Abstract
Background
Social-interpersonal dysfunction increases disability in major depressive disorder (MDD). Here we clarified the durability of improvements in social-interpersonal functioning made during acute-phase cognitive therapy (CT), whether continuation CT (C-CT) or fluoxetine (FLX) further improved functioning, and relations of functioning with depressive symptoms and relapse/recurrence.
Method
Adult outpatients (N=241) with recurrent MDD who responded to acute-phase CT with higher risk of relapse (due to unstable or partial remission) were randomized to 8 months of C-CT, FLX, or pill placebo plus clinical management (PBO) and followed 24 additional months. We analyzed repeated measures of patients’ social adjustment, interpersonal problems, dyadic adjustment, depressive symptoms, and major depressive relapse/recurrence.
Results
Large improvements in social-interpersonal functioning occurring during acute-phase CT (median d=1.4) were maintained, with many patients (median=66%) scoring in normal ranges for 32 months. Social-interpersonal functioning did not differ significantly among C-CT, FLX, and PBO arms. Beyond concurrently measured residual symptoms, deterioration in social-interpersonal functioning preceded and predicted upticks in depressive symptoms and major depressive relapse/recurrence.
Limitations
Results may not generalize to other patient populations, treatment protocols, or measures of social-interpersonal functioning. Mechanisms of risk connecting poorer social-interpersonal functioning with depression were not studied.
Conclusions
Average improvements in social-interpersonal functioning among higher-risk responders to acute phase CT are durable for 32 months. After acute-phase CT, C-CT or FLX may not further improve social-interpersonal functioning. Among acute-phase CT responders, deteriorating social-interpersonal functioning provides a clear, measurable signal of risk for impending major depressive relapse/recurrence and opportunity for preemptive intervention.
Keywords: cognitive therapy, continuation treatment, social-interpersonal functioning, relapse, residual symptoms
Introduction
Major depressive disorder (MDD) a leading cause of disability worldwide, partly due to social-interpersonal dysfunction (e.g., difficulty fulfilling social roles as a worker, parent, or friend) accompanying depressive episodes and residual symptoms (American Psychiatric Association, 2013; Collins et al., 2011; Kessler et al., 2014). During acute-phase cognitive therapy (CT) or pharmacotherapy, social-interpersonal functioning improves, but only about half to three-quarters as much as depressive symptoms decrease (e.g., Hirshfeld et al., 2002; Lin et al., 2015; Renner et al. 2014; Vittengl et al., 2004; Zu et al., 2014). After acute-phase treatment for MDD, continuation and maintenance treatments reduce depressive relapse and recurrence, respectively (Biesheuvel-Leliefeld et al., 2015; Borges et al., 2014; Jarrett, Minhajuddin, Gershenfeld, et al., 2013). However, relatively few social-interpersonal functioning data are available after acute-phase treatment ends. Here we analyzed social-interpersonal functioning among 241 adult outpatients with recurrent MDD who responded to acute-phase CT with higher risk of relapse (due to unstable or partial remission) and were randomized to 8 months of continuation CT (C-CT), fluoxetine (FLX), or pill placebo with clinical management (PBO) and followed 24 additional months (Jarrett, Minhajuddin, Gershenfeld, et al., 2013). We aimed to clarify the durability of initial improvements in social-interpersonal functioning, effects of continuation treatments on functioning, and relations of functioning with depressive symptoms and relapse/recurrence.
Relations between social-interpersonal functioning and depression likely are bidirectional. Depressive symptoms may produce functional impairment via several processes. For example, neurocognitive deficits in depression, including poor attention and memory (Lam, Kennedy, McIntyre, & Khullar, 2014) and impaired social cognition (Weightman, Air, & Baune, 2014) may inhibit or disrupt social-interpersonal functioning. In addition, low positive emotionality (e.g., low energy, diminished enthusiasm), which is characteristic of depression, may reduce reinforcement-seeking behaviors such as socializing and working (Watson, Clark, McIntyre, & Hamaker, 1992). Lower positive emotionality was a risk factor for poorer long-term outcomes among acute-phase CT responders in a previous trial (Vittengl et al., 2010) and in the current dataset (Vittengl et al., 2015a).
Social-interpersonal dysfunction may also lead to onset and maintenance of depression. For example, the absence or end of high-quality social relationships (e.g., due to bereavement, divorce, geographic relocation) predicts poorer physical and mental health (Baumeister & Leary, 1995; Baumeister, 2012) and increased risk for suicide (Tsai et al., 2015). Similarly, social skills deficits (e.g., in speech content and style, facial expressions, gaze) may precede depression and be important treatment targets (Segrin, 2000, 2011). Moreover, social-interpersonal rejection reduces self-esteem (Leary, 2012), which correlates substantially with depression (Sowislo & Orth, 2013). Finally, pre-treatment social-interpersonal dysfunction predicted non-response to acute-phase CT in the current dataset (Jarrett, Minhajuddin, Kangas et al., 2013)
Social-interpersonal functioning improves less, and more slowly, and do depressive symptoms during acute-phase cognitive therapy (Vittengl et al., 2004) or pharmacotherapy (Lin et al., 2015; Zu et al., 2014) for depression. For example, medians of 27% of patients entered and 63% exited acute-phase CT with normal-range social-interpersonal functioning in a previous trial (Vittengl et al., 2004). Further, reductions in depressive symptoms account for most of the pre-post change in social-interpersonal functioning in both acute-phase CT and pharmacotherapy (Hirschfeld et al., 2002; Vittengl et al., 2004). Similar patterns were apparent during acute phase CT in the current dataset (Dunn et al., 2012). In sum, many patients continue to experience significant social-interpersonal impairment at the end of acute-phase CT or pharmacotherapy for depression (Hirschfeld et al., 2002; Kennedy et al., 2007; Vittengl et al., 2004), and this impairment predicts poorer longer-term outcomes (e.g., Solomon et al., 2004; Vittengl et al., 2010).
Less is known about patients’ social-interpersonal functioning months-years after acute-phase treatment for depression, including the effects of continuation and maintenance treatments. Maintenance pharmacotherapy has improved social-interpersonal functioning relative to pill placebo in several trials (e.g., Sambunaris et al., 2014; Trivedi et al., 2010). But maintenance pharmacotherapy infrequently produced normal-range social-interpersonal functioning among patients with chronic depression in one trial (Kocsis et al., 2002). Moreover, patients switched from combined treatment (interpersonal psychotherapy plus pharmacotherapy) to maintenance monotherapy (psychotherapy or pharmacotherapy alone) experienced deteriorating functioning (Lenze et al., 2002). Finally, in a prior study of acute-phase CT responders, improved functioning endured for two years, on average. Among these acute-phase CT responders, C-CT did not improve functioning relative to assessment control, and social-interpersonal functioning deteriorated before major depressive relapse/recurrence (Vittengl et al., 2004, 2009).
In this context, the current analyses aimed to clarify social-interpersonal functioning after response to acute-phase CT for depression. We analyzed data from 241 patients with recurrent MDD who responded to acute-phase CT, were judged to be at increased risk for relapse due to unstable or partial remission, were randomized to 8 months of continuation treatment (C-CT, FLX or PBO), and were assessed up to 24 additional months (Jarrett, Minhajuddin, Gershenfeld et al., 2013). We tested (1) durability of improvements in social-interpersonal functioning made during acute-phase CT, including the proportion of patients with “healthy” or normal-range functioning; (2) whether continuation treatment with CCT or FLX further improved functioning relative to PBO; and (3) whether social-interpersonal dysfunction was a leading indicator of upticks (i.e., “blips” or small increases) in depressive symptoms and of major depressive relapse/recurrence.
Method
Participants
A two-site randomized clinical trial described in detail by Jarrett and Thase (2010) and Jarrett, Minhajuddin, Gershenfeld et al. (2013) provided data in accordance with ethical standards and institutional review board approvals. Outpatients participated if they (a) provided written informed consent; (b) met criteria for recurrent MDD (American Psychiatric Association, 2000) on the Structured Clinical Interview (First et al., 1996); (c) had remitted between previous depressive episodes, had ≥ 1 depressive episode with complete inter-episode recovery, or had antecedent dysthymic disorder; (d) had 17-item Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960) scores ≥14;1 and (e) were ≥18 and ≤70 years old. Exclusionary criteria were (a) poorly controlled or severe concurrent medical disorders possibly causing depression; (b) organic or psychotic mental disorders, active substance dependence, bipolar disorder, or primary eating or obsessive-compulsive disorders; (c) inability to complete questionnaires in English; (d) active suicide risk; (e) history of non-response to ≥8 weeks of CT or 6 weeks of fluoxetine; or (f) current pregnancy or planned pregnancy within 11 months after intake.
The current analyses focused on 241 responders to acute phase CT, who were judged to be at increased risk for relapse/recurrence (Jarrett and Thase, 2010), and who consented to an experimental continuation treatment protocol described following. These 241 patients were M = 43 (SD = 12) years old and had completed M = 16 (SD = 3) years of education; 67% were women; 85% were white, 8% black, and 8% other ethnicities. Patients’ mean age of MDD onset was 21 (SD = 10) years, and their major depressive episode had lasted M = 24 (median = 9, SD = 44) months at intake.
Acute phase
Patients were withdrawn from any psychotropic medications before, and were not prescribed medications during, the acute phase. Cognitive therapists (N = 16) completed ≥1 year of CT training and demonstrated competence via Cognitive Therapy Scale (Young & Beck, 1980) scores ≥40. Therapists submitted session videotapes for review and participated in weekly group supervision/feedback sessions. The acute-phase CT protocol included 16 or 20 CT sessions over 12 weeks, with 2 additional weeks allowed for rescheduling. Participants first received 8 twice-weekly sessions. Patients with ≥40% reduction in HRSD scores then received 8 weekly sessions (16 total sessions), whereas patients with less reduction in HRSD scores received 8 twice-weekly sessions before 4 weekly sessions (20 total sessions).2 More CT sessions for patients with less early improvement was designed to increase patients’ chances of eligibility for the continuation phase.
Continuation Phase
Among 523 consenting to the acute phase, 292 patients responded to acute-phase CT (no major depressive episode and final HRSD ≤12). Among the responders, 241 also met a priori criteria for higher relapse risk due to unstable or partial remission (i.e., at least one of the last 7 approximately weekly acute-phase HRSD scores ≥7; Jarrett & Thase, 2010) and consented to randomization to 8 months of continuation-phase CT (C-CT; n = 86), fluoxetine with clinical management (FLX; n = 86), or pill placebo with clinical management (PBO; n = 69).3
The 10-session C-CT protocol included 4 biweekly then 6 monthly sessions, each approximately 60 minutes (Jarrett et al., 1989; Jarrett et al., 2008). C-CT patients learned to apply compensatory skills to residual and emergent depressive symptoms; to generalize CT skills across problems, situations, and time; and to cope proactively with previously identified cognitive and behavioral risks. Patients’ acute-phase and C-CT therapists were the same, with few exceptions (e.g., due to a therapist’s maternity leave).
Experienced pharmacotherapists provided FLX and PBO. The clinical-management protocol (Fawcett et al., 1987) was double-blinded with 10 sessions on the same schedule as C-CT. Research pharmacies dispensed identical capsules with active fluoxetine or placebo. In the first (30–45 minutes) and subsequent (15–30 minutes) sessions, pharmacotherapists provided supportive contact addressing symptoms and signs of depression, information about depression, and effects of medication. Pharmacotherapists were prohibited from using specific C-CT methods. Patients received 10 mg/day for 2 weeks, then 20 mg/day for 2 weeks, and 40 mg/day thereafter. Although most patients (73%) achieved 40mg/day, pharmacotherapists could decrease doses to reduce side effects, and patients intolerant to ≥10mg/day received only clinical management. All randomized patients are analyzed here.
Follow-up Phase
After the continuation phase, patients entered follow-up. Blinded evaluators assessed patients every 4 months for 24 months. Patients were encouraged to seek interim evaluation from study staff if they experienced depressive symptoms. Across study phases, atients meeting criteria for MDD were referred out for treatment.
Measures
Longitudinal Interval Follow-Up Evaluation. Independent evaluators completed this semi-structured retrospective interview (Keller et al., 1987) every 4 months after acute-phase CT, when patients, therapists, or follow-up evaluators suspected major depressive relapse or recurrence, and at study exit. Weekly psychiatric status ratings (PSR) of DSM-IV MDD of 1 (no symptoms) or 2 (one or two mild symptoms) for ≥ 35 continuous weeks defined recovery. Weekly PSR of 5 (meets MDD criteria) or 6 (meets MDD criteria with severe impairment and/or psychosis) for ≥ 2 weeks defined relapse and recurrence, before and after recovery, respectively (Jarrett & Thase, 2010), and are analyzed together here as relapse/recurrence. Use of potentially mood-altering non-protocol treatment was tracked with the LIFE, as well, and coded as present or absent each study week. Social-interpersonal functioning was scored monthly using the Range of Impaired Functioning Tool (RIFT; Leon et al., 1999). The RIFT includes items addressing functioning at work (employment, household activities, school), in social relationships (spouse, children, other relatives, friends), and in recreational activities, plus overall satisfaction with functioning. Items are rated from 1 (no impairment) to 5 (severe impairment). The highest (most impaired) rating within work and relationships domains is retained. The 4 items are summed to form a total RIFT score, and higher scores reflect greater dysfunction. Among assessments used in the current analyses, median alpha internal consistency and interrater reliability estimates, respectively, were acceptable for the RIFT (.71, .78) and for a monthly depressive symptom (PSR) average that paralleled the RIFT temporally (.97, .95).
Social Adjustment Scale—Self Report (SAS-SR)
The SAS-SR (Weissman and Bothwell, 1976) is a 56-item questionnaire measuring functioning in important social domains. Patients answer sections reflecting their social roles (e.g., parenting and marital functioning is inapplicable to some). We computed the total score as a broad index of functioning. Higher values reflect poorer adjustment. Among assessments used in the current analyses (acute-phase intake, randomization, every 4 months after randomization), alpha reliability for the total score was moderately high (median = .87).
Inventory of Interpersonal Problems (IIP)
The IIP (Horowitz et al., 1988) is a 127-item self-report scale measuring the extent to which specific behaviors, thoughts, and emotions have caused difficulties in social relationships. We analyzed the total scores as broad index of interpersonal functioning. Higher scores values indicate greater problems. Among assessments used in the current analyses (acute-phase intake, randomization, every 4 months after randomization), alpha reliability for the total score was very high (median = .98).
Dyadic Adjustment Scale (DYS)
Patients in marital and similarly committed dyads complete this 32-item self-report questionnaire measuring positive adjustment and satisfaction in the romantic relationship (Spanier, 1976). We analyzed the total score for these patients, whereas patients not in committed romantic relationships had no DYS scores. Higher values mark better overall relationship quality. Among assessments used in the current analyses (acute-phase intake, randomization, every 4 months after randomization), alpha reliability for the total score was high (median = .95).
Depressive symptoms
Patients completed the 21-item Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and 30-item Inventory of Depressive Symptomatology—Self-Report (IDS-SR; Rush, Gullion, Basco, Jarrett, & Trivedi, 1996), and clinicians administered the 17-item HRSD. The BDI, IDS-SR, and HSRD reflect the same depressive symptom construct concurrently and longitudinally and can be aggregated to increase reliability (Vittengl, Clark, Kraft, & Jarrett, 2005). We standardized the BDI, IDS-SR, and HRSD based on their distributions at acute-phase intake and averaged the three measures to form a composite index (Vittengl et al., 2013). Higher composite scores indicate greater residual depressive symptoms. Among assessments used in the current analyses (acute-phase intake, randomization, every 4 months after randomization), alpha reliability computed treating the three scales as items was high (median = .91).
Proportions of Participants with Healthy Social-Interpersonal Functioning
To clarify improvements in functioning, we estimated the proportions of patients with “healthy” or normal-range scores on the SAS-SR, IIP, DYS, and RIFT using available norms. On each measure, we classified patients within 1.28 SD of the normative mean in the pathological direction as healthy, following established cutoffs (Vittengl et al., 2009). Although more conservative than a traditional cutoff of 2 SD from the mean (about 98% healthy and 2% unhealthy in the normative population), the 1.28 SD cutoff (about 90% healthy and 10% unhealthy in the normative population) is more consistent with the higher prevalence of psychopathology resulting in social-interpersonal dysfunction (e.g., Kessler et al., 2005). We used pooled adult norms for the SAS-SR (M = 1.59, SD = 0.33), IIP (M = 0.77, SD = 0.39), and DYS (M = 114.8, SD = 17.8) compiled by Vittengl et al. (2004) and norms for the RIFT (M = 7.4, SD = 1.7) from Judd et al. (2005).
Statistical Analyses
We completed intent-to-treat analyses by including all randomized patients. Supporting comparisons among treatment arms, proportions of patients completing the 139-week study (8-month experimental plus 24-month follow-up phases) did not differ significantly among C-CT (52.3%), FLX (48.8%), and PBO (46.4%) groups, χ2(2) = 0.56, p ≥ 76. Similarly, patients’ average number of study weeks completed did not differ significantly among C-CT (mean = 97.0, SD = 53.1), FLX (mean = 87.6, SD = 59.3), and PBO (mean = 82.1, SD = 61.5) groups, ANOVA F(2,240) = 1.32, p = .27, Kruskal-Wallis χ2(2) = 2.41, p = .30.
We estimated means and proportions and tested differences among treatments with repeated-measures multilevel models using PROC GLIMMIX in SAS 9.3 software (SAS Institute, Inc.). The multilevel models allowed retention of cases with some missing data (as opposed to deletion of cases with any missing data in ordinary least squares models) to produce more powerful and accurate hypothesis tests (e.g., Littell, Milliken, Stroup, & Wolfinger, 1996; Nich & Carroll, 1998). Models of continuous outcomes (e.g., raw SAS-SR scores) assumed a normal distribution and identity link function. Models of dichotomous outcomes (e.g., proportion with “healthy” SAS-SR scores) assumed a binary distribution and logit link function. Models included fixed effects of continuation treatment and assessment time, the continuation treatment × time interaction, and receipt of non-protocol treatment as a covariate. Models included compound symmetric error structures. We computed effect size d and z-scores using least-squares means estimated in multilevel models and measures’ SD at acute-phase intake. We predicted time to relapse/recurrence in Cox regression models using PROC PHREG. Because patients experiencing relapse/recurrence were referred out for treatment, Cox regression models excluded the non-protocol treatment covariate.
Related Findings from the Current Clinical Trial
Related analyses of the current clinical trial dataset addressed different goals. Among all patients entering the trial, poorer social-interpersonal functioning at intake predicted dropout and non-response to the acute phase (Jarrett, Minhajuddin, Kangas et al., 2013); and social-interpersonal functioning improved during the acute phase (Dunn et al., 2012; Renner et al., 2012), although not as much as depressive symptoms improved (Vittengl et al., 2013). Among higher-risk responders to the acute phase, C-CT or FLX reduced relapse (Jarrett, Minhajuddin, Gershenfeld et al., 2013) and residual symptoms (Vittengl et al., 2014) compared to PBO during the experimental phase; and social-interpersonal dysfunction (as the strongest markers of a low positive-emotionality/behavioral activation factor) at the end of the acute phase predicted lower odds of stable remission and recovery from MDD (Vittengl et al., 2015a). Use of potentially mood-altering non-protocol medications was limited (about 15% of patients reported any use, mostly short-term) and did not differ significantly among experimental groups during the 32-month study (Jarrett Minhajuddin, Gershenfeld et al., 2013).
Results
Levels of Social-Interpersonal Functioning
We completed intent-to-treatment analyses of scores on the four social-interpersonal functioning measures (SAS-SR, IIP, DYS, RIFT; see Appendix 1 for descriptive statistics) using repeated-measures multilevel models. Models included the main effects of assessment time (acute-phase CT intake, randomization, and approximately monthly through 32 months on the RIFT or every 4 months on the SAS-SR, IIP, and DYS) and continuation-phase treatment group (C-CT, FLX, or PBO), and the treatment-by-time interaction. As shown in Table 1, the main effect of time was significant in all models. However, the main effect of continuation treatment and the treatment × time interaction were not significant.
Appendix 1.
Descriptive Statistics for Raw Study Measures
| Measure | Assessment | N | M | SD |
|---|---|---|---|---|
| Social Adjustment Scale—Self-report | Acute intake | 234 | 2.54 | 0.40 |
| Post-acute month 0 | 218 | 1.90 | 0.36 | |
| Post-acute month 4 | 161 | 1.88 | 0.37 | |
| Post-acute month 8 | 145 | 1.83 | 0.37 | |
| Post-acute month 12 | 128 | 1.84 | 0.35 | |
| Post-acute month 16 | 119 | 1.78 | 0.41 | |
| Post-acute month 20 | 115 | 1.84 | 0.42 | |
| Post-acute month 24 | 104 | 1.83 | 0.37 | |
| Post-acute month 28 | 102 | 1.79 | 0.38 | |
| Post-acute month 32 | 98 | 1.74 | 0.38 | |
| Inventory of Interpersonal Problems | Acute intake | 237 | 1.68 | 0.52 |
| Post-acute month 0 | 220 | 1.11 | 0.49 | |
| Post-acute month 4 | 163 | 1.06 | 0.49 | |
| Post-acute month 8 | 140 | 1.00 | 0.52 | |
| Post-acute month 12 | 124 | 1.01 | 0.56 | |
| Post-acute month 16 | 120 | 1.00 | 0.57 | |
| Post-acute month 20 | 117 | 1.01 | 0.59 | |
| Post-acute month 24 | 109 | 1.05 | 0.57 | |
| Post-acute month 28 | 102 | 0.93 | 0.54 | |
| Post-acute month 32 | 100 | 0.93 | 0.57 | |
| Dyadic Adjustment Scale | Acute intake | 136 | 87.73 | 22.57 |
| Post-acute month 0 | 120 | 97.88 | 23.16 | |
| Post-acute month 4 | 86 | 98.72 | 21.33 | |
| Post-acute month 8 | 80 | 99.95 | 25.79 | |
| Post-acute month 12 | 66 | 101.62 | 21.24 | |
| Post-acute month 16 | 66 | 102.65 | 24.85 | |
| Post-acute month 20 | 64 | 103.03 | 23.19 | |
| Post-acute month 24 | 56 | 102.36 | 20.64 | |
| Post-acute month 28 | 53 | 101.19 | 22.63 | |
| Post-acute month 32 | 58 | 102.72 | 22.86 | |
| Depressive symptom severity composite | Acute intake | 241 | 48.86 | 9.20 |
| Post-acute month 0 | 241 | 17.57 | 6.99 | |
| Post-acute month 4 | 182 | 18.01 | 10.24 | |
| Post-acute month 8 | 172 | 16.09 | 11.12 | |
| Post-acute month 12 | 146 | 16.40 | 10.21 | |
| Post-acute month 16 | 143 | 16.45 | 12.26 | |
| Post-acute month 20 | 136 | 18.22 | 13.14 | |
| Post-acute month 24 | 131 | 17.06 | 12.36 | |
| Post-acute month 28 | 121 | 16.18 | 11.66 | |
| Post-acute month 32 | 124 | 15.41 | 10.89 | |
| Range of Impaired Functional Tool | Acute intake | 237 | 14.06 | 2.92 |
| Post-acute month 0 | 181 | 9.43 | 2.52 | |
| Post-acute month 4 | 163 | 9.21 | 2.75 | |
| Post-acute month 8 | 146 | 8.79 | 2.52 | |
| Post-acute month 12 | 124 | 8.98 | 2.63 | |
| Post-acute month 16 | 124 | 9.29 | 2.90 | |
| Post-acute month 20 | 120 | 9.41 | 2.77 | |
| Post-acute month 24 | 110 | 9.11 | 2.85 | |
| Post-acute month 28 | 107 | 9.20 | 2.68 | |
| Post-acute month 32 | 101 | 8.83 | 2.62 | |
| Psychiatric Status Ratings of Major Depressive Disorder | Post-acute month 1 | 200 | 2.11 | 1.01 |
| Post-acute month 4 | 196 | 2.05 | 0.98 | |
| Post-acute month 8 | 181 | 1.94 | 1.04 | |
| Post-acute month 12 | 160 | 1.83 | 1.07 | |
| Post-acute month 16 | 154 | 1.76 | 1.12 | |
| Post-acute month 20 | 146 | 1.92 | 1.30 | |
| Post-acute month 24 | 136 | 1.99 | 1.27 | |
| Post-acute month 28 | 130 | 1.69 | 1.09 | |
| Post-acute month 32 | 125 | 1.73 | 1.15 | |
| Non-protocol treatment receipt (1 = any; 0 = none) | Acute intake | 241 | 0.00 | 0.00 |
| Post-acute month 0 | 241 | 0.00 | 0.00 | |
| Post-acute month 4 | 200 | 0.03 | 0.17 | |
| Post-acute month 8 | 191 | 0.08 | 0.28 | |
| Post-acute month 12 | 176 | 0.09 | 0.29 | |
| Post-acute month 16 | 158 | 0.10 | 0.30 | |
| Post-acute month 20 | 152 | 0.09 | 0.28 | |
| Post-acute month 24 | 141 | 0.09 | 0.29 | |
| Post-acute month 28 | 135 | 0.07 | 0.26 | |
| Post-acute month 32 | 129 | 0.05 | 0.23 |
Note. N = 241. Not all post-acute months 1–32 shown for the Range of Impaired Functional Tool and Psychiatric Status Ratings. Tabled values reflect missing data for some measures and assessment times, whereas the intent-to-treat analyses included all patients.
Table 1.
Prediction of Psychosocial Functioning through Acute, Continuation, and Follow-up Phases
| Measure | Assessment time main effect | Continuation treatment main effect | Treatment × time interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | df | F | p | |
| SAS-SR | 9,1157 | 133.92 | <.001 | 2,236 | 0.21 | .809 | 18,1157 | 1.27 | .199 |
| IIP | 9,1164 | 86.64 | <.001 | 2,237 | 1.90 | .152 | 18,1164 | 1.04 | .415 |
| DYS | 9,606 | 8.23 | <.001 | 2,148 | 0.32 | .728 | 18,606 | 1.05 | .401 |
| RIFT | 33,4105 | 38.22 | <.001 | 2,237 | 0.05 | .948 | 66,4105 | 0.97 | .536 |
Note. N = 241 patients who had higher-risk response to acute-phase cognitive therapy and were randomized to 8 months of continuation treatment (cognitive therapy, fluoxetine, or placebo) and followed up to 24 additional months. For the Social Adjustment Scale—Self-Report (SAS-SR), Inventory of Interpersonal Problems (IIP), and Dyadic Adjustment Scale (DYS), 10 assessments occurred at intake to acute-phase cognitive therapy and approximately every 4 months thereafter. For the Range of Impaired Functioning Tool (RIFT), functioning estimates were computed at acute-phase intake, randomization, and for each month 1–32 after randomization. Tabled results are from repeated-measures multilevel models. Multilevel models controlled receipt of non-protocol treatment.
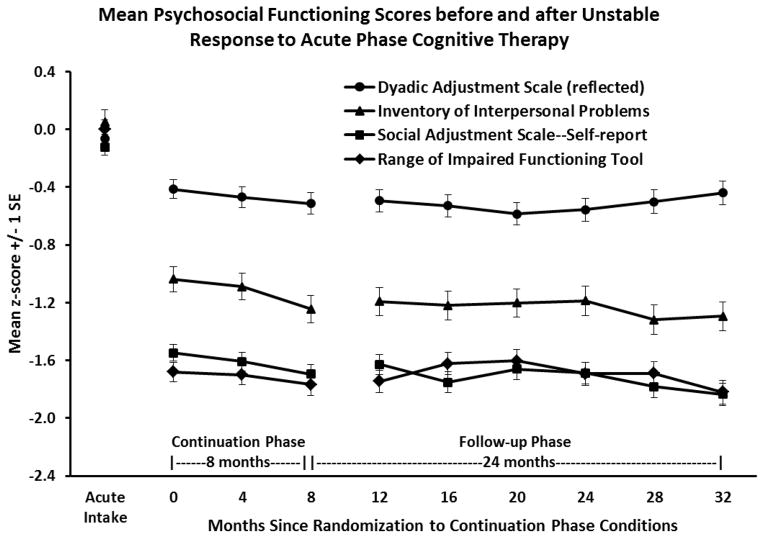
Among acute-phase CT responders, social-interpersonal functioning remained improved at all assessments during experimental and follow-up phases relative to acute-phase intake (all pairwise contrasts p < .01; see Figure 1). Effect sizes (mean d) were large for the SAS-SR (1.57), IIP (1.25), and RIFT (1.70) and moderate for DYS (0.44). The proportions of acute-phase CT responders with healthy-range social-interpersonal functioning during the experimental and follow-up phases (mean percentage months 0–32 vs. acute-phase intake) on the SAS-SR (71.3 vs. 7.8%), IIP (68.2 vs. 20.1%), DYS (64.4 vs. 48.7%), and RIFT (59.4 vs. 5.3%) were uniformly higher than at acute-phase intake.
Figure 1.
Patients with higher-risk response to acute-phase cognitive therapy (N = 241) showed large, relatively stable improvements in social-interpersonal functioning that were maintained through 8 months of continuation phase treatment (randomization at month 0 to cognitive therapy, fluoxetine, or pill placebo) and 24 months of follow-up. Dyadic Adjustment Scale scores are reflected so that lower scores mark improved relationships.
Relative to the end of the acute phase (month 0/randomization), additional improvements were small (mean d) on the SAS-SR (0.16), IIP (0.18), RIFT (0.02), and DYS (0.10) and statistically significant (pairwise contrasts p < .05) only for some measures and months (SAS-SR months 8, 16, 28, 32; IIP months 8–32; DYS month 20; RIFT none). Similarly, relative to the end of the acute phase, mean increases in percentages of patients with healthy-range social-interpersonal functioning were small on the SAS-SR (0.9%), IIP (0.6%), DYS (0.4%), and RIFT (0.4%).
Social-Interpersonal Dysfunction as a Leading Indicator of Depression
We tested the extent to which social-interpersonal dysfunction preceded upticks in depressive symptoms and relapse/recurrence among acute-phase CT responders. First, in a series of repeated-measures multilevel models, we tested relations between depression-symptom-composite scores and the SAS-SR, IIP, and DYS assessed every 4 months. We predicted depressive symptoms at each assessment (t0) from depressive symptoms at the prior assessment (t-1) and social-interpersonal functioning at the prior assessment (t-1). As shown in Table 2, poorer functioning on the SAS-SR (effect size r = .21) and IIP (r = .14) predicted subsequent increases in depressive symptoms significantly. The DYS produced a consistent but smaller result (r = −.09) that did not cross the statistical significance threshold, perhaps because the analysis had less power (only patients in romantic dyads completed the DYS).4
Table 2.
Prediction of Depressive Symptom Changes and Relapse/Recurrence from Prior Social-Interpersonal Functioning
| B | SE | p | |
|---|---|---|---|
| Multilevel models: Predictors | Outcome: Depressive symptoms at time t0 | ||
| 1. Prior depressive symptoms (t-1) | 0.171 | 0.045 | <.001 |
| Prior SAS-SR score (t-1) | 5.952 | 1.302 | <.001 |
| 2. Prior depressive symptoms (t-1) | 0.264 | 0.037 | <.001 |
| Prior IIP score (t-1) | 2.749 | 0.824 | <.001 |
| 3. Prior depressive symptoms (t-1) | 0.378 | 0.049 | <.001 |
| Prior DYS score (t-1) | −0.040 | 0.024 | .094 |
|
| |||
| Cox regression model: Predictors | Outcome: Relapse/recurrence at time t0 | ||
| 1. Prior PSR score (t-1) | 0.512 | 0.160 | .001 |
| Prior RIFT score (t-1) | 0.222 | 0.051 | <.001 |
Note. N = 241 patients with higher-risk response to acute-phase cognitive therapy were randomized to 8 months of continuation treatment (cognitive therapy, fluoxetine, or pill placebo with clinical management) and followed up to 24 additional months. The Social Adjustment Scale—Self-Report (SAS-SR), Inventory of Interpersonal Problems (IIP), and Dyadic Adjustment Scale (DYS) predicted a composite of the Hamilton Rating Scale for Depression, Beck Depression Inventory, and the Inventory for Depressive Symptomatology—Self-report at assessments taken at randomization and every 4 months through 32 months. The Range of Impaired Functioning Tool (RIFT) predicted Psychiatric Status Ratings (PSR) of major depressive disorder at months 1–32 after randomization. Multilevel models controlled receipt of non-protocol treatment, continuation phase group, assessment time, and the group × time interaction. The Cox regression model controlled continuation phase group. Interactions of continuation group with functioning were not significant and were excluded from final models. Significant p values relevant to hypothesis tests are bolded.
Second, in a Cox regression model, we predicted relapse/recurrence (vs. study exit without relapse/recurrence) from monthly assessments of depressive symptoms (PSR scores) and social-interpersonal functioning (RIFT scores). Incremental to prior depressive symptoms (t-1), poorer prior social-interpersonal functioning (t-1) predicted relapse/recurrence at t0 (r = .38, hazard ratio = 1.25; see Table 2). After accounting for residual symptoms, the monthly incidence of relapse/recurrence increased by 25% for each 1 point increase in the prior month’s RIFT.
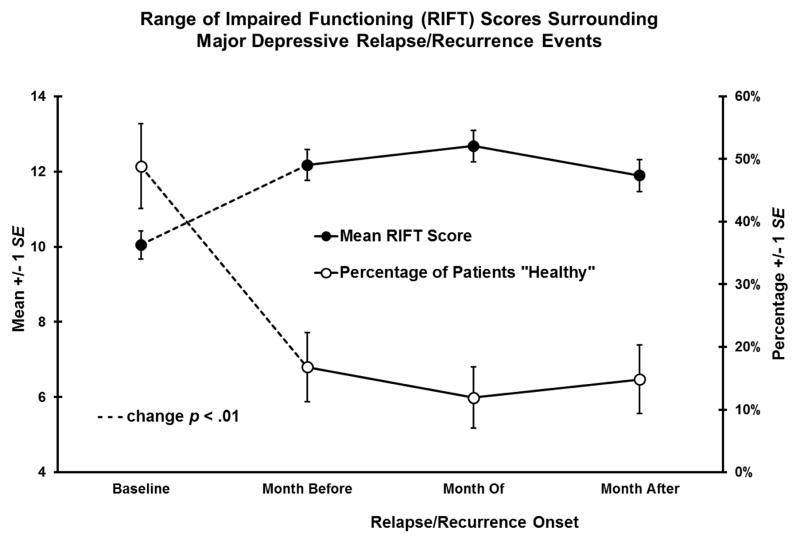
To illustrate this finding, we identified 61 patients who relapsed or recurred between months 3–31 after acute-phase CT. We compared their RIFT scores at baseline (month 1) and in the month immediately before relapse/recurrence onset, the month of relapse/recurrence onset, and the month after relapse/recurrence onset in multilevel models. As shown in Figure 2, from baseline to the month before relapse/recurrence onset, patients’ mean RIFT scores (dysfunction) increased (d = 0.81), and the proportion with “healthy” functioning decreased (48.9 to 16.8%) significantly. Across the subsequent two months, functioning did not change significantly.
Figure 2.
After response to acute-phase CT for depression (baseline), social-interpersonal functioning deteriorated before relapse/recurrence.
Discussion
The current analyses clarified social-interpersonal functioning for 32 months after response to acute-phase CT for MDD, including durability of gains, effects of continuation treatments, and prediction of depressive symptoms and relapse/recurrence. Outpatients with recurrent MDD who responded to acute-phase CT but were judged to be at increased risk for relapse were randomized to 8 months of active continuation treatment (C-CT or FLX) or PBO and followed 24 additional months (Jarrett & Thase, 2010). These patients’ relatively large improvements in social-interpersonal functioning during acute-phase CT (median d = 1.4) were maintained for 32 months on four measures. Proportions of patients scoring in the “healthy” ranges of four functioning measures increased from before (median = 13%) to after (median = 66%) acute-phase CT. These data add to the growing literature regarding the enduring benefits of acute-phase CT on both depression (Cuijpers et al., 2013) and social-interpersonal functioning (Vittengl et al., 2004). Nonetheless, many (median = 34%) of the current higher-risk CT responders continued to show social-interpersonal dysfunction, and active continuation treatments (C-CT or FLX) did not further improve functioning, highlighting substantial room for improving treatments and patients’ lives.
Both continuation-phase treatments (C-CT and FLX) decreased relapse (Jarrett, Minhajuddin, Gershenfeld et al., 2013) and residual symptoms (Vittengl et al., 2014) compared to PBO. Why C-CT and FLX did not also improve social-interpersonal functioning is unknown but replicates a previous trial of patients with recurrent MDD who responded to acute-phase CT and were then randomized to C-CT versus assessment control (Vittengl, Clark, & Jarrett, 2004). One possible explanation is that many patients with recurrent MDD reach a practical “ceiling” for social-interpersonal functioning during response to acute phase CT. Although few observations in the current dataset reached measurement limits for positive functioning (< 1% on the SAS-SR, IIP, and DYS; 2% on the RIFT), patients vulnerable to recurrent MDD may have personal (e.g., temperamental, genetic) and/or environmental (e.g., socioeconomic, familial) liabilities that constrain improvement in social-interpersonal functioning during months-long treatments. Thus, preserving improvements in social-interpersonal functioning made during acute-phase CT response (e.g., 59–71% within healthy ranges) may be the limit without more intensive (e.g., CT plus pharmacotherapy) or longer (e.g., acute, continuation, and maintenance CT) treatments.
Social-interpersonal dysfunction was a clear risk for impending upticks in depressive symptoms and major depressive relapse/recurrence. These findings replicate a prior randomized clinical trial of C-CT versus assessment control for acute-phase CT responders (Vittengl et al., 2009) and, therefore, may now be appropriate for clinical application. After response to acute-phase CT, single-point increases in RIFT scores increased the monthly incidence of relapse/recurrence by 25–29% in the current and earlier (Vittengl et al., 2009) trials. Moreover, increases in RIFT scores between the end of acute-phase CT and the month prior to the onset of relapse were apparent in both studies. It is important to note that prediction from social-interpersonal dysfunction was incremental to residual depressive symptoms, another clear risk for relapse/recurrence (Fava et al., 2007). Thus, complementing assessment of residual depressive symptoms, clinicians should consider restarting, augmenting, or switching treatments when either poor or deteriorating social-interpersonal functioning is evident.
Limitations
The current analyses have important limitations. First, we analyzed patients with recurrent MDD who showed higher-risk response to acute-phase CT delivered by experienced, supervised therapists in a research protocol. The extent to which our results generalize to other patient groups, treatments, and clinical settings requires empirical clarification. Second, our analyses included standard measures of depressive symptoms (HRSD, BDI, IDS-SR) that overlapped conceptually with social-interpersonal functioning. More sensitive tests might be possible using measures of symptoms completely independent of functioning, although such an approach would alter current clinical definitions of “depression” (American Psychiatric Association, 2013; Ro & Clark, 2009). Third, our findings rely on patient and clinician ratings, whereas other measures may offer complementary information (e.g., collateral reports, direct behavioral observations). Fourth, attrition from the 32-month study may have limited detection of some hypothesized effects. Finally, mechanisms of risk connecting poorer social-interpersonal functioning with subsequent increases in depressive symptoms and major depressive episodes were not addressed by our study and are important targets for future research.
Conclusions
Many, if not most, patients with recurrent MDD who respond to acute-phase pharmacotherapy or CT will experience a relapse or recurrence without additional treatment (Biesheuvel-Leliefeld et al., 2015; Vittengl et al., 2007). Providing continuation treatments to higher-risk patients at higher-risk times may improve outcomes, as well as efficiency in environments of limited resources. This strategy requires empirical guidance to identify risks. In the current analyses, we found that average gains in social-interpersonal functioning made during response to acute-phase CT were maintained for 32 months. Despite largely stable averages, downturns in individual patients’ social-interpersonal functioning were leading indicators of upticks in depressive symptoms and major depressive relapse/recurrence, replicating a prior clinical trial (Vittengl et al., 2009). Consequently, social-interpersonal functioning may provide clinicians with a measurable signal of the need for starting or modifying continuation treatment after acute-phase CT. Future research clarifying the mechanisms of risk connecting social-interpersonal dysfunction to depression after response to acute-phase CT may support more specific improvements in treatment protocols.
Appendix 2.
Prediction of Social-Interpersonal Functioning Changes from Prior Depressive Symptoms
| Multilevel models: Predictors | B | SE | p |
|---|---|---|---|
| Outcome: SAS-SR at time t0 | |||
| 1. Prior SAS-SR score (t-1) | 0.558 | 0.039 | <.001 |
| Prior depressive symptoms (t-1) | −0.036 | 0.039 | .354 |
| Outcome: IIP at time t0 | |||
| 2. Prior IIP score (t-1) | 0.959 | 0.015 | <.001 |
| Prior depressive symptoms (t-1) | −0.010 | 0.017 | .566 |
| Outcome: DYS at time t0 | |||
| 3. Prior DYS score (t-1) | 0.960 | 0.021 | <.001 |
| Prior depressive symptoms (t-1) | −0.011 | 0.025 | .669 |
Note. N = 241 patients with higher-risk response to acute-phase cognitive therapy were randomized to 8 months of continuation treatment (cognitive therapy, fluoxetine, or pill placebo with clinical management) and followed up to 24 additional months. The Social Adjustment Scale—Self-Report (SAS-SR), Inventory of Interpersonal Problems (IIP), and Dyadic Adjustment Scale (DYS) were predicted by a composite of the Hamilton Rating Scale for Depression, Beck Depression Inventory, and the Inventory for Depressive Symptomatology—Self-report at assessments taken at randomization and every 4 months through 32 months. Multilevel models controlled receipt of non-protocol treatment, continuation phase group, assessment time, and the group × time interaction.
Highlights.
Social-interpersonal functioning improved during cognitive therapy for depression.
Many responders (median = 66%) showed normal-range functioning for 32 months.
Social-interpersonal functioning deteriorated before major depressive relapse.
Poor or deteriorating functioning may signal need for preventive treatment..
Footnotes
Two patients erroneously entered CT with HRSD = 13 at one of two diagnostic visits due to a scoring error. One patient responded and one dropped out during CT. Following Data Safety and Monitoring Board (DSMB) recommendations, the two patients are analyzed here as they were treated during data collection.
Early symptom reduction levels were misclassified for four patients. Following DSMB recommendations, the patients are analyzed here as they were treated during data collection.
Three non-responders and one lower-risk responder were randomized in error but are included in the intent-to-treat analyses, following Jarrett, Minhajuddin, Gershenfeld et al. (2013).
In contrast to our primary findings, depressive symptoms were not a leading indicator of changes in social-interpersonal functioning (see Appendix 2).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeffrey R. Vittengl, Department of Psychology, Truman State University.
Lee Anna Clark, Department of Psychology, University of Notre Dame.
Michael E. Thase, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania
Robin B. Jarrett, Department of Psychiatry, The University of Texas Southwestern Medical Center.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Baumeister RF. Need-to-belong theory. In: Van Lange PM, Kruglanski AW, Higgins ET, Van Lange PM, Kruglanski AW, Higgins ET, editors. Handbook of Theories of Social Psychology. Vol. 2. Sage; Thousand Oaks, CA: 2012. pp. 121–140. [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol Bull. 1995;117:497–529. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Biesheuvel-Leliefeld KM, Kok GD, Bockting CH, Cuijpers P, Hollon SD, van Marwijk HJ, Smit F. Effectiveness of psychological interventions in preventing recurrence of depressive disorder: Meta-analysis and meta-regression. J Affect Disord. 2015;174:400–410. doi: 10.1016/j.jad.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Borges S, Chen Y, Laughren TP, Temple R, Patel HD, David PA, … Khin NA. Review of maintenance trials for major depressive disorder: A 25-year perspective from the US Food and Drug Administration. J Clin Psychiatry. 2014;75:205–214. doi: 10.4088/JCP.13r08722. [DOI] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, … Stein DJ. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Hollon SD, van Straten A, Bockting C, Berking M, Andersson G. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4) doi: 10.1136/bmjopen-2012-002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TW, Vittengl JR, Clark LA, Carmody T, Thase ME, Jarrett RB. Change in psychosocial functioning and depressive symptoms during acute-phase cognitive therapy for depression. Psychol Med. 2012;42:317–326. doi: 10.1017/S0033291711001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava GA, Ruini C, Belaise C. The concept of recovery in major depression. Psychol Med. 2007;37:307–317. doi: 10.1017/S0033291706008981. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Epstein P, Fiester S, Elkin I, Autry J. Clinical management--imipramine/placebo administration manual. NIMH Treatment of Depression Collaborative Research Program. Psychopharmacol Bull. 1987;23:309–324. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York State Psychiatric Institute, Biometrics Research Department; New York: 1996. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RA, Dunner DL, Keitner G, Klein DN, Koran LM, Kornstein SG, … Keller MB. Does psychosocial functioning improve independent of depressive symptoms? A comparison of nefazodone, psychotherapy, and their combination. Biol Psychiatry. 2002;51:123–133. doi: 10.1016/s0006-3223(01)01291-4. [DOI] [PubMed] [Google Scholar]
- Horowitz LM, Rosenberg SE, Baer BA, Ureño G, Villaseñor VS. Inventory of interpersonal problems: psychometric properties and clinical applications. J Consult Clin Psychol. 1988;56:885–892. doi: 10.1037//0022-006x.56.6.885. [DOI] [PubMed] [Google Scholar]
- Jarrett RB. Cognitive therapy for recurrent unipolar major depressive disorder: The continuation/maintenance phase. 1989. Unpublished treatment manual. [Google Scholar]
- Jarrett RB, Thase ME. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: design of a double-blinded, fluoxetine- and pill placebo-controlled, randomized trial with 2-year follow-up. Contemp Clin Trials. 2010;31:355–377. doi: 10.1016/j.cct.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, Thase ME. Preventing depressive relapse and recurrence in higher risk cognitive therapy responders: A randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA Psychiatry. 2013;70:1152–1160. doi: 10.1001/jamapsychiatry.2013.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Kangas JL, Friedman ES, Callan JA, Thase ME. Acute phase cognitive therapy for recurrent major depressive disorder: Who drops out and how much do patient skills influence response? Behav Res Ther. 2013;51:221–230. doi: 10.1016/j.brat.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Vittengl JR, Clark LA. Preventing recurrent depression. In: Whisman MA, editor. Adapting Cognitive Therapy for Depression: Managing Complexity and Comorbidity. Guilford Press; New York: 2008. pp. 132–156. [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Leon AC, Solomon DA, … Keller MB. Psychosocial disability in the course of bipolar I and II disorders. Arch Gen Psychiatry. 2005;62:1322–1330. doi: 10.1001/archpsyc.62.12.1322. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kennedy N, Foy K, Sherazi R, McDonough M, McKeon P. Long-term social functioning after depression treated by psychiatrists: A review. Bipolar Disord. 2007;9:25–37. doi: 10.1111/j.1399-5618.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, de Jonge P, Shahly V, van Loo HM, Wang PS, Wilcox MA. Epidemiology of depression. In: Gotlib IH, Hammen CL, Gotlib IH, Hammen CL, editors. Handbook of Depression. 3. Guilford; New York: 2014. pp. 7–24. [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-Month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JH, Schatzberg A, Rush AJ, Klein DN, Howland R, Gniwesch L, … Harrison W. Psychosocial outcomes following long-term, double-blind treatment of chronic depression with sertraline vs placebo. Arch Gen Psychiatry. 2002;59:723–728. doi: 10.1001/archpsyc.59.8.723. [DOI] [PubMed] [Google Scholar]
- Lam RW, Kennedy SH, McIntyre RS, Khullar A. Cognitive dysfunction in major depressive disorder: Effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59:649–654. doi: 10.1177/070674371405901206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary MR. Sociometer theory. In: Van Lange PM, Kruglanski AW, Higgins ET, Van Lange PM, Kruglanski AW, Higgins ET, editors. Handbook of Theories of Social Psychology. Vol. 2. Sage; Thousand Oaks, CA: 2012. pp. 151–159. [Google Scholar]
- Lenze EJ, Dew MA, Mazumdar S, Begley AE, Cornes C, Miller MD, … Reynolds CI. Combined pharmacotherapy and psychotherapy as maintenance treatment for late-life depression: Effects on social adjustment. Am J Psychiatry. 2002;159:466–468. doi: 10.1176/appi.ajp.159.3.466. [DOI] [PubMed] [Google Scholar]
- Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impaired Functioning Tool (LIFE-RIFT): A brief measure of functional impairment. Psychol Med. 1999;29:869–878. doi: 10.1017/s0033291799008570. [DOI] [PubMed] [Google Scholar]
- Lin C, Chou L, Chen M, Chen C. The relationship between symptom relief and functional improvement during acute fluoxetine treatment for patients with major depressive disorder. J Affect Disord. 2015;182:115–120. doi: 10.1016/j.jad.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute; Cary, NC: 1996. [Google Scholar]
- Nich C, Carroll K. Now you see it, now you don’t: A comparison of traditional versus random-effects regression models in the analysis of longitudinal follow-up data from a clinical trial. J Consult Clin Psychol. 1998;65:252–261. doi: 10.1037//0022-006x.65.2.252. [DOI] [PubMed] [Google Scholar]
- Renner F, Cuijpers P, Huibers MH. The effect of psychotherapy for depression on improvements in social functioning: A meta-analysis. Psychol Med. 2014;44:2913–2926. doi: 10.1017/S0033291713003152. [DOI] [PubMed] [Google Scholar]
- Renner F, Jarrett RB, Vittengl JR, Clark LA, Thase ME. Interpersonal problems as predictors of therapeutic alliance and symptom improvement in cognitive therapy for depression. J Affect Disord. 2012;138:458–467. doi: 10.1016/j.jad.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Sambunaris A, Gommol C, Chen C, Greenberg WM. Efficacy of levomilnacipran extended-release in improving functional impairment associated with major depressive disorder: Pooled analyses of five double-blind, placebo-controlled trials. Int Clin Psychopharmacol. 2014;29:197–205. doi: 10.1097/YIC.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrin C. Social skills deficits associated with depression. Clin Psychol Rev. 2000;20:379–403. doi: 10.1016/s0272-7358(98)00104-4. [DOI] [PubMed] [Google Scholar]
- Segrin C. Depressive disorders and interpersonal processes. In: Horowitz LM, Strack S, editors. Handbook of Interpersonal Psychology. Wiley; Hoboken, NJ: 2011. pp. 425–448. [Google Scholar]
- Solomon DA, Leon AC, Endicott J, Mueller TI, Coryell W, Shea MT, Keller MB. Psychosocial impairment and recurrence of major depression. Compr Psychiatry. 2004;45:423–430. doi: 10.1016/j.comppsych.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Sowislo JF, Orth U. Does low self-esteem predict depression and anxiety? A meta-analysis of longitudinal studies. Psychol Bull. 2013;139:213–240. doi: 10.1037/a0028931. [DOI] [PubMed] [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. J Mar Fam. 1976;38:15–28. [Google Scholar]
- Trivedi MH, Dunner DL, Kornstein SG, Thase ME, Zajecka JM, Rothschild AJ, … Gelenberg A. Psychosocial outcomes in patients with recurrent major depressive disorder during 2years of maintenance treatment with venlafaxine extended release. J Affect Disord. 2010;126:420–429. doi: 10.1016/j.jad.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Lucas M, Kawachi I. Association between social integration and suicide among women in the United States. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Improvement in social-interpersonal functioning after cognitive therapy for recurrent depression. Psychol Med. 2004;34:643–658. doi: 10.1017/S0033291703001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Moderators of continuation phase cognitive therapy’s effects on relapse, recurrence, remission, and recovery from depression. Behav Res Ther. 2010;48:449–458. doi: 10.1016/j.brat.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: A comparative meta-analysis of cognitive-behavioral therapy’s effects. J Consult Clin Psychol. 2007;75:475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Kraft D, Jarrett RB. Multiple measures, methods, and moments: A factor-analytic investigation of change in depressive symptoms during acute phase cognitive therapy. Psychol Med. 2005;35:693–704. doi: 10.1017/s0033291704004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Nomothetic and idiographic symptom change trajectories in acute-phase cognitive therapy for recurrent depression. J Consult Clin Psychol. 2013;81:615–626. doi: 10.1037/a0032879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Stable remission and recovery after acute-phase cognitive therapy for recurrent major depressive disorder. J Consult Clin Psychol. 2014;82:1049–1059. doi: 10.1037/a0037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Predictors of longitudinal outcomes after unstable response to acute-phase cognitive therapy for major depressive disorder. Psychotherapy. 2015a;52:268–277. doi: 10.1037/pst0000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Improved cognitive content endures for two years among unstable responders to acute-phase cognitive therapy for recurrent major depressive disorder. Psychol Med. 2015b doi: 10.1017/S0033291715001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Jarrett RB, Clark LA. Deterioration in psychosocial functioning predicts relapse/recurrence after cognitive therapy for depression. J Affect Disord. 2009;112:135–143. doi: 10.1016/j.jad.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, McIntyre CW, Hamaker S. Affect, personality, and social activity. J Pers Soc Psychol. 1992;63:1011–1025. doi: 10.1037//0022-3514.63.6.1011. [DOI] [PubMed] [Google Scholar]
- Weightman MJ, Air TM, Baune BT. A review of the role of social cognition in major depressive disorder. Front Psychiatry. 2014:5. doi: 10.3389/fpsyt.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social-adjustment by patient self-report. Arch Gen Psychiatry. 1976;33:1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Young J, Beck AT. Cognitive Therapy Scale: Rating Manual. Center for Cognitive Therapy; Philadelphia, PA: 1980. [Google Scholar]
- Zu S, Xiang Y, Liu J, Zhang L, Wang G, Ma X, Li Z. A comparison of cognitive-behavioral therapy, antidepressants, their combination and standard treatment for Chinese patients with moderate–severe major depressive disorders. J Affect Disord. 2014;152–154:262–267. doi: 10.1016/j.jad.2013.09.022. [DOI] [PubMed] [Google Scholar]