Abstract
Introduction
Variability in surgical and pathological techniques in Western centers may lead to inconsistency in lymph node staging in patients with gastric adenocarcinoma. We hypothesize that ex-vivo dissection (EVD) after gastrectomy for adenocarcinoma increases lymph node yield.
Methods
We retrospectively reviewed 222 consecutive patients who underwent gastrectomy with curative intent for adenocarcinoma between November 2010 and June 2014. In August of 2012, we began performing EVD of nodes in surgical specimens (EVD group, N=111), as opposed to submitting specimens en-bloc with lymph node basins attached to the specimen (No EVD group, N=111). Primary end-point was lymph node yield.
Results
The median number of lymph nodes procured was significantly higher in the EVD compared to the No EVD group (30 vs 21 lymph nodes, respectively; P<0.0001). Moreover, 28% of the No EVD group were not adequately staged (defined by ≤ 15 nodes), compared to 5% of the EVD group (P<0.0001). Stage-for-stage overall survival was not significantly different.
Conclusion
EVD may be a useful tool to maximize lymph node yield. However, this had no impact on staging or survival. This is an interesting finding that warrants further investigation.
Keywords: ex-vivo dissection, gastric cancer, gastrectomy, lymph node, staging
Introduction
Gastric cancer is the fourth leading cause of cancer and cancer-related deaths worldwide [1]. Curative treatment of gastric cancer is surgical resection and lymphadenectomy [2]. Given the high recurrence rates from operation alone, a multimodality approach is preferred for locoregionally advanced non-metastatic gastric cancer [3]. Advances in neoadjuvant and adjuvant treatment modalities in combination with surgical resection have optimized disease-free and overall survival rates [4].
Lymph node metastasis is common (>50%) in primary gastric cancer invading beyond the submucosa (T2) [5] and lymph node status is a key prognostic factor in gastric cancer [6]. According to the 2010 7th edition of the American Joint Committee on Cancer (AJCC) staging manual, at least 16 lymph nodes must be examined to obtain proper oncologic staging [7]. Performance of a D2 lymphadenectomy, which removes perigastric and the first and second tier of nodes along the branches of the celiac axis, splenic vessels, and splenic hilum, optimizes the potential to obtain the recommended number of nodes required for staging. Multiple studies have demonstrated the feasibility of this technique by Western surgeons on Western patients, who typically have higher body mass indices (BMI) and more co-morbidities than Eastern patients [8,9]. Failure to obtain sufficient lymph nodes may lead to under-staging, especially when ten nodes or fewer are examined [10]. Conversely, some studies demonstrated improved prognostic ability in gastric cancer patients who had more than 16 lymph nodes examined [11,12]. We routinely perform D2 lymphadenectomy at our institution, and have previously reported the increase in lymph node yield by a fat clearing technique [13]. Nevertheless, we have not always procured the recommended 16 lymph nodes to properly stage patients. Thus, we sought to examine other techniques to optimize our lymph node counts. Over the last two years, we have adopted the technique of surgeon ex vivo dissection (EVD) to further optimize the number of lymph nodes examined. The purpose of this study is to examine the effect of EVD on the number of lymph nodes examined. Our hypothesis is that EVD optimizes the lymph nodes counts and thus improves staging. In patients who did not receive neoadjuvant therapy, improved nodal staging has the potential to impact decisions about postoperative adjuvant therapy.
Methods
Patients
All patients who underwent a gastrectomy with curative intent for adenocarcinoma at Memorial Sloan Kettering Cancer Center from November 2010 to June 2014 were identified from a prospectively maintained gastric database. The study was conducted according to the institutional human research committee procedures. A total of 222 consecutive patients, who followed our standard treatment algorithm based on the preoperative stage (either neoadjuvant chemotherapy followed by resection for locally advanced tumors, or proceeding immediately to resection for early stage gastric cancer), underwent gastrectomy for adenocarcinoma via the open or minimally invasive approach. Neoadjuvant radiation therapy is not routinely administered according to our standard treatment algorithm. In August of 2012, we began performing ex vivo dissection of nodes in our surgical specimen (EVD group) as opposed to submitting specimens en-bloc with the lymph node basins attached to the specimens (No EVD group). The decision to perform an ex vivo dissection was surgeon specific.
All procedures were performed by two surgeons (VES & DC). Patient-related data included gender, age, and BMI. Tumor-related data included oncologic and histopathologic data, including tumor type, histology, Lauren classification, World Health Organization (WHO) classification, tumor location, TNM staging based on the 7th edition AJCC staging on postoperative evaluation, and number of lymph nodes examined. Treatment-related data included use of neoadjuvant chemotherapy, extent of gastric resection, and extent of lymphadenectomy. The primary end-point was the lymph node yield. The secondary end-point was stage specific survival.
Technique of EVD
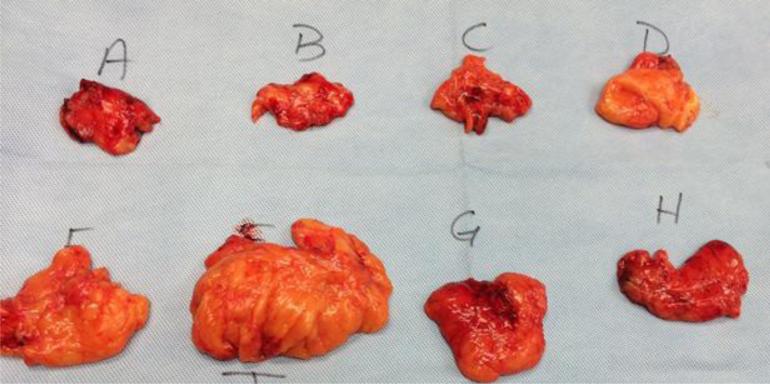
Specimens in the No EVD group were submitted intact to the pathology department for frozen section examination of the margin and further processing as deemed clinically appropriate. Specimens in the EVD group were processed immediately following gastrectomy on the back table in the OR, prior to being sent to pathology for a frozen section of the proximal or distal margin. On the back table, lymph nodes included in the specimen were divided into 10-12 discrete lymph node packets with accompanying adipose tissue, correlating with lymph node stations (Figure 1). This process takes 3-5 minutes. These labeled stations were then processed separately by our pathologists and reported separately in the final pathology report. The pathological evaluation of the specimens are performed by pathology assistants, surgical oncology fellows, or gastrointestinal subspecialty fellows, which are all overseen by the attending pathologist.
Figure 1.
Ex vivo dissection of a specimen into lymph node stations.
Statistical Analysis
Statistical analyses were performed using Graphpad Prism software version 5.03 (GraphPad Software, Inc. La Jolla, CA). Categorical variables were compared using chi-square or Fisher's exact test, whereas continuous variables were compared using Mann-Whitney U-test (two-tailed). Multivariate binary logistic regression analysis was used to identify independent predictors of understaging (procuring <16 lymph nodes). Forward stepwise regression analysis was utilized, including parameters in the model with a P<0.10. Overall survival curves were generated using the Kaplan-Meier method. All results are expressed as mean ± SD, unless specified otherwise. The null hypothesis was rejected when α<0.05.
Results
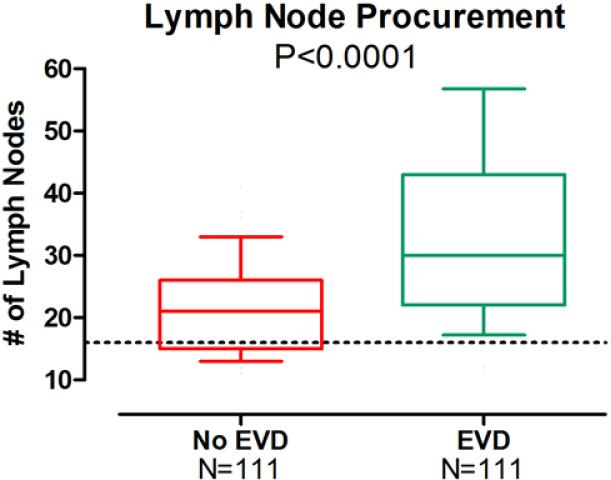
All patient-, tumor-, and treatment-related variables are listed in Table 1. There were no significant differences between the EVD and No EVD groups. However, mean [±SD] lymph node counts were significantly higher in the EVD group compared to the No EVD group (34±15.4 v. 22±8.4, respectively; P<0.0001) (Figure 2). Mean lymph node counts were significantly higher at each individual N status, including N0 (31±15.6 v. 19±7.5, respectively; P<0.0001), N1 (36±16.3 v. 24±7.3, respectively; P=0.01), and N3 (41±15.2 v. 28±7.1, respectively; P=0.003), but not N2 (32±10.7 v. 25±10.4, P=0.09) (Table 2). Although significantly more patients in the EVD group had at least 16 lymph nodes procured (95% v. 72%, respectively; P<0.0001), lymph node positivity rates (positive/total lymph nodes) were not significantly different. Overall AJCC stage distribution was not different comparing the No EVD to the EVD groups.
Table 1.
Patient parameters stratified by ex vivo dissection
| No EVD Group N=111 | EVD Group N=111 | P Value | |
|---|---|---|---|
| Patient-related Variables | |||
| Male, n (%) | 59 (53%) | 60 (54%) | 0.83 |
| Female, n (%) | 52 (47%) | 51 (46%) | |
| Age, years | 62±13.6 | 61±15.6 | 0.97 |
| Body mass Index [BMI] (kg/m2) | 26.9±5.3 | 26.6±4.8 | 0.81 |
| Tumor-related Variables | |||
| Histology, n (%): | |||
| Well-differentiated | 3 (3%) | 7 (6%) | |
| Moderately differentiated | 48 (43%) | 60 (54%) | 0.07 |
| Poorly differentiated | 60 (54%) | 44 (40%) | |
| Lauren classification, n (%): | |||
| Intestinal | 48 (43%) | 58 (52%) | |
| Mixed | 17 (15%) | 16 (14%) | 0.38 |
| Diffuse | 46 (42%) | 37 (34%) | |
| WHO classification, n (%) | |||
| Tubular | 65 (59%) | 57 (51%) | |
| Signet ring | 43 (39%) | 47 (42%) | 0.47 |
| Other/unknown | 4 (4%) | 7 (6%) | |
| Location, n (%): | |||
| Lower third | 51 (46%) | 46 (41%) | |
| Middle third | 34 (31%) | 29 (26%) | 0.32 |
| Upper third | 26 (23%) | 36 (33%) | |
| T status, n (%): | |||
| T0 | 6 (5%) | 7 (6%) | |
| T1a | 19 (17%) | 22 (20%) | |
| T1b | 20 (18%) | 19 (17%) | |
| T2 | 16 (14%) | 12 (11%) | 0.97 |
| T3 | 30 (27%) | 31 (28%) | |
| T4a | 18 (16%) | 19 (17%) | |
| T4b | 2 (2%) | 1 (1%) | |
| N status, n (%): | |||
| N0 | 64 (58%) | 63 (57%) | |
| N1 | 18 (16%) | 19 (17%) | 1.0 |
| N2 | 13 (12%) | 13 (12%) | |
| N3 | 16 (14%) | 16 (14%) | |
| Stage, n (%): | |||
| in situ | 6 (5%) | 6 (5%) | |
| I | 47 (43%) | 45 (41%) | |
| II | 29 (26%) | 31 (28%) | 0.98 |
| III | 28 (25%) | 27 (24%) | |
| IV | 1 (1%) | 2 (2%) | |
| Treatment-related Variables | |||
| Neoadjuvant Chemotherapy, n (%): | |||
| Yes | 50 (45%) | 61 (55%) | 0.18 |
| No | 61 (55%) | 50 (45%) | |
| Total/Subtotal Gastrectomy, n (%) | 44 (40%) | 44 (40%) | 1.0 |
| Distal Gastrectomy, n (%) | 67 (60%) | 67 (60%) | |
| Lymphadenectomy, n (%): | |||
| D1+ | 7 (6%) | 9 (8%) | 1.0 |
| D2 | 104 (94%) | 102 (92%) | |
WHO: World Health Organization
Figure 2.
The box and whisker plots illustrate the 10th, 25th, 50th (median), 75th, and 90th percentiles. The dotted line represents the minimum number of lymph nodes (16) required for proper staging of a patient with adenocarcinoma. The ex-vivo lymphadenectomy technique maximized the number of lymph nodes procured (P<0.0001).
Table 2.
Lymph node counts stratified by ex vivo dissection
| No EVD Group | EVD Group | P Value | |
|---|---|---|---|
| Lymph Nodes Examined | 22±8.4 | 34±15.4 | <0.0001 |
| Male | 20±7.4 | 32±14.8 | <0.0001 |
| Female | 24±8.8 | 35±16.1 | 0.0002 |
| Age<60 years | 23±7.2 | 31±11.5 | 0.0009 |
| Age≥60 years | 21±9.0 | 35±17.4 | <0.0001 |
| BMI<30 kg/m2 | 23±8.3 | 33±15.8 | <0.0001 |
| BMI≥30 kg/m2 | 19±8.2 | 37±14.0 | <0.0001 |
| Well-Moderately differentiated | 19±7.0 | 26±13.5 | 0.02 |
| Poorly differentiated | 24±9.0 | 37±15.0 | <0.0001 |
| Intestinal/Mixed type | 21±8.6 | 31±14.7 | <0.0001 |
| Diffuse type | 23±7.9 | 37±15.9 | <0.0001 |
| Signet ring classification | 23±8.0 | 37±14.7 | <0.0001 |
| Lower third | 20±8.1 | 31±13.5 | <0.0001 |
| Middle third | 22±7.5 | 33±14.9 | <0.0001 |
| Upper third | 24±8.8 | 36±15.0 | <0.0001 |
| T status lymph node yield: | |||
| T0 | 21±5.5 | 27±12.4 | 0.52 |
| T1a | 16±6.0 | 31±16.4 | 0.003 |
| T1b | 22±9.4 | 27±12.4 | 0.12 |
| T2 | 24±10.4 | 36±14.5 | 0.01 |
| T3 | 23±6.7 | 35±16.2 | 0.003 |
| T4 | 23±9.2 | 41±14.7 | 0.0001 |
| N status lymph node yield: | |||
| N0 | 19±7.5 | 31±15.6 | <0.0001 |
| N1 | 24±7.3 | 36±16.3 | 0.01 |
| N2 | 25±10.4 | 32±10.7 | 0.09 |
| N3 | 28±7.1 | 41±15.2 | 0.003 |
| Stage: | |||
| I | 19±7.6 | 29±14.6 | <0.0001 |
| II | 23±9.3 | 36±16.8 | 0.001 |
| III | 25±7.8 | 39±14.4 | <0.0001 |
| Neoadjuvant chemotherapy | 23±8.0 | 33±14.2 | <0.0001 |
| No neoadjuvant chemotherapy | 20±8.5 | 34±16.9 | <0.0001 |
| Total/Subtotal Gastrectomy | 22±7.0 | 35±14.9 | <0.0001 |
| Distal Gastrectomy | 22±9.2 | 33±15.9 | <0.0001 |
| D1+ Lymphadenectomy | 10±5.7 | 32±11.2 | 0.002 |
| D2 Lymphadenectomy | 23±8.0 | 34±15.8 | <0.0001 |
| Lymph Node Positivity Rate (Positive /Total Lymph Nodes) (%) | 9.6±18.4 | 8.8±17.6 | 0.82 |
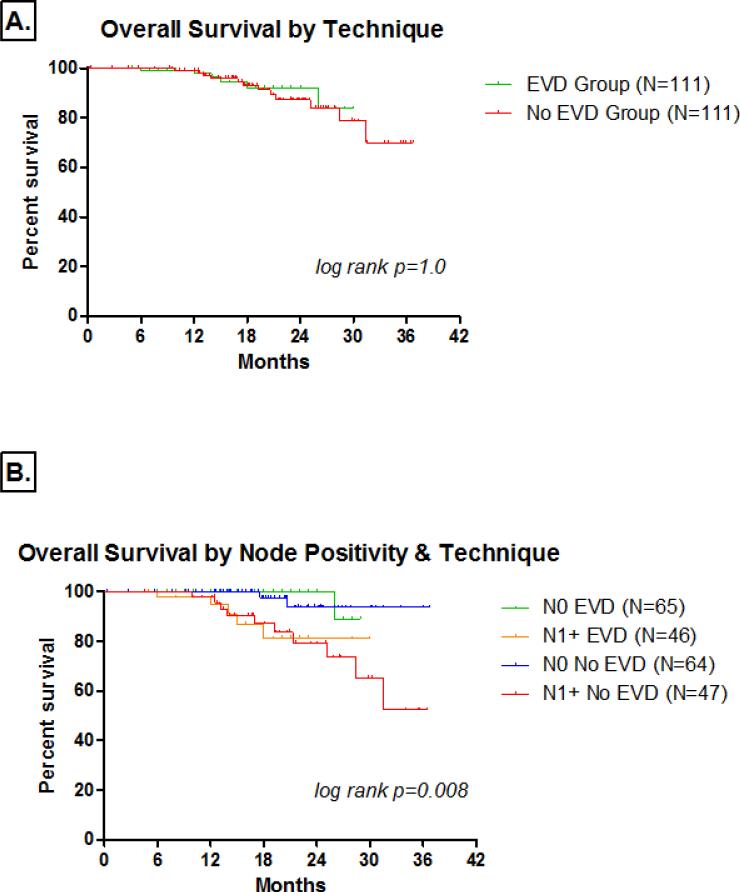
The median follow-up of all patients was 17 months (range 1-38 months). Median follow-up was significantly longer in the No EVD group (19.5 v. 14 months, respectively; P<0.0001). One-year overall survival rates in the EVD and No EVD groups were 97.8% and 99.0% respectively. Two-year overall survival rates were 92.0% and 87.3%, respectively. Three-year overall survival rates for Stage I, II, and III disease were 98.8%, 65.9%, and 43.6%, respectively. There were only three patients with Stage IV disease (all downstaged preoperatively with chemotherapy) and all were alive at last follow-up. Stage-specific overall survival was not significantly different between the two groups for Stage I (log rank p=0.32), Stage II (log rank p=0.18), and Stage III (log rank p=0.80) disease. There was no significant difference in overall survival based on ex vivo dissection technique (P>0.05) (Figure 2A). Moreover, overall survival based on ex vivo dissection technique and nodal positivity were not significantly different (P>0.05) (Figure 2B). There was no significant difference in overall survival when comparing node negative patients in the EVD and No EVD groups (log rank p=0.49) or node positive patients (log rank p=0.92). Finally, there was no significant difference in recurrence-free survival between the EVD and No EVD groups (log rank p=0.56).
Variables postulated to affect adequate staging (≥16 lymph nodes) in gastric cancer are listed in Table 3. Ex vivo dissection was the most significant variable (P<0.0001), followed by the use of neoadjuvant chemotherapy (P=0.02), obesity (P=0.03), and extent of lymphadenectomy (P=0.03). On multivariate analysis, performing an ex vivo dissection was the most significant predictor of adequate staging (odds ratio [OR]: 7.04; 95% confidence interval [CI]: 2.70-18.18; P<0.0001), followed by performing a D2 lymphadenectomy (OR: 5.31; 95% CI: 1.46-19.61; P=0.01), and neoadjuvant chemotherapy use (OR: 2.27; 95% CI: 1.02-5.05; P=0.04).
Table 3.
Adequate Staging
| Percent of patients adequately stage (≥16 lymph nodes) | P Value | |
|---|---|---|
| Male | 98/119 (82%) | 0.43 |
| Female | 88/103 (85%) | |
| Age<60 years | 72/83 (87%) | 0.29 |
| Age≥60 years | 113/139 (81%) | |
| BMI<30 kg/m2 | 145/169 (86%) | 0.03 |
| BMI≥30 kg/m2 | 39/53 (74%) | |
| Neoadjuvant chemotherapy | 99/111 (89%) | 0.02 |
| No Neoadjuvant chemotherapy | 85/111 (77%) | |
| Total/Subtotal Gastrectomy | 76/88 (86%) | 0.33 |
| Distal Gastrectomy | 109/134 (81%) | |
| D1 Lymphadenectomy | 10/16 (63%) | 0.03 |
| D2 Lymphadenectomy | 175/206 (85%) | |
| No EVD | 80/111 (72%) | <0.0001 |
| EVD | 105/111 (95%) | |
BMI: body mass index; EVD: ex vivo dissection
Discussion
Ex vivo dissection increased our lymph node counts, on average, by 12 lymph nodes. Moreover, EVD increased the proportion of patients that were adequately staged according to the AJCC guidelines. Nevertheless, despite these improvements, EVD had no impact on staging or survival.
Various histopathologic techniques have been described to maximize lymph nodes counts in gastric cancer specimens. In 1990 we reported on a series of 11 patients with resectable gastric cancer our institution describing a comprehensive fat-clearing method that doubled lymph node counts (P<0.01) and identified smaller nodes (P<0.001) [13]. Hanna et al reported on a series of 114 gastric cancer specimens that underwent two different techniques to harvest lymph nodes: manual nodal dissection (MND) and systematic fat blocking (SFB) [14]. In SFB all the fat tissue (excluding the greater omentum) and large lymph nodes were removed from the specimen. The remaining fat was divided into blocks of tissue measuring 20×10×5 mm and stained with haematoxylin and eosin then examined for lymph nodes by light microscopy. The SFB technique retrieved significantly more lymph nodes than the conventional MND technique (66±21 vs. 50±20, respectively; P=0.001). Additionally, the SFB technique was better in identifying smaller lymph nodes and consistently produced a cumulative higher percentage of positive lymph nodes compared to MND. Lavy and colleagues describe a technique using acetone as a fat dissolving solution [15]. The acetone group had a higher lymph node count compared to MND (26.1 vs. 19.3, respectively; P=0.003); however, there was no significant difference in the average number of positive lymph nodes (P=0.22).
Other factors affecting lymph node counts in gastric cancer specimens have been studied. For example, Schoenleber et al reviewed 99 consecutive patients who underwent gastrectomy for gastric adenocarcinoma to determine factors that affect lymph node counts [16]. The surgeon (P=0.16) did not affect lymph node counts; however, the senior pathologist (P=0.03) and, more significantly, the pathology technician (P<0.001) had the most significant influence on lymph node counts on multivariate analysis. The study by Cichowitz and colleagues reviewing 88 patients with esophagogastric cancers demonstrated that the pathologist also had a significant influence on lymph node counts, independent of the level of experience [17]. Age, gender, operative approach, and use of neoadjuvant therapy did not impact lymph node counts. Similar findings regarding the influence of the pathologist and lymph node counts have been reported in the colorectal literature [18,19]. In our study we used the same dedicated group of gastric cancer pathologists to review all of our specimens.
Several studies have demonstrated a survival advantage for examining more lymph nodes. This was first described in the East by the Koreans and Japanese [20,21]. Noda and colleagues from Japan demonstrated the importance of examining small lymph nodes (4 mm post-histologic processing) to avoid stage migration [20]. Lee at al from Korea demonstrated a survival advantage associated with obtaining at least 15 nodes [21]. Moreover, stage IIIB patients who had ≥35 nodes procured (compared to <20) also had a stage-specific survival advantage. Karpeh and colleagues from our institution also demonstrated a survival advantage by obtaining a minimum of 15 lymph nodes in gastric cancer [22]. Altorki et al retrospectively reviewed a series of 264 patients with esophageal cancer treated by esophagectomy without neoadjuvant therapy [20]. He demonstrated a death hazard reduction of 34% in patients that had more than 16 nodes resected and a 48% reduction in patients that had more than 25 nodes [23].
Adequate staging can be achieved with ex vivo dissection. Schmidt et al demonstrated that when D2 lymphadenectomy was combined with ex vivo dissection all 331 patients had the recommended minimum of 16 lymph nodes examined [9]. In our study we had 23% more patients meet the minimum required lymph node counts required for proper staging using the ex vivo dissection technique. Cichowitz et al demonstrated a median of 11 more lymph nodes counted with ex vivo dissection [17]. Nevertheless, both of these studies, as well as our current study, did not demonstrate any stage-specific survival advantage associated with ex vivo dissection in gastric cancer.
This study has several limitations, including the inherent biases of a retrospective study. Nevertheless, the sample size is robust with >200 gastric cancer resections that were all done consecutively in a relatively short time period. The short follow-up time, which is under two years in both cohorts, is a limitation that prevents full assessment and comparisons of stage-matched survival. Furthermore, some institutions may have additional costs associated with processing individual lymph node packets. Nonetheless, we've proposed a simplified, modification of the well-described Asian technique to improve lymph node yield for Western patients. It offers the Western surgeon an additional tool that adds no morbidity to patients, and can be done in a relatively short period of time in the operating room. Most importantly EVD allows the surgeon and collaborating pathologist to maximize lymph node yield following lymphadenectomy for gastric cancer.
In summary, our study demonstrates that EVD of regional lymph nodes following curative gastrectomy for gastric cancer is associated with an increase in lymph node counts with surgeon ex vivo dissection. Compared to non-EVD processing of the surgical specimen, EVD was associated with an improvement in the proportion of patients with adequate nodal staging, as defined by the AJCC recommendation of >15 nodes. However, this increase in the number of examined nodes and in adequately staged patients had no impact on overall stage distribution or stage-specific survival.
Figure 3.
The Kaplan-Meier demonstrate the overall survival rates based on [A] ex vivo dissection technique and [B] lymph node positivity and ex vivo dissection technique.
Primary Discussant
John T. Mullen, MD (Boston, MA)
Thank you Dr. Afaneh for your excellent presentation. As you know, over the years there has been much debate as to the proper extent of lymph node dissection for patients with gastric cancer, and there has been much published about the frequent problem of understaging in the West owing to low numbers of examined lymph nodes, including many proposals for new staging systems (e.g., using lymph node ratios, log odds of metastatic lymph nodes, etc.) designed to compensate for these low nodal yields. You and your co-authors are to be congratulated for adopting a technique known to significantly improve lymph node yields which has long been employed in Asia and has also been employed at my institution over the past several years – ex-vivo dissection (EVD). Surprisingly, though you demonstrated much higher lymph node yields with EVD than without EVD, there were no differences in either the stage-specific stratification of the patients or in overall survival. I have two questions for you:
-
1)
The first and most obvious question is how can you explain the fact that examining 12 more lymph nodes in the EVD group than in the non-EVD group led to absolutely zero difference in nodal staging? The percentages of patients in each of the N groupings (i.e., N0, N1, etc.) are virtually identical (there isn't even a trend!), and the P value is 1.0.
-
2)
In light of your findings, will you and your colleagues continue to take the extra time to do ex-vivo dissections as you currently do on patients with gastric cancer, will you modify the procedure in some way to achieve even higher nodal yields (e.g., dissect out individual lymph nodes as opposed to entire packets), or will you abandon it altogether?
Closing Discussant
Dr. Afaneh
-
1)
Dr. Mullen, thank you taking the time to carefully review our manuscript. I think you raise an important question. I believe the reason we found no difference in nodal staging is two-fold. First, at baseline, we are performing an extended lymphadenectomy (D2). The additive effect of ex vivo dissection may be less substantial in these patients. The number of patients undergoing a D1 lymphadenectomy was small and therefore we are unable to make any conclusions in those patients. In hospitals where the median lymph node yield is often below 8-10 nodes, the effect may be more demonstrable. Second, our follow-up is relatively short. The current study has a follow-up period of less than three years, therefore it may be difficult to assess any impact on stage distribution or even survival. The true staging of those patients with inadequate nodal dissection will never really be known.
-
2)
This is an important question given the negative finding of our study. It may be premature to fully abandon this technique as our colleagues in the East have clearly demonstrated a benefit of ex vivo dissection. Reviewing the data at three years would be necessary to assess any impact on survival. Other techniques we are currently considering includes submitting the lymph node stations as they are removed in vivo as separate nodal stations or having the pathologists take additional slides of the packets submitted.
Acknowledgements
We would like to personally thank Dr. Murray Brennan for his careful review of our manuscript. We would also like to thank Marianne Beninati for her help with data collection.
Footnotes
Plenary Presentation: Digestive Disease Week 2015, Society for Surgery of the Alimentary Tract, 56th Annual Meeting, Washington DC (May 2015)
Author Contributions and Conflicts of Interest
CA: Participated in research design, performance of the research, data analysis, and writing of the paper, no conflict of interest
AL: Participated in research design, performance of the research, data analysis, and writing of the paper, no conflict of interest
LS: Participated in research design and critical revision of the paper, approving final version of manuscript, no conflict of interest
GK: Participated in research design and critical revision of the paper, approving final version of manuscript, no conflict of interest
SSY: Participated in research design and critical revision of the paper, approving final version of manuscript, no conflict of interest
LT: Participated in research design and critical revision of the paper, approving final version of manuscript, no conflict of interest
DC: Participated in research design and critical revision of the paper, approving final version of manuscript, no conflict of interest
VES: Participated in research design, data analysis and critical revision of the paper, approving final version of manuscript, no conflict of interest
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Pisters PWT, Kelsen DP, Tepper JE. Cancer of the Stomach. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. Cancer: Principles & Practice of Oncology. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 1043–1078. [Google Scholar]
- 3.D'Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Dang P, Raut CP, Pandalai PK, Maduekwe UN, Rattner DW, Lauwers GY, Yoon SS. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255:478–485. doi: 10.1097/SLA.0b013e31824857e2. [DOI] [PubMed] [Google Scholar]
- 6.Nitti D, Marchet A, Olivieri M, Ambrosi A, Mencarelli R, Belluco C, et al. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077–1085. doi: 10.1245/aso.2003.03.520. [DOI] [PubMed] [Google Scholar]
- 7.AJCC cancer staging manual. 7th edition Springer; New York: 2010. [Google Scholar]
- 8.Degiuli M, Sasako M, Ponti A. Italian Gastric Cancer Study Group. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643–649. doi: 10.1002/bjs.6936. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt B, Chang KK, Maduekwe UN, Look-Hong N, Rattner DW, Lauwers GY, Mullen JT, Yang HK, Yoon SS. D2 lymphadenectomy with surgical ex vivo dissection into node stations for gastric adenocarcinoma can be performed safely in Western patients and ensures optimal staging. Ann Surg Oncol. 2013;20:2991–2999. doi: 10.1245/s10434-013-3019-1. [DOI] [PubMed] [Google Scholar]
- 10.Bouvier AM, Haas O, Pirad F, Roignot P, Bonithon-Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer. 2002;94:2862–6. doi: 10.1002/cncr.10550. [DOI] [PubMed] [Google Scholar]
- 11.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 12.Son T, Hyung WJ, Lee JH, Kim YM, Kim HI, An JY, Cheong JH, Noh SH. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer. 2012;118:4687–4693. doi: 10.1002/cncr.27426. [DOI] [PubMed] [Google Scholar]
- 13.Candela FC, Urmacher C, Brennan MF. Comparison of the conventional method of lymph node staging with a comprehensive fat-clearing method for gastric adenocarcinoma. Cancer. 1990;66:1828–1832. doi: 10.1002/1097-0142(19901015)66:8<1828::aid-cncr2820660830>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Hanna GB, Amygdalos I, Ni M, Boshier PR, Mikhail S, Lloyd J, Goldin R. Improving the standard of lymph node retrieval after gastric cancer surgery. Histopathology. 2013;63:316–324. doi: 10.1111/his.12167. [DOI] [PubMed] [Google Scholar]
- 15.Lavy R, Hershkovitz Y, Kapiev A, et al. A comparative study on two different pathological methods to retrieve lymph nodes following gastrectomy. Int J Surg. 2014;12:725–728. doi: 10.1016/j.ijsu.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 16.Schoenleber SJ, Schnelldorfer T, Wood CM, Qin R, Sarr MG, Donohue JH. Factors influencing lymph node recovery from the operative specimen after gastrectomy for gastric adenocarcinoma. J Gastrointest Surg. 2009;13:1233–1237. doi: 10.1007/s11605-009-0886-7. [DOI] [PubMed] [Google Scholar]
- 17.Cichowitz A, Burton P, Brown W, Smith A, Shaw K, Slamowicz R, Nottle PD. Ex vivo dissection increases lymph node yield in oesophagogastric cancer. ANZ J Surg. 2015;85:80–84. doi: 10.1111/ans.12365. [DOI] [PubMed] [Google Scholar]
- 18.Nathan H, Shore AD, Anders RA, Wick EC, Gearhart SL, Pawlik TM. Variation in lymph node assessment after colon cancer resection: patient, surgeon, pathologist, or hospital? J Gastrointest Surg. 2011;15:471–479. doi: 10.1007/s11605-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valsecchi ME, Leighton J, Jr, Trester W. Modifiable factors that influence colon cancer lymph node sampling and examination. Clin Colorectal Cancer. 2010;9:162–167. doi: 10.3816/CCC.2010.n.022. [DOI] [PubMed] [Google Scholar]
- 20.Noda N, Sasko M, Tamaguchi N, Nakanishi Y. Ignoring small lymph nodes can be a major cause of staging error in gastric cancer. Br J Surg. 1998;85:831–834. doi: 10.1046/j.1365-2168.1998.00691.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee HK, Yang HK, Kim WH, Lee KU, Choe KJ, Kim JP. Influence of the number of lymph nodes examined on staging of gastric cancer. Br J Surg. 2001;88:1408–1412. doi: 10.1046/j.0007-1323.2001.01875.x. [DOI] [PubMed] [Google Scholar]
- 22.Karpeh MS, Leon L, Klimstra D, Brennan MF. Lymph node staging in gastric cancer: is location more important than number? An analysis of 1,038 patients. Ann Surg. 2000;232:362–371. doi: 10.1097/00000658-200009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altorki NK, Zhou XK, Stiles B, Port JL, Paul S, Lee PC, Mazumdar M. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg. 2008;248:221–226. doi: 10.1097/SLA.0b013e31817bbe59. [DOI] [PubMed] [Google Scholar]