Abstract
Proteoglycans (PGs) are one type of non-collagenous proteins in the extracellular matrix of bone, which primarily contain a core protein and glycosaminoglycans (GAGs). GAGs are highly polar and negatively charged, thus having a strong tendency in attracting water molecules into the matrix. We hypothesized in this study that PGs in bone play a pivotal role in sustaining the toughness of the tissue only when water is present. To test the hypothesis, we used a novel nanoscratch test to measure the in situ toughness of human cadaveric bone treated with and without PNGase F, an enzyme that specifically removes the N-linked oligosaccharides of GAGs from core proteins. Cortical bone specimens were prepared from the posterior aspect of mid-diaphyseal femurs of six (N=6) male human donors between 51.5±5.17 years old. Biochemical and histochemical assays were used to verify whether N-linked oligosaccharides were removed from bone matrix by PNGase F. By testing wet and dehydrated bone specimens, the coupling effect between water and PGs on the in situ toughness of bone was investigated. The two-way ANOVA analyses showed that removal of GAGs had significant effects on the in situ toughness of wet bone samples. In contrast, the removal of GAGs did not show significant effects on the toughness of dry bone. The results of this study, for the first time, suggest that GAGs play a pivotal role in the in situ toughness of bone only when water is present, and vice versa water functions as a plasticizer in bone only when GAGs are present.
Keywords: Bone, Proteoglycans, Toughness, Water, Glycosaminoglycans
INTRODUCTION
Age-related bone fragility fractures are one of major health problems in elderly due to its high risk of morbidity and even mortality [1]. For early diagnosis, bone mineral density (BMD) has been currently used as a standard measure for assessing fracture risks [2]. However, use of mineral quantity alone lacks specificity in predicting bone fracture risks [3, 4]. It is not surprising because the mineral phase is only one of the four constituents (i.e. hydroxyapatite crystals, Type I collagen fibrils, non-collagenous proteins, and water) in the highly hierarchical structure of bone. In fact, structural changes at different hierarchies can also significantly affect the mechanical competence of the tissue [5, 6].
Non-collagenous proteins (NCPs), a heterogeneous group of matrix proteins counting only a few percentage of total volume of bone, are dispersed throughout the extracellular matrix of the tissue [7, 8]. Serving as structural proteins, some NCPs have been found to be directly involved in the deformation and failure of bone [9, 10]. Among the NCPs, proteoglycans (PGs) are present mainly in the extrafibrillar matrix and unlikely reside in the mineralized collagen fibrils [11, 12]. These molecules are polar and highly negatively charged, thus possessing a great potential of attracting water into the matrix as observed in articular cartilage [13]. Meanwhile, previous evidence shows that water molecules residing only in very small gaps (<4Å) in bone matrix play a critical role in the plasticity and toughness of bone [14]. More importantly, previous studies show that the amount of bound water in bone changes with age and is correlated with the age-related deterioration of bone toughness [15]. Thus, it could be logically deduced that PGs may be involved in such water-mediated plastic deformation of bone at ultrastructural levels. However, this potential scenario has been largely overlooked.
Hence, we hypothesized in this study that PGs play a crucial role in toughening bone through its ability to retain water molecules in the matrix. To test the hypothesis, we used a novel nanoscratch test to measure the in situ toughness of human cadaveric bone specimens with or without enzymes treatments for removal of the N-linked oligosaccharides in GAGs. By testing the bone specimens under both wet and dehydrated conditions, the results of this study, for the first time, indicated that there was a direct coupling effect between water and GAGs on the in situ toughness of bone.
MATERIALS & METHODS
Bone specimen preparation
Human cadaveric femurs were procured each from six middle aged (51.5±5.17 years old) male donors (N=6) from a research tissue bank (National Disease Research Exchange, Philadelphia, PA), screened for skeletal diseases and/or bone-affecting medications. A total of six (6) cortical bone cubes (4.5mm × 4.5mm × 4.5mm) were prepared from the posterior quadrant of mid-diaphysis of each femur using a low speed diamond saw (IsoMet®, Buehler, Lake Bluff, IL). These cubes were then divided into two groups (wet and dry) for in situ toughness tests using a novel nanoscratch technique in a Nano Indenter XP system (Keysight Technologies, Santa Rosa, CA). The wet bone samples were stored and tested in PBS solution, whereas the dry bone specimens were dehydrated in a vacuum oven at 70°C for 4 hours prior to testing to remove mobile and bound water in the tissue [16]. The test surface was perpendicular to the longitudinal axis of bone and polished to a surface roughness less than 30 nm. Of the three specimens in each group, one was tested without any treatments (untreated), one served as the test specimen treated with pre-heating for protein unfolding and PNGase F, and the remaining one was employed as the control, which was only pre-heated without PNGase F treatment. In addition, separate sets of bone specimens from the same donor and anatomic locations were prepared for both biochemical and histochemical assays.
Peptide-N-glycosidase F (PNGase F) treatment
Specimens were first heated in a denaturing buffer at 100°C for 5 minutes to expose the GAGs and then immersed in 200μl solution containing 2,500 units PNGase F (New England BioLabs, Inc.) at 37°C for 24 hours to remove polysaccharides, with an estimated concentration of 0.077unit/μg bone mass. After the treatment, the specimens were rinsed in distilled water 10 minutes each for three times. The heating process in this study would unlikely induce collagen denaturation since our previous study shows that collagen molecules in bone start to denature only after heating it above 120°C [17]. However, we cannot exclude the possible denaturation of other proteins. In this study, the control samples were used to catch potential effects induced by the heating process.
Biochemical assay for PGs detection
PGs extracts were prepared from separate sets of specimens from the same donor and anatomic locations using the published protocols [18, 19] with modifications. Briefly, human bone tissues were first crushed into powder form in liquid nitrogen and then extracted in buffer I (4M-guanidinium chloride /50mM-sodium acetate buffer, pH5.8). After centrifugation at 10,000g, the pellet was extracted in buffer II (4M-guanidinium chloride/50mM-Tris/HCl buffer, pH 7.4+0.25M-EDTA). After centrifugation at 10,000g, the supernatant was mixed with 9× volume of ethanol to precipitate PGs at 4°C, centrifuged at 10,000g and re-suspended in cold acetone. The precipitates were treated with PNGase F for 2 hrs and subjected to SDS gel electrophoresis assay and the proteins were detected by Commassie Blue staining.
Histochemical assays for PGs detection
5.0mm×5.0mm bone slices (about 800μm thick) were also prepared for histochemical assays to verify the efficacy of PNGase F in removing N-linked oligosaccharides of GAGs. Two staining protocols primarily for GAGs were employed in this study: dimethylmethylene blue (DMMB) (Sigma) and alcian blue (Sigma) on bone samples, respectively. DMMB blue staining was performed with 0.04M dimethylmethylene blue in 0.01M acetic acid for 12 hrs in cold room. For Alcian blue staining, bone slices were incubated in 3% acetic acid for 3min, and then incubated in 1% alcian blue, PH 2.5 for 1 hrs at room temperature using a protocol modified from Luna LG [20], followed by destaining in a solution of ethanol/water/acetic acid (9:10:1) for 20 min.
Nanoscratch tests
All nanoscratch tests were performed using the lateral scratch function on a Nano Indenter XP system (Keysight Technologies, Santa Rosa, CA). This technique was developed in our laboratory to estimate the in situ mechanical properties of bone [21]. Briefly, the polished bone specimens were glued onto a holder and mounted on the sample holder of nanoindenter and a cube-corner diamond tip was used in the test. The region of interest (within lamellae of osteons) was identified under a microscope and a pre-test surface profile was then obtained by running the scratch tip along the scratch path at a 50μN contact force. Then a scratch of 10μm long was made with a constant penetration load (5mN) and a constant scratch velocity (0.5μm/s). The scratch force (Fs) was measured and the longitudinal and cross-sectional profiles were recorded to determine the initial penetration depth (di), average penetration depth (dp) and the average residual scratch depth (ds), and average width (Ws) of the scratch groove. Finally, the in situ toughness (us) of bone was estimated using the following equation:
| (1) |
in which, no elastic strain energy dissipation was considered. On each specimen, measurements in six randomly chosen regions were performed and the average was taken for later analysis in order to alleviate the effect of data scattering within each specimen.
Statistical analysis
Two-factor ANOVA analysis was performed to detect the effect of hydration status (i.e. fully dehydrated and fully hydrated) and treatment (with and without enzyme) on the in situ nanoscratch toughness of bone. The sample size was N=6 and the statistical significance was considered only if p<0.05. Then, post hoc multiple comparisons using Tukey model were conducted to determine the differences between the test groups.
RESULTS
The ANOVA analyses of nanoscratch measurements indicated that dehydration had significant effects on initial penetration depth, elastic recovery depth, and nanoscratch groove depth (p < 0.0002), but had limited effects on the nanoscratch force and nanoscratch groove width (p > 0.15). On the other hand, PNGase F treatment had significant effects only on the depth and width of nanoscratch groove and elastic recovery depth (p < 0.025). These results suggest that the effects of dehydration and PNGase F treatment on the in situ mechanical behavior of bone are dictated by the different underlying mechanisms.
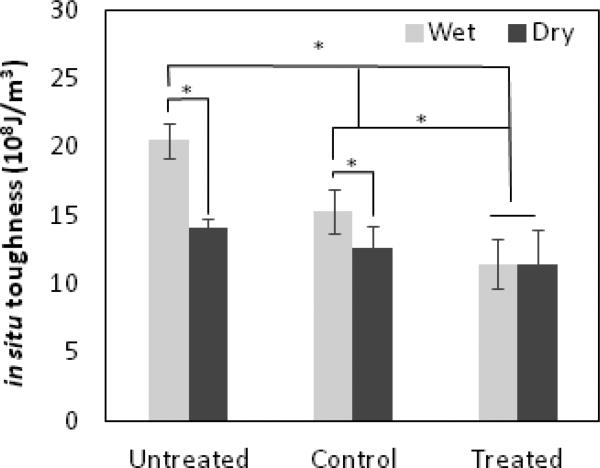
Further analyses indicated that both dehydration (p<0.00001) and PNGase F treatment (p<0.00000001) had significant effects on the in situ toughness of bone. In addition, a strong cross-effect was observed between the two factors (p<0.0003), indicating that the effect of water and GAGs on the toughness of bone was strongly interrelated. The multiple comparisons between the groups indicated that the in situ toughness of wet bone decreased significantly after PNGase F treatments (p<0.02), whereas such differences diminished under dry conditions (p=0.44) (Fig. 1). It was also noted that the in situ toughness of control specimens was greater than that of treated specimens (p<0.01), but less than that of untreated specimens (p<0.0001). This suggests that the denaturing process prior to PNGase F treatments may also induce deterioration in the in situ toughness of bone.
Figure 1. Removal of N-linked oligosaccharides comprised the toughness of wet bone tissues.
In situ toughness of bone of three test groups: bone specimens without any treatment were referred as to untreated group; bone specimens treated with pre-heating but no enzyme treatment were considered as Control group, and bone specimens treated with pre-heating and PNGase F were called Treated group. The data are presented as mean ± SD. n = 6. *P < 0.05.
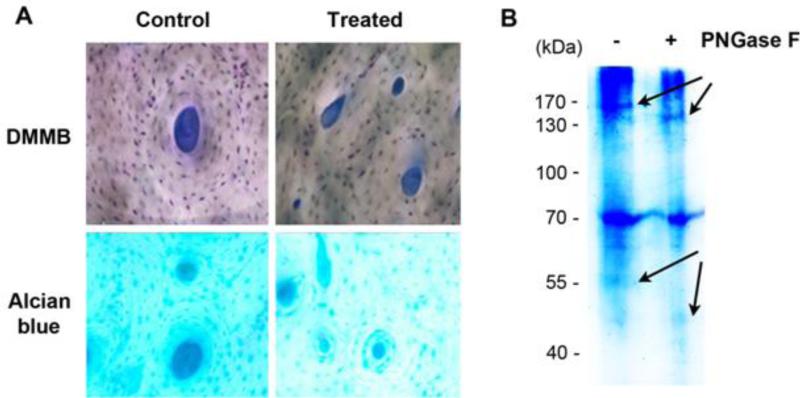
The biochemical and histochemical experiments provided clear evidence that PNGase F enzyme was effective in removing N-linked oligosaccharides from human bone matrix. First, the histochemical assays showed that the staining with DMMB and alcian blue was largely uniform and significantly reduced in treated human bone specimens compared with the control specimens in both staining schemes (Fig. 2A), suggesting that the amount of the GAGs greatly decreased in the treated samples. Second, the SDS-gel electrophoresis assay exhibited two band shifts between 130-170 kDa and 40-55 kDa (arrows), suggesting an increase in mobility of two proteins on the gel after removal of GAGs (Fig. 2B). Thus, we conjecture that those proteins are most likely the core proteins that anchor the N-linked oligosaccharides and GAGs. Also noticeably, DMMB and alcian blue stains were discernible throughout the image of control specimens, suggesting uniform dispersions of PGs in the extracellular matrix of bone.
Figure 2. Removal of GAGs by PNGase F.
The removal of GAGs by PNGase F was detected by (A) SDS-gel electrophoresis and (B) histochemical stained with DMMB and alcian blue as compared to Control bone samples. Arrows indicate the protein band shift after the treatment.
DISCUSSION
PGs contain oligosaccharides, which are connected to core proteins through the N-glycosylated (via asparagine residue) and the O-glycosylated (via serine or threonine residue) linked types. In this study, we used PNGase F, the most effective enzyme that can remove almost all N-linked oligosaccharides, to detach GAGs from the core proteins. The results of this study confirm that PNGase F treatment is effective in removing GAGs from bone matrix.
In addition, the nanoscratch tests used in this study give rise to a novel means for determining toughness of the bone by measuring the total energy dissipation until failure. The measurement was conducted on the surface layer of specimens, within which GAGs can be effectively removed by PNGase F treatments. The results of the nanoscratch tests suggest that the removal of N-linked oligosaccharides may result in significant decreases in the in situ toughness of bone only when water is present. On the other hand, water functions as a plasticizer only if N-linked oligosaccharides are not removed. This coupled effect of PGs with water on the in situ toughness of bone may be one of the ultrastructural origins that dictate the ultrastructural behavior of the tissue at nanoscopic scales. It could be inferred that removal of GAGs from PGs makes bone lose its capability of attracting water, thus diminishing the role of water in plasticizing the tissue.
It is also noteworthy that the denaturation of PGs by heating may also lead to deterioration of bone toughness, suggesting the structure of PGs is also vital for in situ structural integrity of bone. By elucidating the role of PGs in sustaining the in situ toughness of bone, we may decipher one of the underlying mechanisms of bone fragility fractures due to the ultrastructural changes induced by aging or other skeletal disorders.
ACKNOWLEDGEMENT
Research reported in this study was supported by grants from NIH/NIAMS (R21AR066925, XW and JXJ) and Welch Foundation (AQ-1507, JXJ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors’ roles: Study design: XW, JXJ. Study conduct: XW, HX, YH, SG, JXJ. Data collection: HX, YH, SG. Data analysis: XW, HX, YH, SG, JXJ. Data interpretation: XW, HX, YH, SG, JXJ. Drafting manuscript: XW, HX, YH, SG, JXJ. Revising manuscript content: XW, HX, YH, SG, JXJ. Approving final version of manuscript: XW, HX, YH, SG, JXJ. XW and JXJ take responsibility for the integrity of the data analysis.
REFERENCES
- 1.McCalden RW, et al. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75(8):1193–205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Alho A. Bone mineral content and mechanical strength. An ex vivo study on human femora at autopsy. Clinical Orthopaedics and Related Research. 1988;227:292–7. [PubMed] [Google Scholar]
- 3.Hui SL. Age and bone mass as predictors of fracture in a prospective study. Journal of Clinical Investigation. 1988;81(6):1804–9. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aspray TJ, et al. Low bone mineral content is common but osteoporotic fractures are rare in elderly rural Gambian women. J Bone Miner Res. 1996;11(7):1019–25. doi: 10.1002/jbmr.5650110720. [DOI] [PubMed] [Google Scholar]
- 5.Sroga GE, Vashishth D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Current osteoporosis reports. 2012;10(2):141–50. doi: 10.1007/s11914-012-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng L, Jasiuk I. Multi-scale characterization of swine femoral cortical bone. Journal of biomechanics. 2011;44(2):313–20. doi: 10.1016/j.jbiomech.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Urist MR, Strates BS. The classic: Bone morphogenetic protein. Clinical orthopaedics and related research. 2009;467(12):3051–62. doi: 10.1007/s11999-009-1068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derkx P, et al. Immunolocalization and quantification of noncollagenous bone matrix proteins in methylmethacrylate-embedded adult human bone in combination with histomorphometry. Bone. 1998;22(4):367–73. doi: 10.1016/s8756-3282(97)00299-8. [DOI] [PubMed] [Google Scholar]
- 9.Poundarik AA, et al. Dilatational band formation in bone. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(47):19178–83. doi: 10.1073/pnas.1201513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurner PJ, et al. Osteopontin deficiency increases bone fragility but preserves bone mass. Bone. 2010;46(6):1564–73. doi: 10.1016/j.bone.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mbuyi-Muamba JM, Dequeker J, Gevers G. Collagen and non-collagenous proteins in different mineralization stages of human femur. Acta anatomica. 1989;134(4):265–8. doi: 10.1159/000146700. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto Y, et al. EDTA-insoluble, calcium-binding proteoglycan in bovine bone. Calcified tissue international. 1995;56(5):398–402. doi: 10.1007/BF00301609. [DOI] [PubMed] [Google Scholar]
- 13.Han E, et al. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophysical journal. 2011;101(4):916–924. doi: 10.1016/j.bpj.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuel J, et al. Water residing in small ultrastructural spaces plays a critical role in the mechanical behavior of bone. Bone. 2014;59:199–206. doi: 10.1016/j.bone.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyman JS, et al. Partial removal of pore and loosely bound water by low-energy drying decreases cortical bone toughness in young and old donors. Journal of the mechanical behavior of biomedical materials. 2013;22:136–45. doi: 10.1016/j.jmbbm.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, et al. The role of collagen in determining bone mechanical properties. J Orthop Res. 2001;19(6):1021–6. doi: 10.1016/S0736-0266(01)00047-X. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, et al. The role of collagen in determining bone mechanical properties. Journal of Orthopaedic Research. 2001;19(6):1021–1026. doi: 10.1016/S0736-0266(01)00047-X. [DOI] [PubMed] [Google Scholar]
- 18.Franzen A, Heinegard D. Extraction and purification of proteoglycans from mature bovine bone. The Biochemical journal. 1984;224(1):47–58. doi: 10.1042/bj2240047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzen A, Heinegard D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. The Biochemical journal. 1985;232(3):715–24. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luna L. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. McGraw Hill Book.Co.; New York: 1968. [Google Scholar]
- 21.Islam A, Neil Dong X, Wang X. Mechanistic modeling of a nanoscratch test for determination of in situ toughness of bone. Journal of the mechanical behavior of biomedical materials. 2012;5(1):156–64. doi: 10.1016/j.jmbbm.2011.08.019. [DOI] [PubMed] [Google Scholar]