Abstract
Recent findings indicate that pedunculopontine tegmental nucleus (PPTg) neurons encode reward-related information that is context-dependent. This information is critical for behavioral flexibility when reward outcomes change signaling a shift in response patterns should occur. The present experiment investigated whether NMDA lesions of the PPTg affects the acquisition and/or reversal learning of a spatial discrimination using probabilistic reinforcement. Male Long-Evans rats received a bilateral infusion of NMDA (30 nmoles/side) or saline into the PPTg. Subsequently, rats were tested in a spatial discrimination test using a probabilistic learning procedure. One spatial location was rewarded with an 80% probability and the other spatial location rewarded with a 20% probability. After reaching acquisition criterion of 10 consecutive correct trials, the spatial location – reward contingencies were reversed in the following test session. Bilateral and unilateral PPTg-lesioned rats acquired the spatial discrimination test comparable to that as sham controls. In contrast, bilateral PPTg lesions, but not unilateral PPTg lesions, impaired reversal learning. The reversal learning deficit occurred because of increased regressions to the previously ‘correct’ spatial location after initially selecting the new, ‘correct’ choice. PPTg lesions also reduced the frequency of win-stay behavior early in the reversal learning session, but did not modify the frequency of lose-shift behavior during reversal learning. The present results suggest that the PPTg contributes to behavioral flexibility under conditions in which outcomes are uncertain, e.g. probabilistic reinforcement, by facilitating sensitivity to positive reward outcomes that allows the reliable execution of a new choice pattern.
Keywords: reversal learning, brainstem, pedunculopontine tegmental nucleus, reinforcement
1. Introduction
A change in outcomes associated with a particular choice pattern can serve as a salient cue to switch an ongoing strategy. There is substantial evidence that prefrontal cortex, striatal and thalamic circuitry support a behavioral switch when there is a change in outcomes associated with a learned strategy (McBride and Slotnick 1997; Birrell and Brown 2000; Chudasama et al. 2001; Ragozzino et al. 2003; Kim and Ragozzino 2005; Palencia and Ragozzino 2006; Ragozzino and Rozman 2007; Floresco et al. 2008; Brown et al. 2010). Less is known about how different brainstem structures may affect behavioral flexibility when a change in outcomes for a learned choice pattern occurs. The pedunculopontine tegmental nucleus (PPTg) is one brainstem structure that may play a role in behavioral flexibility. The PPTg projects to basal ganglia structures (Martinez-Gonzalez et al., 2011) shown to be involved in behavioral flexibility including the dorsomedial striatum (Baker et al., 2014b), nucleus accumbens core (Floresco et al., 2006) and subthalamic nucleus (Baker et al., 2014a). The PPTg also projects to thalamic subregions that support behavioral flexibility such as the mediodorsal thalamus (Chudasama et al., 2001; Oakman et al., 1999; Parnaudeau et al., 2015) and parafascicular thalamic nucleus (Brown et al., 2010). Further, the PPTg receives input from the medial prefrontal cortex (Semba and Fibiger 1992; Holmstrand and Sesack 2011; Kita and Kita 2011) which is critical for behavioral switching (Baker et al., 2014a,b; Floresco et al., 2008; Reichel et al., 2015). Thus, the PPTg is well positioned to contribute to switches in learned choice patterns with a change in reward contingencies.
Past studies suggest that the PPTg may support a flexible shift in choice patterns with a change in reward contingencies. For example, neurotoxic lesions of the PPTg impair win-shift performance in a radial-arm maze (Taylor et al. 2004). In the delayed win-shift test, PPTg lesions led rats to commit more errors by choosing arms that were rewarded in the training phase, as well as repeat arms already entered in the test phase (Taylor et al. 2004). Even without a delay, PPTg lesions increased errors to maze arms that were never reinforced, as well as caused repeated entries to arms already selected in a session (Keating and Winn 2002). Further, PPTg inactivation was shown to prevent a reduction in lever pressing when conditions switch such that a food reward is no longer contingent on a lever press (Maclaren et al. 2013). Thus, based on lesion or inactivation studies there is evidence that the PPTg supports a shift in choice patterns when there is a change in reward contingencies.
The correlated activity of PPTg neurons during different behavioral tasks also suggest that this brain area may support behavioral flexibility, in part, by the accurate processing of positive reward for a particular context (Norton et al. 2011; Hong and Hikosaka 2014). In non-human primates required to exhibit flexible responding of different saccades, PPTg neurons preferentially respond to information related to positive rewards (Hong and Hikosaka 2014). In rats performing a win-shift task, approximately one-third of PPT neurons exhibited elevated activity during consumption of a liquid reward (Norton et al., 2011). These same PPTg neurons displayed further enhanced activity to positive reward when the context was changed (Norton et al. 2011). This suggests that PPTg neurons integrate reward and context information and may facilitate a shift in choice patterns when a reward is associated with a particular context change. Because a significant proportion of PPTg neurons preferentially encode positive reward, PPTg lesions or inactivation may affect sensitivity to positive reinforcement that affects learning and/or reversal learning.
While these findings suggest that PPTg contributes to behavioral flexibility when learned choice patterns cease from being associated with a positive reward, unknown is whether PPTg supports specific behavioral processes that allow behavioral flexibility. Employment of behavioral paradigms such as set-shifting and reversal learning have been used to reveal whether a brain area supports behavioral flexibility, as well as specifically determine what processes neural systems support to enable behavioral flexibility, i.e. reduced perseveration of an initially learned choice pattern and/or maintenance of a new choice after initially selected (Ragozzino et al. 2003; Floresco et al. 2006; Castane et al. 2010; Brown et al. 2012; Baker and Ragozzino 2014a). Reversal learning that involves probabilistic reinforcement has the added advantage of determining whether a particular brain manipulation affects the sensitivity to positive and/or negative reinforcement (Bari et al. 2010; Brown et al. 2012; Amitai et al. 2014; Dalton et al. 2014). Thus, manipulations of the PPTg during acquisition and reversal learning in a task with probabilistic reinforcement may reveal whether the PPTg supports initial learning and/or reversal learning through specific reward processes.
To examine whether the PPTg is involved in probabilistic reversal learning, the present study investigated the effects of NMDA lesions of the PPTg on acquisition and reversal learning of a spatial discrimination using an 80/20 reinforcement schedule. Specifically, rats chose between two different spatial locations in a modified T-maze such that the ‘correct’ location was reinforced on 80% of trials and the ‘incorrect’ location reinforced on 20% of trials. After learning this discrimination, the reinforcement contingences for the two spatial locations were reversed.
2. Materials and Methods
2.1 Subjects
Adult male Long Evans rats weighing 330–425g served as the subjects for this study. The rats were individually housed in plastic cages (26.5 × 50 × 20cm) in a temperature controlled room at 21–23° Celsius and humidity 30%. They were on a 12 h light/dark cycle. The animals were food restricted to 85–90% of their body weight during the experiment. A total of 28 rats were used in this experiment. Animal care and use was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Institutional Laboratory Animal Care and Use Committee at the University of Illinois at Chicago.
2.2 Apparatus
Behavioral testing occurred in a four-arm maze made from black acrylic. The maze had a center square base (10 × 10 cm) that connected all four arms. Each maze arm contained a base (10 × 55 cm), two side walls (15 × 55cm) and a back wall (8 × 15cm). There was a circular food well (3.2 cm in diameter and 1.6 cm deep) that was placed 3 cm away from the back wall. The maze was located in a room that had various extramaze cues that could be used to spatially navigate in the maze.
2.3 Surgery
Surgery was conducted during the light phase of the light/dark cycle. All rats were anesthetized with xylazine (10mg/kg) and ketamine (100mg/kg). The rats were randomly assigned to one of two groups; a sham group which received 0.25 μl of saline and a lesion group which received 0.25 μl of 4.2 μg of N – methyl-D-aspartate (NMDA) in saline. All surgeries were conducted in a stereotaxic frame with the following coordinates: anterior- posterior (AP) at −7.6mm, mediolateral (ML) at ± 1.6mm from the midline, and dorsoventral (DV) −7.6 from the surface of the skull. The injections were made using a 28 gauge needle connected to a tube that was attached to a 10 μl syringe. The syringe was driven by a microinfusion pump that was programmed to infuse 0.25 μl of volume across 3 minutes. After infusion of saline or NMDA, the needle was left in position for 3 minutes to allow the drug to diffuse. After surgery, rats received a 1mg/kg injection of meloxicam to reduce any pain from the surgery. Following surgery, rats were fed ad libitum for five consecutive days. Subsequently, rats were food restricted to reduce their weight to 85% of their free feed weight. Food restriction and stabilization to 85% of their free feed weight required approximately seven days. Rats were handled daily for 10 minutes during food restriction.
2.4 Maze Training
After recovery from surgery and food restriction, rats received maze training. All training and testing occurred during the light phase of the light/dark cycle. Each rat was exposed to the cross-maze and trained to obtain a half piece of Froot Loops cereal (Kellogg, Battle Creek, MI, USA) from each food well. During training, a rat was also picked up after consuming a cereal piece and placed into a different maze arm. This acclimated a rat to being picked up in the maze as occurred in the test phase. After a rat consumed all four cereal pieces from each food well, it was placed in a holding chamber near the maze. The food wells were rebaited and a new trial was started. This phase of training continued until a rat completed a minimum of five trials in 15 min across two consecutive days. Subsequently, a final day of training occurred in which a black plastic block (9 cm wide × 13 cm high × 1 cm thick) was placed at the entrance of one arm, giving the maze a T-shape. A rat was placed in the stem arm and allowed to enter either choice arm to obtain a cereal piece. After the initial choice, a rat was placed back in the stem arm. If a rat chose the same arm as the initial choice, it was returned to the stem arm until it chose the other arm. Once a rat had selected both arms it was placed on top of its home cage while the two choice-arms were rebaited. The session ended after a rat had completed seven of these trials as in previous experiments (McCool et al. 2008; Baker and Ragozzino 2014b). Testing occurred in the following session. On average, training required five sessions.
2.5 Spatial Discrimination Testing with Probabilistic Reinforcement
Each rat was tested on acquisition and reversal learning of a spatial discrimination over two consecutive days. A probabilistic learning procedure was used in both test sessions. In the acquisition phase, one choice arm was designated as the ‘correct’ arm and contained a half piece of cereal on 80% of the trials. On the other 20% of trials, the ‘incorrect’ arm was baited with a half piece of cereal. The first two trials of the test always contained a reinforcement in the ‘correct’ arm. Acquisition criterion was achieved when a rat entered the ‘correct’ arm for 10 consecutive trials. Thus, a rat learned to always enter the same maze arm based on spatial location for 10 consecutive trials. On the second day of testing (reversal learning), the ‘correct’ and ‘incorrect’ arms were reversed from those on acquisition such that a rat was required to enter the arm opposite to that on acquisition. Thus, the new ‘correct’ arm was reinforced on 80% of the trials and the new ‘incorrect’ arm was reinforced on 20% of the trials. The first two trials of the test always contained a reinforcement in the ‘correct’ arm. The criterion for reversal learning was 10 consecutive trials for entering the new ‘correct’ arm.
An analysis of errors in the reversal learning session was conducted to determine whether PPTg lesions affected the initial inhibition of a learned choice pattern as measured by perseverative errors and/or the maintenance of a new choice after being initially selected as measured by regressive errors (Brown et al. 2010; Amodeo et al. 2012). To determine the number of perseverative errors, trials were separated into consecutive blocks of four trials. Perseveration was defined as initially selecting the arm that was ‘correct’ during acquisition in three or four trials in a block. Thus, if a rat chose the previously ‘correct’ arm on the majority of trials in a block it was considered to be perseverating. Once a rat made two or more choices in the new, ‘correct’ arm in a block, perseveration was no longer considered to occur. All subsequent entries into the previously ‘correct’ arm were defined as regressive errors. Perseveration is considered a measure of the inability to initially inhibit a previously learned choice pattern. Regressive errors determine the ability to maintain a new choice pattern after being initially selected.
An analysis was also performed to determine whether PPTg lesions altered the sensitivity to reinforcement or no reinforcement on ‘correct’ trials (Bari et al., 2010). A rat’s choices in the test were analyzed based on the outcome (reinforcement or no reinforcement) of each preceding trial and expressed as a ratio. For ‘correct’ trials, a win–stay ratio was determined by the number of times a rat received a reinforcement in the ‘correct’ arm and then chose the same ‘correct’ arm on the subsequent trial, divided by the total number of reinforced trials for the ‘correct’ trials only. The lose–shift ratio was determined by the number of times a rat changed its choice after not receiving reinforcement in the ‘correct’ arm on the previous trial, divided by the total number of non-reinforced trials for only ‘correct’ trials.
2.6 Histology
After completion of behavioral testing, rats were given an overdose of sodium pentobarbital. Rats were intracardially perfused with 0.9% phosphate buffered saline followed by 4% formaldehyde solution. The brain was removed and placed in 4% formaldehyde solution overnight. The following day, brains were placed in a 30% sucrose, phosphate buffered saline (PBS) solution and allowed to sink prior to sectioning. Brains were frozen and cut into 40-mm coronal sections on a cryostat. Half of the sections were immediately mounted on slides, dried and stained with cresyl violet. Alternating slices were processed for neuronal nuclear antigen (NeuN MAB 377, Millipore, Temecula, CA) immunohistochemistry according to the following procedure: three 10-minute PBS rinses, 60 minutes in Vectastain® blocking serum (Vectastain® Sheep IgG ABC Kit, Vector Laboratories, Burlingame, CA), 3 days in primary anti-NeuN antibody with regular agitation (400:1), three 10-minute rinses in PBS, 1 day in Vectastain® biotinylated antibody with regular agitation, three 10-minute rinses in PBS, 90 minutes in Vectastain® ABC reagent, three 10-min rinses in PBS, 4 to 10 minutes in diaminobenzidine peroxidase substrate solution, one 5-minute PBS rinse, and two 10-minute rinses in PBS. Lesions were then verified with reference to the stereotaxic atlas of Paxinos and Watson (1998). Sections for cresyl violet staining were also obtained as an alternative to determine the extent of the lesion. Because the NeuN stain provided the most clear extent of neuronal loss NeuN-stained sections were used for the histological analysis.
2.7 Statistical Analysis
A one-way analysis of variance (ANOVA) determined whether there was a significant lesion effect on trials to criterion. Three separate groups were included in this analysis: 1) saline or sham control; 2) bilateral NMDA lesion and 3) unilateral NMDA lesion. A separate ANOVA was conducted for the acquisition phase and reversal learning phase. ANOVA tests were conducted to examine differences among the groups on both perseverative and regressive errors. ANOVA tests were also conducted to determine a treatment effect on win–stay and lose–shift performance. A significant treatment effect was followed by a Newman–Keuls post-hoc test to determine significant differences between treatment groups. An alpha level of 0.05 was set for significance in all of the statistical analyses.
3. Results
3.1 Histology
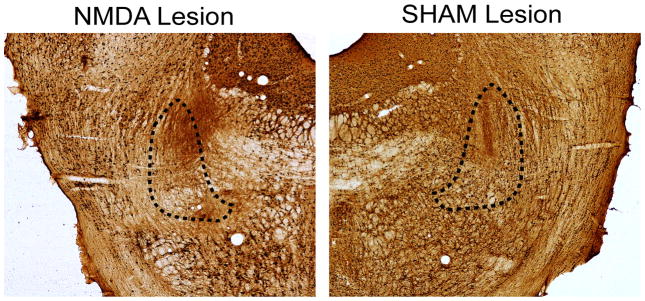
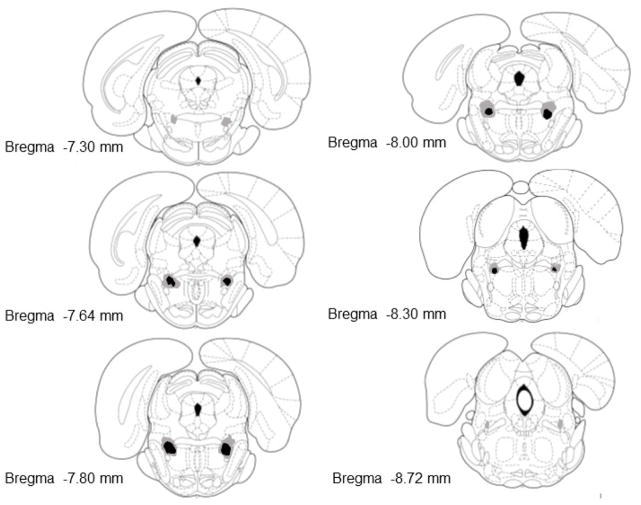
Figure 1 shows a representative example of a NeuN stain in an NMDA lesion and sham control of the PPTg. Only the NeuN stained sections were used for histological analysis. Figure 2 illustrates the extent of the NMDA lesion that was targeted toward the PPTg. Nine rats were included in the bilateral lesion group for behavioral analyses because NMDA injections bilaterally lesioned the PPTg. In these rats, the lesion was concentrated in the posterior PPTg ranging from −7.6 to − 8.3 mm. In the area of the PPTg where the lesion was concentrated there was a near absence of cell bodies (see Figure 1). Rats that had bilateral NMDA lesions principally located in the PPTg also had damage to the regions bordering the PPTg. This included damage dorsally in the microcellular tegmental nucleus, medially in the deep mesencephalic nucleus and/or ventrally in the oral pontine reticular nucleus. The largest lesions extended to the more anterior PPTg or posterior to the rostral portion of the parabrachial nucleus (see Figure 2).
Figure 1.
Photomicrographs of the PPTg region from a NMDA-lesioned rat (left side) and sham lesion rat (right side), approximately 7.8mm posterior to bregma, processed for NeuN immunoreactivity. Approximate borders of the PPTg are shown with dashed lines.
Figure 2.
Representation of lesion damage in rats classified as having bilateral PPTg lesions. Numbers in the bottom left of each section indicate the distance that each section is from bregma (mm). The black shaded areas represents the minimum extent of neuronal loss for all bilaterally lesioned rats. The surrounding grey areas represents the maximum extent of neuronal loss in the bilateral lesion group. Sections were adapted from those in Paxinos & Watson (1998).
Eight other rats injected with NMDA were not included in the bilateral lesion group because histology revealed that there was not a bilateral lesion of the PPTg. Seven of the rats had unilateral damage to the PPTg with an intact PPTg in the other hemisphere. These seven rats were included in the behavioral analyses as a separate unilateral PPTg group. Of the seven rats with unilateral damage, four rats had a cannula placement that was principally located in the superior cerebellar peduncle with some neuronal loss in the subpedencular tegmental nucleus and dorsal portion of the pontine reticular nucleus. However, there was an absence of cell loss in the PPTg in this hemisphere. In the other three rats, there was unilateral damage in the PPTg with neuronal loss in the opposite hemisphere to the medial portion of the microcellular tegmental nucleus without cellular damage to the PPTg. One other rat receiving a NMDA injection had a posterior lesion located in the lateral parabrachial nucleus and did not achieve reversal learning criterion. Because this rat did not achieve reversal learning criterion, the data from this rat was not included in the behavioral analyses. Thus, only the seven rats that had unilateral damage to the PPTg and completed behavioral testing were included in the behavioral analyses as a separate unilateral PPTg lesion group as described below.
Eight rats were included in the control group as they had bilateral cannula placements in the PPTg. There was an additional three saline-injected rats excluded from the analyses because of cannula placements outside of the PPTg. Two rats had an injection cannula located ventral in the pontine reticular nucleus. A third saline-injected rat had cannulae located in the paralemniscal nuclei. This rat did not complete reversal learning.
Overall, of the 28 rats that received stereotaxic surgery 24 rats were included in the behavioral analyses described below. The 4 rats not included in the behavioral analyses was one unilateral PPTg lesioned rat that did not complete reversal learning and 3 rats that received saline injections but had placements outside of the PPTg. The final behavioral analyses included 8 saline-injected controls, 9 bilateral PPTg lesioned rats and 7 unilateral PPTg lesioned rats. The latter group was included to determine whether unilateral damage to the PPTg was sufficient to produce a behavioral effect and whether it was similar or distinct from the bilateral PPTg lesion group.
3.2 Spatial discrimination test: Acquisition and Reversal Learning
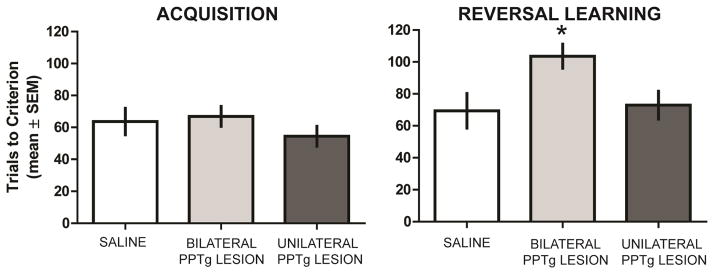
Figure 3 illustrates the results during acquisition of the spatial discrimination. All groups obtained criterion in approximately 60 trials. An ANOVA indicated that the difference in trials to criterion among the groups during acquisition was not significant, F2,21 = 0.83, P = 0.46. In reversal learning, sham controls and the unilateral PPTg group required approximately 70–80 trials to achieve criterion while the bilateral PPTg group required nearly 110 trials to obtain criterion (see Figure 3). The difference in trials to criterion among the groups was significant, F2,21 = 4.16, P = 0.028. Post-hoc analyses revealed that the bilateral PPTg group required significantly more trials to criterion than saline-injected rats or the unilateral PPTg group (P’s < 0.05). There was not a significant difference in achieving reversal learning criterion between the saline group and the unilateral PPTg group (P > 0.05).
Figure 3.
PPTg lesion effect on spatial acquisition and reversal learning. Neither unilateral or bilateral PPTg lesions affected trials to criterion in the acquisition phase. Bilateral PPTg lesions significantly increased trials to criterion in reversal compared to that of sham controls and the unilateral PPTg lesion group. There was not a significant difference in reversal learning performance between sham controls and the unilateral PPTg lesion group. * = p < 0.05 vs sham control and unilateral PPTg lesion group.
3.3 Perseverative and Regressive Errors during Reversal Learning
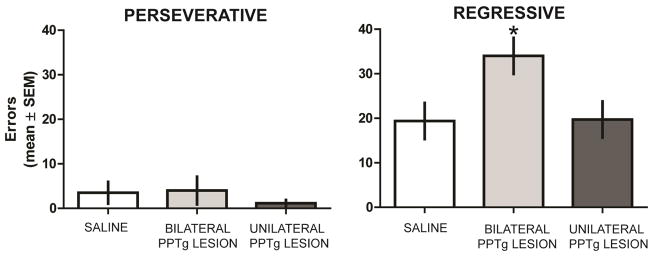
In reversal learning, most rats committed perseverative errors in the first block of trials. Subsequently, rats began to choose the new correct spatial location (see Figure 4). There was no significant difference in the number of perseverative errors among the groups, F2,21 = 0.35, P 0.71. In contrast, there was a significant difference in the number of regressive errors among the groups, F2,21 = 4.39, P = 0.026. The bilateral PPTg group committed significantly more regressive errors than the other two groups (P’s < 0.05). There was not a significant difference in the number of regressive errors committed between the saline and unilateral PPTg groups (P > 0.05).
Figure 4.
Errors committed during probabilistic reversal learning. All groups committed a similar number of perseverative errors during reversal learning. Bilateral PPTg lesions significantly increased regressive errors compared to that of sham controls and unilateral PPTg lesion lesions during reversal. There was not a significant difference in regressive errors between sham controls and the unilateral PPTg lesion group. * = p < 0.05 vs sham control and unilateral PPTg lesion group.
3.4 Win-Stay and Lose-Shift Performance during Reversal Learning
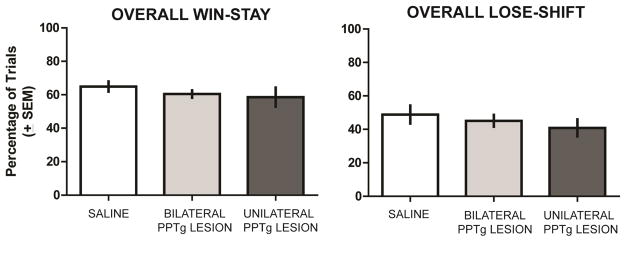
An analysis of overall win-stay/lose-shift performance indicated that all groups made about 60% of possible win-stay choices while committing approximately 50% of possible lose-shift choices (see Figure 5). There was not a significant difference in overall win-stay probabilities among the groups, F2,21 = 0.76, P = 0.48. In addition, there was not a significant difference in overall lose-shift probabilities among the groups, F2,21 = 0.69, P = 0.51.
Figure 5.
Overall win-stay and lose-shift performance during reversal learning. All groups exhibited comparable win-stay performance and lose-shift probabilities.
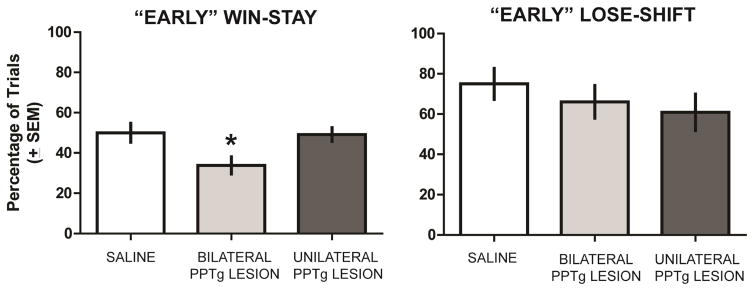
Observation of rats during reversal learning suggested that rats were more likely to make errors earlier in the session than later when they would make several consecutive correct choices in a row and eventually achieve criterion of ten consecutive correct choices. Thus, an analysis of overall win-stay/lose-shift performance may mask a deficit in one or both of these measures that occurs early in a reversal learning session. To test this, we conducted another win-stay and lose-shift analysis, but only up to the point in which a rat achieved 5 consecutive correct trials. This criterion was chosen because on each choice a rat has a 50% probability of obtaining a correct choice based on chance. Five consecutive correct choices, based on chance, is the minimum number of choices in which the probability of achieving is less than 5% (3.12%). The results from this subsequent analysis are shown in Figure 6. For win-stay, there was a significant group effect, F2,21 = 4.86, P = 0.018. Subsequent analyses indicated that the bilateral PPTg group had a significantly lower win-stay probability than the saline and unilateral PPTg groups (P’s < 0.05). In contrast, there was not a significant group effect for lose-shift probabilities, F2,21 = 0.71, P = 0.50.
Figure 6.
“Early” win-stay lose-shift analysis. Win-stay and lose-shift probabilities were analyzed on reversal learning trials until a rat made five consecutive correct choices. Bilateral PPTg lesions led to a significantly lower probability of win-stay trials compared to that of sham and the unilateral PPTg lesion group. There was not a significant difference in win-stay probabilities between the sham and unilateral PPTg lesion groups. All groups exhibited comparable lose-shift probabilities early in reversal learning. * = p < 0.05 vs sham control and unilateral PPTg lesion group.
4. Discussion
The present experiment investigated the effects of a PPTg lesion on spatial acquisition and reversal learning using probabilistic reinforcement. In the spatial discrimination test, the ‘correct’ spatial location was reinforced with 80% probability and the ‘incorrect’ spatial location reinforced with 20% probability. PPTg lesions did not affect the initial learning of the spatial discrimination. However, when the reinforcement contingencies were reversed PPTg lesions impaired probabilistic reversal learning. Bilateral damage to the PPTg was necessary to observe a reversal learning deficit as rats that had unilateral PPTg lesions did not exhibit a probabilistic reversal learning impairment. Because bilateral lesions of the PPTg did not affect initial learning, the deficit observed in reversal learning can not be explained by a fundamental deficit in discrimination learning or motivation consistent with past studies (Taylor et al. 2004; Wilson et al. 2009; Maclaren et al. 2013).
The unilateral PPTg lesion group was comparable to saline-injected controls across all behavioral measures. The similarity in all rats included in the unilateral lesion group was that there was significant cellular loss to the PPTg in only one hemisphere. However, there were rats from this group that had neuronal loss dorsal to the intact PPTg while other rats had neuronal loss ventromedial to the intact PPTg. Despite these lesion differences and the sample size being small, the subgroups exhibited similar performance on the various behavioral measures. The results suggest that unilateral PPTg lesions with damage to areas bordering the PPTg in the opposite hemisphere are not sufficient to impair probabilistic reversal learning.
The bilateral NMDA lesion of the PPTg was more centered in the posterior PPTg. Somewhat comparable to the present findings, a past experiment investigated neurotoxic lesions of the anterior and posterior PPTg on learning to bar press under fixed-ratio and variable-ratio schedules (Wilson et al., 2009). Posterior PPTg lesions led to a transient impairment in learning a fixed-ratio schedule, but when having to switch to learning a variable-ratio schedule posterior PPTg resulted in a more extensive impairment. Thus, when rats had to switch response patterns to a condition in which outcomes were uncertain, posterior PPTg lesions impaired performance. The present study did not attempt to distinguish between the anterior and posterior PPTg, although neuronal loss primarily occurred in the posterior PPTg as defined in previous studies (Wilson et al. 2009; MacLaren et al. 2015a,b). Taken together, the findings from these studies suggest that the posterior PPTg facilitates a switch in response patterns under conditions in which outcomes are uncertain.
The results from probabilistic reversal learning also revealed that PPTg lesions overall did not affect the ability to maintain a correct response immediately following a correct trial in which a food reward was received (win-stay behavior). In addition, PPTg lesions did not affect the probability of switching a response immediately following a correct trial in which no food reward was received (lose-shift behavior). However, when examining the earlier trials of reversal learning, bilateral PPTg lesions significantly reduced the probability that a rat would maintain the correct response immediately following a correct trial in which a food reward was received. These findings suggest that PPTg lesions altered the sensitivity to positive reinforcement particularly when this reinforcement is unexpected early in reversal learning. In fact, neurons within the PPTg respond to unexpected reward as well as reward magnitude which suggests it may be involved in alerting other brain areas of a change in expectations and execution of a response pattern (Norton et al. 2011; Okada and Kobayashi 2013; Thompson and Felsen 2013; Hong and Hikosaka 2014). A change in reward outcomes following a learned response pattern can be essential information to use in order to rapidly switch to an alternative response pattern. If the PPTg is critical for processing unexpected rewards, then the deficit following bilateral NMDA lesions may relate to the change in early win-stay performance observed during reversal learning.
An analysis of errors indicated that bilateral PPTg lesions impaired reversal learning by selectively increasing regressive errors. Thus, bilateral PPTg lesions did not affect the initial inhibition and switch away from a learned choice pattern in reversal learning, but affected the ability to maintain a new choice pattern after being initially selected. The lack of perseveration observed in reversal learning is comparable to a study which found that PPTg lesions can lead to overconsumption of a sucrose solution, but not perseverative bouts (MacLaren et al. 2015a). The increase in regressive errors following PPTg lesions may occur due to disruption of PPTg projections to forebrain areas shown to support maintenance of a new response during reversal learning. In particular, the PPTg projects to various striatal and thalamic subregions (Hallanger and Wainer 1988; Erro et al. 1999; Holmstrand and Sesack 2011). Past studies have demonstrated that the dorsomedial striatum, ventromedial striatum, lateral habenula and parafascicular thalamic nucleus are brain areas that support behavioral flexibility by selectively facilitating a maintenance of a new choice pattern (Ragozzino and Choi 2004; Palencia and Ragozzino 2006; Haluk and Floresco 2009; Brown et al. 2010; Baker et al. 2015). One possibility is that the PPTg is part of a larger neural network along with the striatum, thalamus, and lateral habenula that facilitates the maintenance of a new choice pattern when conditions require a switch away from a previously learned choice pattern.
The finding that bilateral PPTg lesions did not affect initial acquisition but impaired reversal learning when there was probabilistic reinforcement raises possibilities of how the PPTg may modulate other neural mechanisms that support reversal learning. The more posterior region of the PPTg sends strong projections to the ventral tegmental area (VTA) and medial substantia nigra pars compacta (SNc) [Semba and Fibiger 1992; Holmstrand and Sesack 2011]. One possibility is that PPTg directly modifies midbrain dopaminergic neuronal signaling related to positive reward (Pan and Hyland 2005; Bortolanza et al. 2010; Hong and Hikosaka 2014). In monkeys, PPTg neurons which signal reward in a saccade-related task preferentially project to medial and central portions of the SNc (Hong and Hikosaka 2014). The medial portion of the SNc preferentially signals motivational value which is proposed to be critical for changing behaviors when outcomes change (Matsumoto and Hikosaka 2009; Hong and Hikosaka 2014). Further, this region of the SNc sends a strong projection to medial portions of the striatum (Moriizumi et al. 1992; Haber et al. 2000; Joel and Weiner 2000). A previous experiment employing fast-scan cyclic voltammetry showed that a phasic dopamine signal was evoked when task contingencies changed and an unexpected food reward was delivered in both the rat nucleus accumbens core and dorsomedial striatum (Brown et al., 2011). These striatal regions also contribute to reversal learning and even show a similar error profile as that observed in the present study following bilateral PPTg lesions, e.g. increase in regressive errors or decreased win-stay behavior (Ragozzino and Choi 2004; Haluk and Floresco 2009; Dalton et al. 2014). This PPTg-medial SNc-striatal circuit offers one way by which unexpected changes in outcomes can affect changes in action selection during reversal learning. Further, this represents a mechanism by which regional specificity of dopaminergic signaling can influence behavioral flexibility. This more selective influence of the PPTg to the dopaminergic system may be contrasted with recent findings following lateral habenula inactivation during probabilistic reversal learning in which increased lose-shift and decreased win-stay behavior was observed throughout the session (Baker et al. 2015). These findings suggest diverse roles for various inputs into the dopaminergic system during flexible behavior.
Altered PPTg input to midbrain dopamine neurons may, in part, contribute to the neuropathology underlying executive function deficits in Parkinson’s disease. Numerous studies have indicated that Parkinson’s disease patients have degeneration of PPTg neurons (Zweig et al. 1988; Pahapill and Lozano 2000; Braak et al. 2004) and can exhibit deficits in probabilistic reversal learning (Peterson et al. 2009). The combined degeneration of PPTg neurons and midbrain dopamine neurons in Parkinson’s disease may exacerbate deficits in cognitive flexibility. Future studies that examine the effects of combined damage to the PPTg and SNc in tests such as probabilistic reversal learning could be useful in modeling the cognitive deficits in Parkinson’s disease. Probabilistic reversal learning paradigms can have particular appeal because they have shown a significant degree of translatability across species in other disorders (D’Cruz et al. 2011; Amodeo et al. 2012; Ineichen et al. 2012; Buelow et al. 2015). More broadly, modeling neurobiological changes observed in Parkinson’s disease with probabilistic reversal learning may lead to novel diagnostic tools and early prevention critical for advancing treatment of cognitive decline in Parkinson’s disease.
While the PPTg may modulate activity in other midbrain and thalamic areas to facilitate the reliable execution of a new choice pattern, unclear is whether a subset of PPTg neurons may be principally responsible. The PPTg is a neurochemically heterogeneous nucleus that contains cholinergic, glutamatergic and GABAergic neurons (Wang and Morales 2009). Because NMDA lesions non-specifically damage PPTg neurons, it is unknown whether specific neurotransmitter systems that make-up the PPTg play a preferential role in enabling the maintenance of a new response pattern during reversal learning. A recent study found that selective depletion of cholingeric neurons in the posterior PPTg did not affect acquisition of a fixed-ratio schedule or a switch to a variable-ratio schedule suggesting that, at least, removal of cholinergic neurons alone is not sufficient to affect learning of different instrumental conditioning schedules (MacLaren et al., 2015b). Future studies that dissect out the different neurochemical populations of PPTg neurons, including GABAergic neurons, can help address this issue.
Highlights.
PPTg lesions impair probabilistic reversal learning
PPTg lesions do not affect perseverative errors in reversal learning
PPTg lesions increase regressive errors in reversal learning
PPTg lesions alter positive reinforcement sensitivity in reversal learning
Acknowledgments
This research was supported by National Institutes of Health Grant P50 HD055751 to M.E.R. P.M.B. was supported by the University of Illinois at Chicago Center for Clinical and Translational Science (CCTS), Award Number UL1TR000050, from the National Center for Advancing Translational Sciences, National Institutes of Health. The authors would like to thank David Wirtshafter for his help in immunohistochemical staining procedures and Samantha Fortin for assistance in taking the photomicrographs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amitai N, Young JW, Higa K, Sharp RF, Geyer MA, Powell SB. Isolation rearing effects on probabilistic learning and cognitive flexibility in rats. Cognitive, affective & behavioral neuroscience. 2014;14:388–406. doi: 10.3758/s13415-013-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behavioural Brain Research. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Oh SE, Kidder KS, Mizumori SJY. Ongoing behavioral state information signaled in the lateral habenula guides choice flexibility in freely moving rats. Frontiers in Behavioral Neuroscience. 2015;9 doi: 10.3389/fnbeh.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Ragozzino ME. Contralateral disconnection of the rat prelimbic cortex and dorsomedial striatum impairs cue-guided behavioral switching. Learn Mem. 2014a;21:368–379. doi: 10.1101/lm.034819.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Ragozzino ME. The prelimbic cortex and subthalamic nucleus contribute to cue-guided behavioral switching. Neurobiol Learn Mem. 2014b;107:65–78. doi: 10.1016/j.nlm.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolanza M, Wietzikoski EC, Boschen SL, Dombrowski PA, Latimer M, Maclaren DA, Winn P, Da Cunha C. Functional disconnection of the substantia nigra pars compacta from the pedunculopontine nucleus impairs learning of a conditioned avoidance task. Neurobiol Learn Mem. 2010;94:229–239. doi: 10.1016/j.nlm.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Brown HD, Amodeo DA, Sweeney JA, Ragozzino ME. The selective serotonin reuptake inhibitor, escitalopram, enhances inhibition of prepotent responding and spatial reversal learning. J Psychopharmacol. 2012;26:1443–1455. doi: 10.1177/0269881111430749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, Baker PM, Ragozzino ME. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci. 2010;30:14390–14398. doi: 10.1523/JNEUROSCI.2167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow MT, Amick MM, Queller S, Stout JC, Friedman JH, Grace J. Feasibility of use of probabilistic reversal learning and serial reaction time tasks in clinical trials of Parkinson’s disease. Parkinsonism Relat Disord. 2015;21:894–898. doi: 10.1016/j.parkreldis.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Castane A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci. 2001;14:1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- D’Cruz AM, Ragozzino ME, Mosconi MW, Pavuluri MN, Sweeney JA. Human reversal learning under conditions of certain versus uncertain outcomes. Neuroimage. 2011;56:315–322. doi: 10.1016/j.neuroimage.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Phillips AG, Floresco SB. Preferential involvement by nucleus accumbens shell in mediating probabilistic learning and reversal shifts. J Neurosci. 2014;34:4618–4626. doi: 10.1523/JNEUROSCI.5058-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erro E, Lanciego JL, Gimenez-Amaya JM. Relationships between thalamostriatal neurons and pedunculopontine projections to the thalamus: a neuroanatomical tract-tracing study in the rat. Exp Brain Res. 1999;127:162–170. doi: 10.1007/s002210050786. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallanger AE, Wainer BH. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;274:483–515. doi: 10.1002/cne.902740403. [DOI] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Holmstrand EC, Sesack SR. Projections from the rat pedunculopontine and laterodorsal tegmental nuclei to the anterior thalamus and ventral tegmental area arise from largely separate populations of neurons. Brain Struct Funct. 2011;216:331–345. doi: 10.1007/s00429-011-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Pedunculopontine tegmental nucleus neurons provide reward, sensorimotor, and alerting signals to midbrain dopamine neurons. Neuroscience. 2014;282C:139–155. doi: 10.1016/j.neuroscience.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ineichen C, Sigrist H, Spinelli S, Lesch KP, Sautter E, Seifritz E, Pryce CR. Establishing a probabilistic reversal learning test in mice: evidence for the processes mediating reward-stay and punishment-shift behaviour and for their modulation by serotonin. Neuropharmacology. 2012;63:1012–1021. doi: 10.1016/j.neuropharm.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–474. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Keating GL, Winn P. Examination of the role of the pedunculopontine tegmental nucleus in radial maze tasks with or without a delay. Neuroscience. 2002;112:687–696. doi: 10.1016/s0306-4522(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Kita H. Cholinergic and non-cholinergic mesopontine tegmental neurons projecting to the subthalamic nucleus in the rat. Eur J Neurosci. 2011;33:433–443. doi: 10.1111/j.1460-9568.2010.07537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Markovic T, Daniels D, Clark SD. Enhanced consumption of salient solutions following pedunculopontine tegmental lesions. Neuroscience. 2015a;284:381–399. doi: 10.1016/j.neuroscience.2014.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclaren DA, Wilson DI, Winn P. Updating of action-outcome associations is prevented by inactivation of the posterior pedunculopontine tegmental nucleus. Neurobiol Learn Mem. 2013;102:28–33. doi: 10.1016/j.nlm.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Wilson DI, Winn P. Selective lesions of the cholinergic neurons within the posterior pedunculopontine do not alter operant learning or nicotine sensitization. Brain Struct Funct. 2015b doi: 10.1007/s00429-014-0985-4. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat. 2011;5:22. doi: 10.3389/fnana.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SA, Slotnick B. The olfactory thalamocortical system and odor reversal learning examined using an asymmetrical lesion paradigm in rats. Behav Neurosci. 1997;111:1273–1284. doi: 10.1037//0735-7044.111.6.1273. [DOI] [PubMed] [Google Scholar]
- McCool MF, Patel S, Talati R, Ragozzino ME. Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem. 2008;89:114–124. doi: 10.1016/j.nlm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriizumi T, Leduc-Cross B, Wu JY, Hattori T. Separate neuronal populations of the rat substantia nigra pars lateralis with distinct projection sites and transmitter phenotypes. Neuroscience. 1992;46:711–720. doi: 10.1016/0306-4522(92)90157-w. [DOI] [PubMed] [Google Scholar]
- Norton AB, Jo YS, Clark EW, Taylor CA, Mizumori SJ. Independent neural coding of reward and movement by pedunculopontine tegmental nucleus neurons in freely navigating rats. Eur J Neurosci. 2011;33:1885–1896. doi: 10.1111/j.1460-9568.2011.07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Cozzari C, Hartman BK. Characterization of the extent of pontomesencephalic cholinergic neurons’ projections to the thalamus: comparison with projections to midbrain dopaminergic groups. Neuroscience. 1999;94:529–547. doi: 10.1016/s0306-4522(99)00307-3. [DOI] [PubMed] [Google Scholar]
- Okada K, Kobayashi Y. Reward prediction-related increases and decreases in tonic neuronal activity of the pedunculopontine tegmental nucleus. Front Integr Neurosci. 2013;7:36. doi: 10.3389/fnint.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123(Pt 9):1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The effect of N-methyl-D-aspartate receptor blockade on acetylcholine efflux in the dorsomedial striatum during response reversal learning. Neuroscience. 2006;143:671–678. doi: 10.1016/j.neuroscience.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaudeau S, Taylor K, Bolkan SS, Ward RD, Balsam PD, Kellendonk C. Mediodorsal thalamus hypofunction impairs flexible goal-directed behavior. Biol Psychiatry. 2015;77:445–453. doi: 10.1016/j.biopsych.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: 1998. [DOI] [PubMed] [Google Scholar]
- Peterson DA, Elliott C, Song DD, Makeig S, Sejnowski TJ, Poizner H. Probabilistic reversal learning is impaired in Parkinson’s disease. Neuroscience. 2009;163:1092–1101. doi: 10.1016/j.neuroscience.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem. 2004;11:70–77. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav Neurosci. 2003;117:1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Rozman S. The effect of rat anterior cingulate inactivation on cognitive flexibility. Behav Neurosci. 2007;121:698–706. doi: 10.1037/0735-7044.121.4.698. [DOI] [PubMed] [Google Scholar]
- Reichel JM, Nissel S, Rogel-Salazar G, Mederer A, Käfer K, Bedenk BT, Martens H, Anders R, Grosche J, Michalski D, Härtig W, Wotjak CT. Distinct behavioral consequences of short-term and prolonged GABAergic depletion in prefrontal cortex and dorsal hippocampus. Front Behav Neurosci. 2015;8:452. doi: 10.3389/fnbeh.2014.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Taylor CL, Kozak R, Latimer MP, Winn P. Effects of changing reward on performance of the delayed spatial win-shift radial maze task in pedunculopontine tegmental nucleus lesioned rats. Behav Brain Res. 2004;153:431–438. doi: 10.1016/j.bbr.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Felsen G. Activity in mouse pedunculopontine tegmental nucleus reflects action and outcome in a decision-making task. J Neurophysiol. 2013;110:2817–2829. doi: 10.1152/jn.00464.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DI, MacLaren DA, Winn P. Bar pressing for food: differential consequences of lesions to the anterior versus posterior pedunculopontine. Eur J Neurosci. 2009;30:504–513. doi: 10.1111/j.1460-9568.2009.06836.x. [DOI] [PubMed] [Google Scholar]
- Zweig RM, Hedreen JC, Jankel WR, Casanova MF, Whitehouse PJ, Price DL. Pathology in brainstem regions of individuals with primary dystonia. Neurology. 1988;38:702–706. doi: 10.1212/wnl.38.5.702. [DOI] [PubMed] [Google Scholar]