Abstract
Background
Trials demonstrating the efficacy of biologic therapy for moderate to severe hidradenitis suppurativa (HS) have inspired new multidisciplinary treatment strategies. We present our experience with combined biologic and surgical therapy for recalcitrant HS.
Methods
Between 2011 and 2014, 21 patients (57 cases) with Hurley Stage III HS underwent radical resection with delayed primary closure alone, or in combination with adjuvant biologic therapy. Demographic data, treatment regimen, outcomes, and complications were retrospectively reviewed for all cases.
Results
Eleven patients underwent combined surgical and biologic therapy, whereas radical resection alone was performed in 10 patients. The average soft tissue deficit, before closure, for the combined and surgery-only patients was 56 cm2 and 48.5 cm2, respectively (P = 0.66). Biologic agents including infliximab (n = 8) and ustekinumab (n = 3) were initiated 2 to 3 weeks after closure and were continued for an average of 10.5 months. Recurrence was noted in 19% (4/29) and 38.5% (10/26) of previously treated sites for combined and surgery-only patients (P < 0.01). For the combined cohort, the disease-free interval was approximately 1 year longer on average (P < 0.001); however, this difference was reduced to 4.5 months when considering time to recurrence after cessation of biologic therapy (P = 0.09). New disease developed in 18% (2/11) and 50% (5/10) of combined and surgery-only patients, respectively (P < 001). No adverse events were noted among patients who received biologic therapy.
Conclusions
Lower rates of recurrence and disease progression, as well as a longer disease-free interval may be achieved with the use of adjuvant biologic therapy after radical resection for recalcitrant HS.
Keywords: biologic therapy, hidradenitis suppurativa, infliximab, radical resection, recurrence, ustekinumab
Hidradenitis suppurativa (HS) is a chronic, debilitating inflammatory disease of apocrine-bearing skin that affects 1% to 4% of the population.1,2 Patients with this condition often present with painful, recurrent nodules, abscesses, and disfiguring scars along the intertriginous regions of the axillary, breast, and inguinal folds, gluteal cleft, and perineum.3 In moderate to severe cases, destruction of normal skin architecture, over time, results in the formation of interconnecting sinus tracts and fistulae with extensive dermal and subcutaneous fibrosis.4 The invariably painful, unsightly, and malodorous nature of this disease often limits physical activity and serves as a source of significant psychosocial distress, decreased work productivity, and impaired quality of life for affected individuals.5,6
Current evidence supports a complex interplay of mechanical, inflammatory, and infectious etiologies for HS.7,8 Lack of a clear pathogenic mechanism, however, has precluded the development of effective, targeted treatment strategies that consistently achieve disease improvement and/or maintain long-term remission. The Hurley clinical grading system is a useful adjunct in classifying disease severity (ie, presence of recurrence, multifocal involvement, cicatrization) and often forms a basis for the selection of appropriate treatment.9 Topical or systemic antibiotics, antiandrogens, retinoids, immunosuppressive agents, and radiation therapy have been used with variable success in treating mild to moderate disease.9,10 For regions plagued by more extensive involvement, wide local excision mandating advanced reconstruction is often necessary, depending on the location and complexity of the wound as well as the degree of contamination.11–13 Despite significant advances in medical and surgical therapies for HS, satisfaction rates with current treatment modalities tend to be low, secondary to high rates of recurrence (19–74%) and disease progression (20–25%).13–16
More recently, studies investigating the role of biologic therapy have yielded promising results for patients with refractory HS. By suppressing critical inflammatory mediators (ie, tumor necrosis factor [TNF]-α and interleukins [IL]-12/23), these agents act to prevent the uncontrolled, chronic inflammation that underlies many of the sequelae and physical manifestations of this disease. Prospective, open-label or randomized trials evaluating the efficacy and tolerability of a number of these agents have demonstrated substantial improvements in disease activity, patient satisfaction, and quality-of-life scores.17–20 Nevertheless, significant variability in study design, type and duration of therapy, and measured outcomes among reported studies has confounded interpretation of these results and limited recommendations regarding the role of biologic therapy in recalcitrant HS.
When used in combination with radical resection of grossly evident disease, the immunosuppressive effects of biologics could potentially augment the results achievable through surgical intervention alone. To date, however, there have been no trials investigating the role of adjuvant biologic therapy in reducing local recurrence and/or disease progression in patients with recalcitrant HS. At our institution, we have adopted a multidisciplinary treatment strategy that facilitates collaboration between the plastic surgeon and rheumatologist and integrates established surgical principles with therapy that targets underlying proinflammatory mechanisms that contribute to disease pathogenesis. The purpose of this retrospective study was to evaluate our early experience with adjuvant biologic therapy after wide local excision and delayed primary closure in patients with Hurley stage III HS.
PATIENTS AND METHODS
Patient Demographics
Retrospective review of medical records was performed for all consecutive patients who underwent radical resection and delayed primary closure of regions affected by advanced HS, between January 2011 and March 2014. All procedures were performed by the senior author (K.K.E.). Data were collected in accordance with an institutional review board-approved protocol for patients with Hurley stage III disease who were treated surgically, either alone or in combination with biologic therapy (Fig. 1). Radical resection was defined as excision of all hair-bearing skin within an affected region, including a clear margin of 1 cm. Patients who underwent minor drainage procedures or limited excisions, as well as those requiring more advanced reconstruction with grafts, regional flaps, or free tissue transfer were excluded. Demographic data, clinical risk factors, treatment regimen, and outcomes were documented for all cases.
FIGURE 1.
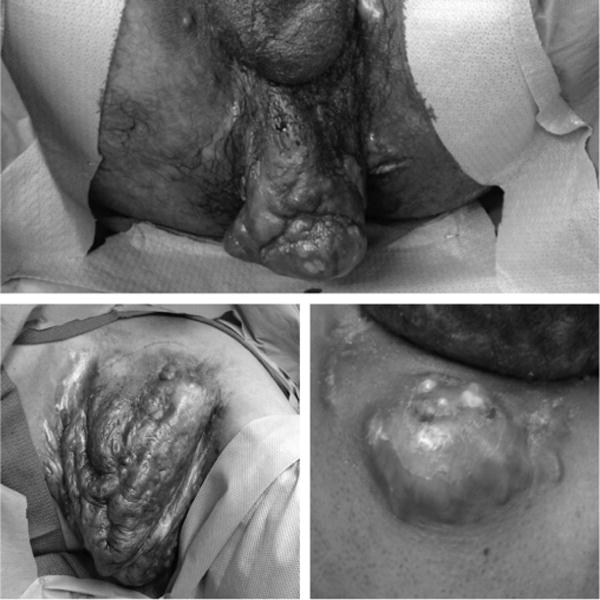
Various examples of Hurley stage III HS demonstrating extensive keloid scarring with multiple interconnected sinus tracts and fistulae. (Above) Advanced HS involving the male perineum. (Below, left) Deforming left axillary HS resulting in distortion of the normal skin architecture with extensive dermal and subcutaneous fibrosis and scarring. (Below, right) Painful HS involving the posterior neck in a young, obese male.
Therapeutic Cohorts and Combined Therapy Protocols
Patients were retrospectively divided into 2 cohorts for purposes of comparison: those treated with (1) radical resection alone or (2) in conjunction with adjuvant biologic therapy. In all cases, patients underwent serial debridement before definitive closure. Intraoperative cultures were obtained after each debridement and were used to guide sensitivity-directed antibiotic therapy as well as the timing of closure. Local wound care with either wet to dry dressing changes or vacuum-assisted closure was provided in the interim between debridements. For patients in both cohorts, the decision to close was guided by the presence of negative cultures, absence of clinical signs of infection, and adequate response to local wound care. When deemed appropriate, all wounds were closed in a delayed primary fashion using nondissolvable, vertical-mattress sutures.
Patients with Hurley stage III HS were considered potential candidates for adjuvant biologic therapy with infliximab (chimeric anti–TNF-α monoclonal antibody). Given the reportedly rare association with drug-induced lupus (0.5% to 1%),21 patients found to be antinuclear or anti-double stranded DNA antibody positive on routine laboratory testing were offered alternate therapy with ustekinumab (common p40 subunit inhibitor of IL-12/23). Allergies to either agent, active systemic infection, advanced heart failure, and/or severe immunocompromise were considered contraindications to treatment with biologics. Patients who opted for adjuvant biologic therapy began treatment 2 to 3 weeks after definitive closure under the direction of a consulting rheumatologist (V.K.S.) (Fig. 2). Infliximab was administered intravenously at 3 mg/kg on weeks 0, 2, and 6, followed by maintenance dosing (5 mg/kg) every 6 weeks. Ustekinumab was administered subcutaneously every 4 weeks, at weight-based doses of either 45 mg (≤100 kg) or 90 mg (>100 kg). Therapy was continued for a minimum duration of 6 months from the time of remission (documented wound healing) and was discontinued at the discretion of the rheumatologist (Fig. 3).
FIGURE 2.
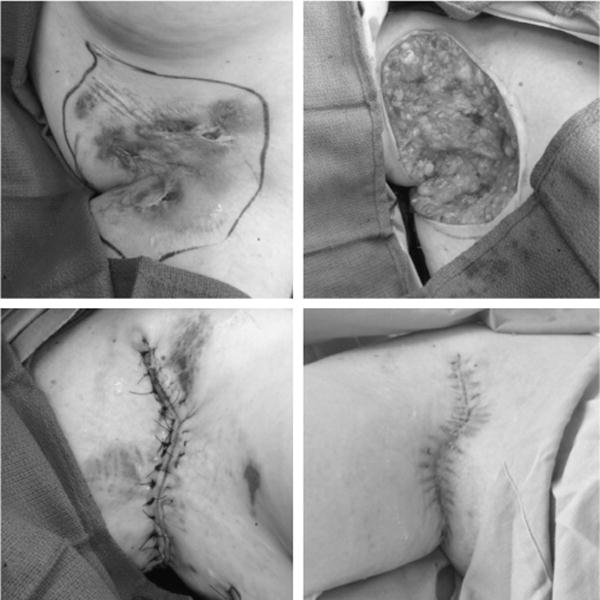
Hurley stage III HS involving the right axilla of a 46-year-old nonsmoking woman with a BMI of 31. (Above, left) Preoperative markings before wide radical excision of all grossly evident disease. (Above, right) Large soft tissue defect of the right axilla after radical resection of HS including 1-cm margins of normal-appearing tissue. The wound was temporized with a vacuum-assisted closure device in the 4-day interim between radical resection and definitive closure. (Below, left) Right axilla immediately after delayed primary closure with non-dissolvable, interrupted, vertical-mattress sutures. (Below, right) Evaluation at 2 weeks, demonstrating complete re-epithelialization of the axillary wound, without dehiscence, before the initiation of infliximab therapy. BMI indicates body mass index.
FIGURE 3.
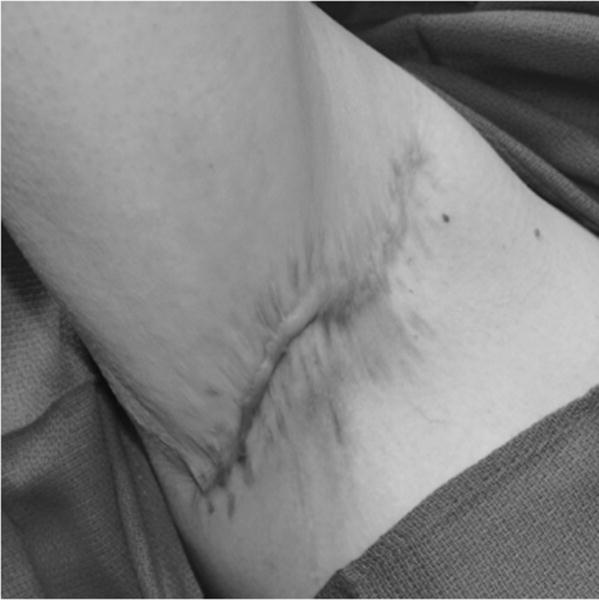
Photograph of the patient in Figure 3 at 6-month follow-up. Immediately after discontinuation of adjuvant infliximab therapy, the right axilla remains completely healed without evidence of recurrence.
Outcomes Analysis
Primary outcomes included rates and timing of disease recurrence and/or disease progression. Recurrence was defined as persistent or newly developed signs of HS appearing in previously treated locations, while the presence of lesions in previously unaffected areas was definitive for disease progression. Complications included any cause of delayed healing or operative revision, as well as any adverse events attributed to the use of biologic therapy. Baseline comparisons between the 2 cohorts were accomplished utilizing the 2-tailed, unpaired t test and the χ2 test for data containing continuous and categorical variables, respectively. A P value less than 0.05 was considered statistically significant.
RESULTS
Over a 3-year period, 21 patients (7 men and 14 women) with a mean age of 32 years (r, 18–66 years) underwent radical resection and delayed primary closure for the management of 57 cases of Hurley Stage III HS. Of these, 11 patients (29 cases of HS) underwent combined treatment with surgical resection of each affected site followed by adjuvant biologic therapy; whereas, the remaining 10 patients (26 cases of HS) underwent radical resection of each site alone. The mean body mass index of the study population was 36 kg/m2 (r, 20–56 kg/m2). Three patients (14%) were diabetic, 7 patients (33%) reported regular or occasional tobacco use, and 7 patients (33%) presented with multiple affected anatomic locations. All patients in this series had documented failure of previously attempted medical and/or minor surgical therapy. There were no significant differences in the demographic data, clinical risk factors, or location of disease between the 2 treatment groups (Table 1).
TABLE 1.
Demographic Data and Disease Characteristics for Hidradenitis Patients in the Combined and Surgical Cohorts
| Combined Cohort (n = 11) | Surgical Cohort (n = 10) | P | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age, yr | |||
| Mean (range) | 31 (19–44) | 33 (18–66) | 0.68 |
| Sex | |||
| % Male | 36 | 30 | 0.88 |
| Race (%) | |||
| Black | 5 (45) | 6 (60) | |
| White | 3 (27) | 3 (30) | |
| Hispanic | 1 (9) | 0 (0) | |
| Asian/Pacific Islander | 1 (9) | 0 (0) | |
| Other | 1 (9) | 1 (10) | |
| BMI, kg/m2 | |||
| Mean (range) | 36 (20–56) | 35.5 (21.5–50) | 0.92 |
| Comorbidities (%) | |||
| Hypertension | 4 (36) | 4 (40) | 0.26 |
| Diabetes mellitus | 1 (9) | 2 (20) | 0.73 |
| Tobacco use | 3 (27) | 4 (40) | 0.74 |
| Failed previous therapy (%) | |||
| Topical/systemic antibiotics | 11 (100) | 10 (100) | |
| Antiandrogens | 7 (64) | 6 (60) | |
| Retinoids | 1 (9) | 0 (0) | |
| Immunosuppressants/steroids | 8 (73) | 6 (60) | |
| Minor excision/drainage | 11 (100) | 10 (100) | |
| Disease characteristics | |||
| Location (%) | |||
| Axilla | 12 (41) | 13 (50) | |
| Inguinal fold | 6 (21) | 2 (8) | |
| Groin/genital/perineal | 5 (17) | 6 (23) | |
| Breast | 4 (14) | 2 (8) | |
| Other (trunk, neck, gluteal cleft) | 2 (7) | 3 (11) | |
| No. affected sites | |||
| Mean (range) | 2.6 (1–4) | 2.6 (1–5) | 0.71 |
| Duration of symptoms, yr | |||
| Mean (range) | 7.5 (2–26) | 7 (2–20) | 0.63 |
Patients in the combined and surgery-only cohorts underwent an average of 2.3 and 2.6 operative debridements, resulting in a mean soft tissue deficit, per site, of 56 cm2 and 48.5 cm2, respectively (P = 0.66). The average interval between initial debridement and delayed primary closure was approximately 4.1 days for patients in the combined cohort and 4.2 days for patients who underwent radical resection alone (P = 0.76). Of the 11 patients in the combined therapy cohort, 8 (73%) received adjuvant biological therapy with infliximab, while 3 patients (27%) underwent treatment with ustekinumab. Biologic therapy was initiated between postoperative days 14 and 20 (mean, day 16) in all cases, after removal of sutures and documentation of complete re-epithelialization. Therapy was continued for a mean duration of 10.5 months (r, 6–15 months) (Fig. 4). Four of the 11 patients (36%) remained on biologic therapy with infliximab at the time of final data collection. Patients in the combined and surgery-only cohorts were followed for an average period of 18 months (r, 6–31 months) and 20.5 months (r, 4–36 months) after radical resection and delayed primary closure, respectively (P = 0.81) (Table 2).
FIGURE 4.
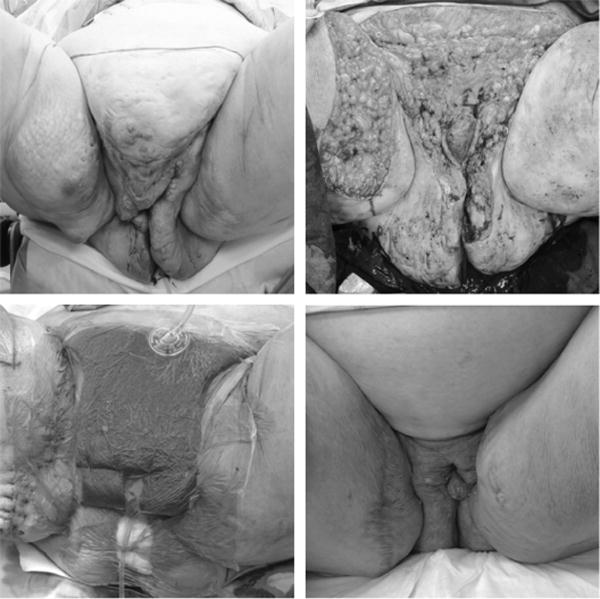
Deforming, recalcitrant Hurley stage III HS involving the perineum, vulva, and suprapubic/lower abdominal region of a 36-year-old obese woman with a BMI of 50. (Above, left) Preoperative markings. (Above, right) Massive soft tissue defect after radical resection of all grossly evident disease, including 1-cm margins of normal-appearing tissue as well as the inferior extent of her abdominal pannus. (Below, left) Postoperative view after the patient’s second debridement, demonstrating delayed primary closure of the abdominal and right proximal thigh wounds. A vacuum-assisted closure device was used to temporize the larger, more contaminated genital/perineal wounds for an additional 4 days, before definitive closure. (Below, right) Evaluation at 18 months. The patient presents 6 months after completion of a 12-month course of adjuvant infliximab therapy with no evidence of recurrence or disease progression.
TABLE 2.
Treatment Characteristics for Patients in the Combined and Surgical Cohorts
| Combined Cohort (n = 11) | Surgical Cohort (n = 10) | P | |
|---|---|---|---|
| Operative management | |||
| No. debridements | |||
| Mean (range) | 2.3 (1–4) | 2.6 (1–3) | 0.71 |
| Defect size, cm2 | |||
| Mean (range) | 56 (24–225) | 48.5 (18–90) | 0.49 |
| Negative pressure (VAC) therapy (%) | 21 (72) | 18 (60) | 0.89 |
| Interval from excision to DPC, d | |||
| Mean (range) | 4.1 (2–10) | 4.2 (2–12) | 0.76 |
| Biologic therapy | |||
| Infliximab/Ustekinumab | 8/3 | N/A | |
| Time from closure to initiation of biologic therapy, d | |||
| Mean (range) | 16 (14–20) | N/A | |
| Duration of biologic therapy, mo | |||
| Mean (range) | 10.5 (6–15)* | N/A | |
| Follow-up, mo | |||
| Mean (range) | 18 (6–31) | 20.5 (4–36) | 0.81 |
Four patients remained on biologic therapy at the time of final follow-up. DPC indicates delayed primary closure; N/A, Not Applicable, VAC, vacuum-assisted closure.
Outcomes for patients in the combined and surgical cohorts are presented in Table 3. For patients in the combined therapy cohort, reoperation for local recurrence was required in 4 of 29 previously treated regions (19%), including 2 cases of labial recurrence, 1 axillary recurrence, and 1 within the perineum. In contrast, recurrence, necessitating reoperation, occurred in 10 of 26 sites (38.5%) among patients treated with surgery alone, including the axilla (n = 3), genitalia (n = 2), inguinal fold (n = 2), breast (n = 2), and perineum (n = 1) (P < 0.01). On average, the disease-free interval between delayed primary closure and recurrence was 18.5 months (r, 4 to 30 months) for patients in the combined cohort versus 6 months (r, 1.5 to 15 months) for patients treated with resection alone (P < 0.001). Of the 4 recurrences in patients treated with adjuvant biologic therapy, 3 (75%) occurred within 15 months after discontinuation of infliximab (n = 2) or ustekinumab (n = 1) (mean, 10.5 months), with the remaining occurring after 4 months of active treatment with infliximab. When compared with the disease-free interval for the surgery-only cohort, the mean duration to recurrence after cessation of biologic therapy was not statistically different (6 versus 10.5 months; P = 0.09). Additionally, we found no significant difference in recurrence rates due to age, sex, duration of symptoms, local wound care, or location of disease among either cohort.
TABLE 3.
Outcomes and Complications Stratified by Treatment Cohort
| Combined Cohort (n = 11) | Surgical Cohort (n = 10) | P | |
|---|---|---|---|
| Outcomes | |||
| Local recurrence (%) | 4 (19) | 10 (38.5) | <0.01 |
| Location of recurrence | |||
| Axilla | 1 | 3 | |
| Inguinal fold | 0 | 2 | |
| Groin/genital/perinea | 3 | 3 | |
| Breast | 0 | 2 | |
| Time to recurrence, from closure, mo | |||
| Mean (range) | 18.5 (4–30) | 6 (1.5–15) | <0.001 |
| Time to recurrence, from cessation of biologics, mo | |||
| Mean (range) | 10.5 (2.5–15)* | N/A | |
| Disease progression/development of new lesions | 2 (18) | 5 (50) | <0.001 |
| Time to progression, from closure, mo | |||
| Mean (range) | 18 (16–20) | 8.5 (4–10.5) | <0.05 |
| Time to progression, from cessation of biologics, mo | |||
| Mean (range) | 10.5 (9–12) | N/A | |
| Complications | |||
| Incisional wound dehiscence | 0 | 1 | |
| Surgical-site infection | 0 | 2 | |
| Delayed wound healing | 2 | 0 | |
| Adverse events related to biologic therapy | 0 | N/A | |
Recurrence occurred in 1 patient after 4 months of active treatment with infliximab. N/A, Not Applicable.
With respect to disease progression, 2 cases (18%) were identified within 12 months of discontinuing infliximab among patients in the combined cohort. For those treated with radical resection alone, progression of HS was observed in 5 of the 10 patients (50%) after a mean duration of 8.5 months (P < 0.001). All cases of recurrence or disease progression were treated with wide re-excision and delayed primary closure without further recurrence.
Complications in this series were minimal and consisted of 1 case of minor incisional dehiscence and 2 surgical site infections among 3 patients in the surgery-only cohort. For 2 patients in the combined cohort, delayed healing after radical resection and closure postponed the initiation of biologic therapy by approximately 1 week. There were no major adverse events related to the use of biologic therapy for any patient in this series (Table 3).
DISCUSSION
Treatment strategies for HS relate to disease severity and include a variety of lifestyle modifications (i.e., weight loss, smoking cessation, meticulous hygiene, and diabetes control), local and systemic therapies, and minor surgical procedures in the setting of mild-to-moderate disease (Hurley stages I and II).22 For patients with more extensive and/or refractory HS (Hurley stage III), conventional medical therapies have proven less effective, and radical resection of all involved tissue may offer the only potential option for a cure (Table 4).13,16,23 Nevertheless, high rates of recurrence and disease progression after wide local excision have led investigators to seek alternative treatment strategies and therapeutic combinations.13–16 Recent trials demonstrating the safety and efficacy of biologic therapy in patients with moderate-to-severe HS have spurred questions regarding the potential utility of these agents as adjuncts to more traditional surgical interventions.
TABLE 4.
Suggested Treatment for Hidradenitis Suppurativa According to the Hurley Stage of Disease
| Stage | Characteristics | Suggested Treatment |
|---|---|---|
| I | Solitary or multiple isolated abscess formation without scarring or sinus tracts | Lifestyle modifications* Topical antibiotics Laser treatment Zinc gluconate (adjunctive therapy) |
| II | Recurrent abscesses, single or multiple widely separated lesions, with sinus tract formation | Oral antibiotics Laser treatment Immunomodulator/biologic therapy Radical excision† |
| III | Diffuse or broad involvement across a regional area with multiple interconnected sinus tracts and abscesses | Radical excision† Immunomodulator/biologic therapy Referral to reconstructive surgeon for wound management and/or closure after regional resection |
Lifestyle modifications (ie, weight loss, smoking cessation, meticulous hygiene practices, glycemic control) are essential in all stages of treatment.
Radical excision of all hair-bearing skin within an affected region is the mainstay of treatment for patients with advanced or refractory disease.
Because the benefit of TNF-α inhibitors in HS was first reported among patients being treated for Crohn disease, increased speculation regarding the role of immunologic dysfunction in the pathogenesis of HS has emerged.24–26 High concentrations of proinflammatory cytokines that directly modulate both innate (IL-1β and TNF-α) and adaptive (IL-10, IL-12, IL-17, and IL-23) immune responses have been identified in HS skin specimens and provide a rationale for the use of various biologics in more advanced forms of this disease.27,28 Inhibition of TNF-α, in particular, has been shown to reduce markers of inflammation that play a central role in the pathologic inflammatory response associated with HS.17 In 2013, Samycia and Brassard29 published a review of nearly 50 limited case series and small clinical trials evaluating the safety and effectiveness of various TNF-α inhibitors in treating HS. Although few well-controlled, prospective studies were identified, both infliximab and adalimumab appeared to be well-tolerated and effective at producing at least temporary improvements in pain, disease severity, and/or quality-of-life for the majority of treated patients.
Encouraging observations with TNF-α inhibitors have paved the way for newer biologics that target both upstream and downstream inflammatory mediators. Specifically, IL-12 and IL-23 are heterodimeric cytokines that share a common p40 subunit and play an important role in the differentiation and proliferation of helper T-cell (Th) subsets 1 and 17, respectively. The IL-12/Th1 and IL-23/Th17 pathways have been implicated in the promotion of massive tissue inflammation, and both are highly expressed by HS lesion-derived macrophages.28,30 Ustekinumab is a monoclonal antibody that targets the common p40 subunit of IL-12/-23 and inhibits both pathways, thereby reducing the expression of downstream cytokines and chemokines, such as TNF-α.28,31 Maintenance therapy with ustekinumab has been shown to significantly improve both disease activity and long-term quality-of-life for patients with recalcitrant HS.32–34 Although favorable responses have been documented for both infliximab and ustekinumab, their role in the treatment of HS is often limited by cost and a lack of predictable long-term efficacy after treatment withdrawal.16,32,33,35
In this study, we observed significantly lower rates of recurrence (19% vs 38.5%; P < 0.01) and disease progression (18% vs 50%; P < 0.001) among patients in whom adjuvant biologic therapy was continued for at least 6 months. These protective benefits also appeared to be longer lasting. For patients in the combined cohort, the disease-free interval between closure and local recurrence was approximately 1 year longer, on average (18.5 vs 6 months; P < 0.001). Interestingly as well, the mean time to recurrence after cessation of biologic therapy was an additional 4.5 months longer than the disease-free interval for patients treated with radical resection alone (10.5 vs 6 months), although this observation was not statistically significant (P = 0.09). Progression of disease was observed in only 2 patients from the combined cohort within 1 year of discontinuing infliximab. Although differences in the efficacy of infliximab and ustekinumab could not be determined—given the infrequency of adverse outcomes and small sample size—our findings support a potential synergistic effect between adjuvant biologic therapy and radical resection in maintaining control of refractory HS.
An overall recurrence rate of 19% among patients receiving adjuvant biologic therapy in this series emphasizes the importance of achieving disease-free margins through wide excision. Studies evaluating different surgical protocols have demonstrated fewer complications, lower recurrence rates, and improved quality-of-life after radical resection of all involved tissue.13,36,37 A retrospective review of 31 patients undergoing minor drainage procedures, limited excisions, or radical resections for HS revealed recurrence rates of 100%, 42.8%, and 27%, respectively, after a mean follow-up of 72 months (P < 0.05).14 Other studies evaluating outcomes with various closure techniques indicate the risk of recurrence is likely influenced more by the extent of excision, degree of contamination, and/or regional distribution of apocrine glands, rather than the method of definitive closure.14,38,39 This may explain why higher rates of recurrence are often seen after attempts at immediate primary closure, where an inadvertent compromise in the margin of excision and/or heavy bacterial burden may diminish the probability of successful wound healing.11,16 Not surprisingly, rates of recurrence in this series were slightly higher after resection of axillary, perineal, and genital HS. This observation likely reflects the increased difficulty of obtaining disease-free margins in regions where functional and aesthetic considerations take priority and often limit the extent of resection.
The timing of wound closure also plays an integral role in ensuring successful outcomes and minimizing complications early on. Wound infection and/or bacterial colonization can preclude successful healing, particularly in patients with extensive anogenital, perineal, and/or axillary involvement. Aggressive attempts at premature closure invariably lead to high rates of dehiscence.40 Serial debridement and appropriate local wound care is, therefore, central in our approach to advanced HS. In the majority of our patients, internal vacuum-assisted therapy is used, before delayed primary closure, which has been shown to improve outcomes when compared to single-stage repair.40 In situations where primary closure is not possible or would otherwise compromise functional or aesthetic integrity, reconstruction with skin grafts, local-regional flaps, and/or free tissue transfer may become necessary.13,41,42 For most patients in this series, however, delayed primary closure and early mobilization permitted cosmetically acceptable results with minimal downtime—obviating the morbidities associated with graft or flap loss and/or donor-site defects.
This study was limited by its retrospective design and small sample size, which narrowed the role of statistical analysis and our ability to control for potential confounders (ie, heterogeneity of treatment regimens and local wound care) or generate substantial comparisons between infliximab and ustekinumab for the treatment of HS. A longer follow-up period would permit a more longitudinal assessment of outcomes, including the risk of recurrence and disease progression after cessation of these agents. Currently, the literature provides little to no guidance regarding the most appropriate timing, dosing frequency, or duration of biologic therapy for refractory HS, and determination of these parameters was not within the capacity of our study. Finally, it is important to recognize that incorporation of biologic therapy does not come without risk. Although no adverse events were associated with the use of infliximab or ustekinumab in this series, rare instances of infection, drug reactions, inflammatory arthropathy, and poor treatment effect have been reported previously.43–45
CONCLUSIONS
This report is the first to describe the use of targeted biologic therapy as an adjunct to radical resection and delayed primary closure for Hurley stage III HS. Our preliminary results with this multimodal approach are encouraging and suggest a potential role for adjuvant biologic therapy in the management of patients with recalcitrant disease. Although no definitive conclusions can be drawn, this study underscores the need for a prospective, randomized, controlled trial that can validate our findings and answer questions regarding the appropriate indications, treatment protocol, safety, and long-term effectiveness of different biologic agents when used as adjuncts in the multidisciplinary treatment of refractory HS.
Footnotes
Conflicts of interest and sources of funding: none declared.
References
- 1.Revuz JE, Canoui-Poitrine P, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa. J Am Acad Dermatol. 2008;59:596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35:191–194. doi: 10.1016/s0190-9622(96)90321-7. [DOI] [PubMed] [Google Scholar]
- 3.Jemec GB. Clinical practice: Hidradenitis suppurativa. N Engl J Med. 2012;366:158–164. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 4.Parks RW, Parks TG. Pathogenesis, clinical features and management of hidradenitis suppurativa. Ann R Coll Surg Engl. 1997;79:83–89. [PMC free article] [PubMed] [Google Scholar]
- 5.Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621–623. doi: 10.1016/j.jaad.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 6.von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol. 2001;144:809–813. doi: 10.1046/j.1365-2133.2001.04137.x. [DOI] [PubMed] [Google Scholar]
- 7.Kurzen H, Kurokawa I, Jemec GB, et al. What causes hidradenitis suppurativa? Exp Dermatol. 2008;17:455–456. doi: 10.1111/j.1600-0625.2008.00712_1.x. [DOI] [PubMed] [Google Scholar]
- 8.Dufour DN, Bryld LE, Jemec GB. Hidradenitis suppurativa complicating naevus comedonicus: the possible influence of mechanical stress on the development of hidradenitis suppurativa. Dermatology. 2010;220:323–325. doi: 10.1159/000287261. [DOI] [PubMed] [Google Scholar]
- 9.Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539–563. doi: 10.1016/j.jaad.2008.11.911. [DOI] [PubMed] [Google Scholar]
- 10.Jemec GB. Medical treatment of hidradenitis suppurativa. Expert Opin Pharmacother. 2004;5:1767–1770. doi: 10.1517/14656566.5.8.1767. [DOI] [PubMed] [Google Scholar]
- 11.Kagan RJ, Yakuboff KP, Warner P, et al. Surgical treatment of hidradenitis suppurativa: a 10-year experience. Surgery. 2005;138:734–741. doi: 10.1016/j.surg.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 12.Buyukasik O, Hasdemir AO, Kahramansoy N, et al. Surgical approach to extensive hidradenitis suppurativa. Dermatol Surg. 2011;37:835–842. doi: 10.1111/j.1524-4725.2011.01961..x. [DOI] [PubMed] [Google Scholar]
- 13.Alharbi Z, Kauczok J, Pallua N. A review of wide surgical excision of hidradenitis suppurativa. BMC Dermatol. 2012;12:9. doi: 10.1186/1471-5945-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritz JP, Runkel N, Haier J, et al. Extent of surgery and recurrence rate of hidradenitis suppurativa. Int J Colorectal Dis. 1998;13:164–168. doi: 10.1007/s003840050159. [DOI] [PubMed] [Google Scholar]
- 15.Harrison BJ, Mudge M, Hughes LE. Recurrence after surgical treatment of hidradenitis suppurativa. BMJ. 1987;294:487–489. doi: 10.1136/bmj.294.6570.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandal A, Watson J. Experience with different treatment modules in hidradenitis suppurativa: a study of 106 cases. Surgeon. 2005;3:23–26. doi: 10.1016/s1479-666x(05)80006-x. [DOI] [PubMed] [Google Scholar]
- 17.Grant A, Gonzalez T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol. 2010;62:205–217. doi: 10.1016/j.jaad.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 18.Miller I, Lynggaard CD, Lophaven S, et al. A double-blind placebo controlled randomized trial of adalimumab in the treatment of hidradenitis suppurativa. Br J Dermatol. 2011;165:391–398. doi: 10.1111/j.1365-2133.2011.10339.x. [DOI] [PubMed] [Google Scholar]
- 19.Blanco R, Martinez-Taboada VM, Villa I, et al. Long-term successful adalimumab therapy in severe hidradenitis suppurativa. Arch Dermatol. 2009;145:580–584. doi: 10.1001/archdermatol.2009.49. [DOI] [PubMed] [Google Scholar]
- 20.Pelekanou A, Kanni T, Savva A, et al. Long-term efficacy of etanercept in hidradenitis suppurativa: results from an open-label phase II prospective trial. Exp Dermatol. 2009;19:538–540. doi: 10.1111/j.1600-0625.2009.00967.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619–625. doi: 10.1111/j.1365-4632.2011.04871.x. [DOI] [PubMed] [Google Scholar]
- 22.Rambhatla PV, Lim HW, Hamzavi I. A systematic review of treatments for hidradenitis suppurativa. Arch Dermatol. 2012;148:439–446. doi: 10.1001/archdermatol.2011.1950. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell KM, Beck DE. Hidradenitis suppurativa. Surg Clin North Am. 2002;82:1187–1197. doi: 10.1016/s0039-6109(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 24.Martinez F, Nos P, Benlloch S, et al. Hidradenitis suppurativa and Crohn’s disease: response to treatment with infliximab. Inflamm Bowel Dis. 2001;7:323–326. doi: 10.1097/00054725-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Rosi YL, Lowe L, Kang S. Treatment of hidradenitis suppurativa with infliximab in a patient with Crohn’s disease. J Dermatolog Treat. 2005;16:58–61. doi: 10.1080/09546630410024547. [DOI] [PubMed] [Google Scholar]
- 26.Roussomoustakaki M, Dimoulios P, Chatzicostas C, et al. Hidradenitis suppurativa associated with Crohn’s disease and spondyloarthropathy: response to anti-TNF therapy. J Gastroenterol. 2003;38:1000–1004. doi: 10.1007/s00535-003-1185-9. [DOI] [PubMed] [Google Scholar]
- 27.van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumor necrosis factor (TNF)-a, interleukin (IL)-1b and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-a and IL-1b. Br J Dermatol. 2011;164:1292–1298. doi: 10.1111/j.1365-2133.2011.10254.x. [DOI] [PubMed] [Google Scholar]
- 28.Schlapbach C, Hanni T, Yawalkar N, et al. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790–798. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Samycia M, Brassard A. Adalimumab in treatment-resistant hidradenitis suppurativa following recurrence after extensive affected area excision: a review of biologics therapy. J Cutan Med Surg. 2013;17:S23–S32. doi: 10.2310/7750.2012.11144. [DOI] [PubMed] [Google Scholar]
- 30.Korn T, Bettelli E, Oukka M, et al. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 31.Toichi E, Torres G, McCormick TS, et al. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J Immunol. 2006;177:4917–4926. doi: 10.4049/jimmunol.177.7.4917. [DOI] [PubMed] [Google Scholar]
- 32.Gulliver WP, Jemec GB, Baker KA. Experience with ustekinumab for the treatment of moderate to severe hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2012;26:911–914. doi: 10.1111/j.1468-3083.2011.04123.x. [DOI] [PubMed] [Google Scholar]
- 33.Sharon VR, Garcia MS, Bagheri S, et al. Management of recalcitrant hidradenitis suppurativa with ustekinumab. Acta Derm Venereol. 2012;92:320–321. doi: 10.2340/00015555-1229. [DOI] [PubMed] [Google Scholar]
- 34.Baerveldt EM, Kappen JH, Thio HB, et al. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis, and hidradenitis suppurativa. Ann Rheum Dis. 2013;72:626–627. doi: 10.1136/annrheumdis-2012-202392. [DOI] [PubMed] [Google Scholar]
- 35.Mekkes JR, Bos JD. Long-term efficacy of a single course of infliximab in hidradenitis suppurativa. Br J Dermatol. 2008;158:370–374. doi: 10.1111/j.1365-2133.2007.08332.x. [DOI] [PubMed] [Google Scholar]
- 36.Büyükaşik O, Hasdemir AO, Kahramansoy N, et al. Surgical approach to extensive hidradenitis suppurativa. Dermatol Surg. 2011;37:835–842. doi: 10.1111/j.1524-4725.2011.01961..x. [DOI] [PubMed] [Google Scholar]
- 37.Wiltz O, Schoetz DJ, Jr, Murray JJ, et al. Perianal hidradenitis suppurativa: the Lahey Clinic experience. Dis Colon Rectum. 1990;33:731–734. doi: 10.1007/BF02052316. [DOI] [PubMed] [Google Scholar]
- 38.Rompel R, Petres J. Long-term results of wide surgical excision in 106 patients with hidradenitis suppurativa. Dermatol Surg. 2000;26:638–643. doi: 10.1046/j.1524-4725.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 39.Bieniek A, Matusiak L, Okulewicz-Gojlik D, et al. Surgical treatment of hidradenitis suppurativa: experiences and recommendations. Dermatol Surg. 2010;36:1998–2004. doi: 10.1111/j.1524-4725.2010.01763.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen YE, Gerstel T, Verma K, et al. Management of hidradenitis suppurativa wounds with an internal vacuum-assisted closure device. Plast Reconstr Surg. 2014;133:370–377. doi: 10.1097/PRS.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 41.Chen E, Friedman HI. Management of regional hidradenitis suppurativa with vacuum-assisted closure and split thickness skin grafts. Ann Plast Surg. 2011;67:397–401. doi: 10.1097/SAP.0b013e3181f77bd6. [DOI] [PubMed] [Google Scholar]
- 42.Menderes A, Sunay O, Vayvada H, et al. Surgical management of hidradenitis suppurativa. Int J Med Sci. 2010;19:240–247. doi: 10.7150/ijms.7.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Rappard DC, Mooij JE, Baeten DL, et al. New-onset polyarthritis during successful treatment of hidradenitis suppurativa with infliximab. Br J Dermatol. 2011;165:194–198. doi: 10.1111/j.1365-2133.2011.10328.x. [DOI] [PubMed] [Google Scholar]
- 44.Fardet L, Dupuy A, Kerob D, et al. Infliximab for severe hidradenitis suppurativa: transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624–628. doi: 10.1016/j.jaad.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 45.Usmani N, Clayton TH, Everett S, et al. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007;32:204–205. doi: 10.1111/j.1365-2230.2006.02272.x. [DOI] [PubMed] [Google Scholar]


