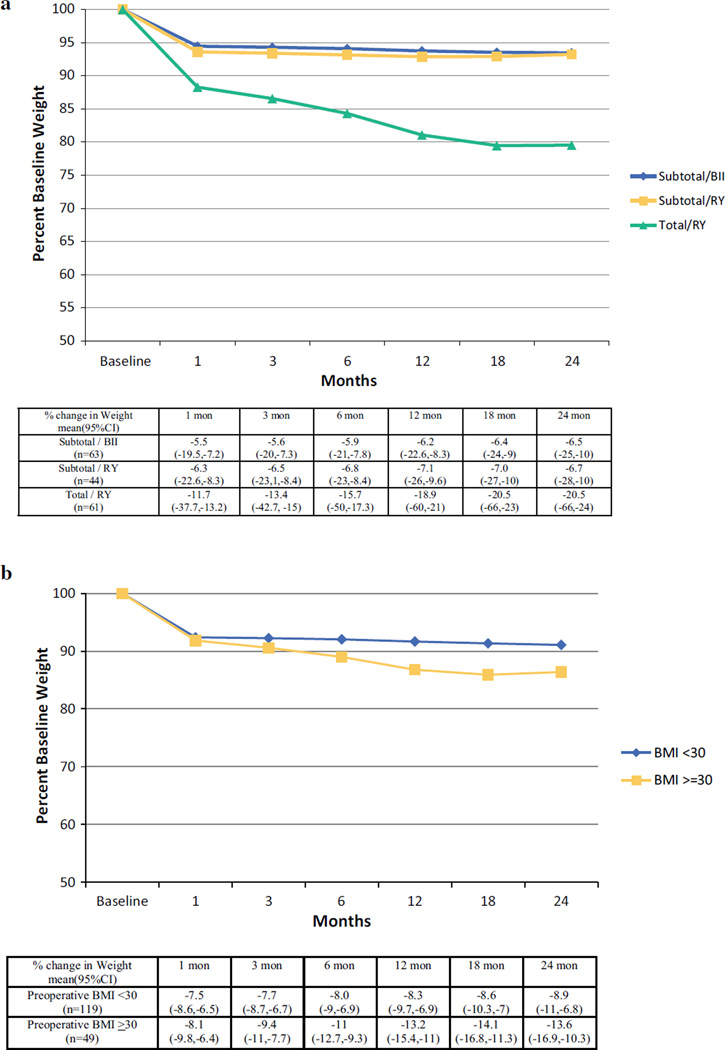
Early-stage patients (n = 155) had a median age of 67.5 years with a postoperative pathologic stage of 0 (n = 16, 10 %) or I (n = 139, 90 %). Fifty-five patients (35 %) underwent a total gastrectomy, and the remainder underwent a subtotal gastrectomy, either with Billroth-II (n = 60, 39 % of all reconstructions) or Roux-en-Y reconstruction (n = 40, 26 % of all reconstructions). Preoperative BMI (p < 0.01) and procedure type (p = 0.01) were independently associated with postoperative weight loss, whereas neither major complications nor neoadjuvant chemotherapy were (Table 2). After adjusting for age and gender, extent of operation (total vs. subtotal gastrectomy, p < 0.01) and preoperative BMI (< 30 vs. ≥30, p < 0.01) were independently associated with the extent of postoperative weight loss. Patients who underwent total gastrectomy lost significantly more weight across all time points than patients who underwent subtotal gastrectomy regardless of reconstruction (p < 0.01) and appear to reach their nadir later compared with patients undergoing subtotal gastrectomy (Fig. 1a). All patients, regardless of baseline BMI or procedure type, lost substantial weight in the first postoperative month. Because our patient population is overweight at baseline (mean BMI: 27.1), we evaluated the effect of BMI using a cutoff of 30. Patients with a starting BMI < 30 lost significantly less weight beyond three months than patients with BMI ≥ 30 (Fig. 1b). At 12 months, the predicted average percent weight change for patients with BMI < 30 and for those with BMI ≥ 30 was −8.3 % (SE: 0.71) and −13.2 % (SE: 1.14), respectively (p = 0.003).
TABLE 2.
Factors associated with change in weight loss over time among early-stage gastric cancer patients
| Factor | Comparison of factors* |
|---|---|
| Age, <65 vs. ≥65 year | p = 0.83 |
| Sex, M vs. F | p = 0.65 |
| Preoperative BMI, <30 vs. ≥30 | p < 0.01 |
| Procedure type, subtotal/BII vs. subtotal/RY vs. total/RY | p = 0.01 |
| Major complication, yes vs. no | p = 0.59 |
| Neoadjuvant chemotherapy, yes vs. no | p = 0.18 |
Models were adjusted for age, sex, and baseline BMI; the model for age was adjusted for sex and baseline BMI; the model for sex was adjusted for age and baseline BMI; the model for BMI was adjusted for age and sex
Comparisons based on average change in percent weight loss over time
FIG. 1.
Estimated average change in weight over time for patients with early-stage gastric cancer by procedure type (a) and BMI (b). Estimates and ranges at specific time points are shown below the figure
Late-Stage Disease
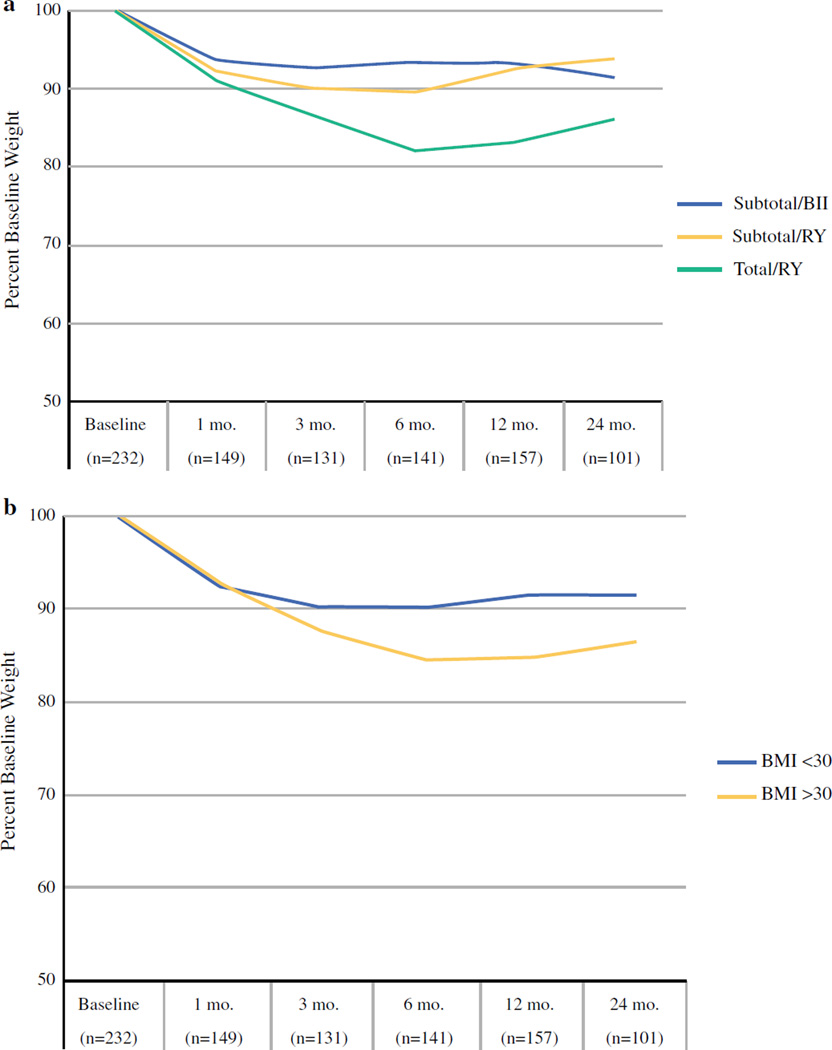
The patterns of weight loss for 221 late-stage patients were similar to those seen in early-stage patients. The nadir in postoperative weight occurred at 6 months and weight appeared to plateau or increase afterward. As expected, late-stage patients had higher rates of neoadjuvant (49 vs. 27 %) and adjuvant chemotherapy (49 vs. 15 %) than early-stage patients. The administration of neoadjuvant chemotherapy was associated with an average percent change in weight from initial evaluation to time of operation of −1.9 % (SD ± 6.4), and adjuvant therapy did not have a statistically significant effect on weight loss. As with early-stage patients, late-stage patients who underwent total gastrectomy reached a nadir in postoperative weight at approximately 6 months (Fig. 2b).
FIG. 2.
Change in weight over time for patients with late-stage gastric cancer by procedure type (a), and baseline BMI (b). The number of patients contributing to calculation of median weight loss value at each time point is shown in parenthesis
The placement of percutaneous endoscopic jejunostomy tubes in the postoperative period was an infrequent occurrence (n = 20, 5 %), predominantly in late-stage patients (n = 14; 70 % of all placements), and always as a result of a complication that prevented oral feeding. Patients who underwent total gastrectomy had postoperative feeding tubes placed more frequently (8 %) compared with subtotal gastrectomy with either reconstruction method (BII, 3 %; RY, 4 %). There was no association between feeding tube placement and weight loss, and there was no difference in either preoperative weight or BMI between patients who did and did not receive postoperative feeding tubes (p = 0.4 and p = 0.06, respectively). The low rate of major complications in early- (n = 20, 13 % of all patients) and late-stage (n = 34, 15 %) patients precluded analysis of the effect of complications on weight loss.
To investigate the effect of extremely low or high BMI on postoperative weight loss, we separately analyzed patients with BMI < 25 and >35. The 145 patients with a BMI < 25 (median 23) had predominantly late-stage disease (n = 86, 59 % of cohort). Overall, 54 patients (37 %) underwent total gastrectomy, 36 patients (25 %) had subtotal/RY, and 55 patients (38 %) had subtotal/BII. Major complications occurred in 12 % (n = 17) of patients and five patients required postoperative feeding tube placement. At 24 months after operation, patients with available follow- up (n = 59) had a median BMI of 20.5 kg.
At the other extreme, 27 patients (7 %) had BMI > 35 (median 37.5). They underwent total gastrectomy (n = 8), subtotal/RY (n = 7), or subtotal/BII (n = 12). Of the 14 late-stage patients, 7 received neoadjuvant chemotherapy, and 13 patients had early-stage disease. Major postoperative complications occurred in 5 patients, and 2 had a feeding tube placed.
DISCUSSION
This study offers the largest description of postoperative weight loss following gastrectomy for gastric cancer at a western center. Weight loss is greatest for patients requiring total gastrectomy and nadirs between 6 and 12 months postoperatively for late-stage patients (Fig. 2) and 12 and 18 months for early-stage patients (Fig. 1). Regardless of procedure, patients with a greater baseline BMI lose significantly more weight than patients with lower BMI while maintaining normal or overweight BMI throughout.
Patients with early-stage disease and BMI < 30 undergoing subtotal gastrectomy, regardless of reconstruction, lost 5–8 % of their baseline weight by 12 months. This is consistent with results of a random assignment trial of Billroth-I compared with Roux-en-Y reconstruction following subtotal gastrectomy in which the average percent body weight loss at 1 year did not differ by reconstruction method (9 vs. 10 %, p = 0.39).13 Approximately two-thirds of those patients had early-stage disease and median preoperative BMI in the normal range.
A trial by Fein et al. compared RY reconstruction with or without pouch after total gastrectomy and reported 15 % weight loss at 3–6 months, which persisted at 5 years for all patients.14 Similarly, we found that patients with early-stage gastric cancer undergoing total gastrectomy with RY reconstruction had an expected weight loss from baseline of 12 and 19 % at 3 and 6 months, respectively. Patients with late-stage disease undergoing total gastrectomy had comparable observed weight loss at 3 and 6 months after operation of 13 and 17 %, respectively.
We hypothesized that differences in preoperative BMI would influence postoperative weight loss and found that the extent of weight loss after gastrectomy for obese patients was significantly greater than that of normal and overweight patients. In other series, substantial postoperative weight loss is associated with a lower likelihood of continuation of systemic chemotherapy and worse overall outcome.7,15 A study of weight loss after gastrectomy by Lee and colleagues aimed to evaluate the association of weight loss with disease recurrence and survival.8 The majority of patients underwent subtotal gastrectomy (71.9 %) for Stage I (59.3 %) gastric cancer. The average weight loss at 12 months normal weight (BMI 20–25) and overweight (BMI > 25) patients was 9.1 and 12.8 %, respectively, similar to our findings. Patients who had ≥30 % weight loss at 6 months had a higher likelihood of early disease recurrence, and there was an association between greater weight loss and poorer survival. We did not incorporate delays in postoperative chemotherapy administration due to excessive weight loss in our analysis. In general, significant weight loss is an ominous sign in cancer patients. It was not our goal to evaluate weight loss as a predictor of disease-specific outcome, and we specifically excluded patients with early disease recurrence to eliminate this confounder.
Our practice is to allow limited liquids by mouth on the first postoperative day if the patient’s vital signs and clinical exam are normal. The diet is advanced daily, and by postoperative day 4 patients may be consuming a post-gastrectomy diet. All patients receive dietary counseling by nursing and nutrition staff prior to discharge. We do not include dietary supplementation as part of routine clinical care. Radiographic assessment of anastomoses is performed when clinically indicated. Because the routine use of feeding tubes is not supported by prospective data and may be harmful, we place them only after a complication that prevents oral feeding.4,5,15,16 Following gastrectomy, concerns about postoperative weight loss and nutritional deficits are motivating factors for the continued study of early postoperative enteral or parenteral nutrition. Our data suggest that not only can we predict significant postoperative weight loss based on the planned operation and baseline BMI, but also that the routine use of enteral feeding tubes is unnecessary.
This study is limited by its retrospective nature and bias that accompanies any single-institution report. We aimed to characterize the impact of the extent of gastric resection and other easily measured treatment-related variables on postoperative weight loss. As such, our study does not attempt to correlate factors, such as weight loss preceding diagnosis, nutritional parameters (e.g., prealbumin), or comorbidities, such as diabetes or tobacco dependence.
The observation of predictable and patterned weight loss based on universally quantifiable factors adds strength to the generalizability of our findings. A major strength of these data is the treatment of consecutive patients in the modern era by surgeons with consistent practice patterns. Postoperative weight loss is clearly dependent upon preoperative BMI and extent of resection. Most patients experience modest weight loss, reach a nadir between 6 and 18 months after operation (depending on clinical stage and the extent of operation), and subsequently stabilize or increase their weight. These data will allow clinicians to identify and counsel patients at risk for significant postoperative weight loss.
Acknowledgments
The authors thank Marianne Beninati for her expertise and dedication in managing the gastric cancer database.
DISCLOSURE This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 2013. [Accessed 1 Dec 2013]; http://globocan.iarc.fr. [Google Scholar]
- 3.Brennan MF, Pisters PW, Posner M, Quesada O, Shike M. A prospective randomized trial of total parenteral nutrition after major pancreatic resection for malignancy. Ann Surg. 1994;220(4):436–441. doi: 10.1097/00000658-199410000-00003. discussion 441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heslin MJ, Latkany L, Leung D, et al. A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Ann Surg. 1997;226(4):567–577. doi: 10.1097/00000658-199710000-00016. discussion 577–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hur H, Kim SG, Shim JH, et al. Effect of early oral feeding after gastric cancer surgery: a result of randomized clinical trial. Surgery. 2011;149(4):561–568. doi: 10.1016/j.surg.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Johnston ID, Welbourn R, Acheson K. Gastrectomy and loss of weight. Lancet. 1958;1(7033):1242–1245. doi: 10.1016/s0140-6736(58)92108-1. [DOI] [PubMed] [Google Scholar]
- 7.Aoyama T, Yoshikawa T, Shirai J, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20(6):2000–2006. doi: 10.1245/s10434-012-2776-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee SE, Lee JH, Ryu KW, et al. Changing pattern of postoperative body weight and its association with recurrence and survival after curative resection for gastric cancer. Hepatogastroenterology. 2012;59(114):430–435. doi: 10.5754/hge09218. [DOI] [PubMed] [Google Scholar]
- 9.Bickenbach KA, Denton B, Gonen M, Brennan MF, Coit DG, Strong VE. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann Surg Oncol. 2013;20(3):780–787. doi: 10.1245/s10434-012-2653-3. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 11.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS® for Mixed Models. 2nd. Cary: SAS Institute Inc; 2006. [Google Scholar]
- 12.Selby LV, Vertosick EA, Sjoberg DD, et al. Morbidity after total gastrectomy: analysis of 238 patients. J Am Coll Surg. 2015;220(5):863.e2–871.e2. doi: 10.1016/j.jamcollsurg.2015.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirao M, Takiguchi S, Imamura H, et al. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013;20(5):1591–1597. doi: 10.1245/s10434-012-2704-9. [DOI] [PubMed] [Google Scholar]
- 14.Fein M, Fuchs KH, Thalheimer A, Freys SM, Heimbucher J, Thiede A. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Ann Surg. 2008;247(5):759–765. doi: 10.1097/SLA.0b013e318167748c. [DOI] [PubMed] [Google Scholar]
- 15.Sierzega M, Choruz R, Pietruszka S, Kulig P, Kolodziejczyk P, Kulig J. Feasibility and outcomes of early oral feeding after total gastrectomy for cancer. J Gastrointest Surg. 2015;19(3):473–479. doi: 10.1007/s11605-014-2720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SH, Kooby DA, Staley CA, 3rd, Maithel SK. An assessment of feeding jejunostomy tube placement at the time of resection for gastric adenocarcinoma. J Surg Oncol. 2013;107(7):728–734. doi: 10.1002/jso.23324. [DOI] [PubMed] [Google Scholar]