Abstract
Introduction
Ischemia causes myocardial infarction and arrhythmias. Up-regulation of cardiac CLC-3 chloride channels is important for ischemic preconditioning-induced second-window protection against myocardial infarction. But its consequences in ischemia-induced electrical remodeling are still unknown.
Methods
The recently-characterized heart-specific overexpression of human short CLC-3 isoform (hsCLC-3OE) mice were used to study the effects of CLC-3 up-regulation on cardiac electrophysiology under ischemia/reperfusion conditions. In vivo surface electrocardiography (ECG) and intracardiac electrophysiology (ICEP) were used to compare the electrophysiological properties of age-matched wild-type (Clcn3+/+) and hsCLC-3OE mice under control and myocardial ischemia-reperfusion conditions.
Results
QT and QTc intervals of hsCLC-3OE mice were significant shorter than those of Clcn3+/+ mice under control, ischemia and reperfusion conditions. In the ICEP, ventricular effective refractory period (VERP) of hsCLC-3OE mice (26.7±1.7 ms, n=6) was significantly shorter than that of Clcn3+/+ mice (36.9±2.8 ms, n=8, P<0.05). Under ischemia condition, both VERP (19.8±1.3 ms) and atrial effective refractory period (AERP, 34.8±2.5 ms) of hsCLC-3OE mice were significantly shorter than those of Clcn3+/+ mice (35.2±3.0 ms and 45.8±1.6 ms, P<0.01, respectively). Wenckebach atrioventricular nodal block point (AVBP, 91.13±4.08 ms) and 2:1 AVBP (71.3±3.8 ms) of hsCLC-3OE mice were significantly shorter than those of Clcn3+/+ mice (102.0±2.0 ms and 84.1±2.8 ms, P<0.05, respectively). However, no differences of ICEP parameters between hsCLC-3OE and Clcn3+/+ mice were observed under reperfusion conditions.
Conclusion
Heart-specific overexpression of hsCLC-3 limited the ischemia-induced QT and ERP prolongation and postponed the advancements of Wenckebach and 2:1 AVBP. CLC-3 up-regulation may serve as an important adaptive mechanism against myocardial ischemia.
Keywords: arrhythmia, torsade de pointes, electrophysiology, ion channels, ECG, ischemia, chloride channels
Introduction
In human and animal models of various species, acute myocardial ischemia (AMI) causes either shortening (especially in larger mammals and human) or progressive prolongation of QT intervals and corresponding action potential duration (APD), which may cause life-threatening arrhythmias and sudden cardiac death[1–5]. Significant inhibition of several outward repolarizing K+ currents due to hypoxia, metabolic blockade, and oxidative stress under ischemia conditions has been considered a major mechanism for the prolongation of action potential duration (APD), effective refractory period (ERP), and QT interval[3].
It is well known that myocardial ischemia usually leads to energy depletion and intracellular acidification, which causes not only a fall in ATP/ADP ratio but also cell swelling[6, 7] and triggers the process of regulatory volume decrease (RVD)[8, 9]. RVD is an adaptive mechanism for the homeostasis of cell size and volume under physiological and pathophysiological conditions through a wide arrays of intracellular signaling cascades[9]. These include activation of osmolyte transporters and ion channels by releasing K+, Cl− and osmatically obligated water from the intracellular compartment of swollen cells and hence restoration of cell volume and cellular function[9, 10]. In cardiac myocytes, activation of CLC-3-related volume-sensitive outwardly-rectifying anion channels (CLC-3/VSOACs) play a very important role in RVD when cell swells during ischemia and reperfusion, which is an important mechanism for ischemic preconditioning (IPC)[11–13], especially the second-window late IPC, induced protection of myocardium from infarction[8, 14]. Theoretically, activation of CLC-3/VSOACs may also perturb APD of the atrial and ventricular myocytes and affect cardiac electrophysiological characteristics [15–17]. The equilibrium potential for Cl− (ECl) is usually between −65 to −40 mV, depending on actual intracellular Cl− concentration ([Cl−]i) and activity (aiCl)[18–21], that is within a membrane potential range more positive than the resting membrane potential (RMP) and can be either negative or positive to the actual membrane potential during the normal cardiac cycle[15]. Thus, compared with many cationic (Na+, K+, Ca2+) channels, Cl− channels have the unique ability to generate both inward and outward currents through the same channels and cause both depolarization and repolarization during the action potential[15, 17, 22]. Therefore, activation of Cl− channels may cause significant abbreviation of APD and depolarization of RMP that can induce early after depolarization (EAD) and cause arrhythmias under pathological conditions[17, 22]. However, the exact roles of Cl− channels in cardiac electrophysiology has never been confirmed due to the lack of specific pharmacological agents for manipulating Cl− channel functions[22, 23]. To overcome this hurdle we recently developed a murine line of heart-specific overexpression of human short CLC-3 isoform (hsCLC-3OE) [14]. Different from the CLC-3 long isoform (hlCLC-3) which is activated by calmodulin kinase II phosphorylation but insensitive to cell volume regulation[24–26], hsCLC-3 lacks an additional 58 amino acids at the amino terminus and is activated by increase in cell volume and inhibited by protein kinase C (PKC) phosphorylation while the hlCLC-3. In the hsCLC-3OE mouse heart CLC-3 protein expression was approximately 65% more than in the wild-type Clcn3+/+ controls as confirmed by Northern blot and Western analysis[14]. Correspondingly, the whole-cell VSOAC current density in the hsCLC-3OE cardiac myocytes was 2–3 fold larger than those of Clcn3+/+ cardiac myocytes under hypotonic cell-swelling conditions. [14]. The objective of this study is to further characterize the in vivo cardiac electrophysiological properties of the hsCLC-3OE mice to gain insights into the functional role of CLC-3/VSOACs in ischemia and reperfusion, which has never been done before [22].
Methods
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication No. 85-23, revised 1996) and was in accordance with the institutional guidelines for animal care and use approved by the University of Nevada, Reno Institutional Animal Care and Use Committee (IACUC).
Experimental Animals
The hsCLC-3OE mice were produced by the Nevada Transgenic Center [14]. Briefly, the cDNA encoding hsClC-3 was amplified using PfuTurbo Polymerase (Stratagene, La Jolla, CA, USA). Primers were designed to introduce a ClaI restriction site for cloning. The amplified hsClC-3 cDNA was subcloned downstream of the α-myosin heavy chain (α-MHC) heart-specific promoter. To make transgenic mice, 3 ng/mL purified DNA construct was microinjected into the male pronucleus of fertilized B6C3F1/5 oocytes. Embryos were placed into the oviduct of a pseudopregnant CD1 female mouse. Resultant pups were screened by polymerase chain reaction on genomic DNA isolated from tail snips using primers that amplify a fragment of DNA between the α-MHC promoter and hsClC-3 cDNA, using Wizard SV Kit with a 2-primer assay (5’-AGACCTCTGACAGAGAAGC-3’, 5’-AGAACTGTTAATGCTGCCTC-3’) resulting in a 220-bp band, as previously described[14]. The overexpression of hsCLC-3 in the heart was confirmed and characterized at both molecular and cellular levels by Northern blot and Western analysis as well as whole-cell patch-clamp previously[14]. The age-matched wide-type C57/Bl6 (Clcn3+/+) mice, from which the transgenic mice had background during manipulation, were obtained from Jacksons Laboratories and used as parallel control under identical conditions in this study. CLC-3 protein expression in hsCLC-3OE heart was 1.65 timesmore than in Clcn3+/+ heart[14].
Surface electrocardiograph (ECG) recordings[23, 27]
ECG recordings were performed on Clcn3+/+ and hsCLC-3OE mice under anesthesia with isoflurane. Body temperature was maintained at 37°C through a heating pad. Six surface leads of ECG (I, II, III, aVR, aVL and aVF) were recorded simultaneously (6-lead ECG amplifier and ACQ-7700 Ponema Physiology Platform, Gould, USA) and analyzed with ecgAUTO2 software (EMKA Technologies, VA, USA). The QT interval was corrected (QTc) for a heart rate (HR) of 600 bpm (R−R=100 ms), a value near the physiologic HR in mice, using the formula of QTc = QT/(RR/100)1/2 as previously described[27–29].
Intracardiac electrophysiological (ICEP) recordings[23]
Mice were anesthetized with isoflurane and placed on a heated pad to maintain body temperature at 37°C. A 1.1 French octapolar electrode catheter (EPR-800, Millar Instruments, Houston, TX) was advanced through the right jugular vein into the right atrium and right ventricle. Programmed electrical stimulation was performed using 2-ms current pulses delivered by an external stimulator (STG3008, Multichannel Systems, Reutlingen, Germany). Surface 6-lead ECG was recorded and monitored throughout the experimental procedures. The 8-lead intracardiac electrograms were recorded and analyzed with a computer-based data acquisition system (IOX-2 acquisition software and ecgAUTO2 software, Emka Technologies, VA, USA).
Right atrial overdrive pacing protocols of either S1S2 or S1S1 stimulus were used to determine atrial effective refractory period (AERP),, Wenckebach point, 2:1 atrioventricular block point or atrial tachycardia, respectively. Right ventricular overdrive pacing protocols of either S1S2 or S1S1 stimulus were performed to determine ventricular effective refractory period (VERP) or inducibility of ventricular tachycardia, respectively..
In vivo ischemia and reperfusion experimental protocols[27]
Mice were intubated and ventilated by a mouse ventilator (Harvard Apparatus, Germany) with isoflurane, oxygen and air (respiration frequency 100 strokes/min). After electrophysiological recordings, a left thoracotomy was performed and the left anterior descending (LAD) artery was occluded with an Ethicon 8.0-silk suture (Ethicon, Inc.) at the site of 2–3mm from the tip of the left atrium. Successful coronary occlusion was verified by the development of a pale color in the distal myocardium through the use of a Surgical Microscope system (Applied Fiberoptics, Southbride, Massachusetts) and by T wave or ST elevation and QRS widening on the ECG. A 25-minute ischemia followed bywith releasing the ligature (reperfusion). Successful reperfusion was confirmed when the bright red color of the left ventricle and the ECG returned close to normal. ECG and ICEP were continuously monitored and recorded during the entire experimental protocols of control, ischemia, and reperfusion. The results shown in Table 1 are from recordings of under control (right before LAD occlusion), ischemia (right after the LAD occlusion to mimic AMI), and successful reperfusion (after releasing the occlusion when the left ventricle turned to bright red color again and the ECG (ST elevation) returned close to normal level
Table 1.
Parameters of surface electrocardiography (ECG) in age-matched (20 weeks old) Clcn3+/+ mice1 and hsCLC-3OE mice2 under control and myocardial ischemia and reperfusion conditions.
| Clcn3+/+ (n=8) | hsCLC-3OE (n=6) | |||||
|---|---|---|---|---|---|---|
| Control | Ischemia | Reperfusion | Control | Ischemia | Reperfusion | |
| ECG (Lead I) |  |
 |
 |
 |
 |
 |
| HR (bpm) | 400.9±0.9 | 379.3±7.4 | 293.2±16.3 | 403.4±1.5 | 427.10±20.6 | 338.6±45.2 |
| P (ms) | 11.3±0.7 | 11.1±0.7 | 13.1±0.6 | 10.2±0.5 | 8.7±0.8 | 10.0±0.8* |
| PR(ms) | 46.0±1.7 | 52.0±3.7 | 69.3±3.2†††, ‡ | 43.5±0.9 | 42.2±2.0 | 48.6±1.8**, † |
| R-R (ms) | 149.7±0.4 | 158.6±3.1 | 206.5±11.1 | 148.7±0.5 | 142.2±7.1 | 185.9±22.1 |
| QRS(ms) | 11.3±0.3 | 13.5±1.6 | 14.0±1.0†† | 10.2±0.6 | 12.3±1.2 | 11.5±0.8 |
| QT (ms) | 26.5±1.2 | 62.4±2.9††† | 33.6±1.1††, ‡‡‡ | 21.7±1.1* | 45.9±4.6**, †† | 23.2±1.4***, ‡‡ |
| QTc (ms) | 21.7±1.0 | 49.7±2.6††† | 23.4±0.7‡‡‡ | 17.8±0.9* | 38.5±3.9*, †† | 17.1±0.7***, ‡‡ |
HR, heart rate; bpm, beats per minute (min).
Clcn3+/+ mice: body weight 32.5±0.9 g;
hsCLC-3OE mice: body weight 29.80±2.38 g;
P<0.05,
P<0.01,
P<0.001vs Clcn3+/+ group;
P<0.05,
P<0.01,
P<0.001 vs control in the same group;
P<0.05,
P<0.01,
P<0.001 vs ischemia in the same group
Statistical analyses
Data were expressed as the means ± standard error (SE). Statistical comparisons were performed either by Student's t test when only two groups were compared or by analysis of variance (ANOVA) with Scheffé contrasts for group data. A two-tailed probability (P) of ≤0.05 is considered statistically significant.
Results
Surface ECG recordings in Clcn3+/+ and hsCLC-3OE mice under control, myocardial ischemia and reperfusion conditions
The molecular and functional overexpression of hsCLC-3 in the heart of hsCLC-3OE mice were confirmed and characterized previously at both molecular and cellular levels by Northern blot, Western analysis, and whole-cell patch-clamp recordings[14]. The hsCLC-3 proteins expressed in the hsCLC-3OE mouse heart were about 1.65 more than in the Clcn3+/+ mouse heart, and correspondingly, the whole-cell VSOAC current density in the hsCLC-3OE cardiac myocytes was 2-fold larger than that of Clcn3+/+ cardiac myocytes under hypotonic cell-swelling conditions. Table 1 summarizes the in vivo electrophysiological phenotypes of the hsCLC-3OE and Clcn3+/+ mice. Under control conditions, although no significant changes in many ECG parameters were observed between hsCLC-3OE and Clcn3+/+ mice, QT of hsCLC-3OE mice was significantly shorter than that of Clcn3+/+ mice (P<0.05). When the heart rate extremes were corrected[27–29] the QTc of hsCLC-3OE mice was also significantly shorter than that of Clcn3+/+ mice (P<0.05).. These results are consistent with our previous preliminary observation of QT shortening in the hsCLC-3OE mice[14].
Myocardial ischemia caused a significant prolongation of QT (P<0.001) and QTc (P<0.001) in the Clcn3+/+ mice. In the hsCLC-3OE mice, however, the ischemia-caused prolongation of both QT and QTc was significantly less than in the Clcn3+/+ mice (Table 1), suggesting that up-regulation of CLC-3 in cardiac myocaytes may limit the ischemia-induced QT prolongation.
Myocardial reperfusion significantly prolonged PR interval (P<0.05) but shortened QT and QTc (P<0.001) in Clcn3+/+ mice, although the QT was still longer than that under control condition. In hsCLC-3OE mice, reperfusion also shortened the QT (P<0.001) and QTc (P<0.001). Both QT and QTc of the hsCLC-3OE mice were significantly (P<0.001) shorter than those of Clcn3+/+ mice under reperfusion conditions and completely recovered to the level of control conditions. The PR interval of the hsCLC-3OE mice was also prolonged during reperfusion (P<0.05). But the P wave and PR interval of the hsCLC-3OE mice were significantly shorter than the P wave (P<0.05) and PR interval (P<0.01) of Clcn3+/+ mice under reperfusion conditions.
Intracardiac Electrophysiology recordings in Clcn3+/+ and hsCLC-3OE mice under control, myocardial ischemia and reperfusion conditions
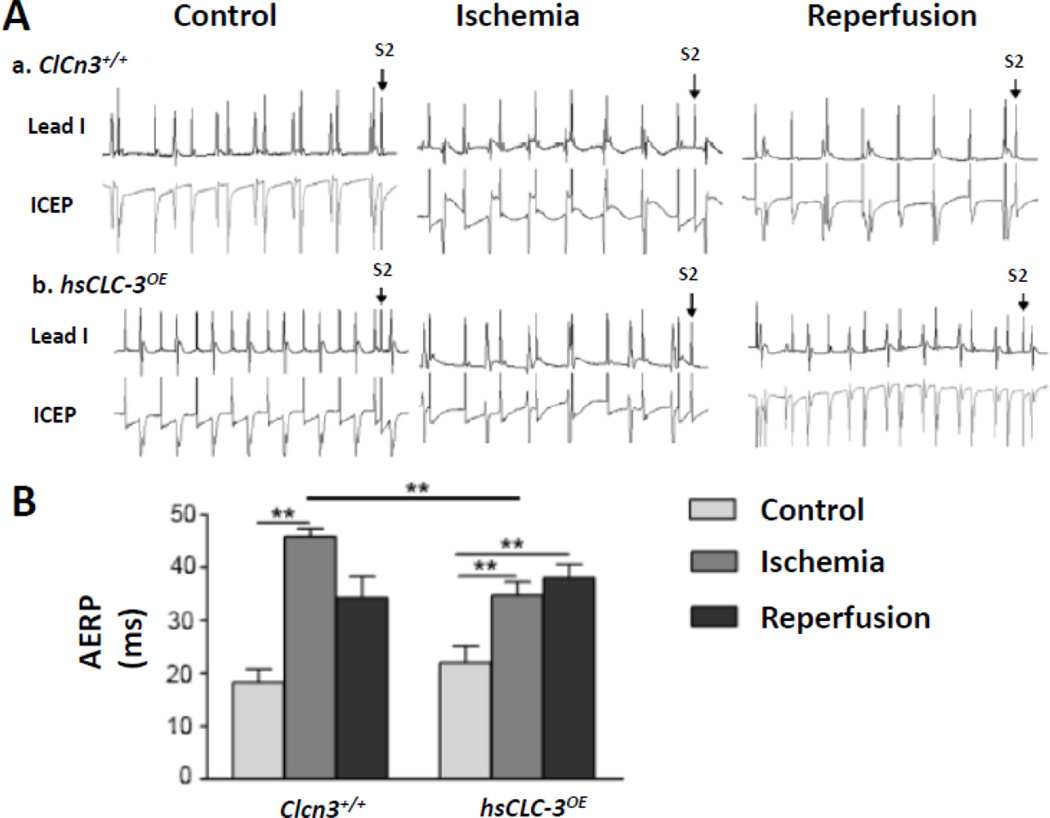
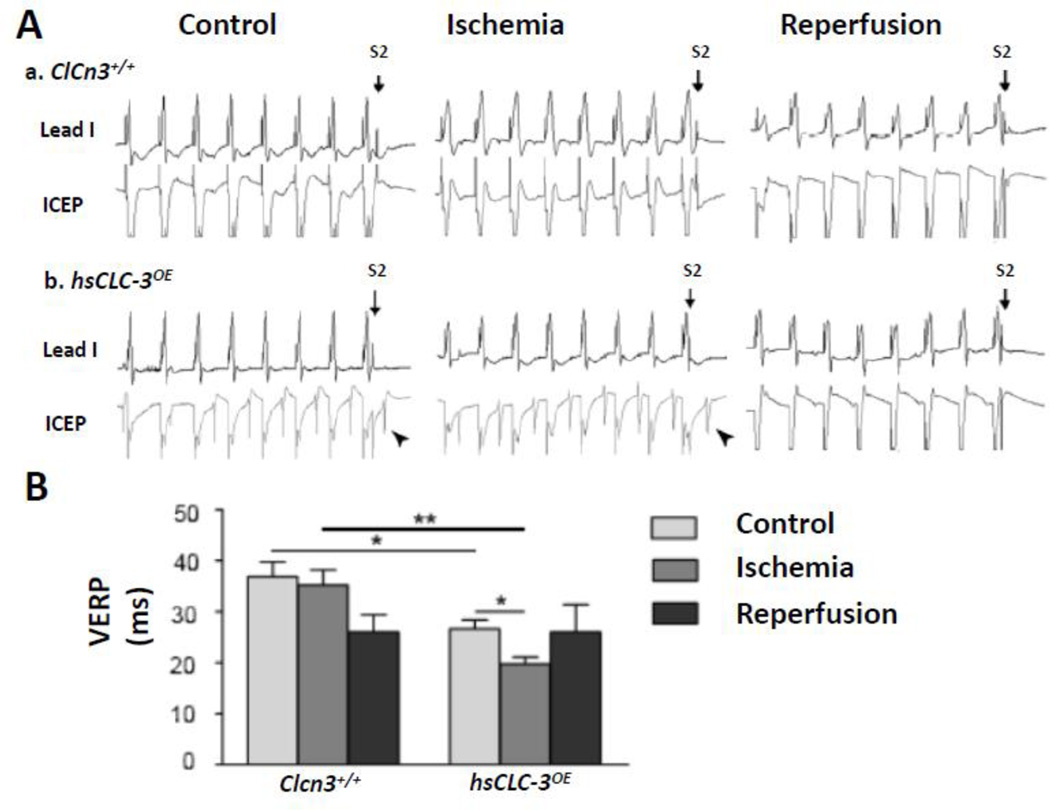
As shown in Figure 1 and 2, under control conditions, the VERP of hsCLC-3OE mice (26.7±1.7 ms, n=6) was significantly shorter than that of Clcn3+/+ mice (36.9±2.8 ms, n=8, P>0.05), while no significant difference in AERP between hsCLC-3OE and Clcn3+/+ mice (22.0±3.2 vs 18.3±2.4 ms, P>0.05) was observed. The shortening of VERP was consistent with the shortening of QT and QTc intervals observed in the surface ECG recordings as described above.
Figure 1. Comparison of atrial effective refractory periods (AERPs) in Clcn3+/+ and hsCLC-3OE mice under control, myocardial ischemia and reperfusion conditions.
A. Representative surface ECG (Lead I) and intracardiac electrophysiology (ICEP) recordings of AERPs in Clcn3+/+ (a) and hsCLC-3OE (b) mice under control, myocardial ischemia and reperfusion conditions. B. Summary of AERPsunder control and myocardial ischemia and reperfusion conditions in Clcn3+/+ and hsCLC-3OE mice. * P<0.05, ** P<0.01
Figure 2. Comparison of ventricular effective refractory periods (VERPs) in Clcn3+/+ and hsCLC-3OE mice under control, myocardial ischemia and reperfusion conditions.
A. Representative surface ECG(Lead I)and intracardiac electrophysiology (ICEP) recordings of VERPs in Clcn3+/+ (a) and hsCLC-3OE (b) mice under control, myocardial ischemia and reperfusion conditions. B. Summary of VERPsunder control and myocardial ischemia and reperfusion conditions in Clcn3+/+ and hsCLC-3OE mice. * P<0.05, ** P<0.01 (oblique arrow-heads pointed to A waves in ICEP recordings)
Myocardial ischemia caused a significant prolongation of AERP from 18.3±2.4 ms to 45.8±1.6 ms (n=8, P<0.01) but no significant changes in VERP (36.9±2.8 vs 35.2±3.0 ms, n=8, P>0.05) in the Clcn3+/+ mice. Interestingly, ischemia caused a significantly less prolongation of AERP (from 22.0±3.2 ms to 34.8±2.5 ms, n=6, P<0.01) and a marked shortening of VERP from 26.7±1.7 ms to 19.8±1.3 ms (n=6, P<0.05) in the hsCLC-3OE mice. Nevertheless, overexpression of CLC-3 in the hsCLC-3OE mouse heart significantly shortened both AERP (34.8±2.5 ms, n=6, P<0.01) and VERP (19.8±1.3 ms, n=6, P<0.05) when compared to those of Clcn3+/+ mice (45.8±1.6 ms and 35.2±3.0 ms, n=8, respectively) under ischemia conditions (Figure 1 and 2), implicating a significant role of CLC-3/VSOACs in the regulation of both AERP and VERP in the ischemic heart.
Myocardial reperfusion of the LV caused no significant changes in the prolonged AERP (45.8±1.6 ms, n=8, vs 34.3±4.1 ms, n=4, P>0.05) and VERP from 35.2±3.0 ms (n=8) to 26.0±3.4 ms (n=4, P>0.05) in the Clcn3+/+ mice. The AERP in hsCLC-3OE mice during myocardial reperfusion remained no change from that during ischemia (38.0±2.6 vs 34.8±2.5 ms, P>0.05) while the VERP during LV reperfusion recovered to the similar level as that under control conditions (26.0±5.4 vs 26.7±1.7 ms, n=6, P>0.05).
It is noteworthy that the changes of AERP were different from those of VERP in the hsCLC-3OE mice during LV ischemia and reperfusion. This can be explained by the differential activation of CLC-3/VSOACs in atria and ventricles. It may also be due to the effects of activation of CLC-3/VSOACs on the conduction in atrioventricular node (AVN). We therefore further examined the role of CLC-3 in the advancements of Wenckebach and 2:1 atrioventricular block point (AVBP) under ischemia and reperfusion conditions (Figure 3). In Clcn3+/+ mice advancements of Wenckebach AVBP occurred during myocardial reperfusion (125.0±2.9 ms vs 94.3±4.0 under control, P<0.01; and 102.0±2.0 ms during ischemia, P<0.05) but not during ischemia (vs 94.3±4.0 under control, P<0.01) and reperfusion (125.0±2.9 ms, P<0.05 vs ischemia). However, no significant differences in Wenckebach AVBP under all three conditions were observed in the hsCLC-3OE mice. Advancements of 2:1 AVBP occurred also during myocardial reperfusion in Clcn3+/+ mice (100.0±1.8 ms vs 74.3±4.0 ms under control, P<0.01; and 84.1±2.8msduring ischemia, P<0.01) and hsCLC-3OE mice (91.3±5.9 ms vs 73.8±3.2 ms under control, P<0.05; and 71.3±3.8 ms during ischemia, P<0.05). However, there were no differences in either Wenckebach AVBP or 2:1 AVBP between control and ischemia conditions in the Clcn3+/+ mice and the hsCLC-3OE mice (Figure 3). Interestingly, under ischemia conditions, Wenckebach AVBP (91.1±4.1 ms) and 2:1 AVBP (71.3±3.8 ms) of hsCLC-3OE mice were significantly shorter than those of Clcn3+/+ mice (102.0±2.0 and 84.1±2.8 ms, respectively, P<0.05). These results suggested that activation of CLC-3 channels in the AVN cells might cause a shortening of the repolarization duration of AVN during myocardial ischemia and reperfusion (Figure 3).
Figure 3. Comparison of Wenckebach and 2:1 block points of atrioventricular node (AVN) in Clcn3+/+ and hsCLC-3OE mice under control, myocardial ischemia and reperfusion conditions.
A. Summary of Wenckebach points of AVN under control, myocardial ischemia and reperfusion conditions in Clcn3+/+ and hsCLC-3OE mice.
B. Summary of 2:1 block points of AVN under control, myocardial ischemia and reperfusion conditions in Clcn3+/+ and hsCLC-3OE mice. * P<0.05, ** P<0.01
Discussion
Since the first discovery of cardiac Cl− currents in 1961 the functional role of Cl− channels in the cardiac electrophysiology in the context of health and ischemic cardiac disease has remained a mystery [22]. In this study, for the first time in literature, we specifically characterized and determined the function of CLC-3 Cl− channels in the regulation of cardiac electrophysiology under control, myocardial ischemia and reperfusion conditions by using the unique hsCLC-3OE mice. We made the following novel findings: 1) Under control conditions, heart-specific overexpression of hsCLC-3 significantly shortened QT, QTc, and VERP; 2) Under ischemia conditions heart-specific overexpression of hsCLC-3 limited the myocardial ischemia-induced prolongation of PR, QT, QTc, AERP, and VERP and significantly delayed Wenckebach point and 2:1 block point in AVN of the hsCLC-3OE mice; 3) Under reperfusion conditions, heart-specific overexpression of hsCLC-3 facilitated the complete recovery of QT, QTc, and PR from ischemia-induced changes and prevented advancements of Wenckebach point in AVN o during myocardial ischemia and reperfusion. These results provided direct experimental evidence that upregulation of cardiac CLC-3/VSOACs may play important functional roles in the in vivo cardiac electrophysiology under ischemia and reperfusion conditions.
Mechanisms for the functional role of CLC-3/VSOACs in cardiac electrophysiology
Previous studies have reported that, depending on actual [Cl−]i and aiCl, ECl in cardiac myocytes is between −65 to −40 mV and within a membrane potential range more positive than the RMP[18–21]. Under physiological conditions the Cl− gradients across cardiac myocytes are asymmetric and activation of CLC-3/VSOACs generates an outwardly rectifying current during the action potential[15–17]. Therefore, activation of CLC-3/VSOACs may cause 1) an acceleration of repolarization and significant abbreviation of APD due to a large outward current (Cl− influx) at membrane potentials more positive than ECl, and 2) a slight depolarization at membrane potentials of more negative than ECl and RMP by conducting a smaller inward current (Cl− efflux)[17, 22]. Previously attempts to define the electrophysiological role of the VSOACs in the heart has been limited at the ex vivo or cellular level [15, 30–33]. But, the lack of specific activators or blockers of the native VSOACs has seriously precluded these studies from obtaining any conclusive evidence for the function of VSOAC activation in cardiac electrophysiology in vivo[15, 22]. The newly developed hsCLC-3OE mice with heart-specific overexpression of hsCLC-3[14] provided us with a unique animal model to specifically address the question about the in vivo electrophysiological function of CLC-3/VSOACs in the heart. In the current study we further compared not only the QT interval but also the QTc between hsCLC-3OE mice and Clcn3+/+ mice and found that both basic QT and QTc of hsCLC-3OE mice were significantly shorter than those of Clcn3+/+ mice. These results are consistent with previous findings [14]. The increased CLC-3 protein expression and cell swelling-induced whole-cell VSOACs in the hsCLC-3OE heart may explain the shortened QT and ERP observed in these mice under basal control condition and ischemia/reperfusion conditions [14, 15, 22]. Similarly, APD shortening (and slight RMP depolarization) due to the increased VSOACs may be responsible for the shortening of AVRP and VERP observed in the hsCLC-3OE mice.
Role of CLC-3/VSOACs in ischemia/reperfusion-induced cardiac arrhythmias
AMI-caused QT shortening or progressive prolongation may induce life-threatening arrhythmias and sudden cardiac death[1–5]. Previous studies on the mechanisms for ischemia-induced QT changes have extensively focused on Na+, K+, Ca2+, and nonspecific cation channels and transporters[1, 3]. Inhibition of outward repolarizing K+ currents by hypoxia, metabolic blockade, and oxidative stress during AMI has been attributed to the major mechanisms for APD and QT prolongation[1–3]. Activation of the ATP-sensitive K+ current (IKATP) under these conditions due to a fall in ATP/ADP ratio has been thought as the major mechanism for the marked APD shortening after an initial APD lengthening. Here, we provided the first direct in vivo evidence that anion channels may also contribute to the AMI-induced changes in ERP and QT intervals.
The basal Cl− current through the CLC-3/VSOACs under physiological conditions is usually small but CLC-3/VSOACs can be further activated by hypoosmotic cell swelling and/or mechanical stretch of cell membrane β1-integrin[24, 33–37]. Hypoxia and ischemia cause intracellular hyperosmosis and cardiac myocytes swelling and the washout of hyperosmotic extracellular fluid after reperfusion induce further cell swelling. Cells in the ischemic zone may also elongate in the longitudinal dimension during active contraction of viable cells surrounding the infarcted zone. Therefore, it is expected that activity of CLC-3/VSOACs may be upregulated and thus contribute more prominently to electrical remodeling under conditions of hypoxia, ischemia and reperfusion[8, 12, 13, 15]. Our previous studies with the CLC-3 knockout (Clcn3−/−) have demonstrated that although targeted disruption of Clcn3 did not cause changes in basic hemodynamic performance and electrophysiological properties it abolished the second-window IPC induced protection of myocardium against infarction and prolonged QT/QTc of Clcn3−/− mice under stressed conditions[8, 14]. In the current study, we used the hsCLC-3OE mice to further directly investigate the role of CLC-3/VSOACs on the in vivo cardiac electrophysiology during ischemia and reperfusion. We found that the ischemia-induced QT/QTC prolongation in the hsCLC-3OE mice was significantly shorter than that in the Clcn3+/+ mice, and reperfusion caused a full recovery of the prolonged QT (QTc) in the hsCLC-3OE mice but only a partial recovery in the Clcn3+/+ mice, indicating that upregulation of CLC-3 activity in the hearts may limit the ischemia-induced QT/QTc prolongation under myocardial ischemia and reperfusion conditions. Thus, activation of CLC-3/VSOACs may be a major adaptive mechanism for compensating the AMI-induced APD and QT prolongation[1–3].
AMI also causes ERP prolongation and it was explained by the slowed recovery of Na+ channels from inactivation secondary to the depolarization and the short-circuiting effect of the opening of K+ channels[2, 3]. In the current study, we found that overexpression of hsClC-3 in the heart lead to a 92% reduction of AERP prolongation in the hsCLC-3OE mice compared to that in the Clcn3+/+ mice and ischemia caused a 150% ERP prolongation in Clcn3+/+ mice but only a 58% ERP prolongation in hsCLC-3OE mice. These results suggest that up-regulation of CLC-3/VSOACs (both overexpression and increased activation of hsCLC-3) Cl− channels under ischemia condition may also contribute to the accelerated repolarization and shortened AERP and VERP.
We also found that LV ischemia and reperfusion caused different changes in AERP and VERP of the hsCLC-3OE mice. This difference may reflect the differential activation of the CLC-3/VSOACs in the atria and ventricles possibly due to the regional differences in actual [Cl−]i and aiCl and the well-known regional changes in membrane potentials and action potential waveforms in the heart [18–21]. Since we were using the coronary artery ligation model, ischemia and reperfusion may cause cell swelling in the affected region of LV myocardium and activation of more CLC-3/VSOACs in ventricles than in the atria. It is reported that severe ischemia could lead to loss of glycogen granules, mitochondrial swelling, and dilatation of sarcoplasmic reticulum vesicles, clumping of nuclear chromatin and ultimately varying degrees of cell swelling in atrioventricular junction. These changes progressed faster in nodal cells than alteration in His bundle/bundle branch cells[38]. Nisbet et al. found that low pH could prolong the atrial Hisian (AH) interval, the AVN effective and functional refractory periods and Wenckebach cycle length significantly in isolated Langendorff-perfused rabbit hearts[39]. However, as far as we have known, few experiments studied the changes of electrophysiological properties in AVN during myocardial ischemia and reperfusion in vivo. Prolongation of AH interval, prolongation of ERP in AVN, and the advancements of Wenckebach and 2:1 block points in AVN implied the abnormality of the AVN function through different aspects. In the current study, we found that Wenckebach AVBP and 2:1 AVBP of hsCLC-3OE mice under ischemia conditions were significantly shorter than those of Clcn3+/+ mice. Overexpression of hsClC-3 channels diminished the advancement of Wenckebach block point but not the advancement of 2:1 block point under reperfusion conditions in hsCLC-3OE mice. These results suggest that up-regulation of CLC-3 channels in the AVN cells might cause a shortening of the repolarization duration of AVN during myocardial ischemia and reperfusion (Figure 3).
Significance and Clinical Relevance Perspective
AMI-caused progressive prolongation of ERP, QT and QTc is one of the major causes of life-threatening arrhythmias and sudden death[1–5]. Treatment of ischemia/reperfusion-induced arrhythmias has been a major challenge owing to the impartial understanding of the molecular mechanisms for the ionic remodeling of the heart in response to myocardial ischemia/reperfusion[1, 3, 4]. The lack of understanding whether and how the APD and QT interval are regulated by Cl− channels may contribute to the ineffective therapy of the life-threatening AMI-induced cardiac arrhythmias. The current study provided novel and compelling evidence for the functional role of the unique CLC-3/VSOACs, which are significantly upregulated during ischemia, in the protection of the heart under stress[15]. Understanding of remodeling of CLC-3/VSOACs may provide new insights into the mechanism for ischemia-induced electrical remodeling and valuable information for more complete understanding of the mechanisms for cardiac electrophysiology in the context of health and disease involving myocardial ischemia and reperfusion. Therefore, these results are of significant importance by providing new therapeutic targets and approaches to the treatment of AMI-induced arrhythmias.
Limitation of the current study
It should be kept in mind, however, the integrated function of Cl− channels may involve multiprotein complexes of the Cl− channel subproteome[22, 40]. Therefore, the current study has the limitations as many other transgenic mouse models [40] that overexpression of CLC-3 in the mouse heart may cause changes in expression and function of other Cl− channels or cation channels, and the consequences of cardiac remodeling on electrophysiology. During ischemia, [Cl−]i can drastically decrease and cause a significant shift in the Em.Cl toward more negative potentials which substantially affects the function of every type of Cl− channels in the heart. CLC-3/VSOACs are also expressed in coronary arteries which may significantly contribute to the changes seen in ischemia-reperfusion. Further phenomic studies are required to systematically unravel the mechanisms for the ischemia/reperfusion-induced changes in the dynamic interactions between the subproteomes of Cl− channels and many other cation channels as well as regulatory proteins[22].
Acknowledgments
Funding Sources: This study was supported by National Heart, Lung, and Blood Institute Grant R01 #HL63914, #HL113598, R21 HL106252, AHA Western State Affiliate Grant-in-Aid #11GRNT7610161 (to D.D.D.), and National Natural Science Foundation of China (#81070154 and #81270258 to Y.G.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
Disclosure: None.
Author contributions: Dayue Darrel Duan and Yi-Gang Li contributed to the conceptual framework of the study. Ying Yu participated in experimental design, data collection and data analysis. Lingyu Linda Ye provided technical support and participated in data collection and analysis. Dean Burking designed and created the hsCLC-3OE mice. Ying Yu drafted the manuscript and all investigators contributed to critical revision of the article.
References
- 1.Antzelevitch C. Ionic, molecular, and cellular bases of QT-interval prolongation and torsade de pointes. Europace. 2007;9(Suppl 4):iv4–iv15. doi: 10.1093/europace/eum166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anumonwo JM, Pandit SV. Ionic mechanisms of arrhythmogenesis. Trends Cardiovasc Med. 2015 doi: 10.1016/j.tcm.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- 4.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasanien AA, Drew BJ, Howie-Esquivel J. Prevalence and prognostic significance of long QT interval in patients with acute coronary syndrome: review of the literature. J Cardiovasc Nurs. 2014;29:271–279. doi: 10.1097/JCN.0b013e31829bcf1a. [DOI] [PubMed] [Google Scholar]
- 6.Steenbergen C, Hill ML, Jennings RB. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ Res. 1985;57:864–875. doi: 10.1161/01.res.57.6.864. [DOI] [PubMed] [Google Scholar]
- 7.Vander Heide RS, Rim D, Hohl CM, Ganote CE. An in vitro model of myocardial ischemia utilizing isolated adult rat myocytes. J Mol Cell Cardiol. 1990;22:165–181. doi: 10.1016/0022-2828(90)91113-l. [DOI] [PubMed] [Google Scholar]
- 8.Bozeat ND, Xiang SY, Ye LL, Yao TY, Duan ML, Burkin DJ, Lamb FS, Duan DD. Activation of volume regulated chloride channels protects myocardium from ischemia/reperfusion damage in second-window ischemic preconditioning. Cell Physiol Biochem. 2011;28:1265–1278. doi: 10.1159/000335858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26:613S–623S. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- 10.Wang GL, Wang GX, Yamamoto S, Ye L, Baxter H, Hume JR, Duan D. Molecular mechanisms of regulation of fast-inactivating voltage-dependent transient outward K+ current in mouse heart by cell volume changes. J Physiol. 2005;568:423–443. doi: 10.1113/jphysiol.2005.091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz RJ, Armstrong SC, Batthish M, Backx PH, Ganote CE, Wilson GJ. Enhanced cell volume regulation: a key protective mechanism of ischemic preconditioning in rabbit ventricular myocytes. J Mol Cell Cardiol. 2003;35:45–58. doi: 10.1016/s0022-2828(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 12.Diaz RJ, Hinek A, Wilson GJ. Direct evidence of chloride ion efflux in ischaemic and pharmacological preconditioning of cultured cardiomyocytes. Cardiovasc Res. 2010;87:545–551. doi: 10.1093/cvr/cvq084. [DOI] [PubMed] [Google Scholar]
- 13.Diaz RJ, Harvey K, Boloorchi A, Hossain T, Hinek A, Backx PH, Wilson GJ. Enhanced cell volume regulation: a key mechanism in local and remote ischemic preconditioning. Am J Physiol Cell Physiol. 2014;306:C1191–C1199. doi: 10.1152/ajpcell.00259.2013. [DOI] [PubMed] [Google Scholar]
- 14.Xiong D, Wang GX, Burkin DJ, Yamboliev IA, Singer CA, Rawat S, Scowen P, Evans R, Ye L, Hatton WJ, Tian H, Keller PS, McCloskey DT, Duan D, Hume JR. Cardiac-specific overexpression of the human short CLC-3 chloride channel isoform in mice. Clin Exp Pharmacol Physiol. 2009;36:386–393. doi: 10.1111/j.1440-1681.2008.05069.x. [DOI] [PubMed] [Google Scholar]
- 15.Duan DD. The ClC-3 chloride channels in cardiovascular disease. Acta Pharmacol Sin. 2011;32:675–684. doi: 10.1038/aps.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan DY, Liu LL, Bozeat N, Huang ZM, Xiang SY, Wang GL, Ye L, Hume JR. Functional role of anion channels in cardiac diseases. Acta Pharmacol Sin. 2005;26:265–278. doi: 10.1111/j.1745-7254.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 17.Yu Y, Zhang H, Li Y-G, Zhang Y, Duan DD. Chloride channels and cardiac arrhythmia: novel therapeutic targets? European Pharmaceutical Review. 2012;17:8–13. [Google Scholar]
- 18.Baumgarten CM, Fozzard HA. Intracellular chloride activity in mammalian ventricular muscle. Am J Physiol. 1981;241:C121–C129. doi: 10.1152/ajpcell.1981.241.3.C121. [DOI] [PubMed] [Google Scholar]
- 19.Caille JP, Ruiz-Ceretti E, Schanne OF. Intracellular chloride activity in rabbit papillary muscle: effect of ouabain. Am J Physiol. 1981;240:C183–C188. doi: 10.1152/ajpcell.1981.240.5.C183. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer KW, Walker JL. Intracellular chloride activity in quiescent cat papillary muscle. Am J Physiol. 1980;238:H487–H493. doi: 10.1152/ajpheart.1980.238.4.H487. [DOI] [PubMed] [Google Scholar]
- 21.Vaughan-Jones RD. Non-passive chloride distribution in mammalian heart muscle: micro-electrode measurement of the intracellular chloride activity. J Physiol (Lond) 1979;295:83–109. doi: 10.1113/jphysiol.1979.sp012956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan DD. Phenomics of cardiac chloride channels. Compr Physiol. 2013;3:667–692. doi: 10.1002/cphy.c110014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Wehrens XH. Programmed electrical stimulation in mice. J Vis Exp. 2010 doi: 10.3791/1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- 25.Duan D, Cowley S, Horowitz B, Hume JR. A serine residue in ClC-3 links phosphorylation-dephosphorylation to chloride channel regulation by cell volume. J Gen Physiol. 1999;113:57–70. doi: 10.1085/jgp.113.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang P, Liu J, Di A, Robinson NC, Musch MW, Kaetzel MA, Nelson DJ. Regulation of human CLC-3 channels by multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 2001;276:20093–20100. doi: 10.1074/jbc.M009376200. [DOI] [PubMed] [Google Scholar]
- 27.Xiang SY, Ye LL, Duan LL, Liu LH, Ge ZD, Auchampach JA, Gross GJ, Duan DD. Characterization of a critical role for CFTR chloride channels in cardioprotection against ischemia/reperfusion injury. Acta Pharmacol Sin. 2011;32:824–833. doi: 10.1038/aps.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang ZM, Prasad C, Britton FC, Ye LL, Hatton WJ, Duan D. Functional role of CLC-2 chloride inward rectifier channels in cardiac sinoatrial nodal pacemaker cells. J Mol Cell Cardiol. 2009;47:121–132. doi: 10.1016/j.yjmcc.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274:H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 30.Decher N, Lang HJ, Nilius B, Bruggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of I(Cl,swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol. 2001;134:1467–1479. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du XY, Sorota S. Cardiac swelling-induced chloride current depolarizes canine atrial myocytes. Am J Physiol. 1997;272:H1904–H1916. doi: 10.1152/ajpheart.1997.272.4.H1904. [DOI] [PubMed] [Google Scholar]
- 32.Hiraoka M, Kawano S, Hirano Y, Furukawa T. Role of cardiac chloride currents in changes in action potential characteristics and arrhythmias. Cardiovasc Res. 1998;40:23–33. doi: 10.1016/s0008-6363(98)00173-4. [DOI] [PubMed] [Google Scholar]
- 33.Vandenberg JI, Bett GC, Powell T. Contribution of a swelling-activated chloride current to changes in the cardiac action potential. Am J Physiol. 1997;273:C541–C547. doi: 10.1152/ajpcell.1997.273.2.C541. [DOI] [PubMed] [Google Scholar]
- 34.Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol. 2003;82:25–42. doi: 10.1016/s0079-6107(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 35.Browe DM, Baumgarten CM. Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J Gen Physiol. 2003;122:689–702. doi: 10.1085/jgp.200308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan D, Hume JR, Nattel S. Evidence that outwardly rectifying Cl-channels underlie volume-regulated Cl-currents in heart. Circ Res. 1997;80:103–113. doi: 10.1161/01.res.80.1.103. [DOI] [PubMed] [Google Scholar]
- 37.Vandenberg JI, Rees SA, Wright AR, Powell T. Cell swelling and ion transport pathways in cardiac myocytes. Cardiovasc Res. 1996;32:85–97. [PubMed] [Google Scholar]
- 38.Armiger LC, Knell CM. Fine structural alteration in the atrioventricular junctional conduction tissues of the dog heart during severe ischaemia. J Submicrosc Cytol Pathol. 1988;20:645–656. [PubMed] [Google Scholar]
- 39.Nisbet AM, Burton FL, Walker NL, Craig MA, Cheng H, Hancox JC, Orchard CH, Smith GL. Acidosis slows electrical conduction through the atrio-ventricular node. Front Physiol. 2014;5:233. doi: 10.3389/fphys.2014.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis J, Maillet M, Miano JM, Molkentin JD. Lost in transgenesis: a user's guide for genetically manipulating the mouse in cardiac research. Circ Res. 2012;111:761–777. doi: 10.1161/CIRCRESAHA.111.262717. [DOI] [PMC free article] [PubMed] [Google Scholar]