Abstract
Purpose
Glioblastoma (GBM) neurosurgical resection relies on contrast-enhanced MRI-based neuronavigation. However, it is well-known that infiltrating tumor extends beyond contrast enhancement. Fluorescence-guided surgery (FGS) using 5-aminolevulinic acid (5-ALA) was evaluated to improve extent of resection (EOR) of GBMs. Pre-operative morphological tumor metrics were also assessed.
Procedures
Thirty patients from a Phase II trial evaluating 5-ALA FGS in newly diagnosed GBM were assessed. Tumors were segmented pre-operatively to assess morphological features as well as post-operatively to evaluate EOR and residual tumor volume (RTV).
Results
Median EOR and RTV were 94.3% and 0.821 cm3, respectively. Pre-operative surface area to volume ratio and RTV were significantly associated with overall survival, even when controlling for the known survival confounders.
Conclusions
This study supports claims that 5-ALA FGS is helpful at decreasing tumor burden and prolonging survival in GBM. Moreover, morphological indices are shown to impact both resection and patient survival.
Keywords: Glioblastoma (GBM), 5-aminolevulinic Acid (5-ALA), Extent of Resection (EOR), Fluorescence-Guided Surgery (FGS), Tumor Segmentation, Tumor Morphology, Safety
Introduction
The goal of surgery in patients with glioblastoma (GBM) is maximum safe resection to relieve tumor mass effect, confirm diagnosis, and enhance the efficacy of adjuvant therapy. In recent years, however, the value of “more complete” resection (i.e. removal of the entire contrast-enhancing tumor) in extending progression-free survival (PFS) and overall survival (OS) has been recognized [1-8]. Consequently, much work has been done to enhance tumor resection, including the development of intraoperative technologies like fluorescence-guided surgery (FGS) [1, 4-5, 7, 9-10]. One of the most well-studied fluorescent markers used for FGS in malignant gliomas is 5-aminolevulinic acid (5-ALA) [11] . 5-ALA is an oral prodrug that is converted to the fluorescent compound protoporphyrin IX (PpIX) during heme biosynthesis in glioma cells, permitting real-time intraoperative visualization of malignant tissue with fluorescence microscopy [12-18]. Though the exact mechanisms of 5-ALA penetration of glioma are still uncertain, it appears that bulk flow of 5-ALA across the leaky GBM vasculature and active transport at the blood-brain and blood-CSF interfaces contribute to 5-ALA-induced fluorescence in both in vitro and in vivo tumor models [16, 19-22]. In a randomized Phase III study, Stummer et al. demonstrated that more extensive GBM resection and higher PFS rates were achievable with 5-ALA FGS compared to conventional resection, leading to multiple follow-up studies on the technique [4-5, 23-32].
One critical, yet often overlooked, component of determining neurosurgical efficacy is the metric(s) by which resection is evaluated [33]. Since convention is to target the enhancing component of contrast-enhanced T1-weighted images (CE-T1w) for resection, most studies utilize extent-of-resection (EOR), a comparison of pre- and post-operative CE-T1w tumor volumes [1, 3-8, 30, 34]. However, recent work suggests that residual contrast-enhancing tumor volume (RTV) more accurately reflects disease burden and patient survival [29]. Furthermore, the measurement of GBM tumor volume at a single time point is known to be inaccurate and irreproducible [35-36]. Taking the quotient of two separate measurements compounds this error, potentially resulting in erroneous relationships between EOR and survival [37].
The method by which tumor volumes are measured is also a potential source of error in resection analyses. Linear measurements are often used to evaluate response to chemoradiation therapy, but they are poorly suited for evaluating the curvilinear tumor remnants around a resection cavity [36, 38]. Modeling RTV with ellipsoids is also known to be largely inaccurate and highly susceptible to intra- and inter-reader variability [36, 39]. Some automated digital-image segmentation techniques have shown promise in measuring pre-operative tumor volumes, but few are designed to evaluate RTV where T1-hyperintense blood products (methemoglobin) and cavity collapse obscure measurements [40-41]. Such structural nuances can be accounted for using manual image contouring; however, this process is time-consuming and suffers from limited reproducibility [39, 42-44]. These limitations often lead to a resection being labeled as a “gross total resection” (GTR, with an RTV of predetermined volume) despite the presence of residual contrast-enhancement, potentially skewing endpoints. Due to the inadequacies of conventional methods, careful consideration of image segmentation techniques – particularly those specially designed for the measurement of pre- and post-operative contrast-enhancing tumor volumes – is essential for the generation of accurate relationships between resection and survival outcomes.
A Phase II study of 5-ALA FGS was initiated in 2011 at Emory University to evaluate its efficacy in the resection of newly diagnosed and recurrent malignant gliomas. To overcome the tumor measurement limitations of previous studies, a rigorously validated, semi-automated segmentation method designed specifically for resection-related outcomes was utilized to measure tumor pre- and post-operative tumor volumes [45]. We report the primary endpoints, EOR and RTV, for a prospective cohort of newly diagnosed GBM patients that have undergone 5-ALA FGS, and interim findings for secondary endpoints including adverse event (AE) rates, PFS, and OS. To the best of our knowledge, this is the largest prospective 5-ALA study in North America utilizing a semi-automated volumetric method designed specifically for tumor resection analysis, and the first to show pre-operative morphological metrics are not only associated with resection outcomes, but also survival outcomes for GBM patients receiving FGS.
Materials and Methods
Patient Selection
All patients included in this analysis were part of a prospective Phase II 5-ALA FGS study at Emory University Hospital Midtown and Emory University Hospital (2011-2014). Patients who had newly diagnosed or recurrent malignant gliomas suspected by MRI were eligible for FGS. The trial included all patients 18 years of age or older with normal bone marrow, renal, and liver function, KPS ≥ 60%, and able to understand and sign an informed consent document before surgery. Patients with midline, basal ganglia, or brainstem tumors, and those receiving any experimental therapies prior to surgery, or with a family history of porphyrias, were excluded. The inability to achieve a GTR was not an exclusion criterion. Demographic, genomic, and clinical values were recorded for each patient as potential covariates for endpoint analyses and included age (continuous and above/below 55 years), KPS before and 6 weeks after surgery, tumor location eloquence, MGMT gene promoter methylation status (methylated versus unmethylated), epidermal growth factor receptor (EGFR) amplification status, and phosphatase and tensin homolog (PTEN) deletion status.
Fluorescence-Guided Surgery
5-ALA (Gliolan®; provided by photonamic GmbH & Co. KG, Wedel, Germany and manufactured by medac Gmbh, Wedel, Germany) was administered to patients orally at 20 mg/kg of bodyweight 3-5 h before induction of general anesthesia. Image-guided microsurgical resection was then carried out using a standard surgical operating microscope adapted for fluorescence excitation and emission at wavelength ranges of 400–410 nm and 480–750 nm, respectively. Fluorescence-guided microsurgical removal of tissue was performed intermittently during the tumor resection, mainly along the contrast-enhancing tumor margins on neuronavigation. Tissue sampled at regions of residual tissue fluorescence was sent for histological confirmation by neuropathology.
Image Acquisition and Analysis
Pre-operative, high-resolution 3D MR images, including 1mm3 T1w magnetization-prepared rapid gradient-echo (3D MP-RAGE) images (TR/TE = 1900/3.52, 256 × 256 matrix, FA = 9°) before and after i.v. administration of gadolinium-based contrast agent (GBCA), were generated ≤ 7 days prior to surgery for neuronavigation and tumor segmentation. The same high-resolution T1w 3D MP-RAGE images were acquired post-operatively, within 48 h (usually within 24 h) of surgery, to evaluate EOR and RTV.
The 510(k) FDA-cleared image analysis platform VelocityAI (Varian Medical Systems, Palo Alto, CA) was used to semi-automatically segment contrast-enhancing tumors with a highly-sensitive fuzzy c-means clustering algorithm (Fuzzy) on pre- and post-operative CE-T1w images as previously described [45]. Spatially co-registered segmentation masks for contrast-enhancing tumor and necrotic tissue were generated for volume and surface area (SA) measurements (Fig. 1). An experienced neuroradiologist confirmed all final segmentations. This method of verification is commonplace for the generation of tumor segmentations for radiotherapy planning and segmentation algorithm validation [41, 46].
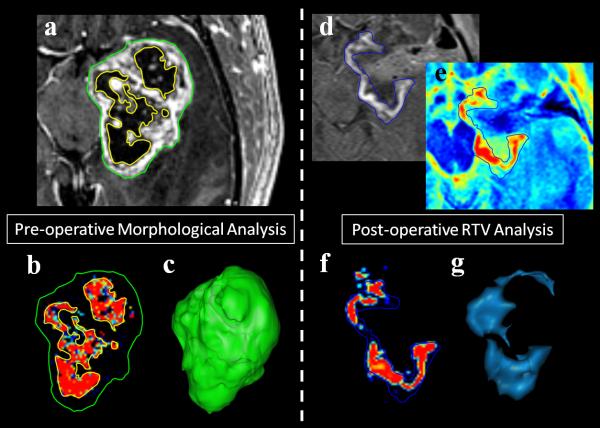
Fig. 1.
Example of semi-automated tumor segmentation results used for volumetric and morphological analysis. Preoperative CE-T1W images of GBM patients are segmented using a semi-automated method to generate volumes for contrast-enhancing (green contour) and necrotic (yellow contour) components (a). Voxels are classified into one of three tissue types (normal, contrast-enhancing, and necrotic) and image masks are generated for volumetric comparison (necrotic class pictured with contours, b). 3-Dimensional tumor surface reconstructions show the structural complexity of each tumor and can be used for surface area measurement (c). Pre-contrast images are subtracted from post-contrast enhancing images (d) to account for nonneoplastic enhancing blood products in the resection cavity (methemoglobin in blood) and to generate T1 difference maps (e). This subtraction image is then segmented using the same semiautomated method to generate contrast-enhancing tumor masks (f) and calculate residual contrast-enhancing tumor volumes (RTVs) (blue contour, d-f). Post-operative tumor morphology can also be visualized using this method (g).
Based on the European Phase III 5-ALA FGS trial criterion, a “complete” resection of the contrast-enhancing tumor (CRET) was defined as ≤ 0.175 cm3 of residual contrast-enhancement after surgery using volumetric assessment [30]. The traditional definition of GTR (residual contrast-enhancing tumor volume ≤ 1 cm3) was also considered. Tumor EOR calculations were determined by comparison of tumor volumes from pre- and post-operative volumetric MRI studies. Morphological indices based on segmentation results were generated as measures of tumor structure and included surface area (SA), percent of necrosis in tumor volume (NV%), and tumor surface area-to-volume ratio (SAVR). Patients were monitored by CE-T1w and T2-weighted, fluid-attenuated inversion recovery (FLAIR) at 3, 6, and 9 months post-surgery, or until tumor progression was confirmed per Response Assessment in Neuro-Oncology (RANO) criteria [36].
Statistical Methods
Statistical analyses were performed with the Statistical Analysis System (version 9; SAS Institute, Cary, NC), were all 2-sided, and with statistical significance set at p < 0.05. Univariate analyses were first used to evaluate the association of individual covariates with EOR and RTV. The impact on primary endpoint of dichotomous covariates was evaluated by conducting paired t-tests on group means and that of continuous covariates was evaluated by testing Pearson's correlation coefficients (ρ) versus no correlation (H0: ρ = 0). Multivariate analysis was then performed using a general linear model to assess the effects of multiple covariates on RTV. As RTV is the summation of contrast-enhancing voxels after surgery, it can be modeled as a Poisson process. Thus, a Poisson distribution was used for regression. A general, step-wise model selection procedure was used to select covariates to be included in the final model with the controlling variables age, pre-operative tumor volume, and MGMT gene promoter methylation status [47].
PFS and OS were evaluated using a right-censored univariate Cox regression model, and corresponding hazard rate ratios (HR) are reported with 95% confidence intervals. Covariates in Cox regression survival analysis were the same as those of the primary endpoint analysis, with the addition of EOR, RTV, pre-operative KPS, and 6 week KPS. The univariate and multivariate analysis procedures used for the survival outcomes were the same as those used for the primary endpoint analysis with controlling variables age, pre-operative KPS, and MGMT gene promoter methylation status. Pre-operative and 6-week KPS values were compared using the Wilcoxon signed-rank test to evaluate post-surgical functional outcomes.
Results
Between October 2011 and December 2014, 56 patients were enrolled in the Phase II 5-ALA FGS trial. Four patients were excluded as histology failed to confirm high-grade glioma (metastasis, n = 2; low-grade glioma, n = 2), and two patients consented for the study, but dropped out before surgery. A total of 31 newly-diagnosed GBM, 7 recurrent GBM, 4 newly-diagnosed WHO grade III (anaplastic) glioma, and 8 recurrent WHO grade III glioma patients were enrolled and completed 5-ALA FGS. Newly-diagnosed GBM patients were the only group analyzed in the currently reported study, as they were the largest, most homogenous group in the cohort. One patient was excluded from endpoint analysis due to an exceptionally large RTV (25.1 cm3) that was found to be an extreme outlier (> 3 interquartile ranges (IQRs) above the third quartile). Pertinent baseline clinical characteristics of analyzed data from 30 patients are shown in Table 1.
Table 1.
Patient and tumor characteristics
| % of patients (n = 30) | |||
|---|---|---|---|
| Gender | Male | 20.0 | 66.7% |
| Female | 10.0 | 33.3% | |
| Age | Range | 24-79 | |
| Median | 60.0 | ||
| SD | 12.2 | ||
| > 55 | 10.0 | 33.3% | |
| ≤ 55 | 20.0 | 66.7% | |
| Preoperative KPS | Range | 60- 90 | |
| Mean | 78 .0 | ||
| SD | 9.5 | ||
| < 80 | 11.0 | 36.7% | |
| 80-100 | 19.0 | 63.3% | |
| Preoperative Tumor Volume (cm3) | Range | 1.6-132 | |
| Mean | 43.9 | ||
| SD | 27.3 | ||
| Tumor Location | Eloquent | 26.0 | 86.7% |
| Non- Eloquent | 4.0 | 13.3% | |
| Histology | Primary | 28.0 | 93.3% |
| Secondary | 2.0 | 6.7% | |
| Therapy after FGS | RT + TMZ | 24 .0 | 80.0% |
| Other Therapy | 4.0 | 13.3% | |
| No TX | 2.0 | 6.7% |
Other therapy includes TMZ only, RT only, experimental treatment, or any combination of these excluding RT + TMZ.
Primary endpoints: EOR and RTV
Median EOR and RTV were found to be 94.8% (IQR: 11.1%; range: 70% - 100%) and 0.76 cm3 (IQR: 5.8 cm3; range: 0 - 15.3 cm3), respectively. CRET resection was achieved in 9 patients (30%), and GTR (≤ 1cm3) was achieved in 16 patients (53.3%). Age (> 55 or ≤55 yrs) was the single strongest covariate for predicting EOR and RTV with p = 0.003 and 0.004, respectively (Table 2). No other covariate was found to be significantly associated with EOR, although NV% exhibited a moderate positive trend. Pre-operative SAVR showed statistically significant negative correlation with RTV (ρ = −0.43, p = 0.02), while pre-operative SA and volume exhibited a positive correlation with RTV (ρ = 0.37, p = 0.04 and ρ = 0.50, p = 0.01).
Table 2.
Univariate analyses of EOR and RTV outcomes.
| EOR (%) | RTV (cm3) | |||||
|---|---|---|---|---|---|---|
| Dichotomous | N | Group Mean (SEM) | p-value | Group Mean (SEM) | p-value | |
| Age | > 55 | 20 | 89.66 (2.23) | <0.01* | 4.78 (1.09) | <0.01* |
| ≤ 55 | 10 | 97.66 (0.68) | 0.88 (0.39) | |||
| Site | Eloquent | 27 | 91.95 (1.80) | 0.44 | 3.66 (0.89) | 0.40 |
| Non-eloquent | 3 | 95.70 (1.27) | 1.87 (0.54) | |||
| MGMT Promoter | Methylated | 20 | 91.61 (2.13) | 0.53 | 4.30 (1.14) | 0.08 |
| Unmethylated | 10 | 93.78 (1.89) | 1.85 (0.50) | |||
| EGFR | Amplification | 14 | 90.51 (2.77) | 0.32 | 5.18 (1.45) | 0.06 |
| No Amplification | 16 | 93.92 (2.09) | 1.99 (0.78) | |||
| PTEN | Deletion | 25 | 92.09 (1.90) | 0.72 | 3.58 (0.93) | 0.77 |
| Intact | 5 | 93.54 (1.51) | 2.97 (0.80) | |||
| Continuous | N | ρ (SEM) | p-value | ρ (SEM) | p-value | |
|---|---|---|---|---|---|---|
| Age (yrs) | 30 | −0.34 (0.18) | 0.07 | 0.26 (0.18) | 0.17 | |
| SA (mm2) | 30 | −0.08 (0.19) | 0.67 | 0.37 (0.18) | 0.04* | |
| Preoperative Tumor Volume (cm3) | 30 | −0.13 (0.19) | 0.49 | 0.50 (0.16) | <0.01* | |
| NV% (%) | 30 | 0.33 (0.18) | 0.07 | −0.12 (0.19) | 0.52 | |
| SAVR (mm2/cm3) | 30 | 0.26 (0.18) | 0.17 | −0.43 (0.17) | 0.02* | |
Significant at p ≤ 0.05.
The final set of variables included in the multivariable RTV model with their coefficients and p-values can be found in Table 3. The controlling variables, although either significant or nearly significant in the univariate analysis, all failed to reach significance when considered with more morphological descriptors. Pre-operative SA and SAVR both retained their significance, with very small weights, in the multivariable model. Notably, although non-significant when analyzed alone, NV% reached significance in this model, exhibiting a very strong, negative correlation with RTV (coefficient = −5.55, p = 1E-5). Taken as a whole, morphological indices describing tumor shape, complexity, and composition appear to have a substantial impact in determining the residual tumor burden after 5-ALA FGS.
Table 3.
Final multivariate, general linear model describing RTV.
| Coefficient (SEM) | p-value | |
|---|---|---|
| Age (yrs)** | 0.01 (0.01) | 0.56 |
| SA (mm2) | 0.01 (0.01) | <0.01* |
| Preoperative Tumor Volume (cm3)** | −0.03 (0.02) | 0.09 |
| NV% (%) | −5.55 (1.03) | <0.01* |
| SAVR (mm2/cm3) | −0.04 (0.01) | <0.01* |
| MGMT Gene Promoter Methylation** | 0.48 (0.38) | 0.21 |
Significant at p ≤ 0.05.
Controlling Variables.
Secondary Endpoint: PFS and OS
Two patients who underwent 5-ALA FGS opted not to have chemoradiation, and were not included in the secondary endpoint analysis (n = 28). The proportions of patients progression-free at 6, 9, and 12 months were 45%, 29%, and 23%, respectively. In the Cox proportional hazard regression of PFS for these patients, MGMT gene promoter methylation decreased the risk of true tumor progression by 75.9% (HR: 0.24, 95% CI: 0.09–0.68, p = 0.01), while no significant effect was found for the previously associated covariates of age and KPS (Table 4). Furthermore, no significant impact of EOR, RTV, or morphological indices was found on PFS. These findings were recapitulated in the multivariate analysis, with MGMT gene promoter methylation status being the only variable significantly associated with PFS (Table 5).
Table 4.
Univariate Cox proportional hazard regression analysis for PFS and OS.
| PFS | OS | ||||
|---|---|---|---|---|---|
| Dichotomous | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | > 55 vs ≤ 55 | 0.97 (0.38, 2.45) | 0.94 | 1.45 (0.54, 3.89) | 0.11 |
| Site | Eloquent vs Non-eloquent | 0.53 (0.15, 1.87) | 0.32 | 0.95 (0.21, 4.25) | 0.94 |
| MGMT Promoter | Methylated vs Unmethylated | 0.24 (0.09, 0.68) | 0.01* | 0.45 (0.16, 1.31) | 0.14 |
| EGFR | Amplification vs No Amplification | 0.78 (0.30, 2.03) | 0.61 | 0.70 (0.26, 1.91) | 0.48 |
| PTEN | Deletion vs Intact | 0.85 (0.19, 3.86) | 0.84 | 0.42 (0.12, 1.54) | 0.19 |
| Continuous | HR (95% CI) | p-value | HR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Age (yrs) | 1.01 (0.97, 1.05) | 0.63 | 1.03 (0.98, 1.07) | 0.24 | |
| KPS before surgery | 0.99 (0.93, 1.04) | 0.61 | 0.93 (0.87, 0.99) | 0.04* | |
| KPS after surgery | 0.99 (0.95, 1.04) | 0.87 | 0.95 (0.92, 0.99) | 0.01* | |
| SA (mm2) | 1.00 (1.00, 1.00) | 0.23 | 1.00 (1.00,1.00) | 0.86 | |
| Preoperative Tumor Volume (cm3) | 0.99 (0.97, 1.01) | 0.12 | 1.00 (0.98, 1.02) | 0.83 | |
| NV% (%) | 0.10 (0.01, 2.98) | 0.18 | 0.04 (0.01, 1.48) | 0.08 | |
| SAVR (mm2/cm3) | 1.00 (1.00, 1.01) | 0.39 | 1.002 (1.00, 1.01) | 0.56 | |
| EOR (%) | 0.99 (0.93, 1.05) | 0.65 | 0.94 (0.89, 0.99) | 0.03* | |
| RTV (cm3) | 0.99 (0.91, 1.08) | 0.82 | 1.09 (1.00, 1.18) | 0.06 | |
Significant at p ≤ 0.05.
Table 5.
Final multivariate Cox proportional hazard regression models of PFS and OS.
| PFS | OS | |||
|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Age (yrs)** | 1.01 (0.97, 1.06) | 0.52 | 1.05 (0.98, 1.12) | 0.14 |
| RTV (cm3) | - | - | 1.17 (1.03, 1.34) | 0.02* |
| SAVR (mm2/cm3) | - | - | 1.01 (1.01, 1.02) | 0.03* |
| NV% (%) | 0.04 (0.01, 1.93) | 0.11 | 0.06 (0.01, 3.33) | 0.17 |
| KPS before surgery** | 1.00 (0.94, 1.06) | 0.99 | 0.95 (0.87, 1.04) | 0.29 |
| MGMT promoter methylation vs no methylation** | 0.19 (0.06, 0.57) | <0.01* | 0.14 (0.03, 0.63) | <0.01* |
Significant at p ≤ 0.05.
Controlling Variables.
The proportion of patients alive at 6, 9, and 12 months was 81%, 52%, and 39%, respectively. Cox proportional hazard modeling showed higher KPS before and 6 weeks after surgery, as well as EOR, all moderately decreased the risk of death in the follow-up period, with RTV trending toward significance (Table 4). When considered in a multivariable model alongside age and morphological measures, pre-operative and 6-week post-operative KPS lost their significant association with OS (Table 5). Instead, MGMT gene promoter methylation, SAVR, and RTV reached significance. Notably, NV% showed strong association with OS in the univariate and multivariate analyses, but fell short of statistical significance in both (HR: 0.04, 95% CI: 0.01-1.48, p= 0.08 and HR: 0.06, 95% CI: 0.01-3.33, p= 0.17, respectively).
Adverse events (AEs)
One severe adverse event potentially attributable to 5-ALA was observed in all 30 patients. A patient with a history of gastrointestinal perforation exhibited a second perforation within 24 h of 5-ALA administration. Surgical correction of the perforation resulted in a return to baseline shortly thereafter.
Substance-specific side effects associated with 5-ALA (cardiac, gastrointestinal, and dermatological) were infrequent (Supplementary Data, Table S1). Dermatological abnormalities were mild and rare (10% of patients) and did not include photodermatosis. Cardiac AEs were slightly more common, yet equally mild, with 5 patients exhibiting arrhythmias (sinus bradycardia, n = 4; sinus tachycardia, n = 1) and 2 patients exhibiting exacerbation of hypertension. Nausea, constipation, and gastroesophageal reflux were the most common gastrointestinal AEs, but were well-managed with standard pharmacotherapy.
Hematological and blood chemistry abnormalities were generally mild, and resolved without treatment or with supportive therapy. Anemia was the most common abnormality, affecting 50% of patients, followed by thrombocytopenia and leukocytosis, affecting 23% and 3% of patients, respectively. Mild supportive therapy restored baseline values for most of these patients within 6 weeks. Aminotransferases (AST and ALT) and alkaline phosphatase (Alk Phos) were elevated in nearly one-third of patients after surgery, but this number dropped substantially when evaluated at 6 weeks (Supplementary Data, Table S2). Hypoalbuminemia was the most common hepatobiliary disturbance observed, but returned to baseline in most patients. Few patients with AST and bilirubin perturbations were observed, most of which normalized to baseline by week 6. Of note, the majority of patients received adjuvant chemoradiation (starting at 4-5 weeks post-surgery) and steroids (immediately after surgery as needed). These therapies are known to cause blood dyscrasias and likely contribute to these hematological and blood chemistry abnormalities.
Neurological AEs made up 14.6% of those identified, varying in severity from digital numbness to hemiplegia, and affected 60% of patients at some point in the follow-up period. Most neurological deficits normalized within the patient's post-operative stay or shortly thereafter, with a single patient exhibiting ongoing deficits (i.e. dysphasia, visual field cut, and gait disturbance). Median pre-operative and 6 week post-operative KPS were found to be significantly different at p = 0.03 (80% [IQR: 20%; range: 60% - 90%] and 70% [IQR: 30%; range: 40%-90%], respectively), implying poor functional outcomes. However, when KPS was stratified (≥ 80% and < 80% groups), no significant difference in medians was observed (p = 0.08). This discrepancy is likely due to the low pre-operative KPS and significant drop at 6 weeks seen in 3 patients with tumors centered in eloquent brain regions.
Discussion
The randomized, phase III European study provided evidence for the efficacy of 5-ALA FGS with more resections of GBM and better PFS in comparison to conventional resection [30]. However, FDA approval of 5-ALA for FGS of malignant gliomas requires separate trials carried out in the United States. Our trial is the first in North America to utilize 5-ALA FGS for malignant gliomas. Our study supports previous results and provides additional spatial metrics to describe the relationship between tumor morphology and resection. Furthermore, we have found that more complete resection of newly diagnosed GBM is associated with better OS, and that pre-operative tumor structure may impact survival by modulating the likelihood of complete resection.
While minimal interaction was found between pre-operative tumor volume measurements and EOR, the association of pre-operative tumor morphological metrics with RTV was notable. Structural indices could outperform current methods for predicting the complete resection of contrast-enhancing tumor. As pre-operative tumor volume is the most salient feature in imaging, it is generally used to determine tumor resectability [48]. However, this single metric can be misleading, particularly in the case of large, high NV% and SAVR tumors, which were shown here to be associated with more complete resections. A more suitable method for determining resectability would require the incorporation of morphological factors describing tumor shape and complexity. A more accurate representation of tumor resectability is possible with the type of segmentation tool used here, and represents a paradigm shift in the pre-operative methods used for evaluating resection feasibility.
Our study also supports previous finding that more complete tumor resections ultimately result in greater OS [1, 4-5, 7-9, 49]. In our sample, we found an increase in risk of death by 17.5% per cm3 of residual tumor. Moreover, morphological analysis allowed the identification of moderate association between SAVR and OS (1.1% increase in risk of death for every mm2 increased at constant volume). Thus SAVR could potentially be interpreted as a quantitative marker of infiltrative capacity, with a large SAVR representing complexity at the tumor margin and an increased interaction of tumor with surrounding tissue. Further, the survival-SAVR association in this study could result from some unknown interaction between biological growth characteristics and morphological features (i.e. high SAVR tumors may be intrinsically more aggressive than low SAVR tumors). This association becomes even more relevant when considered in concert with the strongest potential confounders of this comparison: MGMT gene promoter methylation status and RTV [50]. Also of note is the seemingly protective effect exhibited NV% on OS, a finding largely attributed to gene expression patterns previously [51]. The current work suggests that the protective effect of NV% may also be associated with the increased likelihood of complete resection via NV%'s modulation of RTV. Further studies investigating the differences in morphology and growth patterns among biologically-distinct GBM subtypes could potentially shed light on these biology-resection-survival interactions and should be considered in larger, multi-site FGS trials investigating survival outcomes.
Though our study supports much of what others have set forth concerning the utility and safety of 5-ALA FGS, there are some points of disagreement. The current study exhibited CRET in only 30% of cases, a rate lower than that reported by other studies [30, 34]. Although it is possible these differences are due to surgeon experience and hardware, these differences are likely due to the combination of three unique factors in this study: the high proportion of patients with eloquent tumors, the high post-operative MR image resolution, and the high-sensitivity tumor measurement method. Our study included over 25% more eloquent cases than the European multicenter study (86.7% vs 61.0%, respectively), increasing the likelihood of subtotal resections from the outset. Further, the post-operative MRI voxel volume used in that study (0.175 cm3) was orders of magnitude larger than that in our study (0.001 cm3). With such coarse spatial resolution and the lack of a sensitive segmentation tool, small RTVs approaching the size of a voxel (0.175 – 1 cm3) could be falsely labeled as “no tumor”. Seven patients in our study fall within this RTV range (23%), all of which could have been misclassified as CRET based on lower resolution images. Second, modeling contrast-enhancing tumors with spheroids based on tumor diameters is known to be grossly inaccurate and highly susceptible to intra- and inter-reader variability [36, 39]. The segmentation method used for measurement in this study was validated previously and showed high intra- and inter-reader reproducibility, as well as strong volume and spatial agreement with expert-segmented tumors [45]. Moreover, the combination of high-resolution MRI with voxel-level tissue classification allows the measurement of morphological parameters, like SA, that are highly sensitive to fine changes in tumor structure. This combination bolsters the novelty of the tumor structure/complexity claims in this work, as few studies have described such metrics utilizing measurement methods specifically designed for resection analysis [52-53].
Limitations and Strengths
The absence of a control group, the small sample size, and inhomogeneity in salvage therapies after chemoradiation are clear limitations of this study. Our study does, however, represent one of the largest single-center, prospective studies analyzing resection and survival outcomes of 5-ALA FGS for newly diagnosed GBM in North America using a validated, semiautomated tumor segmentation method. Most studies to date, have used linear diameter or manual segmentation for this end, both of which are heavily user-biased and subject to inaccuracies [30, 36, 38-39, 42]. Lastly, using precision segmentation, this study demonstrates the impact that morphological tumor features (SA, SAVR, and NV%) have on resection and survival outcomes. In a higher-powered study, these indices could be used to evaluate the biology-morphology relationships in distinct GBM subtypes to evaluate the specific impact of tumor structure and resection on survival outcomes in each type.
Conclusions
This clinical study supports the previous randomized, European trial where 5-ALA FGS is helpful at decreasing RTV and providing more complete tumor resections in GBM. We have also found that 5-ALA FGS does increase OS in patients with GBM. Multi-center FGS studies may provide further evidence to support 5-ALA FGS efficacy and safety in a larger, more diverse cohort, and more precisely define the relationship between pre-/post-operative imaging features and resection/survival outcomes in the US to support FDA approval of Gliolan®. Features investigated in these studies should include morphological indices describing tumor complexity and composition, like SA, SAVR, and NV%, as they have been shown here to impact not only tumor resection, but also patient survival.
Supplementary Material
Acknowledgements
This work was supported by National Institute of Health grants R21 CA141836 (CGH/CAH/HS), a predoctoral fellowship F31 CA180319 (JSC), and a research grant from Nx Development Corp. (CGH).
Footnotes
Conflict of Interest:
Dr. Costas Hadjipanayis receives intellectual fees from Nx Development Corp. (Miami, Florida).
References
- 1.Bloch O, Han SJ, Cha S, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012;117:1032–1038. doi: 10.3171/2012.9.JNS12504. [DOI] [PubMed] [Google Scholar]
- 2.Stummer W, Meinel T, Ewelt C, et al. Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J Neurooncol. 2012;108:89–97. doi: 10.1007/s11060-012-0798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardesty DA, Sanai N. The value of glioma extent of resection in the modern neurosurgical era. Front Neurol. 2012;3:140–150. doi: 10.3389/fneur.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, et al. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014;82:e257–265. doi: 10.1016/j.wneu.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16:113–122. doi: 10.1093/neuonc/not137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 7.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–764. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 8.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 9.Vogelbaum MA, Jost S, Aghi MK, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70:234–243. doi: 10.1227/NEU.0b013e318223f5a7. [DOI] [PubMed] [Google Scholar]
- 10.Kubben PL, Scholtes F, Schijns OE, et al. Intraoperative magnetic resonance imaging versus standard neuronavigation for the neurosurgical treatment of glioblastoma: A randomized controlled trial. Surg Neurol Int. 2014;5:70–79. doi: 10.4103/2152-7806.132572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadjipanayis CG, Widhalm G, Stummer W. What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery. 2015 doi: 10.1227/NEU.0000000000000929. [Epub Ahead of Print] DOI: 101227/NEU0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinnen P, de Rooij FW, van Velthuysen ML, et al. Biochemical basis of 5-aminolaevulinic acid-induced protoporphyrin IX accumulation: a study in patients with (pre)malignant lesions of the oesophagus. Br J Cancer. 1998;78:679–682. doi: 10.1038/bjc.1998.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stummer W, Stocker S, Novotny A, et al. In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B. 1998;45:160–169. doi: 10.1016/s1011-1344(98)00176-6. [DOI] [PubMed] [Google Scholar]
- 14.Gibson SL, Havens JJ, Metz L, Hilf R. Is δ-Aminolevulinic Acid Dehydratase Rate Limiting in Heme Biosynthesis Following Exposure of Cells to δ-Aminolevulinic Acid? Photochem Photobiol. 2001;73:312–317. doi: 10.1562/0031-8655(2001)073<0312:iaadrl>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Collaud S, Juzeniene A, Moan J, Lange N. On the selectivity of 5-aminolevulinic acid-induced protoporphyrin IX formation. Curr Med Chem Anticancer Agents. 2004;4:301–316. doi: 10.2174/1568011043352984. [DOI] [PubMed] [Google Scholar]
- 16.Novotny A, Xiang J, Stummer W, Teuscher NS, Smith DE, Keep RF. Mechanisms of 5-aminolevulinic acid uptake at the choroid plexus. J Neurochem. 2000;75:321–328. doi: 10.1046/j.1471-4159.2000.0750321.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu JT, Meza D, Sanai N. Trends in fluorescence image-guided surgery for gliomas. Neurosurgery. 2014;75:61–71. doi: 10.1227/NEU.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivo M, Wilson BC. Mapping ALA-induced PPIX fluorescence in normal brain and brain tumour using confocal fluorescence microscopy. Int J Oncol. 2004;25:37–45. [PubMed] [Google Scholar]
- 19.Rud E, Gederaas O, Hogset A, Berg K. 5-aminolevulinic acid, but not 5-aminolevulinic acid esters, is transported into adenocarcinoma cells by system BETA transporters. Photochem Photobiol. 2000;71:640–647. doi: 10.1562/0031-8655(2000)071<0640:aabnaa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Ennis SR, Novotny A, Xiang J, et al. Transport of 5-aminolevulinic acid between blood and brain. Brain Res. 2003;959:226–234. doi: 10.1016/s0006-8993(02)03749-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhao SG, Chen XF, Wang LG, et al. Increased expression of ABCB6 enhances protoporphyrin IX accumulation and photodynamic effect in human glioma. Ann Surg Oncol. 2013;20:4379–4388. doi: 10.1245/s10434-011-2201-6. [DOI] [PubMed] [Google Scholar]
- 22.Xiang J, Hu Y, Smith DE, Keep RF. PEPT2-mediated transport of 5-aminolevulinic acid and carnosine in astrocytes. Brain Res. 2006;1122:18–23. doi: 10.1016/j.brainres.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanai N. Emerging operative strategies in neurosurgical oncology. Curr Opin Neurol. 2012;25:756–766. doi: 10.1097/WCO.0b013e32835a2574. [DOI] [PubMed] [Google Scholar]
- 24.Della Puppa A, De Pellegrin S, d'Avella E, et al. 5-aminolevulinic acid (5-ALA) fluorescence guided surgery of high-grade gliomas in eloquent areas assisted by functional mapping. Our experience and review of the literature. Acta Neurochir (Wien) 2013;155:965–972. doi: 10.1007/s00701-013-1660-x. [DOI] [PubMed] [Google Scholar]
- 25.Schucht P, Beck J, Abu-Isa J, et al. Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery. 2012;71:927–935. doi: 10.1227/NEU.0b013e31826d1e6b. [DOI] [PubMed] [Google Scholar]
- 26.Schucht P, Seidel K, Beck J, et al. Intraoperative monopolar mapping during 5-ALA-guided resections of glioblastomas adjacent to motor eloquent areas: evaluation of resection rates and neurological outcome. Neurosurg Focus. 2014;37:E16–24. doi: 10.3171/2014.10.FOCUS14524. [DOI] [PubMed] [Google Scholar]
- 27.Keles GE, Chang EF, Lamborn KR, et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006;105:34–40. doi: 10.3171/jns.2006.105.1.34. [DOI] [PubMed] [Google Scholar]
- 28.Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol. 2005;26:2466–2474. [PMC free article] [PubMed] [Google Scholar]
- 29.Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121:1115–1123. doi: 10.3171/2014.7.JNS132449. [DOI] [PubMed] [Google Scholar]
- 30.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 31.Hadjipanayis CG, Jiang H, Roberts DW, Yang L. Current and future clinical applications for optical imaging of cancer: from intraoperative surgical guidance to cancer screening. Semin Oncol. 2011;38:109–118. doi: 10.1053/j.seminoncol.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbosa BJ, Mariano ED, Batista CM, et al. Intraoperative assistive technologies and extent of resection in glioma surgery: a systematic review of prospective controlled studies. Neurosurg Rev. 2015;38:217–226. doi: 10.1007/s10143-014-0592-0. [DOI] [PubMed] [Google Scholar]
- 34.Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93:1003–1013. doi: 10.3171/jns.2000.93.6.1003. [DOI] [PubMed] [Google Scholar]
- 35.van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's Criteria. J Clin Oncol. 2009;27:2905–2908. doi: 10.1200/JCO.2009.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 37.Hughes I, Hase T. Measurements and their Uncertainties: A Practical Guide to Modern Error Analysis. New York. Oxford University Press Inc.; New York: 2010. Error Propagation. pp. 37–52. [Google Scholar]
- 38.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 39.Tofts PS, Collins DJ. Multicentre imaging measurements for oncology and in the brain. BJR. 2011;84:S213–226. doi: 10.1259/bjr/74316620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordillo N, Montseny E, Sobrevilla P. State of the art survey on MRI brain tumor segmentation. Magn Reson Imaging. 2013;31:1426–1438. doi: 10.1016/j.mri.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Bauer S, Wiest R, Nolte LP, Reyes M. A survey of MRI-based medical image analysis for brain tumor studies. Phys Med Biol. 2013;58:R97–129. doi: 10.1088/0031-9155/58/13/R97. [DOI] [PubMed] [Google Scholar]
- 42.White DR, Houston AS, Sampson WF, Wilkins GP. Intra- and interoperator variations in region-of-interest drawing and their effect on the measurement of glomerular filtration rates. Clin Nucl Med. 1999;24:177–181. doi: 10.1097/00003072-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Sorensen AG, Patel S, Harmath C, et al. Comparison of diameter and perimeter methods for tumor volume calculation. J Clin Oncol. 2001;19:551–557. doi: 10.1200/JCO.2001.19.2.551. [DOI] [PubMed] [Google Scholar]
- 44.Chow DS, Qi J, Guo X, et al. Semiautomated volumetric measurement on postcontrast MR imaging for analysis of recurrent and residual disease in glioblastoma multiforme. AJNR Am J Neuroradiol. 2014;35:498–503. doi: 10.3174/ajnr.A3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cordova JS, Schreibmann E, Hadjipanayis CG, et al. Quantitative tumor segmentation for evaluation of extent of glioblastoma resection to facilitate multisite clinical trials. Transl Oncol. 2014;7:40–47. doi: 10.1593/tlo.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang HH, Zhuang AH, Valentino DJ, Chu WC. Performance measure characterization for evaluating neuroimage segmentation algorithms. Neuroimage. 2009;47:122–135. doi: 10.1016/j.neuroimage.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 47.Collett D. Strategy for model selection. In: Chatfield C, Lindsey J, Tanner M, Zidek J, editors. Modeling Binary Data. Chapman & Hall/CRC; Boca Raton, FL: 2003. pp. 91–97. [Google Scholar]
- 48.Orringer D, Lau D, Khatri S, et al. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. 2012;117:851–859. doi: 10.3171/2012.8.JNS12234. [DOI] [PubMed] [Google Scholar]
- 49.Sanai N, Berger MS. Extent of resection influences outcomes for patients with gliomas. Rev Neurol (Paris) 2011;167:648–654. doi: 10.1016/j.neurol.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Paravati AJ, Heron DE, Landsittel D, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma and anaplastic astrocytoma: validation of Radiation Therapy Oncology Group-Recursive Partitioning Analysis in the IMRT and temozolomide era. J Neurooncol. 2011;104:339–349. doi: 10.1007/s11060-010-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutman DA, Cooper LA, Hwang SN, et al. MR imaging predictors of molecular profile and survival: multi-institutional study of the TCGA glioblastoma data set. Radiology. 2013;267:560–569. doi: 10.1148/radiol.13120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crawford FW, Khayal IS, McGue C, et al. Relationship of pre-surgery metabolic and physiological MR imaging parameters to survival for patients with untreated GBM. J Neurooncol. 2009;91:337–351. doi: 10.1007/s11060-008-9719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saraswathy S, Crawford FW, Lamborn KR, et al. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J Neurooncol. 2009;91:69–81. doi: 10.1007/s11060-008-9685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.