Abstract
Hypoparathyroidism (HP) arises most commonly from parathyroid (PT) gland damage associated with neck surgery, and is typically treated with oral calcium and vitamin D. Such treatment effectively increases levels of serum calcium (sCa), but also brings risk of hypercalciuria and renal damage. There is thus considerable interest in using PTH or PTH analogs to treat HP. To facilitate study of this disease and the assessment of new treatment options, we developed two mouse models of acquired HP, and used them to assess efficacy of PTH(1-34) as well as a long-acting PTH analog (LA-PTH) in regulating blood calcium levels. In one model, we used PTHcre-iDTR mice in which the diphtheria toxin (DT) receptor is selectively expressed in PT glands, such that systemic DT administration selectively ablates parathyroid cells. For the second model, we generated GFP-PT mice in which green fluorescent protein (GFP) is selectively expressed in PT cells, such that parathyroidectomy (PTX) is facilitated by green fluorescence of the PT glands. In the PTHcre-iDTR mice, DT injection (2×5 μg/kg, i.p.) resulted in moderate yet consistent reductions in serum PTH and sCa levels. A more severe hypoparathyroid phenotype was observed in GFP-PT mice following GFP-guided PTX surgery. A single subcutaneous injection of LA-PTH increased sCa levels more effectively and for a longer duration (>24hr) than did a ten-fold higher dose of PTH(1-34), without causing excessive urinary calcium excretion. These new mouse models thus faithfully replicate two levels of acquired hypoparathyroidism, moderate and severe, and may be useful for assessing potential new modes of therapy.
Introduction
Hypoparathyroidism (HP) is characterized by inadequate or absent secretion of parathyroid hormone (PTH). Lack of PTH leads to hypocalcemia, hyperphosphatemia, and increased renal calcium excretion [1]. In addition, bone turnover is low and bone microarchitecture is abnormal [2]. Hypoparathyroidism is most often acquired by inadvertent damage or removal of the parathyroid glands during neck surgery [3].
Conventional therapy for hypoparathyroidism consists of oral supplementation of calcium and active vitamin D analogues. Such therapy, however, often fails to keep serum calcium stably within the target range, and can worsen hypercalciuria. Balancing the risk of hypocalcemia with the risk of hypercalciuria is thus a challenging, but important goal for HP therapy [3]. Daily subcutaneous injection of recombinant human (rh) PTH(1-84) has recently been approved by the FDA as adjunct therapy to calcium and vitamin D in patients with hypoparathyroidism [4]. While the approval of rhPTH(1-84) is a major milestone in the development of treatments for the disease, clinical trials did not show lower levels of urinary calcium excretion in the rhPTH(1-84)-treated patients, as compared to placebo-treated controls [5-7]. Novel long-acting PTH analogs and novel modes of delivery of PTH are thus being investigated as potential treatment options for hypoparathyroidism [8-12].
A need to fully understand the mechanisms and consequences of various lines of therapy for HP underlies a demand for more reliable and more accessible animal models of acquired hypoparathyroidism. Existing animals models include genetically modified mice carrying either the PTH-null [13] or GCM2-null [14] alleles. However, unlike in acquired hypoparathyroidism, the most common form of the disease, the HP phenotype in these mice is inherited, and the development of key organs such as the skeleton may be distinctly altered. Furthermore, in patients, hypoparathyroidism typically develops acutely, whereas in the available genetic mouse models, the phenotype is chronic. Moreover, some of the mouse models show early lethality and reduced fertility [13-15], which limits their use. More widely used are animal models generated by surgical parathyroidectomy (PTX), which is most typically performed in rats [16, 17], but also in larger mammals [18-20]. Although the mouse is the most widely used laboratory test animal, it is not generally amenable to PTX surgery, due to the small size of the parathyroid glands and their variable anatomic distribution. As an alternative, thyroparathyroidectomy (TPTX) can be performed in the mouse, but this approach brings the need for thyroid hormone supplementation and the loss of calcitonin made by thyroid glands as added variables [21].
To overcome these problems, we established two new mouse models of acquired hypoparathyroidism. We thus developed the PTHcre-iDTR mouse, in which diphtheria toxin is used to selectively ablate parathyroid cells, and we also developed the GFP-PTX mouse, in which the parathyroid glands have been surgically removed, a process greatly facilitated by green fluorescence of the glands. We characterize the baseline calcium levels in these two mouse models and use them to assess the efficacy of a long-acting (LA) PTH analog, in comparison to PTH(1-34), as a potential treatment for hypoparathyroidism.
Material and Methods
Experimental Animals
The following mice (catalog number of Jackson Laboratory, Bar Harbor, Maine) were obtained: ROSAmT/mG mice (007676) [22], PTH-Cre mice (005989) [23], and iDTR mice (007900) [24]. Genotyping was performed on genomic DNA isolated from tails, using previously published protocols. This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the MGH. Male and female mice were used for experiments. Mice were housed in the Center for Comparative Medicine at Massachusetts General Hospital, at 23°C with 40% humidity in a 12-hour light/dark cycle with free access to water and irradiated diet. Wood bedding and igloo covers were provided for environmental enrichment. Mice were fed a standard chow diet, which contained 1.11% calcium, 0.48% phosphate and 2.5 IU/g vitamin D; or a low calcium diet (Teklad Diets, Madison WI) containing 0.005% Ca, 0.6% phosphate and 0 iU/g vitamin D3, as indicated.
Genome Walking to determine the PTH-Cre integration site
The integration site of the PTH-Cre transgene was determined using the GenomeWalker Universal Kit (Clontech Laboratories, Mountain View, CA). In short, genomic DNA was isolated, digested with the restriction enzymes EcoRV, DraI, or PvuII, and the fragments were purified by phenol-chloroform extraction. The GenomeWalker adaptors were then ligated to the digested fragments. PCR amplification was preformed using a primer to the adaptor sequence and a novel primer, which we designed to the cre portion of the PTH-Cre transgene. A secondary PCR was then performed with nested primers. This yielded a single PCR product that was sequenced and mapped to the mouse genome. With this information, PCR primer pairs were designed to distinguish between mice heterozygous and homozygous for the PTH-Cre transgene. DNA from mice, which were known heterozygous and homozygous for the PTH-Cre transgene by progeny testing, was used to verify the utility of these primers. Based on the integration site we designed PCR primers (forward primer A, CCTGTCAAGGATGTGGAAGA, reverse primer A’, TCAGATCACACCACACAGCA, forward primer B, CAGTTGTCTTTAGTTTACTCAGCATCAG, reverse primer B’, GATAATCGCGAACATCTTCAGGTT) that gave the expected single transgene band in mice homozygous for the PTH-Cre transgene, a single wildtype band in wildtype littermates, and two bands, one wildtype and one transgenic, in mice heterozygous for the PTH-Cre transgene (Suppl. Fig. 2). Mice homozygous for the PTH-Cre transgene were used for subsequent breeding with either homozygous iDTR or homozygous ROSAmTmG mice.
Diphtheria toxin (DT) injection
Diphtheria toxin (Sigma-Aldrich Corporation, St. Louis, MO) was diluted to 0.1-10 μg/ml with saline and injected intraperitoneally (i.p.) in 6-7 weeks old PTHcre-iDTR mice at 10 ml/kg body weight (final concentration=1-100 μg/kg body weight). For control, the same volume of saline was injected i.p. For repetitive DT injection experiments, the same dose was injected every third day up to five times as indicated. Measurements of serum PTH and ionized calcium (Ca++) were performed at baseline, and 3 days after the last injection.
GFP-guided parathyroidectomy (GFP-PTX)
In the anesthetized and surgically prepared animal, a ventral midline incision was made. Under stereomicroscope and UV illumination, GFP-expressing parathyroid glands were visualized and removed in its entirety using fine surgical forceps and scissor, and the incisions were closed with sutures.
Biochemical analyses
For measurements of Ca++, blood was obtained from the tail vein, collected in a capillary tube, and immediately processed using the RapidLab 348 Ca++/pH analyzer (Siemens, Germany).
For other serum biochemical parameter measurements, blood was obtained by cheek bleeding, collected into the CAPIJECT T-MG micro collection tube (Terumo Europe N.V., Belgium), and centrifuged to yield serum that was stored at −80°C until further analysis. Samples were analyzed for phosphate using Phosphor C kit (Wako Pure Chemical Industries, Osaka, Japan), for PTH using mouse PTH 1-84 Elisa kit (Immutopics, San Clemente, CA), and 1,25-dihydroxy vitamin D3 (1,25(OH)2D3) using 1,25-Dihydroxy Vitamin D EIA kit (IDS, Fountain Hills, AZ).
Spot urine was collected every 2 to 4 hours over 24 hours and was stored at −20°C until further analysis. Urinary calcium and creatinine concentrations were determined using the LiquiColor kit (Stanbio Laboratory, Boerne, TX). Urinary calcium concentration (uCa) was expressed as the ratio to the urinary creatinine concentration (uCa/uCr). Urinary cAMP production was measured by RIA as described [8].
Histology and Immunohistochemical staining
Three days after the last DT injection, animals were sacrificed and dissected under the stereomicroscope. The thyroid and parathyroid glands were isolated and removed en bloc, fixed with 4% paraformaldehyde and embedded in paraffin. The long-term effects of DT injection on the parathyroids were studied in animal that also carried the ROSAmTmG transgene for better visualization of parathyroid cells. One month after the last DT injection, mice were sacrificed, a larger portion of the neck region around the thyroid removed en bloc, embedded in OCT and snap frozen in liquid nitrogen. 5 μm frozen sections were obtained and screened for GFP under a fluorescent microscope. To perform immunohistochemistry using a PTH antibody on sections containing GFP-positive cells, sections were incubated in blocking buffer (5% goat serum) for 1 hour at room temperature, before incubation with mouse anti-hPTH antibody (AbD Serotec, Raleigh, NC) in a 1:100 dilution and rabbit anti-GFP antibody (Life Technologies, Grand Island, NY) overnight at 4°C. Sections were stained at room temperature for 2 hours with Alexa Fluor 568/633 goat anti mouse IgG and Alexa Fluor 488 goat anti rabbit IgG antibody (Life Technologies, Grand Island, NY) using a 1:200 dilution. DAPI (Vector Laboratories, Burlingame, CA) was used for nuclear counterstaining.
To evaluate parathyroid cell apoptosis, TUNEL staining was performed on 5 μm paraffin sections of thyroid-parathyroid tissue using In Situ Cell Death Kit (Roche Applied Science, Mannheim, Germany). Antigen retrieval (2.5% proteinase K at 37°C) and staining process were followed by manufacturer's instructions and DAPI was used for counterstaining. The FITC-labeled TUNEL-positive cells were imaged under the fluorescent microscopy.
Peptide injection
Long-acting PTH (LA-PTH) and hPTH(1-34) were synthesized as previously reported [8]. LA-PTH contains the hPTH(1–14) sequence modified with Ala1,3,12, Gln10, Arg11, Trp14, followed by the hPTHrP(15–36) sequence modified with Ala18,22 and Lys26. After synthesis, peptides were diluted in vehicle solution (10 mM citric acid/150 mM NaCl/0.05% Tween-80, pH 5.0), to give a stock solution of 1 μmol/L. Stock solution was further diluted to a final working solution of 5 mL/kg body weight on the day of the injection. A single subcutaneous injection of LA-PTH at a dose of 1.5, 3, 5, 10, 20 nmol/kg, and PTH(1-34) at a dose of 50 nmol/kg was administered to 6-week old DT injected PTHcre-iDTR mice, and to GFP-PTX mice. Animals in the vehicle control group were injected with an equal volume of vehicle solution. Blood and spot urine were collected at 0, 2, 4, 6, 8, 12, 16, 20, 24, 48, 72, 96, 120 hours after injection.
Statistical analyses
Data were processed using Microsoft Excel 2008 and GraphPad Prism 6.0. Comparisons of means were performed by using Student's t test (two-tailed, unequal variances). A p-value smaller than 0.05 was considered statistically significant. Results are expressed as mean ± SD.
Power calculations to determine minimal group size for the experiments testing PTH analogs indicated that with 5 mice in each experimental group we would have 95% power to detect a group mean difference of 13% in ionized calcium assuming a standard deviation of 0.08 (p<0.05).
Results
PTHcre-iDTR mice
Human diphtheria toxin receptors (DTR) were selectively expressed in parathyroid cells by using mice that carry a Cre-inducible diphtheria toxin receptor (iDTR) allele [24]. These mice were mated to mice that express the Cre allele under the control of the human 5.5-kb PTH promoter, which is active only in parathyroid cells [23]. In the resulting PTHcre-iDTR mice, parathyroid cells can be ablated simply by systemic injection of diphtheria toxin (DT), while all other tissues in the mouse are insensitive to DT. To determine the maximally tolerated dose of DT in mice we injected iDTR mice (without the Cre allele) with DT i.p. at ascending doses. In these mice, the loxP-flanked transcriptional STOP cassette upstream of the DTR gene is intact, and therefore DTR is not expressed. At DT doses ≥75μg/kg, we observed death in some animals, likely due to non-specific toxicity (Suppl. Fig. 1). For subsequent experiments in PTHcre-iDTR mice, we therefore chose DT doses ≤50 μg/kg body weight; these doses have also been used by other investigators [25].
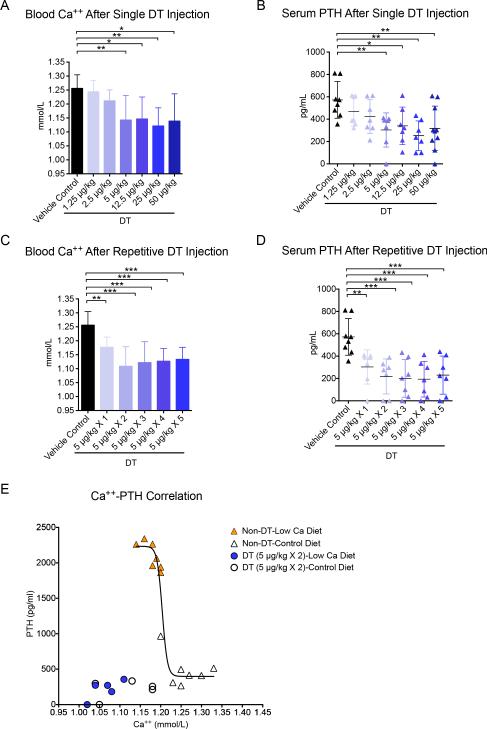
Next, dose finding studies for the ablation of parathyroids were performed in PTHcre-iDTR mice using single i.p. administration of ascending doses of DT. The efficacy was assessed by measuring blood ionized calcium (Ca++) and serum PTH levels three days later. A single dose of 5 μg/kg body weight led to hypocalcemia (Ca++=1.14±0.09 mmol/L) and low PTH levels (PTH=303±153 pg/mL, limit of detection=4 pg/mL) compared to vehicle control (Ca++=1.26±0.05 mmol/L; PTH=572±164 pg/mL) (Fig. 1 a-b). Higher doses of DT did not lead to more severe hypocalcemia. Next, we performed repeated dosing experiments at a fixed dose of 5 μg/kg body weight with 2 to 5 doses given at 3 day intervals. After two DT injections given three days apart, blood Ca++ and serum PTH were lower than after a single injection (Ca++=1.10±0.07 mmol/L; PTH=218±156 pg/mL, p<0.05 vs. single injection), and no further decrease was observed with more frequent administration of DT (Fig. 1 c-d). Doses lower than 5 μg/kg, even when injected multiple times, showed lower efficacy in reducing blood Ca++ and serum PTH (data not shown). Thus we determined that two i.p. injections 3 days apart at 5 μg/kg body weight constitute an optimized dose regimen to induce hypoparathyroidism in this mouse model.
Figure 1.
Dose optimization of DT injection in PTHcre-iDTR mice. (A-B) Blood Ca++ and serum PTH after a single i.p. injection of DT at 5 μg/kg (N=8-12, *p<0.05, **p<0.01, ***p<0.001). (C-D) Blood Ca++ and serum PTH after one to five (as indicated) repetitive doses of DT, each one three days apart, at 5 μg/kg (N=6-12, *p<0.05, **p<0.01, ***p<0.001). (E) Correlation between Ca++ and PTH in hypoparathyroid vs. parathyroid-intact mice. Blood for Ca++ and PTH measurements was collected from PTHcre-iDTR mice 3 days after the last DT injection (2 i.p. injection 3 days apart at 5 μg/kg) on normal chow. The sigmoidal iCa-PTH correlation was obtained from the values in parathyroid-intact, non-DT injected PTHcre-iDTR mice fed a normal or low calcium diet for 2 weeks (N=7-8).
To evaluate the degree to which the PTH response is impaired in our hypoparathyroidism model, we compared it to parathyroid-intact mice which were rendered hypocalcemic by dietary means. In parathyroid-intact control animals (PTHcre-iDTR without injecting DT) fed a low-calcium diet for 2 weeks, hypocalcemia was similar to those in the DT model on regular calcium diet (1.18±0.02 vs. 1.12±0.07 mmol/L), and PTH levels were almost ten times higher than in DT-treated mice (2034±388 vs. 220±131 pg/mL). Plots of Ca++ vs. PTH levels in the parathyroid-intact and DT-ablated animals confirmed the hypoparathyroid phenotype in the DT-treated mice. Challenging DT-treated hypoparathyroid mice by low calcium diet for two weeks led to a further decrease in Ca++ compared to DT-treated mice on regular diet (1.06±0.04 vs. 1.12±0.07 mmol/L) but no additional increase in PTH (217±136 pg/mL vs. 220±131 pg/mL). We conclude that PTH is maximally stimulated in these hypoparathyroid mice (Fig. 1 e).
As expected in hypoparathyroidism, serum Pi levels significantly increased after ablating the parathyroids with DT (Fig. 2 o). Total urinary calcium to urinary creatinine (uCa/uCr) did not decrease in DT-injected mice compared to control mice, despite lower serum calcium and therefore a lower filtered load of calcium (Fig. 2 p). This suggests that the fractional excretion of calcium was increased in DT injected mice, an observation consistent with hypoparathyroidism. The hypoparathyroid phenotype remained stable over 90 days after DT injection (Fig. 2 q).
Figure 2.
Characterization of PTHcre-iDTR mice after DT injection. (A-N) Representative parathyroid gland with surrounding thyroid gland. (A-C): Before DT injection. Intact parathyroid gland with robust PTH staining and no TUNEL positive cells in the parathyroid region. (D-F): 3 days after DT injection (2 i.p. injections at 5 μg/kg 3 days apart). In the parathyroid glands, increased vascularity is observed (D), dramatic decrease in PTH immunoreactivity (E), and abundant apoptotic TUNEL positive cells (F). (G-N): 1 month after vehicle or DT injection (2 i.p. injections at 5 μg/kg 3 days apart). In vehicle control, parathyroid glands (G) shows strong GFP (H) and PTH (I) staining (using Alexa Fluor 488 for GFP and 633 Far-red secondary antibody for PTH). In DT injected mice, the normal structure of the parathyroid glands is lost (K), and most GFP and PTH immunoreactivity no longer detectable. However, a few GFP and PTH double positive cells (L-N) remained. (O) Serum Pi levels in PTHcre-iDTR mice three days after DT or vehicle injection (N=12, ***p<0.001). (P) Urinary calcium excretion in PTHcre-iDTR mice three days after DT or vehicle injection (N=12). (Q) Serum PTH and blood ionized calcium levels in PTHcre-iDTR mice after DT vs. vehicle injection over 3 months. (N=6-8, **p<0.01, ***p<0.001).
To investigate the fate of the parathyroid glands after DT injection, we first examined histological sections of the thyroid and parathyroid tissue obtained from mice three days after the DT injections. The parathyroid gland structure was grossly intact, but there was an increased number of blood vessels, and almost all PTH immunoreactivity had disappeared (Fig. 2 a, b, d, e). In addition, we detected substantial apoptotic cell death in the parathyroid glands by TUNEL staining compared to TUNEL staining that appears negative in normal mice (Fig. 2 c, f). Next, we examined the parathyroid gland one month after DT injections. Because of the difficulties to locate the location of the ablated glands, we performed DT injection in PTHcre-iDTR-mTmG mice, which carry a ROSAmTmG allele and therefore have green fluorescent parathyroid glands. While parathyroid glands could no longer be visualized under the fluorescent dissecting microscope, screening of sections through a larger portion of the neck region around the thyroid under the fluorescent microscope revealed a few dispersed GFP-positive cells. Co-immunostaining with GFP and PTH antibodies demonstrates that the green cells are in fact positive for PTH (Fig. 2 g-n). More injections of DT at later time points (one or 3 months after the 2 doses of 5 μg/kg DT injection) did not further change blood Ca++ and PTH levels of the animals. (data not shown).
GFP-PTX mice
For the second mouse model of acquired hypoparathyroidism, mice homozygous for the double-fluorescent Cre reporter ROSAmT/mG were used [22]. All cells in the ROSAmT/mG mouse express membrane-targeted tandem dimer Tomato (tdTomato), whereas in the presence of an expressed Cre allele, tdTomato is silenced, and membrane-targeted green fluorescent protein is expressed [22]. The ROSAmT/mG mice were mated to homozygous PTH-Cre mice [23] (in which the genotype was confirmed by genome walking; Suppl. Fig. 2), to thus generate PTH-Cre;ROSAmT/mG mice that selectively express GFP in the parathyroid glands. In wildtype mice, the two parathyroid glands were indistinguishable from surrounding tissue (Fig. 3 a), but in PTH-Cre;ROSAmT/mG mice they were easily visualized under UV illumination (Fig. 3 b-c). GFP-guided surgical removal of the parathyroid glands was thus performed using a stereomicroscope under UV illumination (Fig. 3 d-f), to thus generate GFP-PTX mice. Because the GFP greatly facilitated parathyroidectomy, the entire procedure took less than 20 minutes, and was precise, as the thyroid gland was kept intact. Moreover, overall mortality was low, as the GFP-PTX mice had a survival rate at 30 days similar to that of sham-operated mice (90% vs. 95%) (Fig. 3 g). Three days after surgery, GFP-PTX mice displayed marked reductions of blood Ca++ and serum PTH levels, compared to levels found in sham-operated mice (Ca++=1.05±0.40 vs. 1.30±0.03 mmol/L; PTH=32±22 vs. 580±137 pg/mL) (Fig. 3 hi). As expected, serum Pi was significantly increased in GFP-PTX mice (Fig. 3 j). Total urinary calcium excretion did not statistically differ between PTX and sham control mice (Fig. 3 k), again suggesting a higher rate of urinary calcium clearance in the absence of PTH. The hypoparathyroidism and consequent hypocalcemic phenotype was stable over a 3-month observation period (Fig. 3 l). Hypoparathyroid mice of both models were viable and we did not observe seizures.
Figure 3.
Hypoparathyroid phenotype of GFP-PTX mice. (A-F) Photographs of the neck region of PTH-Cre;ROSAmT/mG mice before and after parathyroidectomy. (A) Under halogen light, mouse parathyroid glands cannot easily be distinguished from the surrounding tissue. (B, C) Under UV illumination, GFP-positive parathyroid glands are easily identified. (D-F) Using fine scissors and forceps, green parathyroid glands were selectively removed. (F) Location of thyroid and parathyroids are outlined in red and white, respectively. (G) Survival rate of GFP-PTX mice compared to animals that underwent sham surgery (N=20). (H-K) Blood Ca++, serum PTH, serum Pi, and urinary calcium excretion (uCa/uCr) in GFP-PTX mice 3 days after surgery (N=10-12, ***p<0.001). (L) Serum PTH and blood ionized calcium levels in GFP-PTX vs. sham-operated mice over 3 months (N=6-8, ***p<0.001).
Treatment with long-acting PTH
We then used each of these mouse models of hypoparathyroidism to study the calcium- and phosphate-regulating actions of a long-acting PTH analog LA-PTH, as compared to those of unmodified PTH(1-34) [8]. Each peptide was injected subcutaneously at doses of either 1.5, 5, 10 or 20 nmol/kg for LA-PTH, and at a single dose of 50 nmol/kg for PTH(1-34). In both GFP-PTX and DT-DTR mice, robust, dose-dependent increases in blood Ca++ were observed by two hours post-injection of LA-PTH, and at the highest doses, the effects persisted for as long as 72 hours (Fig. 4 a-b). At the 5 nmol/kg dose, LA-PTH elevated serum calcium levels in GFP-PTX mice to about the normal range for at least 24 hours. In contrast, PTH(1-34) at 50 nmol/kg induced a similar maximal increase in calcium in GFP-PTX mice, but the response peaked at 2 hours and returned to baseline levels by 8 hours (Fig. 4 a-b). LA-PTH also induced a longer-lasting reduction in serum phosphate than did PTH(1-34). Two hours after injection of LA-PTH at doses ranging from 1.5 to 20 nmol/kg, serum Pi levels in both mouse models decreased significantly and remained low for at least 8 hours before returning to baseline levels by 24 hours post-injection. PTH(1-34), by contrast, decreased serum Pi only at the two hour time point, as baseline levels were again observed at 4 hours (Fig. 4 c-d). Spot-urinary calcium levels were unchanged by injection with LA-PTH at any dose (Fig. 4 e-f).
Figure 4.
LA-PTH injection in two mouse models of acquired hypoparathyroidism. Blood Ca++ levels, blood Pi levels and urinary calcium excretion in GFP-PTX (A, C, E) and DT-treated PTHcre-iDTR mice (B, D, F) after a single s.c. injection of different doses of LA-PTH, as indicated, or PTH(1-34). (N=5-7, * p<0.05, **p<0.01, ***p<0.001). Color-shaded curves represent the reference range (mean ± SD) of control animals.
Binding to and activation of the PTH1-receptor by parathyroid hormone leads to increases in cellular cAMP production. We measured urinary cAMP levels after administration of LA-PTH as a direct readout of PTH1R activation in target tissues. Baseline urinary cAMP levels (ucAMP) adjusted for urinary creatine (uCr) were significantly lower in GFP-PTX mice than in sham control mice, consistent with the lower circulating PTH levels (Fig. 5 a). After LA-PTH injection, urinary cAMP increased and peaked at two hours, and the increase was detected for ~16 hours at doses of 10 nmol/kg. In comparison, PTH(1-34) at a dose of 50 nmol/kg increased urinary cAMP only at the two-hour time point, as the levels returned to baseline by 4 hours (Fig. 5 b). The extended urinary cAMP response observed here for LA-PTH in GFP-PTX mice is consistent with observed for this analog previously in wildtype mice [8].
Figure 5.
LA-PTH injection in GFP-PTX mice. Urinary cAMP excretion and serum 1,25(OH)2D3 levels at baseline (A, C) and after a single s.c. injection of different LA-PTH doses as indicated, and PTH(1-34) (B, D). (N=5-8, * p<0.05, **p<0.01, ***p<0.001).
At baseline, serum 1,25(OH)2D3 levels in GFP-PTX mice were lower compared to sham control (Fig. 5 c). LA-PTH administration led to dose-dependent increases in serum 1,25(OH)2D3, which at a dose the 10 nmol/kg, lasted for at least 24 hours, whereas PTH(1-34) at a dose of 24 nmol/kg increased 1,25(OH)2D3 levels maximally at 2 hours, and the levels returned to baseline by 12 hours (Fig. 5 d).
Discussion
We describe two new mouse models for acquired hypoparathyroidism, a disease for which an efficient mouse model was lacking. In the PTHcre-iDTR mice, an inducible human diphtheria toxin receptor (DTR) was used for parathyroid gland cell-specific ablation by diphtheria toxin injection. We determined that two i.p. DT injections at 5 μg/kg body weight resulted in efficient ablation of most of the parathyroid cells, leading to mice with chronic hypoparathyroidism. By three-days post-DT injection we observed abundant cell apoptosis in the parathyroid glands by TUNEL staining, and one month after injection, parathyroid glands could no longer be identified. The hypoparathyroid phenotype was not particularly severe in this model, as mean blood ionized calcium levels were 1.12 vs. 1.26 mmol/L in control animals, yet the low levels were stable over time. One month after the DT ablation, we observed a few dispersed GFP-positive cells in the region of the parathyroids. These cells are likely to be remnants of the parathyroid glands that escaped or resisted DT treatment. As shown by the stable persistence of low blood calcium and PTH levels over 3 months, these cells are not able to regenerate the parathyroids in this time. We conclude, therefore, that DT treatment is not 100% efficient, and that the residual cells are the most likely source of the detectable PTH in the circulation and the milder hypoparathyroid phenotype. Nevertheless, the model is useful as it compares generally to conditions seen in hypoparathyroid patients with mild forms of the disease, for which mean levels of hypocalcemia can vary widely [26]. A noteworthy advantage of this DTR model is that it is simple to induce, as only two intraperitoneal injections of DT are needed to achieve a stable hypoparathyroid state. This enables large numbers of experimental animals to be generated within a few hours, and without the need for surgical procedures.
The second mouse model for acquired hypoparathyroidism is based on surgical parathyroidectomy, the surgery is greatly facilitated, however, by expression of GFP specifically in the parathyroid glands. This modification further allows for preservation of the thyroid gland and thus overcomes a serious limitation of TPTX surgery. The benefit of the GFP-guided approach is further reflected by the short time it takes to accomplish the procedure (<20 minutes per animal), the high efficiency (95% of mice were hypocalcemic), and the stability of the phenotype. The GFP-PTX mice exhibited a more severe phenotype, with mean ionized calcium levels at 1.05 mmol/L, as compared to 1.12 mmol/L in DT-DTR mice, and 1.30 mml/L in sham-operated animals. Depending on the desired severity of the phenotype, and on the resources available for surgical procedures, either of these mouse models could be used as an animal model for acquired hypoparathyroidism.
Finally, the utility of these models was demonstrated by studying the effects of PTH analog administration on calcium and Pi homeostasis. LA-PTH was thus found to be particularly effective in controlling serum Ca and Pi levels. Studies on the mechanism by which LA-PTH and related long-acting analogs induce prolonged responses in vivo suggest that it does not involve enhanced pharmacokinetics, but rather a unique mode of pseudo-irreversible binding to the PTH receptor in target cells, and hence persistent signaling responses, possibly from within internalized cell compartments [8, 27]. We indeed confirmed the persistent cAMP signaling actions of the ligand in the HP mice by measuring urinary cAMP output [8]. This translated into prolonged calcemic and hypophosphatemic responses lasting for over 24 hours, depending on the dose. The analog thus appears to be more efficacious than PTH(1-34) in regulating blood Ca++ and Pi levels in at least these animal models of HP.
An increase in renal calcium reabsorption by LA-PTH could potentially mean an important advantage over oral Ca and vitamin D treatment now used by most HP patients. Because of the small size of mice, however, it is technically challenging to precisely quantify rates of urinary calcium excretion, and so such measurements were not performed in the current studies. Further work is therefore needed to understand better how LA-PTH can alter rates of Ca filtration in the kidney, and how these effects might vary with dose and over time of treatment.
Supplementary Material
Acknowledgment
This work was supported by the NIH grants R01-DK100584 and X01 NS073537-01 (MM), P01-DK11794, project I (TJG), T32DK007028 (KL), and China State Key Laboratory of Oral Diseases Open Funding SKLOD2015OF01 (RB). This research was supported in part by the BrIDGs Program, the NIH Common Fund and the National Center for Advancing Translational Sciences. We thank Wenping Zhao for technical help, Henry Kronenberg, John Potts and Deborah Mitchell for comments on this manuscript, and Elizabeth Monis for proofreading.
References
- 1.Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med. 2008;359(4):391–403. doi: 10.1056/NEJMcp0803050. [DOI] [PubMed] [Google Scholar]
- 2.Underbjerg L, et al. Postsurgical hypoparathyroidism--risk of fractures, psychiatric diseases, cancer, cataract, and infections. J Bone Miner Res. 2014;29(11):2504–10. doi: 10.1002/jbmr.2273. [DOI] [PubMed] [Google Scholar]
- 3.Bilezikian JP, et al. Hypoparathyroidism in the adult: Epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26(10):2317–37. doi: 10.1002/jbmr.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FDA. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm431358.htm.
- 5.Mannstadt M, et al. Efficacy and safety of recombinant human parathyroid hormone (1-84) in hypoparathyroidism (REPLACE): a double-blind, placebocontrolled, randomised, phase 3 study. Lancet Diabetes Endocrinol. 2013;1(4):275–83. doi: 10.1016/S2213-8587(13)70106-2. [DOI] [PubMed] [Google Scholar]
- 6.Sikjaer T, et al. PTH(1-84) replacement therapy in hypoparathyroidism: a randomized controlled trial on pharmacokinetic and dynamic effects after 6 months of treatment. J Bone Miner Res. 2013;28(10):2232–43. doi: 10.1002/jbmr.1964. [DOI] [PubMed] [Google Scholar]
- 7.Rubin MR, et al. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int. 2010;21(11):1927–34. doi: 10.1007/s00198-009-1149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda A, et al. Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc Natl Acad Sci U S A. 2013;110(15):5864–9. doi: 10.1073/pnas.1301674110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farra R, et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci Transl Med. 2012;4(122):122ra21. doi: 10.1126/scitranslmed.3003276. [DOI] [PubMed] [Google Scholar]
- 10.Leone-Bay A, et al. Oral delivery of biologically active parathyroid hormone. Pharm Res. 2001;18(7):964–70. doi: 10.1023/a:1010936227570. [DOI] [PubMed] [Google Scholar]
- 11.Tang J, et al. First in Man Studies of Pharmacokinetic Profiles of a Novel Oral PTH(1-34). [June 22, 2015];J Bone Miner Res. 2014 29(Suppl 1) Available at http://www.asbmr.org/education/AbstractDetail?aid=990476c3-4440-4363-a391-6559b1e59568. [Google Scholar]
- 12.Bryant H, et al. Evolution of a Long-Acting Parathyroid Hormone Analog for the Treatment of Hypoparathyroidism. [June 22, 2015];J Bone Miner Metab. 2014 29(Suppl 1) Available at http://www.asbmr.org/education/AbstractDetail?aid=c32adb0c-27a3-4d2d-91ec-135004cfb35a. [Google Scholar]
- 13.Miao D, et al. Skeletal abnormalities in Pth-null mice are influenced by dietary calcium. Endocrinology. 2004;145(4):2046–53. doi: 10.1210/en.2003-1097. [DOI] [PubMed] [Google Scholar]
- 14.Gunther T, et al. Genetic ablation of parathyroid glands reveals another source of parathyroid hormone. Nature. 2000;406(6792):199–203. doi: 10.1038/35018111. [DOI] [PubMed] [Google Scholar]
- 15.Hough TA, et al. Activating calcium-sensing receptor mutation in the mouse is associated with cataracts and ectopic calcification. Proc Natl Acad Sci U S A. 2004;101(37):13566–71. doi: 10.1073/pnas.0405516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebastian EM, Suva LJ, Friedman PA. Differential effects of intermittent PTH(1-34) and PTH(7-34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone. 2008;43(6):1022–30. doi: 10.1016/j.bone.2008.07.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berdud I, et al. The PTH-calcium relationship during a range of infused PTH doses in the parathyroidectomized rat. Calcif Tissue Int. 1998;62(5):457–61. doi: 10.1007/s002239900460. [DOI] [PubMed] [Google Scholar]
- 18.Finco DR, et al. Selective parathyroidectomy of the dog. Can J Vet Res. 1993;57(4):288–92. [PMC free article] [PubMed] [Google Scholar]
- 19.Gates FL, Grant JH. Experimental Observations on Irradiated, Normal, and Partially Parathyroidectomized Rabbits : I. The Effects of Partial Parathyroidectomy. J Exp Med. 1927;45(1):115–24. doi: 10.1084/jem.45.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox J, et al. Effect of low phosphorus diets on intestinal calcium absorption and the concentration of calcium-binding protein in intact and parathyroidectomized pigs. J Endocrinol. 1978;78(3):379–87. doi: 10.1677/joe.0.0780379. [DOI] [PubMed] [Google Scholar]
- 21.Sakai A, et al. Osteoclast development in immobilized bone is suppressed by parathyroidectomy in mice. J Bone Miner Metab. 2005;23(1):8–14. doi: 10.1007/s00774-004-0534-y. [DOI] [PubMed] [Google Scholar]
- 22.Muzumdar MD, et al. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 23.Libutti SK, et al. Parathyroid gland-specific deletion of the mouse Men1 gene results in parathyroid neoplasia and hypercalcemic hyperparathyroidism. Cancer Res. 2003;63(22):8022–8. [PubMed] [Google Scholar]
- 24.Buch T, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2(6):419–26. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 25.Hatori M, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS One. 2008;3(6):e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell DM, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab. 2012;97(12):4507–14. doi: 10.1210/jc.2012-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okazaki M, et al. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc Natl Acad Sci U S A. 2008;105(43):16525–30. doi: 10.1073/pnas.0808750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.