Abstract
Background
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are widely accepted in Asia for treatment of early gastric cancer (EGC). Few studies have examined lymph node (LN) metastasis for EGC in Western populations. We sought to examine EGC and LN metastasis in a heterogeneous Western population.
Methods
Patients with surgically resected, histologically confirmed AJCC T1a gastric adenocarcinoma were identified in the Surveillance, Epidemiology, and End Results (SEER) database from 2002 to 2012. Patients were excluded if they had stage IV disease, had multiple primary cancers, or received neoadjuvant therapy. Rates of LN metastasis were calculated and survival analyses were performed.
Results
Of 923 patients in the cohort, 72 (7.8%) patients had at least one positive LN on final pathology. When stratified by race, Asian/Pacific Islanders (APIs) demonstrated the lowest rate of LN metastases (n = 17/327; 5.2%), followed by Hispanics (n = 12/171; 7.0%), whites (n = 27/278; 9.7%), and blacks (n = 16/147; 10.9%). The highest rates of Stage IA disease were observed in API (93.9%) and Hispanic (92.4%) patients, followed by white (89.9%) and black (87.1%) patients (p = 0.04). Survival analysis of T1a gastric cancer patients by race/ethnicity showed 5-year overall survival was highest for API (API 88%, Hispanic 81%, black 79%, and white 77%; p<0.01).
Conclusion
The rate of LN metastasis in T1a gastric cancers in the United States is higher than the rates reported in Asia. Survival outcomes in T1a gastric cancers varied significantly by race, suggesting that definitive endoscopic treatment may not be appropriate for all patients in the United States.
INTRODUCTION
In 1962, the Japanese Society of Gastroenterological Endoscopy defined early gastric cancer (EGC) as adenocarcinoma confined to the mucosa and submucosa, regardless of the cancer’s nodal involvement.1 Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) have been developed in Asia for low morbidity treatment of EGC based on the very low node-positive (LN+) rates in large retrospective Asian studies of radical surgical resection of EGC cases.2,3 According to the Japanese Gastric Cancer Association treatment guidelines, gastric adenocarcinomas that are (1) clinically staged as T1a, (2) ≤ 2 cm, (3) well-differentiated, and (4) lack ulceration fall under the standard criteria for EMR/ESD.4 Although EMR/ESD are frequently used in Asia and permit curative treatment of EGC without the morbidity of gastrectomy, the standard of care in Western countries remains formal resection.
There is growing interest in expanding the use of EMR/ESD in Western countries, including the United States. EMR/ESD were initially developed for endoscopic resection of gastric cancer, but now are also being applied to resection of T1a esophageal cancers and colon cancers. Studies including Western data report the rate of lymph node (LN) metastasis for intramucosal esophageal cancer to be 0% to 2%5,6 and 1% to 3%7,8 for colon cancer when the cancer is confined to the top one-third of the submucosa (sm1). However, due to the higher incidence of gastric cancer in Asia, the majority of data regarding LN metastasis rates for T1a disease are based only on Asian cohorts. Whether EMR/ESD can be applied to Western patients using the same criteria based on lymph node LN metastasis rates in Asian T1a gastric cancers has not been well studied. Based on our previous work investigating disparities in gastric cancer outcomes in racial/ethnic groups in the United States, we hypothesized that LN metastasis rates may vary by racial and ethnic groups in a heterogenous Western population.9,10 Therefore, the objective of our study was to investigate the LN metastasis rate and clinical outcomes by race/ethnicity in surgically resected T1a gastric cancers using a large national cancer registry.
METHODS
Patients and Methods
Patient data were obtained from the Surveillance, Epidemiology, and End Results (SEER) website.11 The SEER catchment area covers approximately 28% of the United States; the dataset contains clinicopathologic information, treatment specifics, overall survival (OS) and disease–specific survival (DSS). Tumor location, grade, and histology were coded according to the International Classification of Diseases for Oncology (ICD-O), version 3. Tumor stage was coded according to the American Joint Committee on Cancer (AJCC) TNM staging system, 7th edition.12 SEER requires registries to update disease and vital status on all cases on an annual basis.
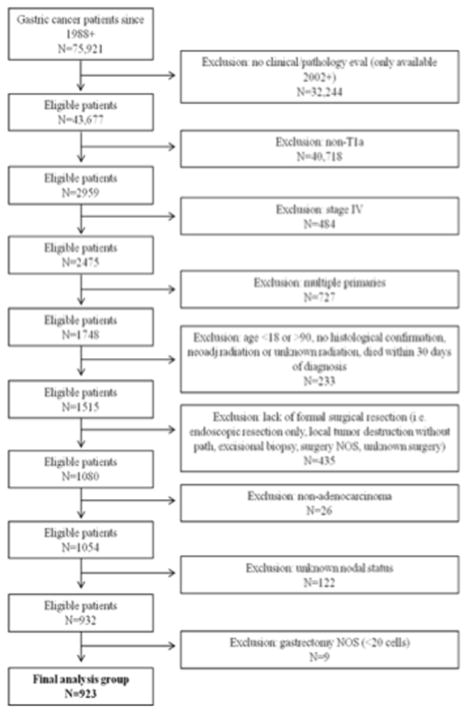
Included in the analyses were surgically resected (codes 30–33, 40–42, 51–52, 61–63), histologically confirmed, AJCC T1a gastric adenocarcinoma patients aged ≥18 years old who were diagnosed from January 2002 to December 2012. As detailed in Figure 1, patients with stage IV disease, multiple primaries, or patients who received neoadjuvant radiation therapy were excluded. Patients treated with endoscopic resection alone as their first course of therapy were not included. ICD-O-3 codes were used to identify patients with adenocarcinoma. Of the 75,921 gastric cancer patients in the SEER registry, our final sample comprised 923 patients; of these 278 were white, 147 were black, 171 were Hispanic, and 327 were Asian/Pacific Islanders (API) (Table 1).
Figure 1.
Patient selection flow sheet.
Table 1.
Patient demographics and clinical characteristics of T1a gastric cancer patients
| Total N=923 N (%) |
LN− N=851 N (%) |
LN+ N=72 N (%) |
P value | |
|---|---|---|---|---|
| Age | 68 (58–76) | 68 (58–76) | 68 (54–75) | 0.62 |
| Median (IQR+) | ||||
|
| ||||
| Age Group | ||||
| 18–64 | 373 (40.4) | 343 (40.3) | 30 (41.7) | 0.82 |
| 65–90 | 550 (59.6) | 508 (59.7) | 42 (58.3) | |
|
| ||||
| Race/Ethnicity | ||||
| Non-Hispanic White | 278 (30.1) | 251 (29.5) | 27 (37.5) | 0.09 |
| Black | 147 (16.0) | 131 (15.4) | 16 (22.2) | |
| Hispanic White | 171 (18.5) | 159 (18.7) | 12 (16.7) | |
| Asian/Pacific Islanders | 327 (35.4) | 310 (36.4) | 17 (23.6) | |
|
| ||||
| Tumor Location | ||||
| Distal | 433 (46.9) | 407 (47.8) | 26 (36.1) | 0.29 |
| Proximal | 29 (3.2) | 26 (3.1) | 3 (4.2) | |
| Middle | 317 (34.3) | 288 (33.8) | 29 (40.3) | |
| Whole | 144 (15.6) | 130 (15.3) | 14 (19.4) | |
|
| ||||
| Grade | ||||
| Well Differentiated | 140 (15.2) | 137 (16.1) | 3 (4.2) | <0.01 |
| Moderately Differentiated | 255 (27.6) | 236 (27.7) | 19 (26.4) | |
| Poorly Differentiated | 408 (44.2) | 361 (42.4) | 47 (65.2) | |
| Undifferentiated | 16 (1.7) | 16 (1.9) | 0 (0.0) | |
| Unknown | 104 (11.3) | 101 (11.9) | 3 (4.2) | |
|
| ||||
| Tumor Size | ||||
| ≤2 cm | 505 (54.7) | 483 (56.8) | 22 (30.5) | <0.01 |
| >2 cm | 244 (26.4) | 205 (24.1) | 39 (54.2) | |
| Unknown | 174 (18.9) | 163 (19.1) | 11 (15.3) | |
|
| ||||
| Surgery Type | ||||
| Total/Near Total | 145 (15.7) | 137 (16.1) | 8 (11.1) | 0.26 |
| Partial | 778 (84.3) | 714 (83.9) | 64 (88.9) | |
|
| ||||
| # of Examined Nodes | ||||
| 1–14 | 590 (63.9) | 548 (64.4) | 42 (58.3) | 0.30 |
| ≥15 | 333 (36.1) | 303 (35.6) | 30 (41.7) | |
IQR=Interquartile Range
Statistical Analysis
Patient demographic and clinical characteristics were compared across groups using the Pearson X2 test for categorical nominal data and the Jonckheere–Terpstra non–parametric test for categorical ordinal data. Univariate and multivariate Cox proportional hazard models identified factors associated with improved DSS and OS, with results reported using hazard ratios (HR) and 95% confidence intervals (CI).
Kaplan–Meier curves were used to calculate median, 3-year and 5-year DSS and OS rates, with the log–rank test used to determine statistical differences across groups. Survival time, in months, was calculated from the date of diagnosis until the date of death. If the patient was alive, the patient was censored at the date of last contact. For the DSS analyses, patients with death due to gastric cancer (SEER cause of death recode 21020 or 50060 or 50300) were identified using cause of death on the death certificate. Patients who died from causes unrelated to their gastric cancer diagnosis were censored at their date of death. Median follow-up time for the 763 patients alive at last contact was 46 months (interquartile range: 19–81 months, mean: 52 months, standard deviation: 36 months). All analyses were performed using SAS, with 2-sided p–values ≤0.05 considered statistically significant.
RESULTS
Patient Demographics and Tumor Characteristics
The final cohort consisted of 923 patients with surgically resected T1a gastric adenocarcinoma (Figure 1). Of those, 851 (92.2%) were node-negative (N-) and 72 (7.8%) were node-positive (LN+). The most common histological subtypes were intestinal (n = 189 of 923, 20.5%), diffuse (n = 39 of 923, 4.2%), signet ring (n = 253 of 923, 27.4%), and adenocarcinoma not otherwise specific (NOS) (n = 357 of 923, 38.7%). LN+ patients tended to have higher frequency of tumors ≥2 cm with higher grade compared with N- patients (Table 1). Twenty-two of 505 (4.4%) tumors ≤2 cm were node-positive, whereas 39 of 244 (16.0%) tumors >2 cm were node-positive. Further details regarding patient demographics, tumor characteristics and surgery characteristics are summarized in Table 1.
Trends in Overall Lymph Node Positivity Rates and Staging by Race
In order to investigate differences by race/ethnicity, LN+ rates for the T1a cohort were examined by race/ethnic groups. We observed lower LN+ rates in API and Hispanics patients (5.2% and 7.0%, respectively) compared with white and black patients (9.7% and 10.9%, respectively; Table 2). API and Hispanic patients had higher rates of stage IA disease compared with whites and blacks, whereas whites and blacks tended to have slightly higher rates of IB and IIA disease (p=0.04, Table 2). API patients tended to have smaller tumors and the highest rate of >15 LNs examined (Table 2).
Table 2.
Patient demographics and clinical characteristics of T1a gastric cancer patients by race
| White N=278 N (%) |
Black N=147 N (%) |
Hispanic N=171 N (%) |
API N=327 N (%) |
P value | |
|---|---|---|---|---|---|
| Age | 71 (58–79) | 62 (54–73) | 67 (57–74) | 70 (60–76) | <0.01† |
| Median (IQR+) | |||||
|
| |||||
| Age Group | |||||
| 18–64 | 101 (36.3) | 83 (56.5) | 75 (43.9) | 114 (34.9) | <0.01 |
| 65–90 | 177 (63.4) | 64 (43.5) | 96 (56.1) | 213 (65.1) | |
|
| |||||
| Grade | |||||
| Well Differentiated | 42 (15.1) | 23 (15.6) | 29 (17.0) | 46 (14.1) | 0.35 |
| Moderately Differentiated | 66 (23.8) | 45 (30.6) | 47 (27.5) | 97 (29.7) | |
| Poorly Differentiated | 121 (43.5) | 67 (45.6) | 74 (43.3) | 146 (44.6) | |
| Undifferentiated | 7 (2.5) | 0 (0.0) | 2 (1.1) | 7 (2.1) | |
| Unknown | 42 (15.1) | 12 (8.2) | 19 (11.1) | 31 (9.5) | |
|
| |||||
| Tumor Size | |||||
| ≤2cm | 138 (49.6) | 72 (49.0) | 94 (55.0) | 201 (61.5) | <0.01 |
| >2cm | 75 (27.0) | 40 (27.2) | 53 (31.0) | 76 (23.2) | |
| Unknown | 65 (23.4) | 35 (23.8) | 24 (14.0) | 50 (15.3) | |
|
| |||||
| Tumor Location | |||||
| Distal | 120 (43.2) | 77 (52.4) | 74 (43.3) | 162 (49.6) | 0.04 |
| Proximal | 13 (4.7) | 4 (2.7) | 6 (3.5) | 6 (1.8) | |
| Middle | 91 (32.7) | 39 (26.5) | 66 (38.6) | 121 (37.0) | |
| Whole | 54 (19.4) | 27 (18.4) | 25 (14.6) | 38 (11.6) | |
|
| |||||
| Surgery Type | |||||
| Total/Near Total | 58 (20.9) | 19 (12.9) | 28 (16.4) | 40 (12.2) | 0.02 |
| Partial | 220 (79.1) | 128 (87.1) | 143 (83.6) | 287 (87.8) | |
|
| |||||
| # of Examined Nodes | |||||
| 1–14 | 188 (67.6) | 95 (64.6) | 120 (70.2) | 187 (57.2) | 0.01† |
| ≥15 | 90 (32.4) | 52 (35.4) | 51 (29.8) | 140 (42.8) | |
|
| |||||
| Positive Node Group | |||||
| 0 | 251 (90.3) | 131 (89.1) | 159 (93.0) | 310 (94.8) | 0.09 |
| ≥1 | 27 (9.7) | 16 (10.9) | 12 (7.0) | 17 (5.2) | |
|
| |||||
| Stage | |||||
| IA (T1aN0) | 250 (89.9) | 128 (87.1) | 158 (92.4) | 307 (93.9) | 0.04†† |
| IB (T1aN1) | 22 (7.9) | 13 (8.8) | 7 (4.1) | 13 (4.0) | |
| IIA (T1aN2) | 6 (2.2) | 6 (4.1) | 5 (2.9) | 5 (1.5) | |
| IIB (T1aN3) | 0 (0) | 0 (0) | 1 (0.6) | 2 (0.6) | |
API=Asian/Pacific Islanders
IQR=Interquartile Range
p-values shown reflect non-parametric testing using the Kruskal Wallis test for continuous data.
p-values shown are based on the Jonckheere-Terpstra test for ordinal data.
Survival Analyses of all T1a Gastric Adenocarcinoma Patients
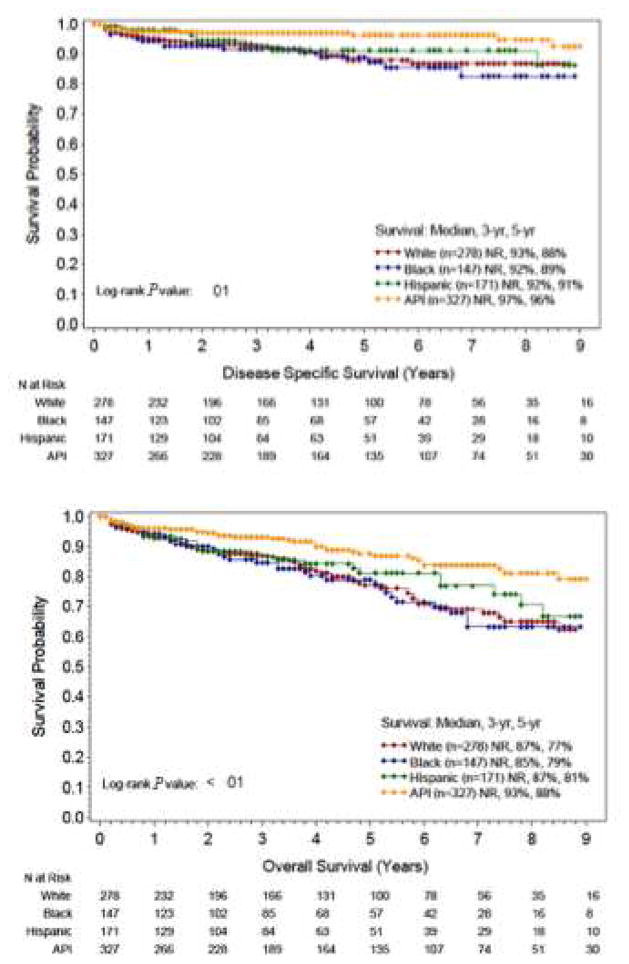
Significant differences were observed in the disease-specific (DSS) and overall survival (OS) of the racial/ethnic groups in the T1a cohort. Kaplan Meier survival curves demonstrate that API had the highest 3-year and 5-year overall survival at 93% and 88%, respectively (Figure 2, p<0.01). Hispanic, white, and black patients had similar 3-year overall survival, with Hispanic patients having slightly better survival after the 5-year mark. A similar trend was observed for disease-specific survival.
Figure 2.
Three-year and 5-year (A) disease-specific survival and (B) overall survival in T1a gastric cancer patients by race/ethnicity.
A multivariate model for OS showed that presence of LN+ disease was the strongest independent predictor of increased mortality (HR, 2.63; 95% CI, 1.70–4.06). Increasing age was also a predictor (HR, 1.07, 95% CI 1.05–1.09). In terms of race/ethnicity, API demonstrated significantly longer OS compared with whites (HR, 0.60; 95% CI, 0.40–0.92), whereas Hispanics (HR, 1.00; 95% CI, 0.63–1.58) and blacks (HR, 1.40; 95% CI, 0.91–2.17) demonstrated similar OS rates compared with whites (Table 3). Similar results were observed in the multivariate analysis for DSS with LN+ disease (HR, 4.58; 95% CI, 2.58–8.13) and age (HR, 1.05; 95% CI, 1.02–1.08) being independent predictors of increased mortality. For race, API demonstrated significantly longer OS rates compared with whites (HR, 0.50; 95% CI, 0.25–1.0) (Table 3).
Table 3.
Predictors of disease-specific and overall survival in T1a gastric cancer patients
| Disease-Specific Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age† | 1.05 (1.03 – 1.07) | <0.01 | 1.05 (1.02 – 1.08) | <0.01 | 1.07 (1.05 – 1.09) | <0.01 | 1.07 (1.05 – 1.09) | <0.01 |
| Median (IQR+) | ||||||||
|
| ||||||||
| Age Group | ||||||||
| 18–64 | (reference) | - | (reference) | - | ||||
| 65–90 | 2.17 (1.23 – 3.81) | 0.01 | 3.00 (2.03 – 4.44) | <0.01 | ||||
|
| ||||||||
| Race/Ethnicity† | ||||||||
| Non-Hispanic White | (reference) | - | (reference) | - | (reference) | - | (reference) | - |
| Black | 1.18 (0.64 – 2.21) | 0.60 | 1.47 (0.76 – 2.83) | 0.25 | 1.01 (0.66 – 1.54) | 0.97 | 1.40 (0.91 – 2.17) | 0.13 |
| Hispanic White | 0.76 (0.38 – 1.55) | 0.45 | 1.00 (0.49 – 2.06) | 0.99 | 0.80 (0.51 – 1.26) | 0.33 | 1.00 (0.63 – 1.58) | 0.99 |
| Asian/Pacific Islanders | 0.39 (0.19 – 0.77) | <0.01 | 0.50 (0.25 – 1.01) | 0.05 | 0.48 (0.32 – 0.72) | <0.01 | 0.60 (0.40 – 0.92) | 0.02 |
|
| ||||||||
| Tumor Location† | ||||||||
| Distal | (reference) | - | (reference) | - | (reference) | - | (reference) | - |
| Proximal | 2.36 (0.91 – 6.13) | 0.08 | 1.94 (0.73 – 5.15) | 0.18 | 1.17 (0.54 – 2.53) | 0.70 | 1.01 (0.46 – 2.21) | 0.97 |
| Middle | 1.22 (0.71 – 2.10) | 0.48 | 1.42 (0.81 – 2.49) | 0.23 | 0.94 (0.66 – 1.34) | 0.73 | 1.05 (0.73 – 1.51) | 0.79 |
| Whole | 0.82 (0.37 – 1.82) | 0.63 | 0.67 (0.30 – 1.51) | 0.34 | 0.97 (0.63 – 1.52) | 0.91 | 0.89 (0.56 – 1.40) | 0.61 |
|
| ||||||||
| Grade† | ||||||||
| Well Differentiated | (reference) | - | (reference) | - | (reference) | - | (reference) | - |
| Moderately Differentiated | 1.96 (0.85 – 4.54) | 0.12 | 1.83 (0.77 – 4.31) | 0.17 | 1.38 (0.85 – 2.23) | 0.19 | 1.41 (0.86 – 2.30) | 0.17 |
| Poorly Differentiated | 1.25 (0.55 – 2.88) | 0.59 | 1.44 (0.60 – 3.45) | 0.41 | 0.75 (0.46 – 1.22) | 0.25 | 1.05 (0.63 – 1.75) | 0.85 |
| Undifferentiated | 1.41 (0.17 – 11.45) | 0.75 | 2.88 (0.34 – 24.56) | 0.33 | 0.95 (0.22 – 4.0.) | 0.94 | 1.91 (0.44 – 8.34) | 0.39 |
| Unknown | 0.83 (0.26 – 2.62) | 0.75 | 0.80 (0.24 – 2.63) | 0.72 | 0.89 (0.48 – 1.63) | 0.70 | 1.15 (0.61 – 2.17) | 0.67 |
|
| ||||||||
| Surgery Type | ||||||||
| Total/Near Total | (reference) | - | (reference) | - | ||||
| Partial | 1.12 (0.55 – 2.26) | 0.75 | 1.31 (0.81 – 2.11) | 0.27 | ||||
|
| ||||||||
| Tumor Size† | ||||||||
| ≤2 cm | (reference) | - | (reference) | - | (reference) | - | (reference) | - |
| >2 cm | 2.13 (1.17 – 3.87) | 0.01 | 1.25 (0.66 – 2.34) | 0.49 | 1.73 (1.20 – 2.50) | <0.01 | 1.16 (0.79 – 1.70) | 0.45 |
| Unknown | 2.68 (1.47 – 4.89) | <0.01 | 2.17 (1.16 – 4.09) | 0.02 | 1.76 (1.20 – 2.59) | <0.01 | 1.51 (1.00 – 2.27) | 0.05 |
|
| ||||||||
| # LN Examined | 0.97 (0.94 – 1.00) | 0.05 | 0.96 (0.94 – 0.98) | <0.01 | ||||
| Median (IQR+) | ||||||||
|
| ||||||||
| # of Examined Nodes† | ||||||||
| 1–14 | (reference) | - | (reference) | - | ||||
| ≥15 | 0.40 (0.21 – 0.76) | <0.01 | 0.53 (0.26 – 1.05) | 0.07 | 0.49 (0.33 – 0.73) | <0.01 | 0.71 (0.47 – 1.08) | 0.11 |
|
| ||||||||
| Positive Node Group† | ||||||||
| 0 | (reference) | - | (reference) | - | (reference) | - | (reference) | - |
| ≥1 | 5.07 (2.97 – 8.67) | <0.01 | 4.58 (2.58 – 8.13) | <0.01 | 2.64 (1.76 – 3.97) | <0.01 | 2.63 (1.70 – 4.06) | <0.01 |
Follow-up through December 31, 2012.
Included in multivariate model.
API=Asian/Pacific Islander
DISCUSSION
It is recognized that EGC is more commonly diagnosed in Asia compared with Western countries. In Japan, EGC comprises approximately 60% of all diagnosed gastric cancers,13 whereas in Western countries the incidence of EGC is 10% to 20%.14 Because EGC is not necessarily synonymous with early stage gastric cancer, the number of involved LNs is an important determinant of outcome, particularly because the use of EMR/ESD is increasing in the West. The incidence of LN involvement in T1a gastric cancers in large Asian series is reported to be 2% to 5%.2,3,15 Though these are the largest studies reported, the populations are relatively homogenous in terms of race/ethnicity. Our study shows an LN+ rate of 7.8% in surgically resected T1a gastric cancers in the US SEER database. Our results fall within previously published Western series that have reported LN+ rates ranging from 4% to 13%.16–21 In addition to having the largest sample size of Western T1a gastric cancers, our study also examines the relationship between race/ethnicity and LN+ disease in the setting of growing enthusiasm to adopt EMR/ESD as a curative therapy for EGC in Western countries.
Just as the results of gastric cancer adjuvant therapy trials conducted in Asia may not be applicable to Western patients, can the standard criteria for EMR/ESD developed in Asia be generalized to Western ECG on the basis of the Asian EGC LN+ rates? Our study indicates that LN+ disease varies by race/ethnicity in the United States. Although the API LN+ rate of 5.2% in our study is comparable with that of large Asian series, LN+ disease rates for white (9.6%) and black (10.9%) patients is almost double that of API patients. This suggests that some racial/ethnic groups may have an unacceptably high rate of LN metastasis in T1a gastric cancer and may not be appropriate candidates for emerging minimally invasive technologies such as EMR/ESD. There are very few studies investigating this topic in the U.S. literature. Fukuhara and colleagues performed a single institution series using a racially and ethnically diverse population in urban New York.17 Although their sample size was limited, they reported a 7.1% LN+ rate in T1a gastric cancers for the entire cohort, with Asians having a lower LN+ rate compared with non-Asians (4.4% vs 9.1%). Our study not only corroborates their previous findings, but we also show that survival outcomes mirror the nodal status of the racial/ethnic groups, with API and Hispanics demonstrating improved survival compared with whites and blacks.
Large retrospective Asian series have provided the data showing low node-positive rates for T1a gastric cancers, thus forming the basis for curative endoscopic resection for early gastric cancer. In our study, Asians had the highest rate (42.8%) of having ≥15 lymph nodes examined while also having highest disease-specific and overall survival. In contrast, white patients had a lower rate (32.4%) of having ≥15 lymph nodes examined and they also had poorer survival. However, the association between lymph nodes examined and survival is still not clear, as Hispanic patients had the lowest rate (29.8%) of having ≥15 lymph nodes examined yet they had the second highest survival rate. Biffi et al22 also reported improved survival in patients having ≥15 lymph nodes examined compared with <15 lymph nodes in T1 gastric cancer patients. However, they did not distinguish T1a from T1b tumors, which is an important distinction when considering rates of lymph node metastasis because the node-positive rate for T1a gastric cancer is 2% but it is 18% in T1b gastric cancer.23
The etiologies underlying outcomes disparities in gastric cancer by race/ethnicity remain poorly defined. In addition to existing reports on the biological differences between Asian and Western gastric cancers,24,25 recently published data by The Cancer Genome Atlas classifying gastric cancer into 4 distinct molecular subtypes may provide a more specific roadmap to further investigate potentially inherent molecular differences between racial/ethnic groups.26 It is conceivable that these molecular differences may be linked to differences in observed biological aggressiveness in gastric cancer and perhaps even the propensity for earlier nodal spread in different racial/ethnic groups, although this area remains largely unexplored.
There are limitations to our current investigation that warrant discussion. We have used SEER for our study, which lacks data on medical comorbidities, disease progression after diagnosis, and details on endoscopic resection techniques. Additionally, the SEER patient population represents only 28% of the U.S. population leaving the possibility of sampling error in determining the rates of node-positivity with T1a gastric cancers.27 Furthermore, SEER does not contain data on receipt of chemotherapy. Thus, unlike radiation therapy, we were unable to exclude patients who may have received neoadjuvant chemotherapy. Before the publication of the MAGIC trial, the standard of care in the United States was adjuvant chemoradiation according to the INT 0116 study results.28,29 Since MAGIC’s publication in mid-2006 established perioperative chemotherapy as an alternative regimen, it is feasible that a small proportion of patients may have received neoadjuvant chemotherapy. In order to investigate how the adoption of the MAGIC protocol might have affected our results, we examined the number of patients diagnosed with T1a gastric cancer by race/ethnicity in 2002 to 2006 versus 2007 to 2012. Although there were fewer LN+ patients being diagnosed in the later time period (Supplemental Table 1), when these LN+ patients were examined by race/ethnicity, there was no significant difference in LN+ rates between the 2 time periods that would explain improved survival in API and Hispanics patients compared with whites and blacks (Supplemental Table 2). Rates of API LN+ were the same and rates of Hispanic LN+ actually increased in the later time period.
Despite increasing interest in advancing the use EMR/ESD for the treatment of EGC in the West, factors related to race/ethnicity appear to impact LN+ rates in a heterogenenous US T1a gastric cancer population, suggesting that endoscopic resection of EGC may not be appropriate for all patients. Although API patients demonstrated similar LN+ rates as retrospective Asian series and could be considered appropriate EMR/ESD candidates, white and black patients had higher LN+ rates that may require radical surgical resection. Although the etiological factors underlying the differences in LN+ rates between racial/ethnic groups warrants further study, our data suggests that definitive endoscopic treatment of T1a gastric cancer should be considered carefully in heterogenous Western populations.
Supplementary Material
Acknowledgments
Dr. Chao’s efforts in manuscript preparation were supported by the National Cancer Institute of the National Institutes of Health under award number NIH 5K12CA001727-20. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acronyms
- AJCC
American Joint Committee on Cancer
- API
Asian/Pacific Islanders
- CI
Confidence intervals
- DSS
Disease–specific survival
- EGC
Early gastric cancer
- EMR
Endoscopic mucosal resection
- ESD
Endoscopic submucosal dissection
- HR
Hazard ratios
- IQR
Interquartile Range
- LN
Lymph node
- N−
Node-negative
- N+
Node-positive
- NOS
Not otherwise specific
- OS
Overall survival
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Author contributions: Conception and design: AH Choi, Joseph Kim. Analysis and interpretation of data: AH Choi, RA Nelson, Joseph Kim. Drafting of article: AH Choi, RA Nelson. Critical revision for intellectual content: AH Choi, RA Nelson, S Merchant, Jae Kim, J Chao, Joseph Kim. Final approval: AH Choi, RA Nelson, S Merchant, Jae Kim, J Chao, Joseph Kim.
Presentations: 2015 Digestive Disease Week, American Society of Gastrointestinal Endoscopy, Washington, D.C.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murakami T. Definition and gross classification of early gastric cancer. Gann Monogr Cancer Res. 1971;11:53–55. [Google Scholar]
- 2.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 3.Nam MJ, Oh SJ, Oh CA, et al. Frequency and predictive factors of lymph node metastasis in mucosal cancer. J Gastric Cancer. 2010;10:162–7. doi: 10.5230/jgc.2010.10.4.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–23. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 5.Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271–8. doi: 10.1097/SLA.0b013e3181fbad42. [DOI] [PubMed] [Google Scholar]
- 6.Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg. 2010;210:418–27. doi: 10.1016/j.jamcollsurg.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 7.The Paris endoscopic classification of superficial neoplastic lesions. esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3–43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 8.Nascimbeni R, Burgart LJ, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200–6. doi: 10.1007/s10350-004-6147-7. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Sun CL, Mailey B, et al. Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann Oncol. 2010;21:152–60. doi: 10.1093/annonc/mdp290. [DOI] [PubMed] [Google Scholar]
- 10.Nelson R, Ko EB, Arrington A, et al. Race and correlations between lymph node number and survival for patients with gastric cancer. J Gastrointest Surg. 2013;17:471–81. doi: 10.1007/s11605-012-2125-x. [DOI] [PubMed] [Google Scholar]
- 11.SEER*Stat. SEER*Stat Database Sub vintage 2009 Pops (1992–2009)<Katrina/Rita Population Adjustment>-Linked to Country Attributes, (ed Released April 2012, based on the November 2011 submission), 2011
- 12.American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 7. New York, NY: Springer-Verlag; 2010. [Google Scholar]
- 13.Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–9. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett SM, Axon AT. Early gastric cancer in Europe. Gut. 1997;41:142–50. doi: 10.1136/gut.41.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang WL, Huang KH, Lan YT, et al. The Risk Factors of Lymph Node Metastasis in Early Gastric Cancer. Pathol Oncol Res. 2015 doi: 10.1007/s12253-015-9920-0. [DOI] [PubMed] [Google Scholar]
- 16.Bravo Neto GP, dos Santos EG, Victer FC, et al. Lymph node metastasis in early gastric cancer. Rev Col Bras Cir. 2014;41:11–7. doi: 10.1590/s0100-69912014000100004. [DOI] [PubMed] [Google Scholar]
- 17.Fukuhara S, Yabe M, Montgomery MM, et al. Race/Ethnicity is predictive of lymph node status in patients with early gastric cancer. J Gastrointest Surg. 2014;18:1744–51. doi: 10.1007/s11605-014-2590-5. [DOI] [PubMed] [Google Scholar]
- 18.Holscher AH, Drebber U, Monig SP, et al. Early gastric cancer: lymph node metastasis starts with deep mucosal infiltration. Ann Surg. 2009;250:791–7. doi: 10.1097/SLA.0b013e3181bdd3e4. [DOI] [PubMed] [Google Scholar]
- 19.Milhomem LM, Cardoso DM, Mota ED, et al. Frequency and predictive factors related to lymphatic metastasis in early gastric cancer. Arq Bras Cir Dig. 2012;25:235–9. doi: 10.1590/s0102-67202012000400005. [DOI] [PubMed] [Google Scholar]
- 20.Nieminen A, Kokkola A, Yla-Liedenpohja J, et al. Early gastric cancer: clinical characteristics and results of surgery. Dig Surg. 2009;26:378–83. doi: 10.1159/000226765. [DOI] [PubMed] [Google Scholar]
- 21.Pelz J, Merkel S, Horbach T, et al. Determination of nodal status and treatment in early gastric cancer. Eur J Surg Oncol. 2004;30:935–41. doi: 10.1016/j.ejso.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Biffi R, Botteri E, Cenciarelli S, et al. Impact on survival of the number of lymph nodes removed in patients with node-negative gastric cancer submitted to extended lymph node dissection. Eur J Surg Oncol. 2011;37:305–11. doi: 10.1016/j.ejso.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Gotoda T, Iwasaki M, Kusano C, et al. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868–71. doi: 10.1002/bjs.7033. [DOI] [PubMed] [Google Scholar]
- 24.Qin XP, Zhou Y, Chen Y, et al. XRCC3 Thr241Met polymorphism and gastric cancer susceptibility: a meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:226–34. doi: 10.1016/j.clinre.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Theuer CP, Al-Kuran R, Akiyama Y, et al. Increased epithelial cadherin expression among Japanese intestinal-type gastric cancers compared with specimens from American patients of European descent. Am Surg. 2006;72:332–8. [PubMed] [Google Scholar]
- 26.Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine MN, Julian JA. Registries that show efficacy: good, but not good enough. J Clin Oncol. 2008;26:5316–9. doi: 10.1200/JCO.2008.18.3996. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 29.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.