Abstract
The assessment of fracture risk often relies primarily on measuring bone mineral density, thereby accounting for only a single pathology: the loss of bone mass. However, bone’s ability to resist fracture is a result of its biphasic composition and hierarchical structure that imbue it with high strength and toughness. Reference Point Indentation (RPI) testing is designed to directly probe bone mechanical behavior at the microscale in situ, although it remains unclear which aspects of bone composition and structure influence the results at this scale. Therefore, our goal in this study was to investigate factors that contribute to bone mechanical behavior measured by cyclic reference point indentation, impact reference point indentation, and three-point bending. Twenty-eight female cadavers (age 57–97) were subjected to cyclic and impact RPI in parallel at the unmodified tibia mid-diaphysis. After RPI, the middiaphyseal tibiae were removed, scanned using micro-CT to obtain cortical porosity (Ct.Po.) and tissue mineral density (TMD), then tested using three-point bending, and lastly assayed for the accumulation of advanced glycation end-products (AGEs). Both the indentation distance increase from cyclic RPI (IDI) and bone material strength index from impact RPI (BMSi) were significantly correlated with TMD (r = −0.390, p = 0.006; r = 0.430, p = 0.002; respectively). Accumulation of AGEs was significantly correlated with IDI (r = 0.281, p = 0.046), creep indentation distance (CID, r = 0.396, p = 0.004), and BMSi (r = −0.613, p < 0.001). There were no significant relationships between tissue TMD or AGEs accumulation with the quasi-static material properties. Toughness decreased with increasing tissue Ct. Po. (r = −0.621, p < 0.001). Other three-point bending measures also correlated with tissue Ct. Po. including the bending modulus (r = −0.50, p < 0.001) and ultimate stress (r = −0.56, p < 0.001). The effects of Ct.Po. on indentation were less pronounced with IDI (r = 0.290, p = 0.043) and BMSi (r = −0.299, p = 0.037) correlated modestly with tissue Ct. Po. These results suggest that RPI may be sensitive to bone quality changes relating to collagen.
Keywords: Bone mechanics, reference point indentation, aging, advanced glycation end-products, bone viscoelasticity, damage mechanics
1. Introduction
The primary mechanical functions of bone are to withstand loads and resist fracture. These are derived from structural adaptations, such as the orientation of the trabecular architecture1, and material level toughening mechanisms, such as material plasticity and micro-cracking2,3. The biphasic composition of mineral and proteins in bone also imbues it with a unique combination of high strength and toughness4,5. Although strength dictates the maximum load that bone can withstand, toughness determines bone’s ability to dissipate energy, resist structural failure6, and form micro-damage that subsequently invokes bone remodeling to repair the damaged bone tissue7. The toughness of bone declines with aging8,9 and disease10,11 and subsequently increases the risk of fracture. The ability to monitor the changes in bone toughness with aging, disease, and therapies, is thus critical for an informed understanding fracture risk.
Measuring the functional material behavior of bone such as strength and toughness has traditionally required the preparation of uniform geometric samples and testing under well-defined conditions to obtain constitutive stress-strain relationships under quasi-static conditions12. However, these approaches typically do not allow the in situ characterization of bone mechanical behavior in its native environment, e.g. with the surrounding soft tissues and body fluid hydration. To complicate matters further, bone is viscoelastic and exhibits strain- and loading- rate dependence in its material behavior13–15, and a quasi-static characterization of bone mechanical behavior may not capture the dynamic nature of a fracture16–18. Given this, an improved understanding of bone material properties and its relationship to fracture risk, particularly in living humans, requires accounting for these environmental and boundary conditions.
Reference point indentation allows the testing of bone in situ by penetrating the soft tissues encasing the bone and then making indentation measurements using a test probe at the site of interest19,20. These indentations are applied to the bone tissue at either cyclically at 40 N/s (Biodent21) or a single impact at 120,000 N/s (Osteoprobe RUO22), and impact indentation in particular has successfully discriminated diseased cohorts such as diabetes and fragility fractures in humans10,23. Yet even as impact indentation detects differences in the material aspects of bone quality in these groups, it remains unknown which tissue-level compositional factors contribute to changes in impact indentation derived measurements, and whether these measurements are influenced by cortical porosity. We thus sought to investigate the contributions of tissue mineralization, degree of non-enzymatic crosslinking, and microCT-derived cortical porosity on the mechanical behavior of bone tissue measured from three-point bending, cyclic RPI, and impact RPI in an intact cadaver model.
2. Materials and Methods
2.1. Human Cadaver Tissue Samples
Lower limbs were removed from fresh frozen human female cadavers (n = 28, Ages: 57–97) with no known history of metabolic disorders. These tissues were dissected and stored at −80C until respective experiments are conducted. Both the left and right limbs are used for all mechanical and biochemical assays. Indentation testing was performed (section 2.2) with all soft tissues intact, e.g. skin, flesh, fat, and periosteum. The anterior-medial quadrant of tibiae were then isolated and dissected out, and 100 mm-long sections distally from proximal mid-diaphysis starting from the longitudinal midpoint of tibiae, going 50 mm distally and proximally from this location, were sectioned using a bandsaw and a low-speed diamond wafered saw, and then used for three-point bending (Figure 1).
Figure 1.

Study design. Material properties were obtained at three different loading rates for a mid-diaphysis section of the anterior tibia using three-point bending, cyclic reference point indentation, and impact reference point indentation. Compositional data including cortical porosity, tissue mineral density, and accumulation of advanced glycation end-products were also acquired.
2.2. Indentation
2.2.1. Cyclic reference point indentation (low loading rate)
Cyclic RPI (BioDent; Active Life Scientific, Santa Barbara, CA) was performed on each cadaver limb using the manufacturer provided stand. The indentations were performed beginning at the midpoint between the patella and ankle joint, and seven indentations each 5 mm apart were obtained in a linear fashion both proximally and distally from the first indentation to yield a total of 15 sites/limb. Indentations were made using a probe assembly consisting of a beveled reference probe with blunted end (~5 mm cannula length) and test probe with spherical tip (2.5 μm radius point) that tapers from a 90° cone shape to cylindrical shaft (BP2 probe, Active Life Scientific, Santa Barbara, CA). The reference probe was inserted through the skin with the reference probe allowed to make contact with the bone surface. The stand was then lowered such that the instrument head was allowed to rest on the bone periosteal surface. The indentation probe was first allowed to perform preconditioning cycles at 4 Hz that incrementally increase up to a threshold force of order 2.5 N, and the probe is then cycled to 10 N for 20 cycles at 2 Hz (40 N/s). Each probe was allowed to make up to 150 measurements as per manufacturer’s specifications and then a new probe would be used. The following parameters were returned or computed from the system: indentation distance increase (IDI [μm]; difference in indentation depth between the 1st and 20th cycle), total indentation distance (TID [μm]; maximum indentation depth achieved), energy dissipation (ED [μJ]; average area under the curve for each loading cycle), creep indentation distance (CID [μm]; average creep distance during the hold phase at peak force of the loading cycle), loading slope (LS [N/μm]; average slope of the load/displacement data during loading), and unloading slope (US [μm]; average slope of the load/displacement data during unloading). The system was calibrated using the manufacturer-provided poly-methylmethacrylate (PMMA) block before and after the measurement of each subject.
2.2.2. Impact reference point indentation (high loading rate)
Impact RPI testing (Osteoprobe RUO; Active Life Scientific, Santa Barbara, CA) was performed in parallel with cyclic RPI at sites 5 mm adjacent to each indent location. The standard Osteoprobe probe was used: 90-degree conical tip with a diameter of about 375 mm and a tip radius of <10 mm. Similar to cyclic RPI, the indentation probe was inserted through the skin perpendicularly, hand-held against the periosteal surface, pre-loaded to 10 N and then impacted with an additional 30 N of force delivered over 2.5 ms (120,000 N/s). A new test probe was used for each subject as to minimize the effects of blunting on measurements. Following testing, indentation normalization was performed per manufacturer’s recommendations by indenting a block of PMMA five times. Results for the average Bone Material Strength index (BMSi), a normalized dimensionless measurement defined by 100 times the ratio of the average IDI of the PMMA block divided by the IDI of the sample, were then returned.
2.3. Micro-Computed Tomography
The tibial sections (100-mm-long and approximately 20-mm-wide) harvested from the region of micro-indentation were scanned (VivaCT 40; Scanco Medical AG, Bruttisellen, Switzerland). Settings were 30 μm3 voxels with an x-ray tube potential of 70 kVp, intensity of 114 mA, 1000 projections, and 150 ms integration time. A hydroxyapatite phantom calibration with the manufacturer’s specifications was performed weekly for this system. Cortical porosity (Ct.Po) and tissue mineral density (TMD) were measured using an automated segmentation routine adapted from Buie and colleagues 24 (Figure S1). Automatic contouring was used to analyze all slices with a lower/upper threshold of 180/1000 (600 mg HA/cm3, selected based on visual inspection) to segment bone. Briefly, dilation and erosion was done with a (5, 5, 1) kernal size. Tissue mineral density (TMD) was defined as the mean of volumetric bone mineral density for all voxels assigned to the matrix (voxels with a BMD more than 600 mgHA/cm3). Intracortical porosity (Ct.Po) was computed as the ratio of the voxels with a BMD less than 600 mgHA/cm3 per total number of voxels in the VOI (noise filter set to sigma = 2.0 and support = 2).
2.4. Quasi-Static three-point bending testing
The 100 mm sections, were further cut into 50 x 4 x 4 mm planks and tested in three- point bending (conforming to ASTM D790 and ISO 178 standards) under constant hydration in an environmental chamber (5866; Instron, Norwood, MA) to determine the material properties. Samples were placed on a span of 40 mm, preloaded to 10 N, and then displaced at 1 mm/min until failure. The bending axis was aligned with the radial direction of the tibiae. Force-displacement data was recorded through the duration of testing. The precise dimensions of each plank were determined using a micrometer, and these dimensions were used to apply standard beam theory to obtain stress and modulus values. Toughness is calculated as the area under the resulting stress-strain curve.
2.5. Advanced Glycation End-products (AGEs)
Small pieces of bone (approximately 4x4x4 mm) from the same region of bone as the indentation testing were assayed for (AGEs) content. The samples were first demineralized using ethylenediaminetetraacetic acid and then hydrolyzed in 6N hydrochloric acid at 110°C for 24 hours. The samples were then centrifuged and the supernatant collected and then plated in triplicate with quinine sulphate standards. Fluorescence emission was read at a wavelength of 440 nm with an excitation wavelength of 370 nm using a micro-plate photometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA). The AGEs content was then normalized to the amount of collagen present in each sample which was quantified using a chloramine T colorimetric assay and standardized using hydroxyproline3,34.
2.6. Statistics
Data sets were tested for a Gaussian distribution using a D’Agostino & Pearson omnibus normality test. The majority of the data were not normally distributed, therefore, Spearman correlations, with the left/right sides as a blocking variable [38], were used to establish the relationships examined within this study. The similarity between indentation modalities was examined using a Bland-Altman plot for the TID returned by both devices. All statistics were performed using GraphPad Prism Software (Prism 6.00, GraphPad Software, La Jolla, CA, USA).
3. Results
The mechanical testing modalities differentially captured the age-related changes in bone. Cyclic indentation derived IDI correlated with increasing age (r = 0.347, p = 0.009). Impact indentation-derived BMSi did not correlate with age (p = 0.246). Three-point bending revealed that ultimate stress (r = −0.319, p = 0.022), toughness (r = −0.377, p = 0.006), and modulus (r = −0.288, p = 0.040) correlated with age. There are age-related structural changes as tissue Ct. Po. increased with age (r = 0.297, p = 0.038) as well as TMD (r = −0.311, p = 0.030). However, there was no observable increase in the accumulation of AGEs (p = 0.63) with age in this cohort (Table 1).
Table 1.
Correlation of measured bone mechanical, structural, and compositional properties with age.
| Correlation Coefficient | p-value | |
|---|---|---|
| IDI | 0.347 | 0.009 |
| BMSi | −0.161 | 0.246 |
| Ult. Stress | −0.319 | 0.022 |
| Toughness | −0.377 | 0.006 |
| Elastic Modulus | −0.288 | 0.040 |
| Ct. Po. | 0.297 | 0.038 |
| TMD | −0.311 | 0.030 |
| AGEs | 0.079 | 0.583 |
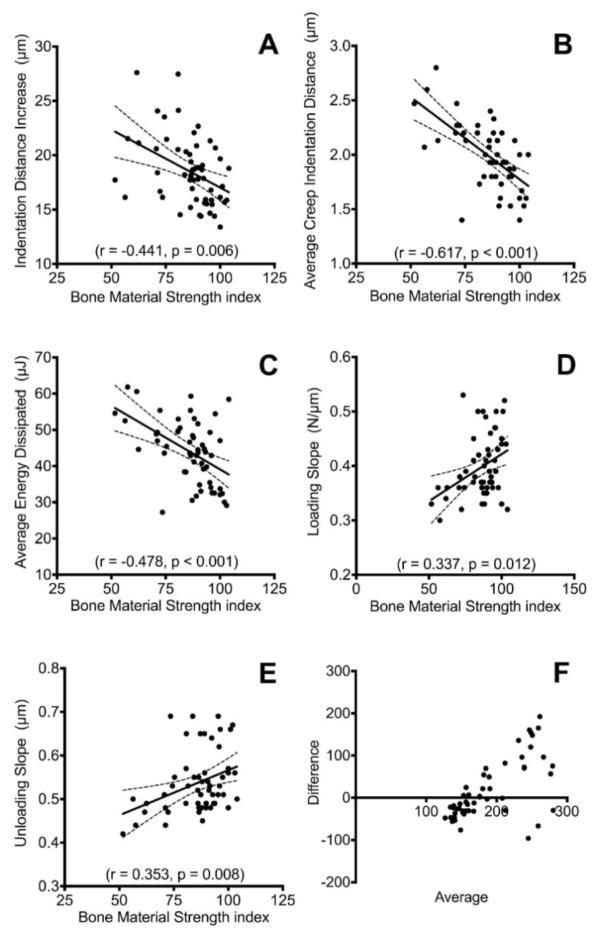
The damage-based indentation testing techniques were correlated with one another. BMSi and IDI, methodologically the two most closely related parameters, showed a modest association with one another (r = −0.441, p = 0.006). However, the strongest association between the impact and cyclic based systems was BMSi and aCID (r = −0.617, p < 0.001). Additionally, ED (r = −0.478, p < 0.001), US (r = 0.353, p = 0.008), and LS (r = 0.337, p = 0.012) were significantly correlated with BMSi (Figure 2). The Bland-Altman plot shows that the scatter between the two devices increases as the bone declines in measured quality (Figure 2F).
Figure 2.
Relationship between low- and high-strain rate indentation testing techniques. The impact indentation produced Bone Material Strength index (BMSi) was significantly correlated with A) indentation distance increase, B) average creep indentation distance, C) average energy dissipated, D) loading slope, and E) unloading slope. Panels A–E show Spearman’s correlations. F) Bland-Altman plot of the total indentation distance for both devices. The scatter between devices increases with decreasing bone quality as indicated by an increased average.
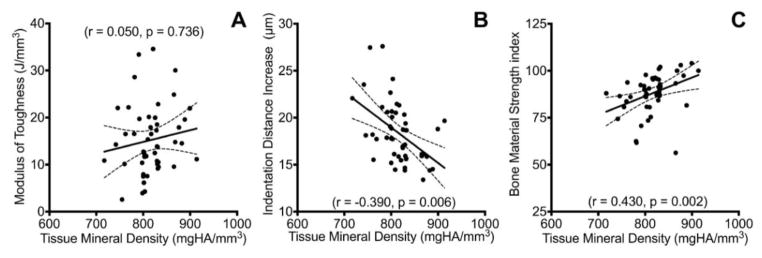
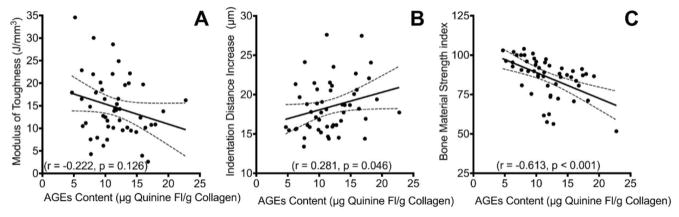
Tissue composition was more strongly correlated with the indentation measured bone properties than the quasi-static beam bending derived properties. Both the IDI and BMSi were significantly correlated with the tissue mineral density (r = −0.390, p = 0.006; r = 0.430, p = 0.002; respectively, Figure 3) to a similar degree. Furthermore, accumulation of AGEs was also significantly correlated with IDI (r = 0.281, p = 0.046), CID (r = 0.396, p = 0.004), and BMSi (r = −0.613, p < 0.001, Figure 4). However, there were no significant relationships between tissue TMD or AGE accumulation with the quasi-static material properties.
Figure 3.
Tissue mineral density relationships. A) TMD was not significantly correlated with toughness. B&C) Both indentation techniques were significantly correlated with TMD.
Figure 4.
Accumulation of advanced glycation end-products relationships. A) Modulus of toughness was not significantly correlated with AGEs content. B) IDI from cyclic reference point indentation testing was only moderately correlated with AGEs. However, C) BMSi from impact reference point indentation was most strongly correlated with AGEs.
Conversely, cortical porosity was more strongly correlated with the quasi-static material properties than either of the indentation techniques. Specifically, the modulus of toughness decreased with increasing tissue Ct. Po. (r = −0.621, p < 0.001). Other three-point bending measures also correlated with tissue Ct. Po. including the bending modulus (r = −0.50, p < 0.001) and ultimate stress (r = −0.56, p < 0.001). The effects of tissue porosity on cyclic indentation and impact indentation were less pronounced with IDI (r = 0.290, p = 0.043) and BMSi (r = −0.299, p = 0.037) correlated modestly with tissue Ct. Po. (Figure 5).
Figure 5.
Cortical porosity relationships. All mechanical testing were correlated with Ct.Po. with the A) modulus of toughness being the strongest. B, C) Indentation testing techniques were only modestly correlated.
4. Discussion
Aging can bring a multitude of changes in bone at the tissue and structural levels that contribute to the general decline of mechanical performance and subsequent increase in fracture risk. Many associations have been identified for the age-related changes of bone25–27, 34–36. The tissue’s ability to resist fracture is defined by its specific composition and structure. In fact, for our sample group, we observed age-associated decline in structure and function. However, the specificity of aging as a predictor of mechanical performance was quite low, and this may be attributed to the relatively old age of the donors as well as the lack of young donors in this group. Therefore, it is critical to understand the relationships of different testing modalities and their sensitivity to tissue variation. This study examined three mechanical testing techniques for bone tissue at different loading rates: quasi-statically by three-point bending, cyclic indentation at 40 N/s, and impact indentation at 120,000 N/s. Since bone is a biphasic viscoelastic material with time-dependent mechanical behavior13–15, the diverse aspects of material composition and structure contribute to toughness and energy dissipation.
We found modest correlations between cyclic and impact RPI measures, and they appear to assess different aspects of bone’s material behavior. This appears consistent with the conclusions of Karim et al who did not observe any significant correlations (p < 0.05) between BMSi and cyclic RPI properties28. Since there are many aspects to bone quality that are declining at different rates with age, and different modes RPI modalities may be probing different aspects of these age-related declines. Consistent with this idea, the Bland-Altman plot of the two devices (Figure 2F) shows increasing divergence between the two indentation-measures with declining bone quality. When comparing three-point bending to the RPI techniques, we found that none of the properties obtained were significantly correlated. Based on our evaluations of structure and composition, this is not surprising as three-point bending was more sensitive to variations in structure, while RPI was more strongly correlated with composition (Figures 3–5). Although we followed ASTM and ISO guidelines for three-point bending, we cannot rule out that shear stresses in the planks influenced our results. Moreover, it is also important to acknowledge that indentation, particularly damage-based reference point indentation, provides a unique mechanical assessment of bone that differs from quasi-static and traditional methods. It is worth noting that the age group in the present study does not include young- and middle- aged individuals and thus may not be revealing of age-related changes. A number of studies have examined the relationship between age and reference point indentation in humans bones with varying degrees of association9,28,37. In general, cyclic RPI appear to be more successful in identifying changes associated with chronological age in the current study and others28,37. Impact RPI however do not seem to correlate with age in this study and others9.
Classic techniques of diagnosing bone health incorporate measures of quantity, specifically areal bone mineral density, which creates a 2d projection that blends structural and compositional data. When parsing out these data we found distinctly different correlations with each mechanical assay. At the tissue-level, TMD was correlated only with indentation. Previous investigations have established a relationship between indentation testing techniques and tissue mineral density at several length scales. Houde et al. have shown femoral neck areal BMD and indentation depth to be inversely correlated in both human and bovine samples29. Examining in closer detail Leong & Morgan observed that within the fracture callus of rats the average TMD and nano-indentation modulus were directly correlated, however this was not true when comparing the area directly under the location of indentation30. Previously, we have shown that microCT-derived TMD in the bone volume directly surrounding the indent site did not influence cyclic RPI measurements8. Additionally, there was only a small difference in the relative correlations between the low- and high-rate testing, which may be due to the predominantly elastic behavior of the mineral phase31.
Similar to TMD, the tissue-level AGEs was significantly correlated with only the cyclic and impact indentation damage behavior. In particular, the strong correlation of BMSi measured by impact RPI and the accumulation of non-enzymatic crosslinks suggests that the high-loading rate method elicits a response that may be more sensitive to collagen modifications. Likewise, the creep behavior, one of the hallmarks of viscoelasticity, indicated by CID as measured by cyclic indentation was also strongly correlated with AGEs. These findings are also supported by work done by Farr and colleagues that demonstrate a reduction of BMSi in diabetic patients who are highly susceptible to accumulate AGEs10, especially since AGES have been shown to specifically modify the viscoelastic behavior of bone32–34. Although AGEs are expected to increase with age34, these effects in some instances require the stratification of age groups to become more apparent35.
At the microstructural level, cortical porosity was the strongest correlate with three-point bending material behavior. This is expected as it has been implied that microstructure, rather than composition, predominantly drives the tissue behavior at this scale for three-point bending in cortical samples36. It is also worth noting that the effects of tissue Ct. Po. on three-point bending behavior are more apparent at values greater than 5%, suggesting that the role of material level composition remain important at low or modest ranges of tissue Ct. Po, with implications that disparate populations with normal BMDs may benefit more from material level measurements such as reference point indentation. Although microCT resolution limits the resolutions of cortical pores of less than 30um, we believe that the larger pores play a more significant role on the bone’s mechanical behavior. One reason RPI may not be correlated with Ct.Po. is that once a pore becomes large enough to influence the measurement, it may produce an unsuccessful indent. We have shown previously that the local porosity measured at the current resolution in the volume immediately surrounding the site of indentation do not seem to have a significant effect on the cyclic RPI measured properties8.
Since bone fracture resistance can be influenced by so many factors it is critically important to understand the underlying compositional mechanisms that drive the subsequent manifestations of mechanical behavior. Our results here suggest that at the tissue level, cyclic and impact RPI may be more sensitive than three-point bending in predicting volumetric TMD variations. While impact RPI may provide robust measurements relating to changes in the collagen phase, it may be less sensitive to the mineral or microstructural changes in the bone. Regardless of the mechanism, given that most fractures in humans are the result of dynamic events, the aspects of bone quality measured by impact indentation may provide more physiologically relevant insights of fracture resistance than quasi-static methods.
Supplementary Material
Highlights.
Bone was mechanically tested at several loading rates using reference point indentation and three-point bending to understand factors that contribute to fracture resistance at multiple length scales.
Tissue mineral density contributes to reference point indentation measured tissue properties.
Cortical porosity correlates negatively with toughness obtained from quasi-static three-point bending.
Advanced glycation end-products were negatively associated with bone material strength index obtained from impact reference point indentation.
Acknowledgments
Funding Information:
This study was funded in part, with no direct involvement, by the National Institutes of Health (NIH T32 AR060719, NIH R43 AG060607, NIH P30 AR057235).
We gratefully acknowledge Daniel Lieb for assistance for the micro-CT analyses. This study was funded in part by the Washington University Institutional Metabolic Skeletal Disorders Training Program (NIH T32 AR060719), the Washington University Musculoskeletal Research Center (NIH P30 AR057235), and the National Institutes of Health Small Business Innovation Research Program (NIH R43 AG060607).
Abbreviations
- BMD
Bone mineral density
- RPI
Reference point indentation
- TID
Total Indentation Distance
- IDI
Indentation Distance Increase
- CID
Creep Indentation Distance
- ED
Energy Dissipated
- LS
Loading Slope
- US
Unloading Slope
- Ct.Po
Cortical Porosity
- TMD
Tissue Mineral Density
- BMSi
Bone Material Strength index
- AGEs
Advanced Glycation End-Products
Footnotes
Disclosures:
The authors have no applicable disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keaveny TM, Morgan EF, Niebur GL, Yeh OC. Biomechanics of trabecular bone. Annu Rev Biomed Eng. 2001;3:307–333. doi: 10.1146/annurev.bioeng.3.1.307. [DOI] [PubMed] [Google Scholar]
- 2.Vashishth D, Behiri JC, Bonfield W. Crack growth resistance in cortical bone: concept of microcrack toughening. J Biomech. 1997;30(8):763–769. doi: 10.1016/s0021-9290(97)00029-8. http://www.ncbi.nlm.nih.gov/pubmed/9239560. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann EA, Schaible E, Bale H, et al. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci U S A. 2011;108(35):14416–14421. doi: 10.1073/pnas.1107966108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritchie RO. The conflicts between strength and toughness. Nat Mater. 2011;10(11):817–822. doi: 10.1038/nmat3115. [DOI] [PubMed] [Google Scholar]
- 5.Gupta HS, Seto J, Wagermaier W, Zaslansky P, Boesecke P, Fratzl P. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc Natl Acad Sci. 2006;103(47):17741–17746. doi: 10.1073/pnas.0604237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burr DB. The contribution of the organic matrix to bone’s material properties. Bone. 2002;31(1):8–11. doi: 10.1016/s8756-3282(02)00815-3. http://www.ncbi.nlm.nih.gov/pubmed/12110405. [DOI] [PubMed] [Google Scholar]
- 7.Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15(1):60–67. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 8.Abraham AC, Agarwalla A, Yadavalli A, McAndrew C, Liu JY, Tang SY. Multiscale Predictors of Femoral Neck In Situ Strength in Aging Women: Contributions of BMD, Cortical Porosity, Reference Point Indentation, and Nonenzymatic Glycation. J Bone Miner Res. 2015;30(12):2207–2214. doi: 10.1002/jbmr.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granke M, Makowski AJ, Uppuganti S, Does MD, Nyman JS. Identifying Novel Clinical Surrogates to Assess Human Bone Fracture Toughness. J Bone Miner Res. 2015;30(7):1290–1300. doi: 10.1002/jbmr.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farr JN, Drake MT, Amin S, Melton LJ, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29(4):787–795. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carriero A, Zimmermann EA, Paluszny A, et al. How tough is brittle bone? Investigating osteogenesis imperfecta in mouse bone. J Bone Miner Res. 2014;29(6):1392–1401. doi: 10.1002/jbmr.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14(4):595–608. doi: 10.1016/8756-3282(93)90081-k. http://www.ncbi.nlm.nih.gov/pubmed/8274302. [DOI] [PubMed] [Google Scholar]
- 13.Robertson DM, Smith DC. Compressive strength of mandibular bone as a function of microstructure and strain rate. J Biomech. 1978;11(10–12):455–471. doi: 10.1016/0021-9290(78)90057-X. [DOI] [PubMed] [Google Scholar]
- 14.Currey JD. The effects of drying and re-wetting on some mechanical properties of cortical bone. J Biomech. 1988;21(5):439–441. doi: 10.1016/0021-9290(88)90150-9. http://www.ncbi.nlm.nih.gov/pubmed/3417696. [DOI] [PubMed] [Google Scholar]
- 15.Carter DR, Hayes WC. The compressive behavior of bone as a two-phase porous structure. J Bone Joint Surg Am. 1977;59(7):954–962. http://www.ncbi.nlm.nih.gov/pubmed/561786. [PubMed] [Google Scholar]
- 16.Brauer CA. Incidence and Mortality of Hip Fractures in the United States. JAMA. 2009;302(14):1573. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks R, Allegrante JP, Ronald MacKenzie C, Lane JM. Hip fractures among the elderly: causes, consequences and control. Ageing Res Rev. 2003;2(1):57–93. doi: 10.1016/s1568-1637(02)00045-4. http://www.ncbi.nlm.nih.gov/pubmed/12437996. [DOI] [PubMed] [Google Scholar]
- 18.Jackson RD, Mysiw WJ. Insights into the epidemiology of postmenopausal osteoporosis: the Women’s Health Initiative. Semin Reprod Med. 2014;32(6):454–462. doi: 10.1055/s-0034-1384629. [DOI] [PubMed] [Google Scholar]
- 19.Hansma PK, Turner PJ, Fantner GE. Bone diagnostic instrument. Rev Sci Instrum. 2006;77(7):075105. doi: 10.1063/1.2221506. [DOI] [Google Scholar]
- 20.Diez-Perez A, Güerri R, Nogues X, et al. Microindentation for in vivo measurement of bone tissue mechanical properties in humans. J Bone Miner Res. 2010;25(8):1877–1885. doi: 10.1002/jbmr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansma P, Turner P, Drake B, et al. The bone diagnostic instrument II: Indentation distance increase. Rev Sci Instrum. 2008;79(6):064303. doi: 10.1063/1.2937199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bridges D, Randall C, Hansma PK. A new device for performing reference point indentation without a reference probe. Rev Sci Instrum. 2012;83(4):044301. doi: 10.1063/1.3693085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malgo F, Hamdy NAT, Papapoulos SE, Appelman-Dijkstra NM. Bone material strength as measured by microindentation in vivo is decreased in patients with fragility fractures independently of bone mineral density. J Clin Endocrin Metabol. 2015;100(5):2039–2045. doi: 10.1210/jc.2014-4346. http://doi.org/10.1210/jc.2014-4346. [DOI] [PubMed] [Google Scholar]
- 24.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41(4):505–515. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Burstein AH, Reilly DT, Martens M. Aging of bone tissue: mechanical properties. J Bone Joint Surg Am. 1976;58(1):82–86. http://www.ncbi.nlm.nih.gov/pubmed/1249116. [PubMed] [Google Scholar]
- 26.McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75(8):1193–1205. doi: 10.2106/00004623-199308000-00009. http://www.ncbi.nlm.nih.gov/pubmed/8354678. [DOI] [PubMed] [Google Scholar]
- 27.Boskey AL, Coleman R. Aging and Bone. J Dent Res. 2010;89(12):1333–1348. doi: 10.1177/0022034510377791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karim L, Van Vliet M, Bouxsein ML. Comparison of cyclic and impact-based reference point indentation measurements in human cadaveric tibia. Bone. 2015 doi: 10.1016/j.bone.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houde J, Marchetti M, Duquette J, et al. Correlation of bone mineral density and femoral neck hardness in bovine and human samples. Calcif Tissue Int. 1995;57(3):201–205. doi: 10.1007/BF00310259. http://www.ncbi.nlm.nih.gov/pubmed/8574937. [DOI] [PubMed] [Google Scholar]
- 30.Leong PL, Morgan EF. Correlations between indentation modulus and mineral density in bone-fracture calluses. Integr Comp Biol. 2009;49(1):59–68. doi: 10.1093/icb/icp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta HS, Wagermaier W, Zickler GA, et al. Nanoscale deformation mechanisms in bone. Nano Lett. 2005;5(10):2108–2111. doi: 10.1021/nl051584b. [DOI] [PubMed] [Google Scholar]
- 32.Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986;314(7):403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- 33.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. [Accessed October 27, 2014];Bone. 2001 28(2):195–201. doi: 10.1016/s8756-3282(00)00434-8. http://www.ncbi.nlm.nih.gov/pubmed/11182378. [DOI] [PubMed] [Google Scholar]
- 34.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40(4):1144–1151. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31(1):1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 36.Choi K, Kuhn JL, Ciarelli MJ, Goldstein SA. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J Biomech. 1990;23(11):1103–1113. doi: 10.1016/0021-9290(90)90003-l. http://www.ncbi.nlm.nih.gov/pubmed/2277045. [DOI] [PubMed] [Google Scholar]
- 37.Milovanovic P, Zimmermann EA, Riedel C, Scheidt AV, Herzog L, Krause M, et al. Multi-level characterization of human femoral cortices and their underlying osteocyte network reveal trends in quality of young, aged, osteoporotic and antiresorptive-treated bone. Biomaterials. 2015;45:46–55. doi: 10.1016/j.biomaterials.2014.12.024. http://doi.org/10.1016/j.biomaterials.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Taylor JMG. Kendall’s and Spearman’s Correlation Coefficients in the Presence of a Blocking Variable. Biometrics. 1987;43(2):409. http://doi.org/10.2307/2531822. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.