Abstract
A slurry-based method was developed for the entrapment of alpha1-acid glycoprotein (AGP) for use in high-performance affinity chromatography to study drug interactions with this serum protein. Entrapment was achieved based on the physical containment of AGP in hydrazide-activated porous silica supports and by using mildly oxidized glycogen as a capping agent. The conditions needed for this process were examined and optimized. When this type of AGP column was used in binding studies, the association equilibrium constant (Ka) measured by frontal analysis at pH 7.4 and 37°C for carbamazepine with AGP was found to be 1.0 (± 0.5) × 105 M−1, which agreed with a previously reported value of 1.0 (± 0.1) × 105 M−1. Binding studies based on zonal elution were conducted for several other drugs with such columns, giving equilibrium constants that were consistent with literature values. An entrapped AGP column was also used in combination with a column containing entrapped HSA in a screening assay format to compare the binding of various drugs to AGP and HSA. These results also agreed with previous data that have been reported in literature for both of these proteins. The same entrapment method could be extended to other proteins and to the investigation of additional types of drug-protein interactions. Potential applications include the rapid quantitative analysis of biological interactions and the high-throughput screening of drug candidates for their binding to a given protein.
Keywords: Alpha1-acid glycoprotein, High-performance affinity chromatography, Drug-protein binding, Entrapment, Human serum albumin
1. Introduction
The interactions between drugs and serum proteins are known to affect such properties as the transport, excretion and metabolism of drugs in the body [1,2]. One serum protein that is involved in many of these interactions is alpha1-acid glycoprotein (AGP) [3–6]. AGP is a major protein constituent in plasma, with a typical concentration in humans of 0.5–1.0 mg/mL [3]. Human AGP contains a single polypeptide chain of 183 amino acids and has an isoelectric point of 2.8–3.8 [4]. This glycoprotein has a carbohydrate content of 45% and an average molar mass of 41 kDa [4,6]. As a transport protein, AGP is known to bind to many basic and neutral drugs in the blood stream [4,5].
Various techniques have been used to examine the binding of drugs with serum proteins. These techniques have included equilibrium dialysis, ultrafiltration, capillary electrophoresis, and various spectroscopic techniques, including surface plasmon resonance [2,7–11]. High-performance affinity chromatography (HPAC) has also been used in such work [1,12,13]. This method makes use of a biologically-related binding agent (e.g., a serum protein) as a stationary phase in an HPLC column, which can then be utilized to examine binding by this agent to drugs or other targets that are applied to the column [12]. This method has been found to have many advantages as a tool for drug-protein binding studies, including its speed, good precision, ease of automation, compatibility with a variety of detectors, and ability to work with small amounts of a drug or binding agent (e.g., through the use of affinity microcolumns) [1,12,13].
When HPAC is used to study the interactions between a drug and an immobilized protein, one important consideration is the choice of the immobilization method that is used to place the protein in the column [14–16]. Many past reports using HPAC for drug binding studies have employed covalent immobilization (e.g., the Schiff base method) [16–23], as has been used with transport proteins such as human serum albumin (HSA) and various lipoproteins [16–18,20–23]. AGP has been immobilized for use in chiral separations by employing amine-based crosslinking or coupling through thiol groups [16,24,25]. AGP has also been immobilized for drug binding studies by having the carbohydrate groups on this glycoprotein undergo mild oxidation, followed by coupling of the resulting aldehyde groups with a hydrazide-activated support [16,19,26–28]. Many of these approaches give an immobilized protein that has good correlation with the behavior seen for the same protein in a soluble form; however, this is not always the case [16,17,19,20,23,25]. For instance, covalent immobilization can produce improper orientation, steric hindrance or multi-site attachment for an immobilized binding agent if proper coupling conditions are not selected. These effects, in turn, can lead to a change in actual or apparent activity for an immobilized protein [14,16,19,25,29,30].
Entrapment is an alternative approach for immobilization that can avoid many of these problems. This is a non-covalent method based on the physical containment of a binding agent, as may occur within a porous support or in a cross-linked polymer network [14,30–34]. Sol-gel encapsulation and hydrogel entrapment have been utilized to immobilize proteins and other agents for applications such as protein-based photonic devices, biosensors, chromatographic columns, and enzyme reactors [31–34]. Other entrapment methods have been developed for use with proteoliposomes or red blood cells through the placement of these agents within gels made from dextran or derivatized acrylamide monomers [35,36]. However, many of the materials that are employed in these methods have pressure or flow rate restrictions that make them difficult to use as HPLC supports [30,35,36]. In addition, slow mass transfer effects can be created as a result of such entrapment processes [34].
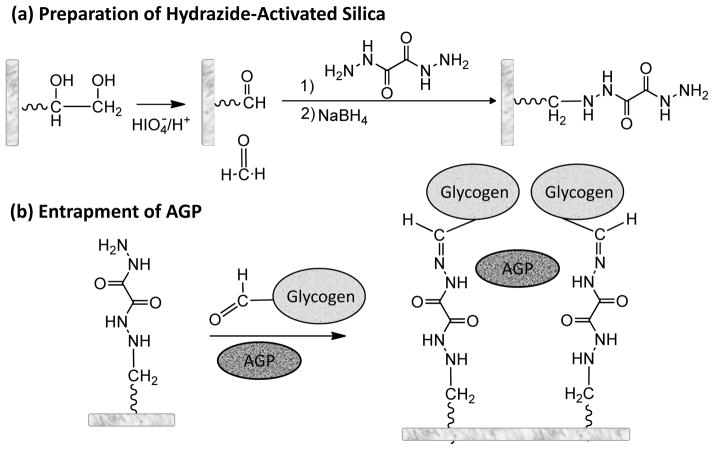
An alternative entrapment approach has recently been described that can be used with standard HPLC supports, as has been demonstrated in work with human serum albumin (HSA) and modified forms of this protein [14,30]. This method (see Figure 1) involves the entrapment of a soluble protein on a hydrazide-activated support by using an oxidized form of glycogen as a capping agent [30]. One useful feature of this method is that it can be used directly with HPLC-grade supports such as porous silica. This feature allows this approach to overcome the pressure and flow restrictions that are seen with many sol gels, hydrogels and low-performance chromatographic supports such as dextran or acrylamide-based gels. The fact that the protein or entrapped agent is held within the pores of the support or at its surface also allows this method to avoid the slow mass transfer properties that are often encountered with other entrapment methods [34]. This method has been shown in preliminary work to be suitable for use with binding agents that span a relatively wide range of sizes (i.e., 5.8–150 kDa) [30]. In addition, this approach has been found to provide good agreement between the binding properties of an entrapped protein like HSA and the soluble form of this protein [14,30].
Figure 1.
Reactions involved in (a) the preparation of a hydrazide-activated support and (b) the entrapment of alpha1-acid glycoprotein (AGP) onto this support in the presence of oxidized glycogen.
This report will examine the optimization and extension of this entrapment technique to the immobilization of AGP and the use of such a support in small HPAC columns for the study of drug-protein binding. The conditions needed for the entrapment of AGP will be examined and optimized. The binding behavior of the resulting AGP columns will be evaluated by using various model drugs and both frontal analysis and zonal elution experiments. The combined use of entrapped AGP and HSA columns to screen drug interactions with these serum proteins will also be considered. The results should make it possible to determine how this entrapment technique can be used in future work with other proteins or in applications such as the high-throughput screening of drugs or the rapid characterization of biological interactions.
2. Materials and methods
2.1. Reagents
The AGP (from pooled human serum, 99% pure), periodic acid reagent (H5IO6), glycogen (bovine liver), HSA (Cohn fraction V, 99% globulin free, 99% fatty acid free), amitriptyline, carbamazepine, chloramphenicol, chlorpromazine, disopyramide, imipramine, lidocaine, nortriptyline, S-propranolol, and quinidine were from Sigma-Aldrich (St. Louis, MO, USA). The Nucleosil silica (7 μm particle diameter, 100 or 300 Å pore size) was obtained from Macherey Nagel (Düren, Germany). Reagents for the micro bicinchoninic (BCA) protein assay were from Pierce (Rockford, IL, USA). All other chemicals were of the purist grades available. All solutions were prepared using water from a Barnstead NANOpure system (Dubuque, IA, USA) or a Milli-Q Advantage A10 purification system (Millipore, Billerica, MA, USA) and were filtered through 0.20 μm GNWP nylon membranes from Millipore.
2.2. Apparatus
The chromatographic system consisted of a series 200 micro pump and absorbance detector from Perkin Elmer (Shelton, CT, USA); or a Jasco PU-980i intelligent HPLC isocratic pump (Tokyo, Japan), a Rheodyne Advantage PF ten-port valve (Cotati, CA), and a Jasco UV-975 UV/Vis detector. Injections were carried out by using a six-port Rheodyne Lab Pro valve (Cotati, CA, USA) and a 5 or 20 μL sample loop. The temperature of the columns and mobile phases were controlled by using a Millipore Waters TCM temperature control module, or a PolyScience circulating VWR circulating water bath (Buffalo Grove, IL, USA) and a water jacket from Alltech (Deerfield, IL, USA). The chromatographic data were collected and processed using in-house programs written in LabView 5.1 (National Instruments, Austin, TX, USA). Centrifugation was conducted by using an Eppendorf 5702 RH centrifuge (Hamburg, Germany). The columns were packed using an HPLC column slurry packer from ChromTech (Apple Valley, MN, USA).
2.3. Column preparation
Nucleosil was first converted to diol-bonded silica, followed by conversion of this support into an aldehyde-activated form; this material was then treated with oxalic dihydrazide to form hydrazide-activated silica, as described previously [15]. One factor that was varied was the amount of oxalic dihydrazide that was added during the last step of this reaction. A mole ratio of 5:1 was initially used for the oxalic dihydrazide versus the aldehyde groups on the support (i.e., where the aldehyde content was approximately equal to the original number of diol groups) [15], as utilized in prior reports with this entrapment approach and in the work conducted in Section 3.6 [14,30]. This ratio was later varied from 5:1 to 0.5:1, with a ratio of 1:1 being used in the remainder of this report.
A 17 mg portion of glycogen was added to 4.0 mL of a pH 5.0, 20 mM sodium acetate buffer that also contained 15 mM sodium chloride and 135 mg of the periodic acid reagent [30]. After mixing this solution for 18 h while shaking at room temperature, the oxidized glycogen was purified by using either ultrafiltration or size exclusion chromatography. In the ultrafiltration method, the oxidized glycogen solution was placed in an Amicon Ultra centrifugal filtration device (30 kDa cutoff, Millipore) and washed three times with water, followed by another three times using pH 5.0, 0.10 M potassium phosphate buffer. Each centrifugation step was carried out for 15 min at 20 °C and 4400 rpm. The oxidized glycogen fraction that remained in the filtration device was removed and diluted to 4 mL by adding pH 5.0, 0.10 M potassium phosphate buffer. The method based on size exclusion chromatography was carried out as described earlier [30] by using an Econo-Pac 10 DG desalting column (6 kDa cutoff, Bio-Rad, Hercules, CA, USA) and pH 5.0, 0.10 M potassium phosphate buffer as the mobile phase.
HSA was entrapped within Nucleosil Si-300 silica as described previously [14,30]. A similar entrapment method was adapted for use with AGP. In this method, 500 μL of 20 mg/mL AGP and 380 μL of 4.25 mg/mL oxidized glycogen, which was used as a capping agent, were mixed for 2 h at room temperature; this mixture was then combined with 80 mg of hydrazide-activated silica at 4 °C for 16 h. Control supports were made in the same manner but with no AGP or HSA being added during the entrapment process. The total protein content of each support was measured directly and in triplicate by using a micro BCA assay, with soluble AGP or HSA being used as the standard and the control support being used as the blank. The AGP, HSA and control supports were packed at 4000 psi (27.6 MPa) into separate 1.0 cm × 2.1 mm i.d. stainless steel columns using pH 7.4, 0.067 M potassium phosphate buffer as the packing solution. All columns and supports were stored at 4 °C in the same pH 7.4 buffer. All experiments were performed within 6 months of column preparation and using less than 400 injections per column. No significant changes being observed under these conditions in either the analyte retention or the column’s binding properties.
2.4. Chromatographic studies
A pH 7.4, 0.067 M potassium phosphate buffer was used as the application buffer throughout this report. Solutions of each drug and the void time marker (sodium nitrate) were prepared in this mobile phase. All chromatographic experiments were carried out in triplicate at 37 °C and pH 7.4 at a typical flow rate of 0.50 mL/min; the use of slightly faster or slower flow rates (i.e., 0.25–0.75 mL/min) did not produce any significant changes in the measured breakthrough volumes or retention factors (RSD < 10%). The wavelengths used for absorbance detection were as follows: amitriptyline, 209 nm; carbamazepine, 285 nm; chloramphenicol, 204 nm; chlorpromazine, 253 nm; disopyramide, 260 nm; imipramine, 249 nm; lidocaine, 207 nm; propranolol, 214 or 225 nm; nortriptyline; 209 nm; quinidine, 234 nm; and sodium nitrate, 205 nm.
The frontal analysis experiments were carried out by continuously applying solutions containing 1–20 μM carbamazepine to an AGP column or a control column in the presence of pH 7.4, 0.067 M potassium phosphate buffer. The retained carbamazepine was later eluted by applying only pH 7.4, 0.067 M potassium phosphate buffer onto these columns. The mean point of each breakthrough curve was determined by using PeakFit 4.12 (Jandel Scientific, Rafael, CA) with a first derivative Savitzky-Golay filter and an exponentially-modified Gaussian (EMG) fit. The resulting data were then fit to various binding models by using DataFit 8.1 software (Oakdale Engineering, Oakdale, PA).
In the zonal elution studies, a 5 or 20 μL sample containing 5–20 μM of a drug or sodium nitrate was injected onto an AGP or HSA column and a control column. In each case, no significant changes occurred in the measured retention with a decrease in the concentration of the injected drug, indicating that linear elution conditions were present. For most of these studies a flow rate of 0.50 mL/min was used; however, for solutes with weak retention, flow rates of 0.10 or 0.30 mL/min were also employed. The void time of each column was determined by making triplicate injections of sodium nitrate. The void time of the system was determined by injecting sodium nitrate and by using a zero-volume union in place of a column. The retention time of each peak were found by using PeakFit 4.12 with a linear progressive baseline correction and an EMG fit.
3. Results and discussion
3.1. Effect of support pore size
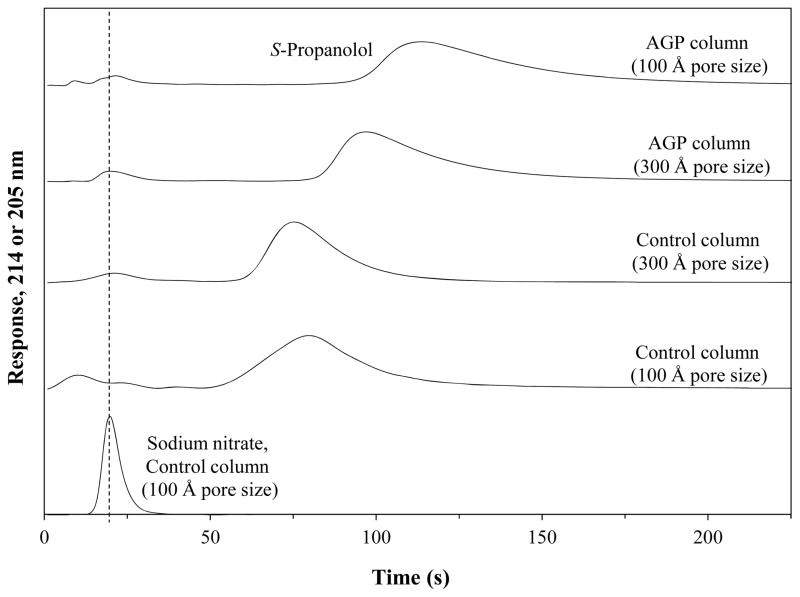
It has been found with HSA that the pore size of a support can affect the amount of protein that is entrapped when using the scheme shown in Figure 1 [30]. HSA, which has a molar mass of 66.5 kDa [37], gives the highest binding capacity when using supports with pore sizes of 100 to 300 Å, with optimum results being obtained at 300 Å [30]. This range of pore sizes was also considered in this report for use with AGP, which has a molar mass that is about two-thirds that of HSA [4,6]. The entrapped AGP supports were compared based on both their protein content and their retention for a drug known to bind to AGP. Figure 2 shows some results that were obtained for injections of S-propranolol onto AGP columns or control columns that were prepared using silica with a pore size of 100 Å or 300 Å. These supports were made by adding a 5:1 mole ratio of oxalic dihydrazide versus aldehyde groups during the support activation step. Sodium nitrate was also injected onto each column as a non-retained solute.
Figure 2.
Typical chromatograms obtained for S-propranolol and sodium nitrate (i.e., a non-retained solute) at 0.50 mL/min and in the presence of a pH 7.4 application buffer at 37 °C when applied to 1.0 cm × 2.1 mm i.d. AGP columns and control columns that contained silica supports with various pore sizes. The results for S-propranolol were obtained by monitoring the absorbance at 214 nm, while the data for sodium nitrate were obtained at 205 nm. The dashed line shows the approximate location of the void time in each of these chromatograms.
It was found that an AGP column made with 100 Å pore size silica gave higher retention for S-propranolol than a column that was prepared with 300 Å pore size silica (overall retention factors, 19.2 (± 0.3) versus 15.8 (± 0.2), where the values in parentheses represent ± 1 S.D.). However, the control column made with the 100 Å pore size support also had larger non-specific binding for S-propranolol than the control column made with 300 Å pore size silica (retention factors, 15.6 (± 0.5) versus 12.6 (± 0.4)). This was due to the larger surface area of the 100 Å pore size support. The difference in the retention factors on the AGP columns and control columns for the two types of supports, which gave values of 3.6 (± 0.5) versus 3.2 (± 0.4), may have represented a small increase in the specific retention for S-propanolol on the AGP column that was made from the 100 Å pore size silica; however, the result for this type of support was not significantly different (at the 95% confidence level) from the value obtained with the 300 Å pore size silica. These results indicated that either support could be used to entrap AGP.
Similar results were acquired when a protein assay was used to determine the amount of the AGP that was entrapped in each of these supports. In this assay, the 100 Å pore size support was found to contain 63.6 (± 7.4) mg AGP/g silica, while the 300 Å pore size support contained 49.1 (± 5.1) mg/g silica when prepared under otherwise identical conditions. Based on this information, silica with a pore size of 100 Å was used with AGP in most of the following studies. However, a 300 Å pore size support was also acceptable and was used in the experiments that compared the binding of various drugs to either entrapped AGP and HSA columns (see Section 3.6).
3.2. Purification of oxidized glycogen
Both ultrafiltration and size exclusion chromatography were evaluated for the purification of oxidized glycogen prior to its use in entrapping AGP. This step was used to remove any remaining periodic acid or soluble oxidation products. Oxidized glycogen that was purified by using size exclusion chromatography [30] was found to sometimes precipitate after overnight storage at 4°C. Although this solution was clear immediately after purification, a precipitate did sometimes appear shortly after this step, as has been noted previously with antibodies that have been oxidized under similar conditions with periodate [38]. However, washing with water before the addition of pH 5.0 buffer was found to greatly reduce this effect.
It was possible to use several of these washing steps within a short time period by employing ultrafiltration as an alternative approach for purifying the oxidized glycogen. The oxidized glycogen that was purified by this approach was stable over several days even when it was stored at 4°C. Furthermore, ultrafiltration allowed for a much higher recovery of the oxidized glycogen than could be obtained by size exclusion chromatography because all of the glycogen remained in the ultrafiltration device during the purification process. This feature also made it possible to use a smaller volume of the original oxidized glycogen solution for the entrapment process. In addition, it was possible during ultrafiltration to concentrate the oxidized glycogen before it was used for entrapment. All of these factors lead to ultrafiltration being used as the purification method of choice for most of the following work that involved the entrapment of AGP.
3.3. Preparation of hydrazide-activated silica
The results in Figure 2 show that S-propranolol had significant non-specific binding on the initial supports that were used to prepare the AGP columns, as indicated by the retention seen for this drug on the control columns. The non-specific binding for this drug was about 80–81% of the total retention measured for the AGP columns that were made using 100 and 300 Å pore size supports and a ratio of 5:1 mol/mol for the oxalic dihydrazide that was added versus the aldehyde groups that were present. Some other drugs that were tested on these columns (see Sections 3.4–3.6) gave similar behavior. For instance, carbamazepine and chlorpromazine were also found to have significant non-specific interactions with this type of column (i.e., 80% or 85% of the total binding, respectively), while the other drugs that were tested had much less retention on the control columns when compared to the entrapped AGP columns (non-specific binding, 6–58%). This non-specific binding is believed to be mainly due to interactions between these drugs and the hydrazide groups on the activated support [39], because many of the same drugs did not have this level of non-specific binding when the support was in a diol-bonded form [16,19,26–28].
To reduce this non-specific binding and optimize the specific retention of the AGP columns, various amounts of oxalic dihydrazide were used during the activation of the supports. The non-specific binding of these supports and their specific retention after the entrapment of AGP was evaluated by making injections of S-propranolol under conditions similar to those used in Figure 2. Table 1 summarizes the results that were obtained. As is shown in this table, the specific retention for S-propranolol on the AGP columns containing 100 Å pore size silica increased significantly when going from a mole ratio of 5:1 to 3:1 or 1:1 for the oxalic dihydrazide that was added versus aldehyde groups during the activation step. The same drug showed a much lower level of specific binding when a mole ratio of 0.5:1 was used. Thus, a mole ratio of 1:1 for the oxalic dihydrazide versus aldehyde groups was utilized in the most of the following studies when preparing a hydrazide-activated support. However, Table 1 confirms that other mole ratios could also have been used, such as were employed with HSA in this study and in previous work with this protein [14,30].
Table 1.
Retention measured for S-propranolol at 37 °C and pH 7.4 on entrapped AGP columns prepared by using various mole ratios of oxalic dihydrazide versus aldehyde groups during the preparation of hydrazide-activated silicaa
| Ratio of oxalic dihydrazide Specific vs. aldehyde groups (mol/mol)b retention time (s)c | Retention time, AGP column (s) | Retention time, control column (s) |
|---|---|---|
| 5:1 | 131.5 (± 0.5) | 97.3 (± 1.6) |
| 34.2 (± 1.7) | ||
| 3:1 | 371.8 (± 1.6) | 114.6 (± 0.2) |
| 257.2 (± 1.6) | ||
| 1:1 | 414.3 (± 4.6) | 104.2 (± 0.3) |
| 310.1 (± 4.6) | ||
| 0.5:1 | 117.8 (± 3.8) | 102.6 (± 0.1) |
| 15.2 (± 3.8) |
Each of the listed retention factors is the average for a triplicate set of injections. Each value in parentheses is a range of ± 1 S.D.
The mol/mol ratios are based on an estimated initial diol content of 920 μmol/g silica, with the assumption that essentially all of these diol groups were converted to aldehydes when reacted with an excess of periodic acid [15].
The specific retention time is equal to the difference in the retention times for the AGP column and control column, and represents the retention of the injected solute due to only AGP.
A closer examination of the data in Table 1 reveals two interesting trends. First, the level of non-specific binding by S-propranolol on the control columns showed only a relatively small variation as the mole ratio of oxalic dihydrazide versus aldehyde groups was changed from 5:1 to 0.5:1. This trend suggested that the given range of mole ratios may not have been sufficient to change the total amount of hydrazide groups that were on the final support. This can be explained by the fact that the amount of added hydrazide groups (i.e., oxalic dihydrazide being a bifunctional agent) was still present in an excess or at an equimolar level with the aldehyde groups throughout this range of mole ratios. The second trend was the large increase in the specific retention that was seen when using intermediate values for this mole ratio (i.e., 3:1 or 1:1). This increase is believed to be due to changes in the level of bifunctional linking of the dihydrazide groups with aldehyde groups on the support, which could have changed the relative spacing of the free hydrazide groups that could couple with oxidized glycogen. This effect has been noted in prior work using hydrazide-activated silica to immobilized oxidized antibodies [15], and may have led in this current study to more effective conditions for the entrapment of AGP.
3.4. Frontal analysis studies
Frontal analysis was next used to evaluate the activity of the entrapped AGP columns. This technique has often been used in HPAC for the characterization of the binding of drugs with immobilized proteins [40–42]. Figure 3(a) shows some typical results that were obtained when this method was used with an entrapped AGP column. In frontal analysis, a known concentration of the target analyte (e.g., a drug) is continuously applied to a column containing an immobilized binding agent (e.g., AGP), while the amount of the analyte that passes through the column is monitored. As the binding agent becomes saturated with the analyte, a breakthrough curve is produced which can provide information on the amount of active binding agent in the column and the association equilibrium constants for the analyte with this binding agent [41].
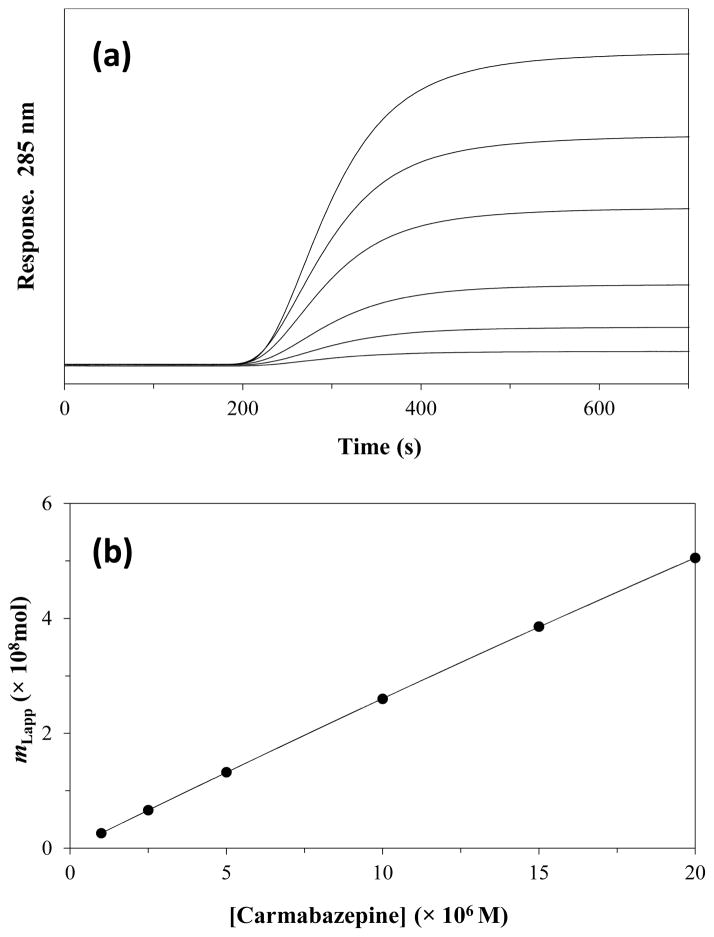
Figure 3.
(a) Typical chromatograms obtained during frontal analysis at 0.50 mL/min using the application of carbamazepine to a 1.0 cm × 2.1 mm i.d. entrapped AGP column at pH 7.4 and 37 °C, and (b) fit of the frontal analysis data for carbamazepine on the entrapped AGP column to a two-site model. The concentrations of applied carbamazepine in (a) were (from top to bottom) 20, 15, 10, 5, 2.5, and 1 μM, respectively. The support used in the AGP column had a pore size of 100 Å. The best-fit line in (b) was obtained by using Eqn. (2) and fixed values for Ka2 and mL2, as based on results that were obtained for carbamazepine on the control column. The correlation coefficient for the fit shown in (b) was 0.9999 (n = 6).
For a relatively fast, reversible interaction at a single type of binding region between an applied target analyte (A) and the ligand or binding agent (L) that is immobilized in the column, Eqn. (1) can be used to describe the relationship between the apparent moles of the target analyte that are needed to reach the mean point of the breakthrough curve (mLapp) as a given molar concentration of the applied analyte, [A] [1,17,27].
| (1) |
In this relationship, Ka is the association equilibrium constant for the binding of A to L, and mL is the total moles of active binding agent that are present in the column. According to Eqn. (1), a system with single-site binding would be expected to produce a linear response for a plot of 1/mLapp vs. 1/[A], where the values of mL and Ka can then be determined from the slope and the intercept of this plot [27,41].
If there are two or more types of binding sites present in the column, alternative equations to Eqn. (1) can be used [17,27,41]. For instance, Eqn. (2) relates mLapp to [A] for a system in which two types of independent binding sites (L1 and L2) are present for A in the column [27,41].
| (2) |
In this equation, mL1 and mL2 are the moles of L1 and L2 in the column, and Ka1 and Ka2 are the association equilibrium constants for A with L1 and L2, respectively. Eqn. (2) can be used to obtain information for a two-site system on the amount of each site that is present (i.e., mL1 and mL2) and the association equilibrium constants for these sites (Ka1 and Ka2) by using a non-linear fit to a plot of mLapp vs. [A] [27,41].
An entrapped AGP column was examined through frontal analysis by using carbamazepine as a model drug. It has been reported that carbamazepine has only one binding site on AGP, but with this drug also having some non-specific binding with hydrazide-activated silica [27]. A correction for these non-specific interactions was made by conducting frontal analysis experiments for carbamazepine on both a control column and an entrapped AGP column. For the control column, the frontal analysis data for carbamazepine gave a good fit with a single-site binding model, as described by Eqn. (1) (see Supplementary Material). The association equilibrium constant and binding capacity measured by this approach for carbamazepine on the control column were 1.8 (± 0.3) ×103 M−1 and 1.4 (± 0.2) ×10−6 mol, respectively.
The results obtained on the control column were next used to correct for non-specific binding by carbamazepine with the support in the entrapped AGP column. The plot of mLapp vs. [carbamazepine] that was obtained for carbamazepine on the AGP column is shown in Figure 3(b). This plot was found to give a good fit to a two-site model, in which one type of interaction was represented by the non-specific binding of carbamazepine to the support and the second interaction was represented by the binding of carbamazepine to AGP. To examine these data, non-linear regression was used along with Eqn. (2) and the values for Ka2 and mL2 that had previously been obtained for carbamazepine on the control column. This approach gave values for Ka1 and mL1, which represented the binding of carbamazepine with AGP, of 1.0 (± 0.5) × 105 M−1 and 1.2 (± 0.3) × 10−9 mol at pH 7.4 and 37°C. This Ka1 value was equivalent at the 95% confidence level with a previously-reported value of 1.0 (± 0.1) × 105 M−1 for carbamazepine with AGP at the same pH and temperature [27].
3.5. Zonal elution studies
Zonal elution was also used to evaluate the binding of drugs with the entrapped AGP columns. In this approach, a narrow plug of a drug was injected onto a column containing the immobilized binding agent [1,12]. The retention time or retention volume for the drug was then determined. Eqns. (3) and (4) show how the retention factor (k) for the injected compound would be related to its association equilibrium constant (Ka) or global affinity constant (nKa′) for its binding to an immobilized protein [41,43].
| (3) |
| (4) |
In these two equations, k is the retention factor for the solute (e.g., after correcting for non-specific interactions with the support), mL is the total moles of active binding sites for the solute on the immobilized protein, and VM is the void volume of the column. The retention of a solute with a single type of binding site is described by Eqn. (3), while the retention of a solute that has multiple but independent binding sites is represented by Eqn. (4). In the case of Eqn. (4), the terms Ka1 through Kan are the association equilibrium constants for the solute at sites 1 through n, while n1 through nn are the relative moles (e.g., mol/mol binding agent) for each type of site in the column. For an individual column, VM can be measured by injecting a non-retained compound. Also, the value of mL can be determined by using frontal analysis, as described in Section 3.4. By using this additional information, Eqn. (3) can then be used with the measured retention factor for a drug on an immobilized protein column, such as one containing entrapped AGP, to obtain the value of Ka or nKa′ for the drug-protein interaction [41].
Zonal elution experiments were conducted with entrapped AGP columns to measure the retention factors for several drugs that are known to bind AGP. The difference in the retention factors for these drugs on the AGP column versus the control column were then found to provide the specific retention factors due to the entrapped AGP. These values were used with Eqn. (3) to estimate the equilibrium constants for the entrapped AGP with these drugs. Table 2 shows the results that were obtained and compares these with previous values that have been reported in the literature [16,19,26,27,44–50]. Most the resulting binding constants had relative precisions of ± 9–11% (carbamazepine being the only exception) and all were statistically identical to the literature values at the 99% confidence level. This agreement indicated that the zonal elution approach and entrapped AGP column could be used to determine the binding constants for drugs with this protein. This approach was also relatively fast, with the retention factor measurements being made for the given drugs within 1–5 min of sample injection at 0.50 mL/min.
Table 2.
Measured retention factors and estimated binding constants (Ka or nKa) at pH 7.4 and 37 °C for various drugs injected onto entrapped AGP columnsa
| Drug | Specific retention factor, k | Estimated Ka or nKa′ (M−1) | Literature values for Ka or nKa′ (M−1)
|
|
|---|---|---|---|---|
| Literature value(s) | Method and conditions [Ref.] | |||
| Carbamazepine | 2.1 (± 0.9) | 6.4 (± 2.7) × 104 | 1.0 (± 0.1) × 105 | HPAC (37°C, pH 7.4) [27] |
| 1.7 × 104 | Equilibrium dialysis (37°C, pH 7.4) [40] | |||
| Disopyramide | 35.7 (± 2.4) | 1.1 (± 0.1) × 106 | 1.0 × 106 | Equilibrium dialysis (37°C, pH 7.4) [41] |
| 2.53 (± 0.28) × 106 | Equilibrium dialysis (4°C, pH 7.4) [42] | |||
| Imipramine | 24.6 (± 2.2) | 7.5 (± 0.7) × 105 | 4.1 × 105 | Equilibrium dialysis (25°C, pH 7.4) [43,44] |
| 1.31 (± 0.53) × 106 | Equilibrium dialysis (4°C, pH 7.4) [45] | |||
| Lidocaine | 5.7 (± 0.7) | 1.8 (± 0.2) × 105 | 1.1–1.7 × 105 | HPAC (37°C, pH 7.4) [26] |
| 1.3 × 105 | Equilibrium dialysis (37°C, pH 7.4) [46] | |||
| S-Propranolol | 69.4 (± 5.6) | 2.1 (± 0.2) × 106 | 4.2 (± 0.3) × 106 | HPAC (37°C, pH 7.4) [16] |
| 1.4 (± 0.7) × 106 | HPAC (37°C, pH 7.4) [19] | |||
Each of the retention factors shown is the average for a triplicate set of injections. Each value in parentheses is a range of ± 1 S.D.
3.6. Use of entrapped protein columns to screen drug interactions with AGP and HSA
The last section of this study examined the use of entrapment to make both AGP and HSA columns for screening and comparing the binding of various drugs with these serum proteins. Both proteins were entrapped onto the same type of support and using the same entrapment conditions, based on prior work that has been conducted with HSA [14,30]. A variety of drugs with known binding constants for AGP and HSA (see Table 3) were then injected onto each column. A correction for non-specific binding was also made by injecting these compounds onto a control column that was made under the same conditions as the AGP and HSA supports but with no soluble protein being added to the reaction mixture.
Table 3.
Retention factors and binding constants measured for various drugs on columns containing entrapped AGP or HSA
| Drug | Protein | (Specific retention factor)/(protein content)a | Estimated Ka or nKa′ (M−1) | Literature value (M−1)b |
|---|---|---|---|---|
| Amitriptyline | AGP | 5.66 (± 0.06) | 7.1 (± 0.3) × 105 | 3.4 × 105 (37 °C) [51] 5.3 × 105 (25 °C) [52] |
| HSA | 0.45(± 0.04) | 5.6 (± 0.2) × 104 | 2.12–6.22 × 104 [53] | |
| Chloramphenicol | AGP | 0.015 (± 0.007) | 1.9 (± 0.5) × 103 | Not reported |
| HSA | 0.018 (± 0.006) | 1.6 (± 0.5) × 103 | 8.2 × 103 [54] | |
| Chlorpromazine | AGP | 12.19 (± 0.05) | 1.5 (± 0.4) × 106 | 1 × 106 [55,56] |
| HSA | 0.9 (± 0.1) | 1.2 (± 0.2) × 105 | 1.68–3.3 × 105 (20 °C) 3.46–4.33 × 105 (28 °C) [57] |
|
| Nortriptyline | AGP | 5.91 (± 0.06) | 7.4 (± 0.2) × 105 | 6.7 × 105 [5,58] |
| HSA | 0.50 (± 0.05) | 6.2 (± 0.3) × 104 | 1.81 × 104 [59] | |
| Quinidine | AGP | 2.40 (± 0.05) | 3.0 (± 0.3) × 105 | 2.9 × 105 [60] |
| HSA | 0.17 (± 0.03) | 2.1 (± 0.4) × 104 | 7.7 × 103 (25 °C) [61] 4.78 × 104 (37 °C) [62] |
Each value in parentheses represents a range of ± 1 S.D., as based on triplicate measurements made over five flow rates (n = 15). The normalized retention factors were calculated by dividing the specific retention factors by the total protein content for each given support, as measured in triplicate and using a value of 21 (± 1) mg AGP/g silica or 37 (± 1) mg HSA/g silica. The specific retention factor was obtained by taking the difference between the overall retention factor on an entrapped protein column and the retention factor due to non-specific interactions for the same solute on the control column, with the latter values being as follows: amitriptyline, 71.7 (± 0.5); chloramphenicol, 0.67 (± 0.02); chlorpromazine, 177 (± 3); nortryptyline, 62.5 (± 0.1); and quinidine, 28.3 (± 0.2).
Most of the literature values were determined at pH 7.4 and 37°C [51,53–56,59,62]. The values from Refs. [52,61] were measured at pH 7.4 and 25 °C. The values of 3.46–4.33 × 105 M−1 from Ref. [57] were measured at pH 7.4 and 28 °C, while the values of 1.68–3.3 × 105 M−1 from the same reference were measured at pH 7.4 and 20 °C. The pH and temperature were not provided in Refs. [5,58,60].
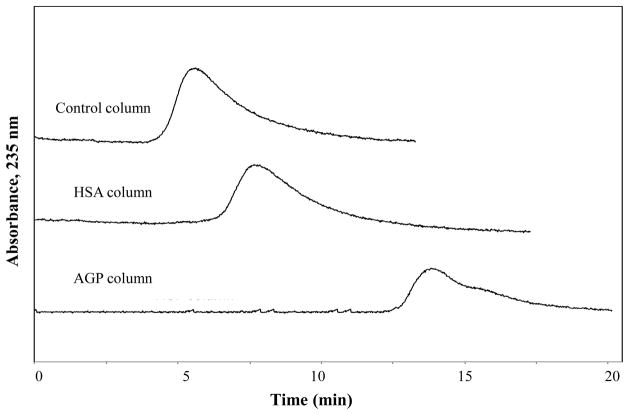
Figure 4 shows a typical set of chromatograms that were obtained on these entrapped protein columns, based on the data that were obtained for chlorpromazine. In this example, it was possible to both determine whether chlorpromazine was able to bind AGP or HSA and to compare the overall extent of this binding by simply comparing the retention times for this drug on each column. For instance, the data in Figure 4 indicate that chlorpromazine had significant binding to both proteins, with the AGP columns having the strongest retention. These results were obtained for chlorpromazine within 8–15 min at 0.50 mL/min. Similar results were obtained for the other drugs that are listed in Table 3, which eluted within 1–9 min at 0.50 mL/min. The same columns could be used at flow rates up to at least 2.0 mL/min, which gave retention times for these drugs of only 0.25–4 min.
Figure 4.
Chromatograms obtained for injections of chlorpromazine at 0.50 mL/min and in the presence of a pH 7.4 application buffer at 37 °C onto 1.0 cm × 2.1 mm i.d. columns containing a control support or entrapped HSA or AGP. Each of these columns was prepared using Nucleosil Si-300 silica.
Although chromatograms like those in Figure 4 provided an initial estimate of how strongly a drug could bind to AGP versus HSA, it was necessary to correct for the different protein contents of the AGP and HSA columns to obtain a more direct comparison. This was done by first calculating the specific retention factor for each drug (i.e., in which the retention factor on the control column was subtracted from the total retention factor on the protein column) and dividing this value by the protein content of the AGP or HSA support (e.g., as previously measured on a separate portion of the same material). The resulting ratios are shown in Table 3 and were used with Eqn. (4) to also obtain the association equilibrium constant or global affinity constant for each drug and protein combination. The results obtained for both the entrapped AGP and HSA columns were in good agreement with previous literature values for the same drug-protein systems [5,51–62]. Based on these experiments, chlorpromazine was found to have the strongest binding to AGP (i.e., a Ka or nKa′ value of 1.5 × 106 M−1), with a 13-fold difference in its affinity for AGP versus HSA. Amitriptyline and nortriptyline both had affinities for AGP in the range of 7–8 × 105 M−1 and which were 12- to 13-fold larger than the values measured for these drugs with HSA. Quinidine had an affinity for AGP of 3.0 × 105 M−1, which was 14-fold larger than its affinity for HSA. Chloramphenicol had weak retention on both AGP and HSA columns, with affinities in the range of 1.6–1.9 × 103 M−1 for these proteins. The equilibrium constants that were estimated for these interactions by using the entrapped protein columns had relative precisions of ± 2.7–27% (average, ± 14%) for AGP and ± 3.6–31% (average, ± 15%) for HSA.
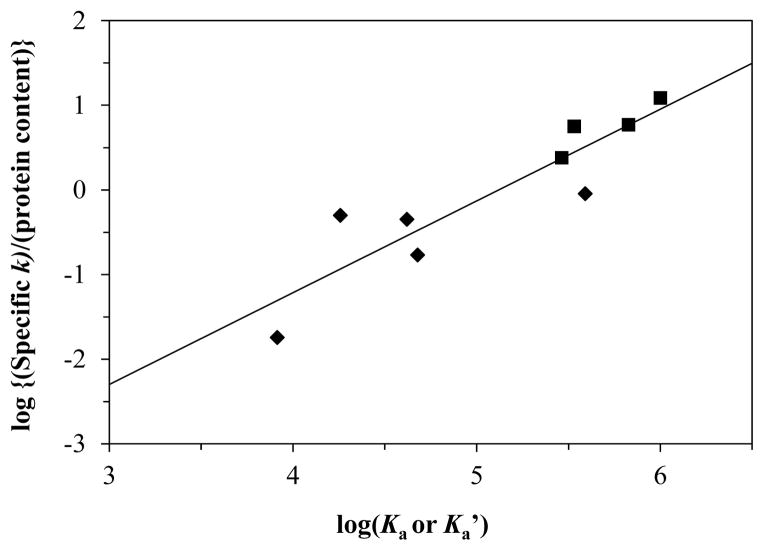
The results from the entrapped protein columns were further compared with the literature values by plotting the (specific retention factor)/(protein content) ratio for each drug and protein combination versus the previously reported binding constants for these interactions. Figure 5 shows the result that was obtained when a log-log plot was used for this comparison. This graph gave a good linear fit, with a correlation coefficient of 0.9057 (n = 9) for binding constants that spanned almost three orders of magnitude (i.e., 103–106 M−1). This result confirmed that the specific retention factors that were measured on the entrapped protein columns could be used to compare the binding strength of drugs to proteins such as AGP and HSA and to provide a relatively easy way of estimating the affinities of these interactions.
Figure 5.
Correlation of log[(specific retention factor)/protein content] versus the logarithm of the binding constants that have been reported for various drugs with AGP (■) or HSA (◆). The equation for the best-fit line was y = [1.08 (± 0.19)] x − [5.55 (± 0.40)], with a correlation coefficient of 0.9057 (n = 9). If a drug had a range of binding constants listed in Table 3 at pH 7.4 and 37 °C, the average of that range was used in this plot. If values were listed for the drug in Table 3 at various temperatures, the value that corresponded to 37 °C or the closest temperature to 37°C was used to construct this plot.
4. Conclusion
A slurry-based method for the entrapment of AGP in HPLC-graded silica was optimized and evaluated for use in studying drug interactions with AGP. Parameters that were considered during this entrapment process including the effects of the support’s pore size, the method used for purifying the oxidized glycogen, and the effect of varying the amount of oxalic dihydrazide versus aldehyde groups that was used to prepare the hydrazide-activated silica. The behavior of the entrapped AGP was examined by means of frontal analysis and zonal elution experiments using model drugs, and was found to give good agreement with the binding behavior that has been reported for AGP in the literature. Columns containing entrapped AGP or HSA were then both used in screening the binding of these proteins with various drugs. There was again good agreement between the results that were obtained on the entrapped protein columns and previous binding constants that have been reported for the same drugs with AGP and HSA. These results indicate that columns containing entrapped AGP and HSA should be useful in applications such as the high-throughput screening of drug candidates and the chromatographic-based analysis of drug-protein binding [14,16,30,33,34,63]. This same entrapment method could also be extended to other proteins and could be applied to the analysis of other biological interactions [14,30].
Supplementary Material
Highlights.
An entrapment method was developed for alpha1-acid glycoprotein (AGP).
This method was used to make HPLC affinity columns for drug binding studies.
The AGP columns were tested in both frontal analysis and zonal elution formats.
Both entrapped AGP and human serum albumin were used to screen drug interactions.
The measured binding constants agreed well with literature values.
Acknowledgments
This work was supported by National Institutes of Health under grant R01 GM044931. These studies were conducted in facilities that were renovated under NIH grant RR015468.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hage DS, Anguizola J, Barnaby O, Jackson A, Yoo MJ, Papastavros E, Pfaunmiller E, Sobansky M, Tong Z. Characterization of drug interactions with serum proteins by using high-performance affinity chromatography. Curr Drug Metab. 2011;12:313. doi: 10.2174/138920011795202938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen Q, Wang L, Zhou H, Jiang HD, Yu LS, Zeng S. Stereoselective binding of chiral drugs to plasma proteins. Acta Pharmacol Sin. 2013;34:998. doi: 10.1038/aps.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceciliani F, Pocacqua V. The acute phase protein alpha1-acid glycoprotein: a model for altered glycosylation during diseases. Curr Prot Pept Sci. 2007;8:91. doi: 10.2174/138920307779941497. [DOI] [PubMed] [Google Scholar]
- 4.Fournier T, Medjoubi-N N, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482:157. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 5.Israili ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab Rev. 2001;33:161. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 6.Schmid K, Nimerg RB, Kimura A, Yamaguchi H, Binette JP. The carbohydrate units of human plasma alpha1-acid glycoprotein. Biochim Biophys Acta. 1977;492:291. doi: 10.1016/0005-2795(77)90080-0. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Li S, Zhang J, Chen X. Flow injection-capillary electrophoresis frontal analysis method for the study of the interactions of a series of drugs with human serum albumin. J Chromatogr B. 2009;877:3144. doi: 10.1016/j.jchromb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Varghese SJ, Johny SK, Paul D, Ravi TK. In vitro interaction study of retinoic acid isomers with telmisartan and amlodipine by equilibrium dialysis method using UV spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2011;79:384. doi: 10.1016/j.saa.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 9.Vuignier K, Veuthey JL, Carrupt PA, Schappler J. Characterization of drug-protein interactions by capillary electrophoresis hyphenated to mass spectrometry. Electrophoresis. 2012;33:3306. doi: 10.1002/elps.201200116. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Wang Q, Yuan Z, Liu W, Gu J, Zhang L. Drug–protein-binding determination of stilbene glucoside using cloud-point extraction and comparison with ultrafiltration and equilibrium dialysis. Drug Develop Indust Pharm. 2010;36:307. doi: 10.1080/03639040903154192. [DOI] [PubMed] [Google Scholar]
- 11.Waters NJ, Jones R, Williams G, Sohal B. Validation of a rapid equilibrium dialysis approach for the measurement of plasma protein binding. J Pharm Sci. 2008;97:4586. doi: 10.1002/jps.21317. [DOI] [PubMed] [Google Scholar]
- 12.Hage DS. High-performance affinity chromatography: a powerful tool for studying serum protein binding. J Chromatogr B. 2002;768:3. doi: 10.1016/s0378-4347(01)00482-0. [DOI] [PubMed] [Google Scholar]
- 13.Hage DS, Anguizola JA, Jackson AJ, Matsuda R, Papastavros E, Pfaunmiller E, Tong Z, Vargas-Badilla J, Yoo MJ, Zheng X. Chromatographic analysis of drug interactions in the serum proteome. Anal Methods. 2011;3:1449. doi: 10.1039/C1AY05068K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson AJ, Anguizola J, Pfaunmiller EL, Hage DS. Use of entrapment and high-performance affinity chromatography to compare the binding of drugs and site-specific probes with normal and glycated human serum albumin. Anal Bioanal Chem. 2013;405:5833. doi: 10.1007/s00216-013-6981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruhn PF, Garver S, Hage DS. Development of dihydrazide-activated silica supports for high-performance affinity chromatography. J Chromatogr A. 1994;669:9. doi: 10.1016/0021-9673(94)80332-3. [DOI] [PubMed] [Google Scholar]
- 16.Xuan H, Hage DS. Immobilization of alpha(1)-acid glycoprotein for chromatographic studies of drug-protein binding. Anal Biochem. 2005;346:300. doi: 10.1016/j.ab.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Sobansky MR, Hage DS. Analysis of drug interactions with high-density lipoprotein by high-performance affinity chromatography. Anal Biochem. 2010;397:107. doi: 10.1016/j.ab.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loun B, Hage DS. Chiral separation mechanisms in protein-based HPLC columns. 1. Thermodynamic studies of (R)- and (S)-warfarin binding to immobilized human serum albumin. Anal Chem. 1994;66:3814. doi: 10.1021/ac00093a043. [DOI] [PubMed] [Google Scholar]
- 19.Mallik R, Xuan H, Guiochon G, Hage DS. Immobilization of alpha1-acid glycoprotein for chromatographic studies of drug-protein binding II. correction for errors in association constant measurements. Anal Biochem. 2008;376:154. doi: 10.1016/j.ab.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiel JE, Ohnmacht CM, Hage DS. Measurement of drug-protein dissociation rates by high-performance affinity chromatography and peak profiling. Anal Chem. 2009;81:4320. doi: 10.1021/ac9000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobansky MR, Hage DS. Identification and analysis of stereoselective drug interactions with low-density lipoprotein by high-performance affinity chromatography. Anal Bioanal Chem. 2012;403:563. doi: 10.1007/s00216-012-5816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sobansky MR, Hage DS. Analysis of drug interactions with very low density lipoprotein by high-performance affinity chromatography. Anal Bioanal Chem. 2014;406:6203. doi: 10.1007/s00216-014-8081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong Z, Schiel JE, Papastavros E, Ohnmacht CM, Smith QR, Hage DS. Kinetic studies of drug–protein interactions by using peak profiling and high-performance affinity chromatography: examination of multi-site interactions of drugs with human serum albumin columns. J Chromatogr A. 2011;1218:2065. doi: 10.1016/j.chroma.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allenmark SG. Chromatographic Enantioseparation: Methods and Applications. Ellis Horwood; Chichester: 1988. [Google Scholar]
- 25.Jewell RC, Brouwer KLR, McNamara PJ. Alpha 1-acid glycoprotein high-performance liquid chromatography column (EnantioPAC) as a screening tool for protein binding. J Chromatogr. 1989;487:257. doi: 10.1016/s0378-4347(00)83035-2. [DOI] [PubMed] [Google Scholar]
- 26.Soman S, Yoo MJ, Jang YJ, Hage DS. Analysis of lidocaine interactions with serum proteins using high-performance affinity chromatography. J Chromatogr B. 2010;878:705. doi: 10.1016/j.jchromb.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xuan H, Joseph KS, Wa C, Hage DS. Biointeraction analysis of carbamazepine binding to alpha1-acid glycoprotein by high-performance affinity chromatography. J Sep Sci. 2010;33:2294. doi: 10.1002/jssc.201000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo MJ, Hage DS. Use of peak decay analysis and affinity microcolumns containing silica monoliths for rapid determination of drug-protein dissociation rates. J Chromatogr A. 2011;1218:2072. doi: 10.1016/j.chroma.2010.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustavsson PE, Larsson PO. Support materials for affinity chromatography. In: Hage DS, editor. Handbook of Affinity Chromatography. CRC Press; Boca Raton: 2006. pp. 15–33. [Google Scholar]
- 30.Jackson AJ, Xuan H, Hage DS. Entrapment of proteins in glycogen-capped and hydrazide-activated supports. Anal Biochem. 2010;404:106. doi: 10.1016/j.ab.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betancor L, Luckarift HR. Bioinspired enzyme encapsulation for biocatalysis. Trends Biotechnol. 2008;26:566. doi: 10.1016/j.tibtech.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Livage J, Coradin T, Roux C. Encapsulation of biomolecules in silica gels. J Phys Condens Matter. 2001;13:R673. [Google Scholar]
- 33.Besanger TR, Brennan JD. Entrapment of membrane proteins in sol-gel derived silica. J Sol-Gel Sci Technol. 2006;40:209. [Google Scholar]
- 34.Monton MRN, Forsberg EM, Brennan JD. Tailoring sol-gel-derived silica materials for optical biosensing. Chem Mater. 2012;24:796. [Google Scholar]
- 35.Yang Q, Lundahl P. Immobilized proteoliposome affinity chromatography for quantitative analysis of specific interactions between solutes and membrane proteins. Interaction of cytochalasin B and D-glucose with the glucose transporter Glut1. Biochemistry. 1995;34:7289. doi: 10.1021/bi00022a001. [DOI] [PubMed] [Google Scholar]
- 36.Zeng CM, Zhang Y, Lu L, Brekkan E, Lundqvist A, Lundahl P. Immobilization of human red cells in gel particles for chromatographic activity studies of the glucose transporter Glut1. Biochim Biophys Acta. 1997;1325:91. doi: 10.1016/s0005-2736(96)00247-7. [DOI] [PubMed] [Google Scholar]
- 37.Peters T., Jr . All About Albumin: Biochemistry, Genetics, and Medical Applications. Academic Press; San Diego, California: 1996. [Google Scholar]
- 38.Keener CR, Wolfe CA, Hage DS. Optimization of oxidized antibody labeling with lucifer yellow CH. BioTechniques. 1994;16:894. [PubMed] [Google Scholar]
- 39.Elder DP, Snodin D, Teasdale A. Control and analysis of hydrazine, hydrazides and hydrazones — genotoxic impurities in active pharmaceutical ingredients (APIs) and drug products. J Pharm Biomed Anal. 2011;54:900. doi: 10.1016/j.jpba.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Chaiken IM. Analytical Affinity Chromatography. CRC Press; Boca Raton: 1987. [Google Scholar]
- 41.Hage DS, Chen J. Quantitative affinity chromatography: practical aspects. In: Hage DS, editor. Handbook of Affinity Chromatography. CRC Press; Boca Raton: 2006. pp. 595–628. [Google Scholar]
- 42.Tweed SA, Loun B, Hage DS. Effects of ligand heterogeneity in the characterization of affinity columns by frontal analysis. Anal Chem. 1997;69:4790. doi: 10.1021/ac970565m. [DOI] [PubMed] [Google Scholar]
- 43.Zheng XW, Podariu M, Bi C, Hage DS. Development of enhanced capacity affinity microcolumns by using a hybrid of protein cross-linking/modification and immobilization. J Chromatogr A. 2015;1400:82. doi: 10.1016/j.chroma.2015.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacKichan JJ, Zola EM. Determinants of carbamazepine and carbamazepine 10,11-epoxide binding to serum protein, albumin and alpha 1-acid glycoprotein. Br J Clin Pharmacol. 1984;18:487. doi: 10.1111/j.1365-2125.1984.tb02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lima JJ, Boudoulas H, Blanford M. Concentration-dependence of disopyramide binding to plasma protein and its influence on kinetics and dynamics. J Pharmacol Exp Ther. 1981;219:741. [PubMed] [Google Scholar]
- 46.Herve F, Duche JC, d’Athis P, Marche C, Barre J, Tillement JP. Binding of disopyramide, methadone, dipyridamole, chlorpromazine, lignocaine and progesterone to the two main genetic variants of human alpha 1-acid glycoprotein: evidence for drug-binding differences between the variants and for the presence of two separate drug-binding sites on alpha 1-acid glycoprotein. Pharmacogenetics. 1996;6:403. doi: 10.1097/00008571-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Bree F, Eap CB, Baumann P, Duche JC, Tillement JP. Comparison of drug binding capacities of two AAG peptidic variants of human origin. Prog Clin Biol Res. 1989;300:399. [PubMed] [Google Scholar]
- 48.Bree F, Rouzeau JD, Durand G, Gardier A, Tillement JP. Comparison of drug binding capacities of three AAG glycan variants of human origin. Prog Clin Biol Res. 1989;300:405. [PubMed] [Google Scholar]
- 49.Herve F, Gomas E, Duche JC, Tillement JP. Evidence for differences in the binding of drugs to the two main genetic variants of human alpha 1-acid glycoprotein. Br J Clin Pharmacol. 1993;36:241. doi: 10.1111/j.1365-2125.1993.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNamara PJ, Slaughter RL, Pieper JA, Wyman MG, Lalka D. Factors influencing serum-protein binding of lidocaine in humans. Anesth Analg. 1981;60:395. [PubMed] [Google Scholar]
- 51.Brinkschulte M, Breyer-Pfaff U. The contribution of alpha 1-acid glycoprotein, lipoproteins, and albumin to the plasma binding of perazine, amitriptyline, and nortriptyline in healthy man. Naunyn Schmied Arch Pharmacol. 1980;314:61. doi: 10.1007/BF00498432. [DOI] [PubMed] [Google Scholar]
- 52.Yasgar A, Furdas SD, Maloney DJ, Jadhav A, Jung M, Simeonov A. High-throughput 1,536-well fluorescence polarization assays for alpha(1)-acid glycoprotein and human serum albumin binding. PLoS One. 2012;7:e45594. doi: 10.1371/journal.pone.0045594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan AB, Khan JM, Ali MS, Khan RH, Din K. Spectroscopic approach of the interaction study of amphiphilic drugs with the serum albumins. Coll Surf B Biointer. 2011;87:447. doi: 10.1016/j.colsurfb.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Vodrazka Z, Jandova D, Grafnetterova J, Schuck O, Kalousek I, Tomasek R, Lachmanova J. The binding of chloramphenicol to albumin of normal and uremic sera. Biochem Pharmacol. 1978;27:1717. doi: 10.1016/0006-2952(78)90545-2. [DOI] [PubMed] [Google Scholar]
- 55.Herve F, Urien S, Albengres E, Duche JC, Tillement JP. Drug binding in plasma. A summary of recent trends in the study of drug and hormone binding. Clin Pharmacokinet. 1994;26:44. doi: 10.2165/00003088-199426010-00004. [DOI] [PubMed] [Google Scholar]
- 56.Verbeeck RK, Cardinal JA, Hill AG, Midha KK. Binding of phenothiazine neuroleptics to plasma proteins. Biochem Pharmacol. 1983;32:2565. doi: 10.1016/0006-2952(83)90019-9. [DOI] [PubMed] [Google Scholar]
- 57.Sharples D. The binding of chlorpromazine to human serum albumin. J Pharm Pharmacol. 1974;26:640. doi: 10.1111/j.2042-7158.1974.tb10679.x. [DOI] [PubMed] [Google Scholar]
- 58.Tinguely D, Baumann P, Conti M, Jonzier-Perey M, Schopf J. Interindividual differences in the binding of antidepressives to plasma proteins: the role of the variants of alpha 1-acid glycoprotein. Eur J Clin Pharmacol. 1985;27:661. doi: 10.1007/BF00547045. [DOI] [PubMed] [Google Scholar]
- 59.Khan AB, Khan JM, Ali MS, Khan RH, Din K. Interaction of amphiphilic drugs with human and bovine serum albumins. Spectrochim Acta A Mol Biomol Spectrosc. 2012;97:119. doi: 10.1016/j.saa.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 60.Essassi D, Zini R, Tillement JP. ANS-drug interactions to AAG and HSA fluorescence and equilibrium dialysis studies. Prog Clin Biol Res. 1989;300:423. [PubMed] [Google Scholar]
- 61.Conn HL, Jr, Luchi RJ. Some quantitative aspects of the binding of quinidine and related quinoline compounds by human serum albumin. J Clin Invest. 1961;40:509. doi: 10.1172/JCI104278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ueda CT, Makoid MC. Quinidine and dihydroquinidine interactions in human plasma. J Pharm Sci. 1979;68:448. doi: 10.1002/jps.2600680414. [DOI] [PubMed] [Google Scholar]
- 63.Keeling-Tucker T, Brennan JD. Fluorescent probes as reporters on the local structure and dynamics in sol-gel-derived nanocomposite materials. Chem Mater. 2001;13:3331. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.