Summary
Objectives
We aimed to determine whether implementation of a structured multi-disciplinary EEG monitoring pathway improved the timeliness of anti-seizure medication administration in response to electrographic seizures in encephalopathic critically ill children.
Methods
A multidisciplinary team developed a pathway to standardize EEG monitoring and seizure management in encephalopathic critically ill children, aiming to decrease the time from electrographic seizure onset to anti-seizure medication administration. Data was collected to inform the team of improvement opportunities which were then provided by an institutional pathway, staff education, and streamlined communication. Measurements were obtained prior to and after pathway implementation to assess for improvement.
Results
We collected data on 41 patients before and 21 after pathway implementation. There were no differences between the baseline and pathway groups in demographic characteristics, acute encephalopathy etiologies, or anti-seizure medications utilized. The median duration from seizure onset to anti-seizure medication administration was shorter for patients treated with the pathway (64 minutes [50, 101]) compared to patients treated prior to pathway implementation (139 minutes [71, 189]) (p=0.0006). The interval from seizure onset to anti-seizure medication order was shorter for the pathway group (31 minutes [20, 49]) than the baseline group (71 minutes [33, 131]) (p=0.003). The interval from anti-seizure medication order to administration was shorter for the pathway group (30 minutes [19, 40]) than the baseline group (40 minutes [17, 68]) (p=0.047). Seizure termination was more likely to occur following initial anti-seizure medication administration in the pathway than baseline group (67% vs. 27%, p=0.002).
Significance
Implementation of the pathway resulted in a significant reduction in the duration between electrographic seizure onset and anti-seizure medication administration, and a significant increase in the rate of electrographic seizure termination following an initial anti-seizure medication. Further study is needed to determine whether these changes are associated with improved outcomes.
Keywords: Seizure, critical care, anti-seizure medication, pathway, quality improvement
Introduction
Electrographic seizures and status epilepticus have been reported in 10–40% of children in pediatric intensive care units (ICU) who underwent clinically indicated continuous EEG monitoring (cEEG).1–14 In addition to serving as biomarkers of brain injury and dysfunction, electrographic seizures and status epilepticus are associated with higher mortality and worse neurobehavioral outcomes among survivors.1; 9; 10; 12; 15–20 Further, the cost of identifying a child experiencing electrographic seizures is modest,21 and data obtained from cEEG often impact clinical management.22 In the context of these data, physicians report increasing cEEG use in pediatric ICUs,23; 24 and recent guidelines and consensus statements recommend performing cEEG in many critically ill children with acute encephalopathy to identify electrographic seizures and status epilepticus.25–27
Seizures and status epilepticus are among the most common neurologic conditions managed in pediatric ICUs by multi-disciplinary teams of intensivists and neurologists.28; 29 Unfortunately, while seizures are often encountered, substantial variability exists in management, and treatment delays have been reported.30–33 Further, convulsive status epilepticus management delays are common and associated with more prolonged seizures and lower anti-seizure medication responsiveness.15; 34–37
As this new evidence became available, an increase in the number of patients undergoing cEEG in our pediatric ICU was noted based on the hypothesis that electrographic seizure identification and management might reduce secondary brain injury and improve outcomes. Since several observational studies indicated that brief exposures to electrographic seizures are not associated with worse outcomes while longer exposures are associated with worse outcomes,17–19 we expected rapid intervention would be required if we aimed to improve outcomes by identifying and managing electrographic seizures. To improve electrographic seizure management, a multi-disciplinary group developed a pathway for EEG monitoring in critically ill patients based on current evidence and guidelines. This detailed plan of care was then implemented with the goal of reducing time to seizure treatment. As described below, data was collected before and then after implementation of the pathway. In this report, we describe our initial performance data, the implemented improvement strategy, and our subsequent performance data.
Methods
We collected data regarding electrographic seizure management prior to this work (January 2010–September 2011), then developed and implemented a pediatric ICU cEEG pathway, and finally collected data during a subsequent time period (September 2014–June 2015). Publication of data from the quality improvement project did not require full review by the Institutional Review Board.
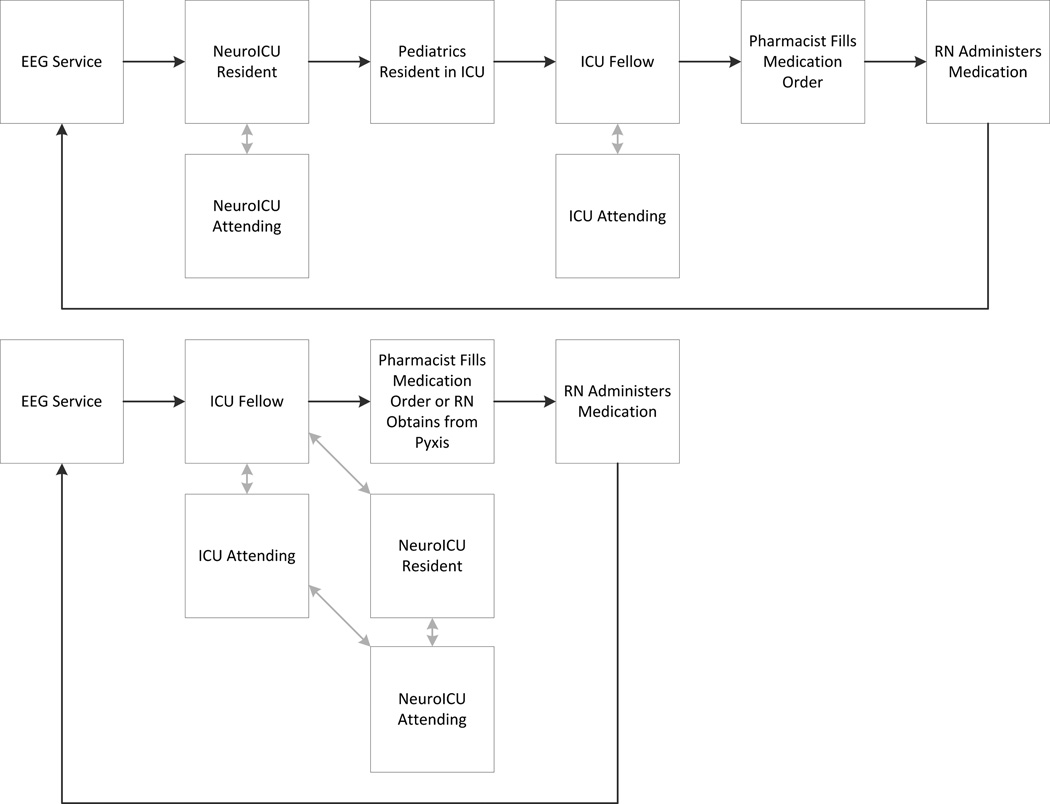
Prior to pathway implementation, consultation/communication and seizure management were individualized without a specified system. Numerous phone calls were required among busy front line clinicians. An example workflow process is provided in Figure 1 (top). After initial management steps were completed the cEEG tracing would be reviewed again to evaluate the impact of the medication and the entire communication cycle would repeat.
Figure 1.
Multi-disciplinary communication structure before (top) and after (bottom) the intensive care EEG monitoring pathway was implemented. In each pathway, following anti-seizure medication administration, the EEG would be rechecked to see if seizures persisted and the workflow would repeat.
Following review of this process and the initial time data, a multi-disciplinary group convened. The multi-disciplinary group was composed of electroencephalographers, registered EEG technologists, critical care neurology consultation physicians, critical care medicine physicians, critical care nurses, a pharmacist, and quality improvement experts. Over six months it reviewed evidence and current processes related to cEEG in critically ill children with the goal of developing a pathway which detailed the care processes for EEG monitoring and seizure management. The team aimed to reduce the time between electrographic seizure onset and anti-seizure medication administration, identified potential sources of variability and management delay (Table 1), and then worked to develop and implement an ICU EEG monitoring pathway. An initial draft was reviewed by all important stakeholders and necessary revisions were incorporated, and the final pathway was posted on the institution pathway website which is available online38 with key components provided in Table 2). It included educational resources regarding EEG monitoring and seizure management, and it provided a streamlined process for results communication and seizure management. An example workflow of the pathway guided communication strategy is provided in Figure 1 (bottom).
Table 1.
Potential Sources of Management Variability and Delay.
Staffing and Personnel:
|
Structural:
|
Knowledge Based:
|
Table 2.
Components of the Intensive Care Unit Continuous EEG Monitoring Pathway Implementation.
Multi-disciplinary Team Development
|
Staff Education
|
Daily Workflow Modifications
|
Although the pathway was implemented for all patient care, we selected a specific patient cohort for measurement to assess whether the implemented changes resulted in improvement. This included patients with the following criteria: (1) age < 18 years, (2) admitted to the pediatric ICU, (3) not admitted for epilepsy surgery admission, (4) electrographic seizures identified by cEEG, (5) no clinical seizures while in the ICU undergoing EEG monitoring since these would be apparent to bedside caregivers and impact medication timing apart from EEG monitoring guided management, (6) no pre-existing diagnosis of epileptic encephalopathy (i.e. hypsarrhythmia or slow spike-wave) which would require neurologist guided individualized epilepsy management, and (7) electrographic seizures for which management was definitely indicated based on standard care at our institution. Management was definitely indicated for patients with electrographic status epilepticus or when multiple >1 minute electrographic seizures occurred within an hour of recording. Patients with only one seizure or several brief seizures might not have had anti-seizure medications initiated based on a clinical decision that treatment was not necessary.
Data were gathered from review of the electronic medical record (clinical characteristics, medication orders, and administration timing) as well as EEG reports and tracings (seizure onset timing, seizure cessation timing). Data gathered included age, sex, dates of admission to the hospital and ICU, dates of discharge from the ICU, dates and duration of cEEG, presence of clinically evident seizures prior to initiation of cEEG, acute encephalopathy etiology category (epilepsy, acute structural, acute non-structural), electrographic seizure characteristics, initial anti-seizure medication administered for electrographic seizures identified by cEEG, whether electrographic seizures terminated for at least 12 hours after the initial anti-seizure medication was administered, and the total number of anti-seizure medications administered for electrographic seizures. Dates and times collected included electrographic seizure onset date/time, anti-seizure medication order date/time, and anti-seizure medication administration date/time. These timing data allowed calculation of (1) total time from electrographic seizure onset to medication administration, (2) time from electrographic seizure onset to medication order, and (3) time from medication order to medication administration.
Statistical analyses were performed using Stata 12 (College Station, Texas). The central tendency of continuous data are reported as median [interquartile range, IQR]. The Wilcoxon rank sum, chi square, and log rank tests were performed to compare characteristics of the baseline and pathway groups. Timing data are displayed using a Kaplan-Meier survival curve showing the time between two events (electrographic seizure onset and anti-seizure medication administration, electrographic seizure onset and anti-seizure medication order, anti-seizure medication order and anti-seizure medication administration).
Results
The overall structure of the clinical services did not change substantially during the period under investigation. Patients were cared for in a closed pediatric ICU composed of a board certified pediatric intensivist, fellows, frontline residents and nurse practitioners. All medications were ordered by pediatric ICU providers. Patients with any type of acute neurologic problem, including seizures, were also cared for by the critical care neurology consultation service. Most attending physicians on both services only performed 2–3 months of service coverage per year. Further, both services were staffed by residents and fellows who were either part of our institution’s training programs or rotating for brief periods from other institutions. Both before and after pathway initiation, patients with any type of acute neurologic condition and encephalopathy underwent 1–2 days of cEEG to identify electrographic seizures as part of our pediatric ICU’s standard care, consistent with recommendations from the Neurocritical Care Society’s Guideline on the Evaluation and Management of Status Epilepticus25 and the American Clinical Neurophysiology Society’s Consensus Statement on Continuous EEG Monitoring in Critically Ill Children and Adults.26; 27 Registered EEG technologists were continuously in the hospital, and they initiated monitoring and performed periodic screening for electrographic seizures at least every six hours. Pediatric EEG fellowship trained physicians interpreted the EEG tracing formally every morning with written reports. Additionally, they viewed the EEG tracing at any time if the clinical teams or EEG technologists noted any changes. Remote access was available. Quantitative EEG seizure identification techniques were not used at bedside for seizure identification. Although the overall pharmacy structure did not change, as part of the pathway implementation levetiracetam was provided in the pediatric ICU Pyxis medication dispensing machines so it could be administered without needing preparation and delivery from the pharmacy.
The study evaluated 41 patients before and 21 patients after pathway implementation. There were no differences between the baseline and pathway groups in demographic characteristics including male sex (baseline 61%, pathway 57%, p=0.77) and median age (baseline 14 months [4, 71], pathway 32 months [10, 78], p=0.32). The primary acute encephalopathy etiology categories were not significantly different between groups (p=0.24) which included epilepsy (24% baseline, 42% pathway), hypoxic ischemic encephalopathy (20% baseline, 4% pathway), stroke (15% baseline, 10% pathway), traumatic brain injury (20% baseline, 10% pathway), central nervous system infection (10% baseline, 5% pathway), metabolic-systemic disorders (7% baseline, 14% pathway), and other structural disorders (5% baseline, 14% pathway). There was no difference in the presence of electrographic status epilepticus between groups (93% baseline, 81% pathway) (p=0.17).
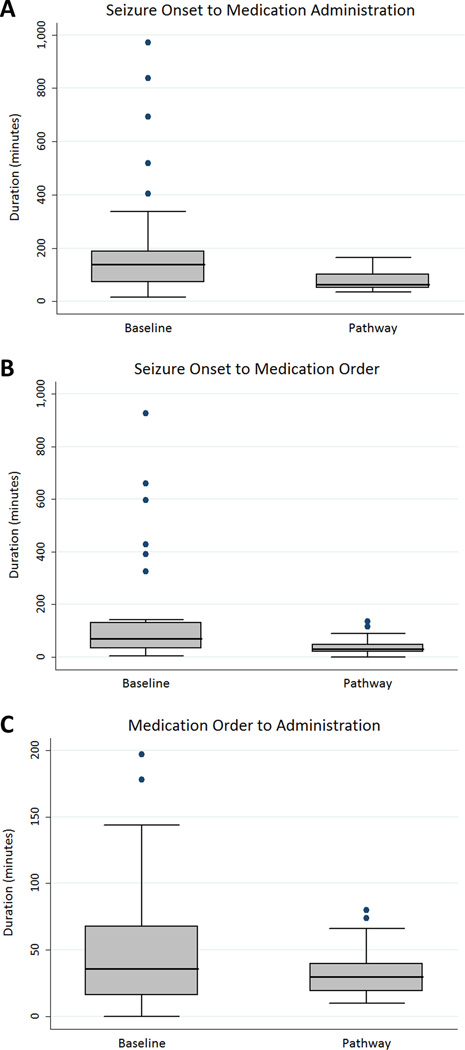
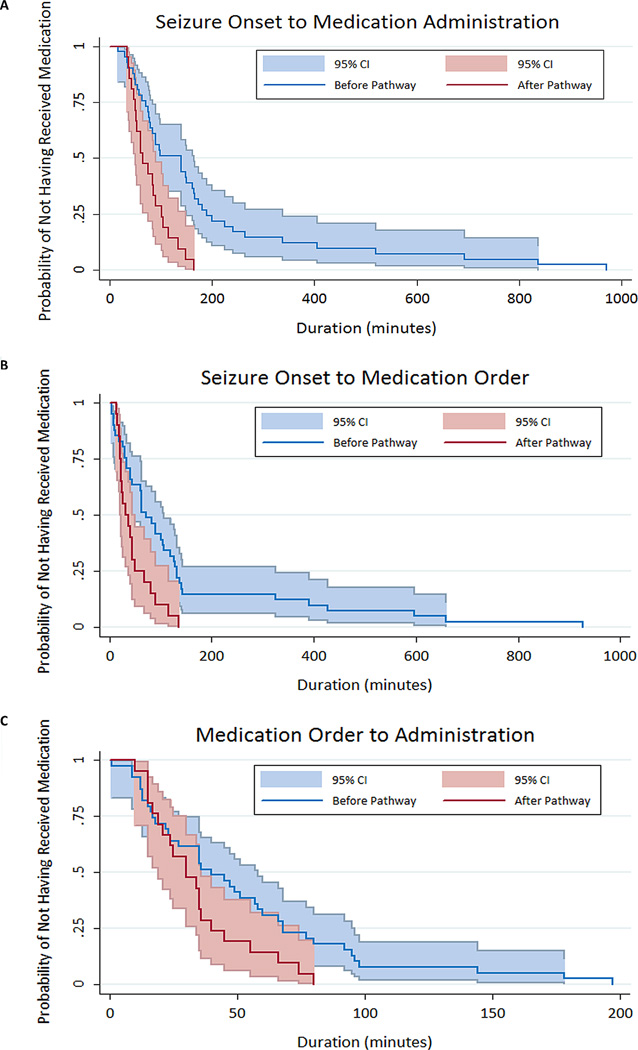
The median time from seizure onset to anti-seizure medication administration was shorter in the pathway group (64 minutes [50, 101]) than in the baseline group (139 minutes [71, 189]) (log-rank test for equality of survivor functions p=0.0006). The seizure onset to anti-seizure medication administration interval can be divided into two sub-intervals: (1) seizure onset to medication order, and (2) medication order to administration. The median interval from seizure onset to anti-seizure medication order was shorter for the pathway group (31 minutes [20, 49]) than the baseline group (71 minutes [33, 131]) (log-rank test for equality of survivor functions p=0.003). The median interval from anti-seizure medication order to administration was shorter for the pathway group (30 minutes [19, 40]) than the baseline group (40 minutes [17, 68]) (log-rank test for equality of survivor functions p=0.047). Box-and-whisker plots are provided in Figure 2. Kaplan-Meier survival curves are provided in Figure 3. All subjects experienced an outcome (3a and 3c= received an anti-seizure medication; 3b = anti-seizure medication was ordered).
Figure 2.
Box-and-Whisker plots comparing patients before and after the intensive care unit EEG monitoring pathway was implemented in terms of (a) the total duration from seizure onset to anti-seizure medication administration, (b) the duration from seizure onset to anti-seizure medication order, and (c) the duration from anti-seizure medication order to medication administration. Each box plot shows the median, upper and lower quartiles (top and bottom of the shaded box), the maximum and minimum values excluding outliers (whiskers), and the outliers (individual dots).
Figure 3.
Kaplan-Meier Survival Curve comparing patients before (blue) and after (red) the intensive care unit EEG monitoring pathway was implemented in terms of (a) the total duration from seizure onset to anti-seizure medication administration, (b) the duration from seizure onset to anti-seizure medication order, and (c) the duration from anti-seizure medication order to medication administration.
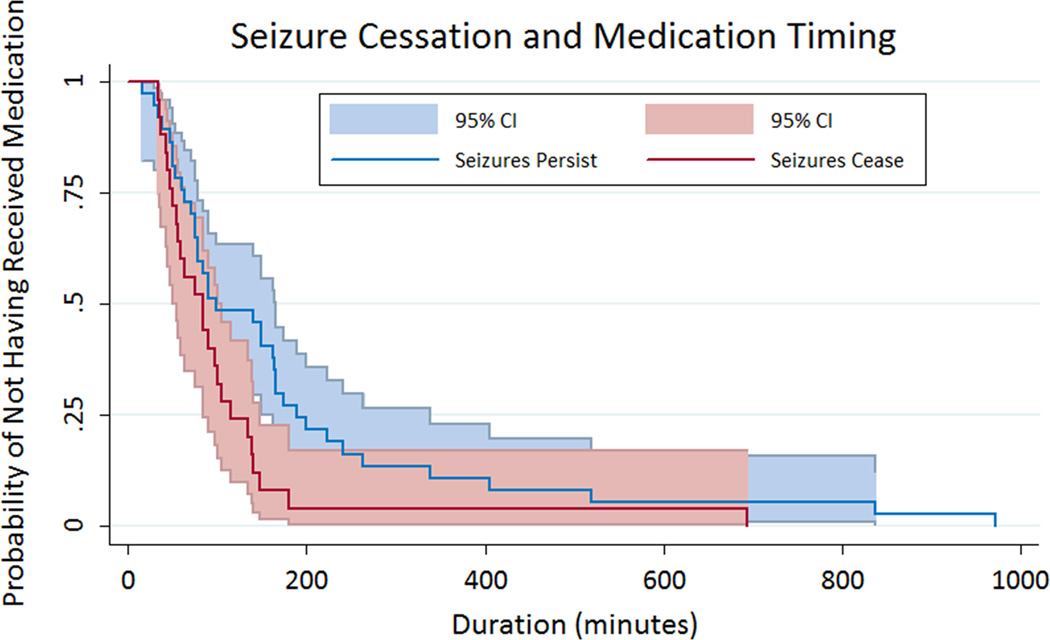
While the sample size was small, we also evaluated relationships between anti-seizure medication administration timing and seizure cessation after the initial medication. The most commonly administered initial anti-seizure medications were levetiracetam in 33 patients (53%), phenobarbital in 12 patients (19%), and phenytoin in 10 patients (16%), and these did not differ between the baseline and pathway groups (p=0.17). The median number of anti-seizure medications utilized did not differ in the baseline group (2 [1, >5] and pathway groups (1 [1, 3]) (p=0.32). The initial anti-seizure medication was less likely to be followed by seizure termination in the baseline group (11 of 41 patients, 27%) than the pathway group (14 of 21 patients, 67%) (p=0.002). The Kaplan-Meier survival curve is provided in Figure 4 and indicates that patients with electrographic seizure cessation after the initial anti-seizure medication were more likely to have received their initial anti-seizure medication sooner after electrographic seizure onset (p=0.03). However, there was no difference between the median duration from seizure onset to anti-seizure medication administration for patients in whom seizures terminated after the first medication was administered (84 minutes [51, 115]) than in patients in whom seizures persisted requiring additional anti-seizure medications (99 minutes [63, 189]) (p=0.07).
Figure 4.
Kaplan-Meier Survival Curve comparing patients in whom seizures persist (blue) and cease (red) after the initial anti-seizure medication was administered.
Discussion
In this quality improvement study, we found that implementation of a multi-disciplinary pathway led to more rapid administration of anti-seizure medications after onset of electrographic seizures. The median duration from electrographic seizure onset to anti-seizure medication administration decreased significantly from 139 minutes in patients in the baseline group compared to 64 minutes in the pathway group. Most of the reduction occurred in the interval from electrographic seizure onset to anti-seizure medication order (median of 71 decreased to 31 minutes) which is consistent with our intervention strategy which mostly targeted steps between seizure identification and order placement. There was also a reduction in the duration from order to medication administration (median of 40 decreased to 30 minutes) which may be due to the addition of premixed bags of levetiracetam to the Pyxis machines in the ICU. Although this was a small single-center study, it provides some initial evidence that our process management did yield more rapid intervention. Interestingly, more rapid anti-seizure medication administration was associated with a significantly greater likelihood of seizure cessation. Further study is needed to determine whether this type of intervention is actually associated with reduced electrographic seizure exposure and whether reduced electrographic seizure exposure is associated with more favorable neurobehavioral outcomes.
Several studies have described associations between convulsive status epilepticus management delays and more prolonged seizures, as well as lower anti-seizure medication efficacy. One study of children with convulsive status epilepticus found that for every minute delay between status epilepticus onset and Emergency Department arrival there was a 5% increase in the risk of having status epilepticus last more than 60 minutes.34 A study of children with continued clinical seizures after the first and second-line anti-seizure medications reported that seizures were terminated in 100% of subjects when a third medication was administered within one hour but only 22% of subjects when the third medication was administered after 1 hour.15 Similarly, a study of children documented that the first and second-line medications terminated convulsive status epilepticus in 86% when administered in less than 15 minutes but only 15% when administered in more than 30 minutes.35 A study of children with convulsive seizures lasting longer than 5 minutes found that treatment delays of more than 30 minutes were associated with seizure control delays.36 Finally, midazolam efficacy has been found to be significantly lower when treatment was initiated more than three hours after seizure onset, and there was a trend toward reduced efficacy even at one hour.37 All of these data indicate that delays in status epilepticus management may be associated with reduced medication efficacy. However, it remains unclear to what extent data from convulsive status epilepticus studies can be extrapolated to studies of electrographic seizures as evaluated in this study.
Studies of children with status epilepticus indicate management delays and variability are common. A retrospective multi-center study reported that even once in the Emergency Department, the median time to administer a second-line anti-seizure medication to a seizing child was 24 minutes.39 Benzodiazepine dosing has been reported to be outside usual dosing guidelines in 23% of children with status epilepticus,33 and excess benzodiazepine dosing contributes to respiratory insufficiency and need for ICU admission.31–33 Extending these mostly Emergency Department data to the critical care setting, a recent multi-center study of children who eventually experienced refractory status epilepticus found that the median time from seizure onset to medication administration was 28 minutes for the first medication, 40 minutes for the second medication, and 59 minutes for the third medication.30 These data indicate that delays are common in managing convulsive status epilepticus. This problem may be even more pronounced for seizures in critically ill patients which are often EEG-only (non-convulsive, subclinical) and therefore require cEEG review for identification.1–14 This is particularly problematic since even at large pediatric institutions, cEEG monitoring is often only reviewed several times per day.24
Several studies have identified associations between electrographic seizure exposures and unfavorable neurobehavioral outcomes, particularly when the seizure exposure is high.1; 9; 10; 12; 15–20 Thus, if rapid identification and management of electrographic seizures actually reduced electrographic seizure exposure, this might be a useful neuroprotective strategy. Our initial data suggest that a multi-disciplinary pathway can lead to more rapid management initiation. While it remains unclear whether this strategy actually leads to improved outcome, it may provide a useful strategy in developing subsequent clinical trials. Electrographic seizure exposure and neurobehavioral outcomes might be compared in patients receiving standard (slower) care and pathway (rapid) care. This strategy might provide a mechanism to evaluate the impact of electrographic seizure identification and management in which all patients receive at least “standard” care, therefore making the investigation ethical and feasible.
Recent studies describing the development of pediatric neurocritical care services indicate that seizures are one of the most commonly encountered problems.28; 29 It is becoming increasingly important to document quantitatively that these services provided added value to patients. Our data indicate that a multi-disciplinary team approach led to more rapid intervention of a potential neuroprotective strategy. If further studies indicate this intervention yields lower electrographic seizure exposures and improved outcomes, this may prove to be an important achievement of multi-disciplinary pediatric neurocritical care programs.
This study has multiple limitations. First, it was intended as a single-center quality improvement study and was therefore smaller and allowed less detailed analysis than if it had been designed as a prospective clinical trial. While there were no known major changes in overall pediatric critical care during the period under investigation, the baseline and pathway groups extended over several years and unknown factors may have changed during this time. Second, we have not evaluated whether more rapid intervention reduces seizure exposure or improves neurobehavioral outcome. If this strategy yields more rapid intervention but doesn’t actually reduce seizure exposure, then it is unlikely to improve outcomes. However, the fact that the strategy did lead to more rapid intervention and was associated with a greater likelihood of seizure cessation indicates that further study is warranted. Finally, while the strategy did reduce the duration from seizure onset to medication administration, even after the strategy was implemented the median duration from electrographic seizure onset to anti-seizure medication administration remained 64 minutes. Possibly, more rapid intervention might be needed in some patients for this strategy to be effective in improving outcomes. Two main components may be targeted for additional improvement. More frequent EEG review might lead to more rapid seizure identification. Additionally, having an anti-seizure medication pre-ordered and available at bedside might lead to more rapid medication administration.
In summary, implementation of a multi-disciplinary pathway that streamlined critical care EEG monitoring workflow significantly reduced the duration from electrographic seizure onset to anti-seizure medication administration. Additionally, our data suggest that seizures may cease more often when the initial anti-seizure medication is administered more rapidly. Further study is needed to determine whether this approach improves neurobehavioral outcomes.
Key Points.
A multidisciplinary team developed a pathway to standardize the process of EEG monitoring and seizure management in encephalopathic critically ill children.
The median duration from seizure onset to anti-seizure medication administration was significantly shorter for patients treated with the pathway.
Seizure termination was more likely to occur following initial anti-seizure medication administration for patients treated with the pathway.
Acknowledgments
Dr. Abend is funded by NIH grant NS076550.
Dr. Dlugos is funded by NIH grant U01NS077276 (Epi4K) and by the Epilepsy Study Consortium.
Dr. Topjian has received support from NINDS (K23).
Dr. Abend serves as a site primary investigator for Pfizer and has served as a scientific advisor for Turing Pharmaceuticals and Marinus Pharmaceuticals. He has given expert testimony in medical-legal cases.
Dr. Dlugos serves as a DSMB member on a study sponsored by Sage Therapeutics. He has given expert testimony in medical-legal cases.
Footnotes
Disclosure of Conflicts of Interest
None of the remaining authors report any conflicts of interest.
Ethical Issues Involved in Publishing
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: Cohort study of risk factors and mortality. Neurology. 2013;81:383–391. doi: 10.1212/WNL.0b013e31829c5cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosain SA, Solomon GE, Kobylarz EJ. Electroencephalographic patterns in unresponsive pediatric patients. Pediatr Neurol. 2005;32:162–165. doi: 10.1016/j.pediatrneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Jette N, Claassen J, Emerson RG, et al. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–1755. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- 4.Abend NS, Dlugos DJ. Nonconvulsive status epilepticus in a pediatric intensive care unit. Pediatr Neurol. 2007;37:165–170. doi: 10.1016/j.pediatrneurol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Tay SK, Hirsch LJ, Leary L, et al. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia. 2006;47:1504–1509. doi: 10.1111/j.1528-1167.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 6.Shahwan A, Bailey C, Shekerdemian L, et al. The prevalence of seizures in comatose children in the pediatric intensive care unit: A prospective video-EEG study. Epilepsia. 2010;51:1198–1204. doi: 10.1111/j.1528-1167.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- 7.Abend NS, Topjian A, Ichord R, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology. 2009;72:1931–1940. doi: 10.1212/WNL.0b013e3181a82687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011;52:1130–1136. doi: 10.1111/j.1528-1167.2011.03070.x. [DOI] [PubMed] [Google Scholar]
- 9.Greiner HM, Holland K, Leach JL, et al. Nonconvulsive status epilepticus: the encephalopathic pediatric patient. Pediatrics. 2012;129:e748–e755. doi: 10.1542/peds.2011-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med. 2012;38:853–862. doi: 10.1007/s00134-012-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arango JI, Deibert CP, Brown D, et al. Posttraumatic seizures in children with severe traumatic brain injury. Childs Nerv Syst. 2012;28:1925–1929. doi: 10.1007/s00381-012-1863-0. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber JM, Zelleke T, Gaillard WD, et al. Continuous video EEG for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care. 2012;17:31–38. doi: 10.1007/s12028-012-9715-z. [DOI] [PubMed] [Google Scholar]
- 13.McCoy B, Sharma R, Ochi A, et al. Predictors of nonconvulsive seizures among critically ill children. Epilepsia. 2011;52:1973–1978. doi: 10.1111/j.1528-1167.2011.03291.x. [DOI] [PubMed] [Google Scholar]
- 14.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071–1077. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambrechtsen FA, Buchhalter JR. Aborted and refractory status epilepticus in children: a comparative analysis. Epilepsia. 2008;49:615–625. doi: 10.1111/j.1528-1167.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- 16.Gwer S, Idro R, Fegan G, et al. Continuous EEG monitoring in Kenyan children with non-traumatic coma. Arch Dis Child. 2012;97:343–349. doi: 10.1136/archdischild-2011-300935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Crit Care Med. 2013;31:215–223. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–1438. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014;82:396–404. doi: 10.1212/WNL.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abend NS, Wagenman KL, Blake TP, et al. Electrographic status epilepticus and neurobehavioral outcomes in critically ill children. Epilepsy Behav. 2015;49:238–244. doi: 10.1016/j.yebeh.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abend NS, Topjian AA, Williams S. How much does it cost to identify a critically ill child experiencing electrographic seizures? J Clin Neurophysiol. 2015;32:257–264. doi: 10.1097/WNP.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abend NS, Topjian AA, Gutierrez-Colina AM, et al. Impact of continuous EEG monitoring on clinical management in critically ill children. Neurocrit Care. 2011;15:70–75. doi: 10.1007/s12028-010-9380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abend NS, Dlugos DJ, Hahn CD, et al. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010;12:382–389. doi: 10.1007/s12028-010-9337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez SM, Carpenter J, Chapman KE, et al. Pediatric ICU EEG Monitoring: Current Resources and Practice in the United States and Canada. J Clin Neurophysiol. 2013;30:156–160. doi: 10.1097/WNP.0b013e31827eda27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 26.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, Part I: Indications. J Clin Neurophysiol. 2015;32:87–95. doi: 10.1097/WNP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman ST, Abend NS, Bleck TP, et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part II: Personnel, Technical Specifications, and Clinical Practice. J Clin Neurophysiol. 2015;32:96–108. doi: 10.1097/WNP.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell MJ, Carpenter J, Au AK, et al. Development of a Pediatric Neurocritical Care Service. Neurocrit Care. 2008 doi: 10.1007/s12028-008-9061-3. [DOI] [PubMed] [Google Scholar]
- 29.LaRovere KL, Graham RJ, Tasker RC. Pediatric neurocritical care: a neurology consultation model and implication for education and training. Pediatr Neurol. 2013;48:206–211. doi: 10.1016/j.pediatrneurol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez Fernandez I, Abend NS, Agadi S, et al. Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology. 2015;84:2304–2311. doi: 10.1212/WNL.0000000000001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin RF, Verhulst L, Neville BG, et al. Inappropriate emergency management of status epilepticus in children contributes to need for intensive care. J Neurol Neurosurg Psychiatry. 2004;75:1584–1588. doi: 10.1136/jnnp.2003.032797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tirupathi S, McMenamin JB, Webb DW. Analysis of factors influencing admission to intensive care following convulsive status epilepticus in children. Seizure. 2009;18:630–633. doi: 10.1016/j.seizure.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Tobias JD, Berkenbosch JW. Management of status epilepticus in infants and children prior to pediatric ICU admission: deviations from the current guidelines. South Med J. 2008;101:268–272. doi: 10.1097/SMJ.0b013e318164e3f0. [DOI] [PubMed] [Google Scholar]
- 34.Chin RF, Neville BG, Peckham C, et al. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol. 2008;7:696–703. doi: 10.1016/S1474-4422(08)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewena S, Young S. When benzodiazepines fail: how effective is second line therapy for status epilepticus in children? Emerg Med Australas. 2006;18:45–50. doi: 10.1111/j.1742-6723.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson K, Metsaranta P, Huhtala H, et al. Treatment delay and the risk of prolonged status epilepticus. Neurology. 2005;65:1316–1318. doi: 10.1212/01.wnl.0000180959.31355.92. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi K, Osawa M, Aihara M, et al. Efficacy of Intravenous Midazolam for Status Epilepticus in Childhood. Pediatr Neurol. 2007;36:366–372. doi: 10.1016/j.pediatrneurol.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 38.CHOP. Critical Care Pathway for EEG Monitoring. 2014 Available at: http://www.chop.edu/clinical-pathway/critical-care-pathway-eeg-monitoring-clinical-pathways. [Google Scholar]
- 39.Lewena S, Pennington V, Acworth J, et al. Emergency management of pediatric convulsive status epilepticus: a multicenter study of 542 patients. Pediatr Emerg Care. 2009;25:83–87. doi: 10.1097/PEC.0b013e318196ea6e. [DOI] [PubMed] [Google Scholar]
- 40.American-Society-of-Electroneurodiagnostic-Technologists. National competency skill standards for ICU/cEEG monitoring. Am J Electroneurodiagnostic Technol. 2008;48:258–264. [PubMed] [Google Scholar]