Summary
Objective
To describe the natural history of EEG changes in patients with Benign Epilepsy with Centrotemporal Spikes (BECTS) over 1 year.
Methods
Centrotemporal spikes (CTS) were visually evaluated based on 24-hour ambulatory EEGs to determine the total, left, right, and bilateral CTS while awake and asleep. These CTS rates were then used to compare the entire night of sleep to the first 2 hours of sleep, the repeatability of spike frequency over two recordings (done within days to weeks), and longitudinal changes in CTS rate over 6 and 12 months.
Results
19 children with newly diagnosed and untreated BECTS were included in this analysis. An excellent correlation was found between the CTS rate during the entire duration of sleep and the first 2 hours of sleep (intraclass correlation (ICC) = 0.87, 95% CI = 0.67–0.95). An excellent correlation was also found between two recordings completed an average of 23 days apart while asleep (ICC = 0.92, 95% CI = 0.80–0.97) and lower, but still good correlation while awake (ICC = 0.70, 95% CI = 0.39–0.87). The average change in CTS rate between recordings at baseline and 6 months was a decrease of 64.7% (range −100% to +51.5%, p=0.01) and the average change in CTS rate between recordings at baseline and 12 months was a decrease of 57.7% (range −100% to +29.1%, p=0.01). Additionally, within 6 months, most children had decreased CTS rates with 30% of children being spike free. This absence of spikes did not continue in all children since the majority (60%) had some CTS at 1 year following diagnosis.
Significance
CTS rates during sleep are stable when compared over days to weeks, however, when comparing spike rates over months there is a larger degree of variability.
Keywords: Electroencephalography, BECTS, BRE, Epilepsy
Introduction
Benign epilepsy with centrotemporal spikes (BECTS) is a common pediatric epilepsy syndrome which is thought to account for 15–23% of all childhood epilepsies.1 Seizures typically begin between 3 and 13 years of age with a peak at 7–8 years.2 Seizures typically occur shortly after the child falls asleep or just before waking with the majority of patients having only a single seizure with relatively few (<10%) being reported to have frequent seizures.3, 4 The characteristic EEG pattern of BECTS consists of a normal background and high voltage, blunted spikes in the centro-temporal head regions that can be unilateral or bilateral and become more frequent during drowsiness and non-REM sleep. The localization is most often unilateral although bilateral synchronous and asynchronous discharges can be seen and the localization of spikes can vary over time in the same patient.
There have been many studies which suggest a possible negative impact of CTS, especially during sleep, on cognition and behavior but these findings are difficult to interpret without knowing how the spikes vary over the time scales of weeks, months, and years. In the initial description of BECTS, Beaussart reports spike frequency as 5–10 per minute and several other studies since then have reported spike frequencies averaging between 22 and 55.6 per minute during slow wave delta sleep.3, 5–11 Longitudinal changes in CTS rates have been reported in previous small samples but the results are difficult to interpret since the patient populations were either all treated with antiepileptic drugs (AEDs) or there were heterogeneous groups with some treated and others untreated.7, 8, 11 Kanemura et al quantified CTS frequency during serial 20 to 40 minute EEGs and reported a peak of 60 spikes per minute at 12 months and continued spikes of 10 per minute at 48 months post-diagnosis, however the authors did not distinguish between awake and sleep states.7, 8 Ebus et al also collected 24 hour EEGs at baseline, 6 months, and 2 years and calculated the percent of seconds with CTS in 30 random 10 second pages.11 The results were reported as being stable, improved, or worsened over this time frame which limited the usefulness.
The purpose of our study is to describe the longitudinal EEG changes seen in children with BECTS who were not treated with AEDs, determine the repeatability of spike rate over two recordings, and assess the accuracy of spike rate during a portion of sleep compared to the entire night. Spike rates and laterality were quantified during 24 hour EEGs completed at diagnosis as well as 6 months and 12 months post-diagnosis. This information is important for planning and interpreting any future studies that aim to relate CTS rate with clinical phenotype.
Methods
All study procedures were approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
Participants
Nineteen children (ages 5–12, 8 females) with one or more complete 24 hour EEG recordings were selected from our ongoing study of children with BECTS. All participants were consecutively recruited from Neurology Clinics at Cincinnati Children’s Hospital Medical Center.2 These children were recruited at the time of BECTS diagnosis, had a history of 1–9 seizures at time at enrollment, and none were taking anti-epileptic medications at the time of study enrollment. All children were native speakers of English with no history of neuropsychological or learning disorders (based on parent report). Informed consent was obtained from a parent/guardian for all participants, including written assent from participants age 11 and up. Formal inclusion and exclusion criteria are listed in Supplementary Table 1.
Electroencephalographic (EEG) Evaluation
Centrotemporal spikes (CTS) were evaluated based on a 24-hour ambulatory EEG recording with electrodes in standard 10–20 positioning. CTS were counted visually by a pediatric epileptologist (JRT) and noted as originating from left or right centrotemporal regions. Periods of wakefulness and sleep (designated by the first sleep spindle) were also noted. When analyzing spike rate repeatability and change in spike rate over 1 year, CTS were counted during the first 2 hours of sleep, starting with the first sleep spindle. In the instances where the entire night of sleep was compared to the initial 2 hours of sleep, the entire night of sleep encompassed the first sleep spindle until the first occurrence of a posterior dominant rhythm. During sleep there were often brief transitions between sleep and wakefulness. These periods were all considered “sleep” unless the period of wakefulness was greater than 5 minutes. Since there were no eye leads periods of rapid eye movement (REM) sleep were quantified the same as those during non-REM sleep. A spike rate was calculated by taking left, right, and total spikes divided by the duration of the awake period, asleep period, and/or total recording time. If CTS appeared generalized or bilaterally symmetric, either during waking or sleep, then they were marked as bilateral if the amplitude difference between the right and left sides was less than 30%. In general, this occurred infrequently. Otherwise the CTS were marked as unilateral. A lateralization index (LI) was calculated in order to assess the degree of asymmetry [(left sided spike rate − right sided spike rate)/(left sided spike rate + right sided spike rate)] so that LI=1 is completely left sided spikes, LI=−1 is completely right sided spikes, and LI=0 is equal left and right spike rates.
A total of 19 children were included in this study (Table 1). The entire sleep period from a 24 hour recording was examined in the first 9 children who participated (14 recordings) and compared to the first two hours of sleep in the same children. Spike rates during two recordings, done within approximately 3 weeks of each other, were compared in 17 children (2 participants did not complete a second baseline 24-hour EEG due to family scheduling concerns). Spike rates during EEGs done at the time of diagnosis, approximately 6 months post-diagnosis, and approximately 12 months post-diagnosis were compared in 10 children. Sixteen participants had finished the 1 year EEG recording, but six of the children were excluded from this analysis because they were started on antiepileptic medication following the baseline recording.
Table 1.
Participant Demographics.
Child demographics. Child number, gender, age at diagnosis, and duration of epilepsy (when enrolled in the study) are listed for each of the separate analyses which were completed (spike rate agreement, spike rate repeatability, and spike rate change over 1 year).
| Spike Rate Agreement (Entire Sleep vs. 2 hr sleep) Analysis | |||
|---|---|---|---|
| Participant | Gender | Age at Diagnosis (years) | Duration of Epilepsy (months) |
| 1 | M | 9.6 | 12.0 |
|
| |||
| 2 | M | 9.1 | 4.0 |
|
| |||
| 3 | M | 5.5 | 0.0 |
|
| |||
| 4 | F | 9.2 | 0.0 |
|
| |||
| 5 | M | 10.6 | 8.0 |
|
| |||
| 6 | F | 9.9 | 0.0 |
|
| |||
| 7 | F | 9.4 | 0.0 |
|
| |||
| 8 | M | 5.5 | 0.0 |
|
| |||
| 9 | F | 5.2 | 0.0 |
|
| |||
| Average ± SD | 8.2 ± 2.1 | 2.7 ± 4.5 | |
| Participant Demographics – Spike Rate Repeatability Analysis | |||
|---|---|---|---|
| Participant | Gender | Age at Diagnosis (years) | Duration of Epilepsy (months) |
| 2 | M | 9.1 | 4.0 |
|
| |||
| 3 | M | 5.5 | 0.0 |
|
| |||
| 5 | M | 10.6 | 8.0 |
|
| |||
| 6 | F | 9.9 | 0.0 |
|
| |||
| 7 | F | 9.4 | 0.0 |
|
| |||
| 8 | M | 5.5 | 0.0 |
|
| |||
| 9 | F | 5.2 | 0.0 |
|
| |||
| 10 | F | 5.4 | 0.0 |
|
| |||
| 11 | F | 9.2 | 0.0 |
|
| |||
| 12 | M | 10.4 | 0.0 |
|
| |||
| 13 | F | 7.5 | 0.0 |
|
| |||
| 14 | M | 7.9 | 4.0 |
|
| |||
| 15 | M | 6.8 | 1.0 |
|
| |||
| 16 | M | 8.3 | 0.0 |
|
| |||
| 17 | F | 12.6 | 0.0 |
|
| |||
| 18 | M | 10.6 | 0.0 |
|
| |||
| 19 | M | 9.3 | 0.0 |
|
| |||
| Average ± SD | 8.4 ± 2.2 | 1.0 ± 2.2 | |
| Participant Demographics – Spike Rate Change Over 1 Year Analysis | |||
|---|---|---|---|
| Participant | Gender | Age at Diagnosis (years) | Duration of Epilepsy (months) |
| 2 | M | 9.1 | 4.0 |
|
| |||
| 3 | M | 5.5 | 0.0 |
|
| |||
| 4 | F | 9.2 | 0.0 |
|
| |||
| 5 | M | 10.6 | 8.0 |
|
| |||
| 8 | M | 5.5 | 0.0 |
|
| |||
| 9 | F | 5.2 | 0.0 |
|
| |||
| 10 | F | 5.4 | 0.0 |
|
| |||
| 11 | F | 9.2 | 0.0 |
|
| |||
| 12 | M | 10.4 | 0.0 |
|
| |||
| 13 | F | 7.5 | 0.0 |
|
| |||
| Average ± SD | 7.8 ± 2.2 | 1.2 ± 2.7 | |
Statistical Analysis
Spike rate agreement between the entire night of sleep and the first 2 hours of sleep was compared using an intraclass correlation (ICC). Similarly, spike rate repeatability between two closely completed EEGs was determined using the ICC. The ICC values were categorized as excellent (0.8–1), good (0.6–0.79), fair (0.4–0.59), and poor (0–0.39). The change in spike rates between the baseline and 6 months, the baseline and 12 months, and 6 months and 12 months was compared using a Wilcoxon signed-rank test. All analysis were carried out using SAS v9.3 (SAS Institute, Cary, NC).
Results
Spike rate agreement between entire night and 2 hours of sleep
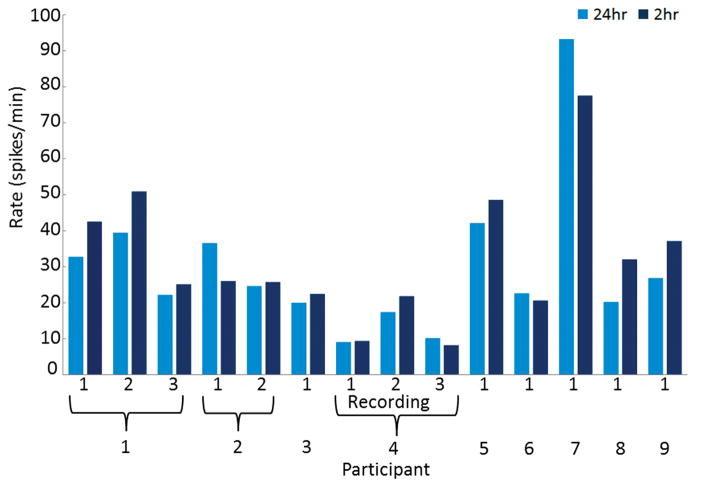
Fourteen recordings from 9 children (average age 8.2 years, range 5.2–10.6 years, 4 females) were included in the analysis of CTS rate agreement between the entire sleep period of a 24 hour recording and the first 2 hours of sleep. This first 2 hours of sleep was chosen as an interval in an attempt to determine the number of CTS over an entire sleep cycle, which is approximately 90 minutes. The entire duration of sleep for a 24 hour recording lasted on average 516 minutes (range 381–627 minutes). The average CTS rate during the entire sleep duration was 25.4 per minute (range 0 to 93.2 per minute) while the CTS rate during the first 2 hours of sleep was 29.9 per minute (range 0–77.6 per minute) (Figure 1). There was excellent correlation between the total CTS rate during the entire duration of sleep and the first 2 hours of sleep (ICC = 0.87, 95% CI = 0.67–0.95). There was also excellent correlation in terms of laterality with left sided and right sided CTS rates having ICC of 0.89 (95% CI = 0.73–0.96) and 0.93 (95% CI = 0.83–0.98), respectively.
Figure 1.
Comparison of spike rates between an entire night of sleep (24hr) and the first two hours of sleep (2hr). Ten children were included in this analysis and three of these children (1, 2, and 4) had multiple recordings which were analyzed. An excellent correlation was found between the CTS rate during the entire duration of sleep and the first 2 hours of sleep (ICC = 0.87, 95% CI = 0.67–0.95).
Spike rate repeatability
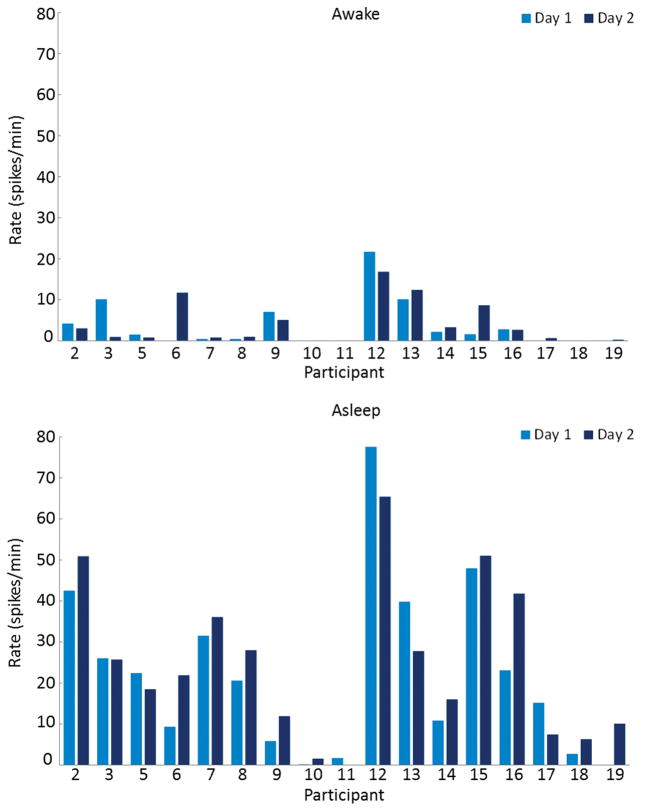
Seventeen children (average age 8.4 years, range 5.2–12.6 years, 7 females) were included in the analysis of CTS rate repeatability. The two recordings were separated by an average of 23 days (range 7–58 days). On the first recording, the average CTS rate while awake was 3.7 per minute (range 0–21.7 per minute) and 22.2 per minute while asleep (range 0–77.6 per minute). On the second recording, the average CTS rate while awake was 4.0 per minute (range 0–16.8 per minute) and 24.8 per minute while asleep (range 0–65.4 per minute) (Figure 2). There was excellent correlation between the two recordings while asleep (ICC = 0.92, 95% CI = 0.80–0.97) and lower, but still good correlation while awake (ICC = 0.70, 95% CI = 0.39–0.87). In addition, when looking specifically at the correlation for left or right sided spikes between the two recordings, while awake the correlation was fair (left ICC = 0.52 (95% CI = 0.11–0.78), right ICC = 0.55 (95% CI = 0.15–0.80) and during sleep it was good for the left and excellent for the right (left ICC = 0.75 (95% CI = 0.47–0.90), right ICC = 0.940 (95% CI = 0.85–0.98)). Two children (11.8%) switched laterality from completely right sided to completely left sided CTS during the recordings and nine children (52.9%) had persistently unilateral CTS over the two recordings (Table 2). The remaining six children (35.3%) had bilaterally independent CTS during both recordings.
Figure 2.
Comparison of spike rates between two recordings (Day 1 and Day 2) done an average of 23 days apart to determine the repeatability of spike frequency. CTS rates were compared while awake (top) and asleep (bottom) in seventeen children. An excellent correlation was found between the two recordings while asleep (ICC = 0.92, 95% CI = 0.80–0.97) and lower, but still very good correlation was found while awake (ICC = 0.70, 95% CI = 0.39–0.87).
Table 2.
Lateralization indices for the spike rate repeatability analysis. Lateralization index (LI) for each child is shown for CTS while awake and asleep.
| Participant | Awake | Asleep | ||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 1 | Day 2 | |
| 2 | 0.6 | 0.6 | −0.2 | 0.1 |
| 3 | 1.0 | 1.0 | 1.0 | 1.0 |
| 5 | −1.0 | −1.0 | −1.0 | −1.0 |
| 6 | −1.0 | −1.0 | −1.0 | 1.0 |
| 7 | −1.0 | −0.9 | −1.0 | −1.0 |
| 8 | 1.0 | 1.0 | 1.0 | 1.0 |
| 9 | −0.2 | −0.8 | 0.0 | −0.7 |
| 10 | −1.0 | −1.0 | −1.0 | −1.0 |
| 11 | −1.0 | § | −0.8 | −1.0 |
| 12 | § | § | −1.0 | −0.2 |
| 13 | 1.0 | 1.0 | 1.0 | 1.0 |
| 14 | −1.0 | −1.0 | −0.8 | −0.2 |
| 15 | 1.0 | 1.0 | 1.0 | 1.0 |
| 16 | 0.6 | 0.2 | 0.0 | 0.2 |
| 17 | § | 1.0 | 1.0 | 1.0 |
| 18 | § | −1.0 | −1.0 | −1.0 |
| 19 | −0.7 | 1.0 | −1.0 | 1.0 |
A LI = 1 indicates completely left sided spikes and LI = −1 indicates completely right sided spikes.
indicates that no spikes were recorded. Two children switched laterality from completely right sided to completely left sided CTS during the recordings and nine children had persistently unilateral CTS over the two recordings.
Change in spike rates over 1 year
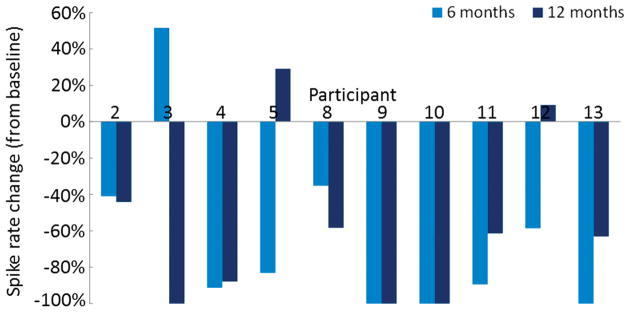
Ten children (average age 7.8 years, range 5.2–10.6 years, 5 females) were included in the analysis of CTS rate change (during sleep) over 1 year. The average duration between the first and second recordings was 172 days (range 144–251 days) and average duration between the first and third recordings was 363 days (range 335–397 days). The average change in CTS rate between recordings at baseline and 6 months was a decrease of 64.7% (range −100% to +51.5%) (Figure 3). At the 6 month time point 9 of 10 children (90%) had decreased spike rate and 1 child (10%) had an increased spike rate. The average change in CTS rate between recordings at baseline and 12 months was a decrease of 57.7% (range −100% to +29.1%). At the 12 month time point, 2 of 10 children (20%) had increased CTS rate compared to baseline. Three children (30%) had no spikes during the 6 month recording and two of these also had no spikes during the 12 month recording. The third child with no spikes at 6 months had spikes during the 12 month recording. One additional child had no spikes during the 12 month recording, after having had an increase in CTS rate during the 6 month recording (Child 2). There were statistically significant changes in spike rates when comparing the baseline and 6 months (p=0.01) as well as the baseline and 12 months (p=0.01). However, there was no significant change in spike rate when comparing those at 6 months to 12 months (p=0.11). Lateralization indices over these recordings show 3 children with persistently unilateral CTS (children 2, 7, 11) (Table 3).
Figure 3.
Comparison of spike rates at baseline, 6 months, and 12 months in ten children. These are expressed as the percent change in CTS rate at 6 months and 12 months compared to baseline, so that a change of −100% indicates an absence of all spikes compared to baseline. The change in CTS rate was statistically significant when comparing baseline to 6 months (p=0.01) and baseline to 12 months (p=0.01) but not when comparing 6 months to 12 months (p=0.11).
Table 3.
Lateralization Indices. Spike Rate Change Over 1 Year Analysis
Lateralization indices for CTS rates at baseline (0 months), 6 months, and 12 months. Lateralization index (LI) for each child is shown for CTS while awake and asleep.
| Participant | 0 months | 6 months | 12 months |
|---|---|---|---|
| 2 | −0.2 | −0.3 | 0.3 |
| 3 | 1.0 | 1.0 | § |
| 4 | 0.3 | 1.0 | −1.0 |
| 5 | 1.0 | −1.0 | 0.3 |
| 8 | −1.0 | −1.0 | § |
| 9 | 1.0 | 1.0 | 1.0 |
| 10 | −0.8 | § | § |
| 11 | −1.0 | § | § |
| 12 | 0.0 | 0.4 | −0.1 |
| 13 | −0.2 | § | 1.0 |
A LI = 1 indicates completely left sided spikes and LI = −1 indicates completely right sided spikes.
indicates that no spikes were recorded. Three children were found to have persistently unilateral CTS over the three recordings.
Discussion
The goals of this study were to describe the longitudinal EEG changes seen in drug-naïve children with BECTS, determine the repeatability of spike rate over two recordings, and assess the accuracy of spike rate during a portion of sleep compared to the entire night. These are important issues for clinicians when counseling patients and families as well as researchers who would like to quantify interictal spikes.
It is clearly demonstrated in our study that CTS rates during sleep are similar when compared over days to weeks, in the same child. However, when looking over months there is a larger degree of variability in CTS rate. Within 6 months, most children had decreased CTS rates with 30% of children being spike free. However, having a 24 hour EEG free of CTS did not universally mean that CTS would not occur as there was one child where this occurred. CTS were shown to persist in the majority (60%) of children at 1 year following diagnosis. Our findings differ from a previous report demonstrating large variability of interictal spike counts in a heterogenous group of children with focal epilepsy (4 with BECTS) when two consecutive nights were compared.12 These authors reported a 42% mean difference in spike frequency between the two consecutive nights, when they counted over the first 20 minutes of sleep. It may be that as a whole, spike rates are variable in children with epilepsy but more stable when looking within a specific epilepsy syndrome such as BECTS. It is also possible that counting spikes over a longer time frame of hours versus minutes reveals greater stability of spike rates between nights.
We have also shown that there is good agreement between an entire night of sleep and the first two hours of sleep. This is similar to findings reported in children with focal nocturnal epileptiform activity (5 with BECTS) showing that the spike index of sleep deprived morning EEGs was similar, but lower than the spike index during a full night recording.13 Our findings also support the results reported in children with continuous spikes and waves during sleep (CSWS) that a sampling of the spike frequency during the first 5 minutes of non-rapid eye movement (NREM) sleep is similar to that seen during the entire NREM period.14
We have shown that spike rates can be very high with some patients having more than one spike per second, throughout sleep. These patients would be consistent with a diagnosis of electrical status epilepticus during sleep (ESES) or CSWS.15, 16 This is consistent with previous reports suggesting a spectrum disorder with BECTS on the mild end and CSWS, ESES, and Landau-Kleffner syndrome on the severe end.17, 18 Our findings and that of others indicate that there is only a partial relationship between awake or sleep CTS rates and seizure frequency or CTS rates and neuropsychological outcomes.19, 20 However, there have been many reports of subtle neuropsychological abnormalities in patients with BECTS and it is difficult to imagine that CTS do not, in any way, contribute to the development of these deficits. Is it possible that spike location or laterality is more important than the absolute spike rate? In our study, a significant proportion of children (30%) had persistent unilaterality of CTS, when followed over 1 year. Several previous reports have indicated that while CTS can be unilateral during one or more recordings, that over time each hemisphere is expected to be similarly involved.9, 21, 22 Is it possible that these patients with persistently unilateral spikes will have a different neuropsychological outcome than patients with bilaterally independent hemisphere involvement? Do patients with EEG dipoles in the same hemisphere have different outcomes from those with dipoles which cross the hemispheres? We plan to explore these hypotheses further in future analyses.
There are several limitations of the current study to consider. We have a limited number of children who have had longitudinal follow-up of CTS rate so definitive conclusions are difficult to make. The majority of children continued to have CTS at 1 year post-diagnosis so it is not possible to say when EEG normalization occurs in “most” patients. Also, CTS frequency was determined using a rate (spikes per minute) rather than the traditional spike wave index (SWI).23 This was done to provide more accurate quantification of spikes but makes the data somewhat more difficult to compare to previous studies for ESES and CSWS where SWI was the primary measure. However, it has previously been shown that spike per minute rates can be converted to spikes per 100 seconds by multiplying by 100/60 (1.67) which is a spike measure that is more comparable to the SWI.14 Finally, all spike counts were completed by one reader without any intra-reader reliability measures.
Despite these limitations, this study provides a natural history of EEG characteristics in children with the most common focal epilepsy syndrome, BECTS. It is our expectation that these findings will assist clinicians with their management and counseling of patients with BECTS and drive further research to understand the relationship between frequent interictal abnormalities and neuropsychological functioning.
Supplementary Material
Supplementary Table 1. Formal inclusion and exclusion criteria for BECTS participants. ILAE – International League Against Epilepsy; ADD/ADHD – Attention deficit disorder/Attention deficit hyperactivity disorder.
Key Points.
At 6 months post-diagnosis, most children with BECTS had decreased spike rates, with 30% of children being spike free.
Spike rates during the first 2 hours of sleep are equivalent to spike rates over the entire night of sleep.
There is stability of spike rates between two consecutive EEGs done weeks apart from one another.
There is large variability in spike rates when comparing over 6 to 12 month time periods.
Acknowledgments
This study was funded by NIH/NINDS R01 NS065840 (Vannest).
Footnotes
Conflict of interest
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure
None of the authors has any conflict of interest to disclose which are relevant to this research activity.
References
- 1.Cavazzuti GB. Epidemiology of different types of epilepsy in school age children of Modena, Italy. Epilepsia. 1980;21:57–62. doi: 10.1111/j.1528-1157.1980.tb04044.x. [DOI] [PubMed] [Google Scholar]
- 2.Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 3.Beaussart M. Benign epilepsy of children with Rolandic (centro-temporal) paroxysmal foci. A clinical entity. Study of 221 cases. Epilepsia. 1972;13:795–811. doi: 10.1111/j.1528-1157.1972.tb05164.x. [DOI] [PubMed] [Google Scholar]
- 4.Loiseau P, Duche B, Cordova S, Dartigues JF, Cohadon S. Prognosis of benign childhood epilepsy with centrotemporal spikes: a follow-up study of 168 patients. Epilepsia. 1988;29:229–235. doi: 10.1111/j.1528-1157.1988.tb03711.x. [DOI] [PubMed] [Google Scholar]
- 5.Bernardina BD, Beghini G. Rolandic spikes in children with and without epilepsy. (20 subjects polygraphically studied during sleep) Epilepsia. 1976;17:161–167. doi: 10.1111/j.1528-1157.1976.tb03393.x. [DOI] [PubMed] [Google Scholar]
- 6.Clemens B, Majoros E. Sleep studies in benign epilepsy of childhood with rolandic spikes. II. Analysis of discharge frequency and its relation to sleep dynamics. Epilepsia. 1987;28:24–27. doi: 10.1111/j.1528-1157.1987.tb03617.x. [DOI] [PubMed] [Google Scholar]
- 7.Kanemura H, Sano F, Aoyagi K, Sugita K, Aihara M. Do sequential EEG changes predict atypical clinical features in rolandic epilepsy? Developmental medicine and child neurology. 2012;54:912–917. doi: 10.1111/j.1469-8749.2012.04358.x. [DOI] [PubMed] [Google Scholar]
- 8.Kanemura H, Sano F, Ohyama T, Sugita K, Aihara M. Sequential EEG characteristics may predict seizure recurrence in rolandic epilepsy. Seizure. 2014;23:646–650. doi: 10.1016/j.seizure.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Kellaway P. The electroencephalographic features of benign centrotemporal (rolandic) epilepsy of childhood. Epilepsia. 2000;41:1053–1056. doi: 10.1111/j.1528-1157.2000.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 10.Berroya AM, Bleasel AF, Stevermuer TL, Lawson J, Bye AM. Spike morphology, location, and frequency in benign epilepsy with centrotemporal spikes. Journal of child neurology. 2005;20:188–194. doi: 10.1177/08830738050200030401. [DOI] [PubMed] [Google Scholar]
- 11.Ebus SC, DMIJ, den Boer JT, et al. Changes in the frequency of benign focal spikes accompany changes in central information processing speed: a prospective 2-year follow-up study. Epilepsy & behavior: E&B. 2015;43:8–15. doi: 10.1016/j.yebeh.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Libenson MH, Haldar A, Pinto AL. The stability of spike counts in children with interictal epileptiform activity. Seizure. 2014;23:454–456. doi: 10.1016/j.seizure.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Larsson PG, Evsiukova T, Brockmeier F, Ramm-Pettersen A, Eeg-Olofsson O. Do sleep-deprived EEG recordings reflect spike index as found in full-night EEG recordings? Epilepsy & behavior: E&B. 2010;19:348–351. doi: 10.1016/j.yebeh.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez IS, Peters JM, Hadjiloizou S, et al. Clinical staging and electroencephalographic evolution of continuous spikes and waves during sleep. Epilepsia. 2012;53:1185–1195. doi: 10.1111/j.1528-1167.2012.03507.x. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez IS, Chapman KE, Peters JM, et al. The tower of Babel: survey on concepts and terminology in electrical status epilepticus in sleep and continuous spikes and waves during sleep in North America. Epilepsia. 2013;54:741–750. doi: 10.1111/epi.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tassinari CA, Rubboli G, Volpi L, et al. Encephalopathy with electrical status epilepticus during slow sleep or ESES syndrome including the acquired aphasia. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2000;111(Suppl 2):S94–S102. doi: 10.1016/s1388-2457(00)00408-9. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez Fernandez I, Chapman K, Peters JM, et al. Treatment for continuous spikes and waves during sleep (CSWS): survey on treatment choices in North America. Epilepsia. 2014;55:1099–1108. doi: 10.1111/epi.12678. [DOI] [PubMed] [Google Scholar]
- 18.Scheltens-de Boer M. Guidelines for EEG in encephalopathy related to ESES/CSWS in children. Epilepsia. 2009;50(Suppl 7):13–17. doi: 10.1111/j.1528-1167.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 19.Ebus SC, Overvliet GM, Arends JB, Aldenkamp AP. Reading performance in children with rolandic epilepsy correlates with nocturnal epileptiform activity, but not with epileptiform activity while awake. Epilepsy & behavior: E&B. 2011;22:518–522. doi: 10.1016/j.yebeh.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Vannest J, Tenney JR, Gelineau-Morel R, Maloney T, Glauser TA. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy & behavior: E&B. 2015;45:85–91. doi: 10.1016/j.yebeh.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Guerrini R, Pellacani S. Benign childhood focal epilepsies. Epilepsia. 2012;53(Suppl 4):9–18. doi: 10.1111/j.1528-1167.2012.03609.x. [DOI] [PubMed] [Google Scholar]
- 22.Panayiotopoulos CP, Michael M, Sanders S, Valeta T, Koutroumanidis M. Benign childhood focal epilepsies: assessment of established and newly recognized syndromes. Brain: a journal of neurology. 2008;131:2264–2286. doi: 10.1093/brain/awn162. [DOI] [PubMed] [Google Scholar]
- 23.Aeby A, Poznanski N, Verheulpen D, Wetzburger C, Van Bogaert P. Levetiracetam efficacy in epileptic syndromes with continuous spikes and waves during slow sleep: experience in 12 cases. Epilepsia. 2005;46:1937–1942. doi: 10.1111/j.1528-1167.2005.00337.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Formal inclusion and exclusion criteria for BECTS participants. ILAE – International League Against Epilepsy; ADD/ADHD – Attention deficit disorder/Attention deficit hyperactivity disorder.