Abstract
Microscopy and field adaptable rapid diagnostic tests (RDTs) are not sensitive and specific in certain conditions such as poor training of microscopists, lack of electricity, or the inability to detect non-falciparum malaria. More sensitive point of care testing (POCT) would reduce delays in diagnosis and initiation of therapy. In the current study, we have evaluated the efficacy of non-instrumented nucleic acid amplification (NINA) coupled with LAMP for detection of traveler’s malaria (n = 140) in comparison with microscopy, nested PCR, and the only FDA-approved rapid diagnostic test. NINA-LAMP was 100% sensitive and 98.6% specific when compared to nested PCR. For non-falciparum detection, NINA-LAMP sensitivity was 100% sensitive compared to nested PCR whereas RDT sensitivity was 71%. LAMP is highly sensitive and specific for symptomatic malaria diagnosis regardless of species in a POCT setting.
Keywords: malaria, point of care test, LAMP
Introduction
Due to the effective implementation of Malaria Control Programs (MCPs), global malaria cases, and the associated mortality have decreased by 26% and 46% respectively since 2000 (42). As evidence of progress, 39 countries have now refocused their programs toward elimination (12). However, artemisinin resistant Plasmodium falciparum has already emerged in South-east Asia and has become a major concern for global health (3, 23). Gold standard microscopy can only achieve a maximum of 80–90% accuracy when used in the field and only when conducted by expert malaria microscopists (34). Failure to detect submicroscopic infections results in untreated patients can be a driving force for the spread of resistant and slow clearing parasites throughout South-east Asia and promulgates the risk of the global spread of drug resistant malaria. Similarly, RDTs currently used in field settings also have limitations such as low sensitivity, false positivity due to cross reactivity, and variable stability at the field site (2, 25). RDTs have especially poor performance in the detection of non-falciparum malaria (7, 39). Although PCR based methods are accurate for low parasitemia Plasmodium infection detection, logistical issues often make this technique incompatible as a POCT (24). The number of malaria cases imported into non-endemic countries such as Canada, the US and Europe continues to increase, likely as a result of globalization and changing immigration and travel patterns (8, 19, 29). In 2009, 35% of Canadian travelers bound to destinations other than the United States travelled to regions with a risk of malaria, a 131% increase from 2000 (11). In addition to the increased incidence of malaria cases seen in Canada, the number of cases severe malaria due to P. falciparum is also increasing each year (19, 21). Travelers to malaria endemic areas may not always appreciate their risk of malaria and do not take malaria prophylaxis or use personal protective measures against mosquito bites (5). Travel for the purpose of “visiting friends and relatives” (VFR) represents a particularly high-risk group of travelers for the acquisition of malaria and is becoming an increasingly important group of travelers (4, 10, 18). Even in well-resourced settings adverse outcomes including death due to malaria may still occur. Misdiagnosis or delay in diagnosis leading to delay in initiating appropriate anti-malarial therapy are factors that contribute to adverse outcomes (26). Diagnosis of malaria in non-endemic settings such as Canada may also be challenging because physicians and laboratory personnel outside of major tertiary care centers may have limited access to gold standard testing and rely on RDTs.
Loop Mediated Isothermal Amplification (LAMP) as an approach for isothermal nucleic acid amplification was first developed in 2000 (28). The LAMP assay for P. falciparum was introduced in 2006 (33) and subsequently a genus and species specific LAMP assay was developed by Han and co-authors targeting 18S rDNA sequence (13). The visual detection system of LAMP amplified nucleic acids was improved by pre-addition of calcein and Hydroxynaphtholblue (HNB) to the LAMP reaction mixture (24, 41). A mitochondrial DNA LAMP assay for Plasmodium species was also developed to increase the sensitivity (32). Along with in-house methods, a commercial kit for LAMP assay was developed by Eiken Chemical Company, Japan and evaluated (14, 31). Real-time fluorescence LAMP with a portable, rechargeable fluorescence reader was also used for malaria detection in the field (30). Importantly, the LAMP method is robust and tolerant to amplification in the presence of blood impurities and therefore does not require pure RNA/DNA like PCR. Hemoglobin, antibodies, and other blood content that strongly inhibit common commercial PCR DNA polymerases (e.g. Taq, Pfu) do not have as strong an effect on Bst DNA polymerase adapted for LAMP reactions. Bst polymerase amplifies DNA with great accuracy at constant temperature via inherent strand displacement activity, which eliminates the requirement for thermal cycling (1).
Despite these developments in nucleic acid based detection of malaria parasites, LAMP assays, including sample preparation and amplification detection, still require a heat block or water bath, a centrifuge, and optionally an expensive fluorescent reader, all of which often require an electricity supply. Program for Appropriate Technology in Health (PATH) has developed non-instrumented nucleic acid amplification (NINA) platform heater (Figure 1) to facilitate isothermal amplification of nucleic acids at the point of care. This heater does not require any electricity or other instrumentation as heat generation derives from Mg-Fe exothermic chemical reaction and is tuned to the target isothermal assay temperature via coupling to an engineered phase change material (PCM) (16, 17, 36, 37, 40). Previously our group evaluated NINA-LAMP performance in Ethiopia for symptomatic malaria diagnosis in comparison with microscopy and nested PCR (35). In the current study, we sought to answer two questions: (1) How does the performance of the latest version of the NINA heater compare to a standard PCR thermo-cycler for detecting malaria parasite infection in returning travelers using microscopy and nested PCR as gold standard, and (2) How does LAMP compare to the BinaxNOW malaria RDT head-to-head in order to determine the feasibility of the LAMP as a POCT in the non-endemic setting for returning travelers.
Figure 1.
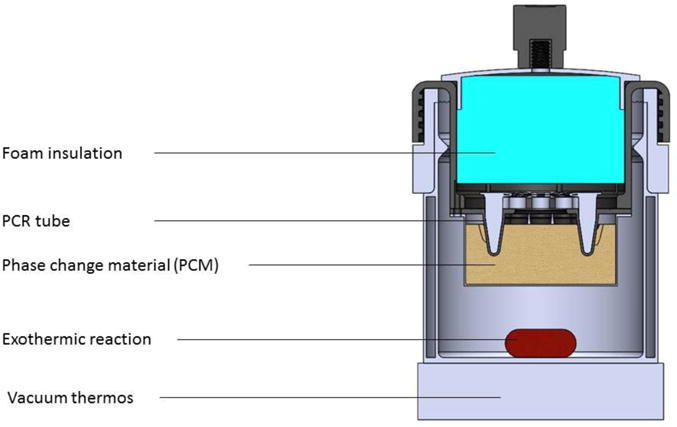
Depiction of the Non-instrumented nucleic acid amplification (NINA) device used in the present study.
Materials and Methods
Sample collection
The returning traveler samples used in the present study were previously collected, curated, and stored at Calgary Laboratory Service, Alberta, Canada (CLS). Individuals who travel to malaria endemic regions may become infected by the Plasmodium parasite and develop the characteristic febrile symptoms of infection only following their returning to the Calgary region. Based on clinical symptoms and CLS standard procedure, travelers returning to Calgary from malaria endemic areas are routinely evaluated for malaria parasite infection via microscopy and the BinaxNOW malaria RDT. All samples were collected from 2003 to 2014. Whole blood samples were collected and aliquoted in EDTA tubes and stored at −80°C until use. The sample size was pre-calculated assuming 90% sensitivity and specificity according to the method described by Malhotra et al., 2010 using a two-sided z-test at a significance level of 0.05 by considering precision at 10% (20). As a result, it was determined that at least 69 positive and 69 negative samples were needed to achieve significance in the analysis. Therefore, a total 69 microscopy positive and 71 microscopy negative samples were included.
Microscopy and RDT
Microscopy slides were prepared immediately after blood collection using standard methods. The Giemsa stained slides were then used to assess the proportion of red blood cells that were infected. Parasitaemia were determined by the proportion (%) of the red blood cells (RBC) that were infected and number of RBCs per micro-liter of blood. BinaxNOW malaria RDT tests were carried out following two different schemes. For samples collected from 2010–2014, RDT testing was performed immediately after collection. For previously collected samples (before 2010), RDT testing was carried out on frozen whole blood stored at −80°C according to the manufacturer’s instructions.
DNA extraction
Two different protocols were followed to isolate DNA for amplification. For nested PCR amplification, pathogen DNA was isolated using the QiaAmp blood DNA extraction kit (Qiagen, Germany) to elute an equal volume of DNA from whole blood. For LAMP amplification, the boil and spin method was used (32). Briefly, 20μL of the whole blood specimen was mixed with 20μL of extraction buffer (40 mM Tris (pH 6.5)-0.4% sodium dodecyl sulfate (SDS)) followed by incubation at 95°C for 5 minutes. The boiled specimen was then centrifuged at 10,000g for 10 minutes. Fifteen μL of the supernatant was diluted 10 times with distilled water and directly used for LAMP amplification. Figure 2 details the work flow used in the evaluation of all four methods performed in this study and described below.
Figure 2.
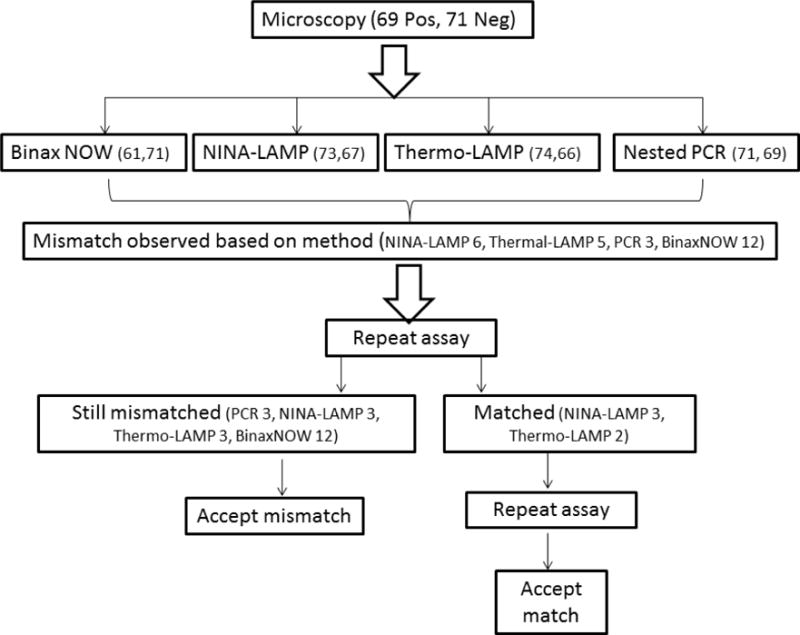
Workflow used in this study when resolving discordance (mismatch results) between the NINA-LAMP, thermal-LAMP (performed in a thermocycler), BinaxNOW (RDT), and nested PCR compared to initial microscopy for the detection of malaria parasites. Number of positive (pos) and negative (neg) results are indicated in parentheses.
Nested PCR
Nested PCR was carried out on QiaAmp extracted DNA according to the method described by Snoununu (38) with a minor modification. One micro-liter of DNA was used as template for the first amplification step. That amplification product was then diluted 10× and 2 μL was used for the next round of amplification. Taq DNA polymerase and other reagents from New England Biolabs (NEB) were used for amplification and products were visualized via SYBR Safe staining after amplicon separation on a 2% agarose gel.
LAMP assay
The LAMP assay was carried out using the Loopamp Pan/Pf Malaria detection kit (stable at room temperature) produced by Eiken Chemical Company (Taito-ku, Tokyo, Japan). Thirty μL of extracted and diluted DNA was added to each reaction tube. Duplicate reaction tubes were prepared for each sample for Pan/Pf detection according to the manufacturer’s instructions. One tube was heated in the PCR thermo-cycler (Eppendorf Mastercycler, Eppendorf, Germany) set at 65°C and the other placed inside the NINA platform heater preheated to 65°C. LAMP reactions were carried out for 40 minutes. After 40 minutes, turbidity was measured by naked eye by three independent readers. Consensus from any two readers was considered as the final result. One P. falciparum (initial parasitaemia 125,000/μL) and one P. vivax (initial parasitaemia 25,000/μL) positive whole blood specimen (collected from two different patients) were diluted with uninfected blood to 10000, 1000, 100, 10, 5, and 1 parasite(s)/μL. Subsequently, the LAMP assay was carried out by NINA and PCR machine.
Results
In the case of serially diluted specimens, the LAMP assay in NINA and thermo-cycler were equally successful in detecting P. falciparum up to a lower limit of five parasites /μL for both Pan and Pf primer sets. On the other hand, for P. vivax minimum level of detection was one parasite/μL for both LAMP platforms. LAMP data, whether obtained from isothermal DNA amplification in the NINA platform heater or in the thermo-cycler, were consistent with the exception of one false negative with the LAMP platform. However, both NINA-LAMP and thermo-cycler-LAMP resulted in 2 false positive P. falciparum cases (negative by microscopy and nested PCR) each along with one false negative (positive by microscopy and nested PCR) by NINA-LAMP. Pan LAMP assay results were consistent on all platforms throughout the study (Table 1). Importantly, nested PCR detected two additional positive cases from microscopy negatives and also three mixed infections. The results of the discordant analysis resolved are depicted in Figure 2. A mismatch where one or more assays differed from the original microscopic diagnosis was observed with NINA-LAMP 3 times, LAMP performed on the thermo-cycler (3), nested PCR (3), and RDT (12). Ability to detect Plasmodium genus (Pan) was considered in the overall accuracy analysis of LAMP performance. LAMP detected all microscopy positive samples resulting in an overall sensitivity of 100% (95% CI, 93.4–100; 69/69) and an overall specificity of 98.6% (95% CI, 91–99) compared to nested PCR considered as gold standard (Table 2). In contrast, the BinaxNOW malaria RDT had an overall sensitivity of 85.9% (95% CI, 74.5–92.4; 59/69) and specificity 98.6% (95% CI, 91.1–99.9) compared to nested PCR.
Table 1.
Microscopy, nested PCR, NINA-LAMP and BinaxNOW malaria RDT results for 140 patients suspected of having malaria.
| Result | Microscopy | Nested PCR | NINA-LAMP | LAMP-thermocycler | RDT (BinaxNOW) |
|---|---|---|---|---|---|
| Total Positive | 69 | 71 | 72 | 72 | 61 |
| P. falciparum | 38 | 40 (42 inc. mixed) | 41 | 41 | 38 |
| Total non-falciparum | 31 | 28 (31 inc. mixed) | 31 | 31 | 23 |
| P. vivax | 25 | 24 (26 inc. mixed) | N/A | N/A | N/A |
| P. ovale | 5 | 4 (5 inc. mixed) | |||
| P. malariae | 1 | 0 (1 inc. mixed) | |||
| Mixed | 0 | 3: P. falciparum + P. vivax (1) P. falciparum + P. malariae (1) P. vivax + P. ovale (1) |
NA | NA | 0 |
| Negative | 71 | 69 | 68 | 68 | 79 |
Table 2.
Sensitivity and Specificity of LAMP and BinaxNOW in comparison with microscopy and nested PCR.
| Test | Nested PCR | ||
|---|---|---|---|
| Sensitivity (%)(95% CI) | Specificity (%)(95% CI) | ||
| LAMP | Overall | 100(93.6–100) | 98.6(91.1–99.9) |
| P. falciparum | 97.6(85.9–99.9) | 100(95.3–100) | |
| non-falciparum | 100(86.3–100) | 99.1(94.3–100) | |
| BinaxNOW | Overall | 85.9(75.2–92.7) | 98.6(91.1–99.9) |
| P. falciparum | 90.5(76.5–96.9) | 100(95.3–100) | |
| non-falciparum | 71.0(51.8–85.1) | 99.1(94.3–100) | |
Discussion
The data presented in the current study demonstrate the excellent accuracy of the LAMP assay. The results of LAMP were equally accurate whether conducted in the electricity-free NINA platform heater or the PCR thermo-cycler. Irrespective of the standard (microscopy or nested PCR), the accuracy of the NINA-LAMP assay was satisfactory for all species. Performance of BinaxNOW RDT against non-falciparum cases was less successful with sensitivity of 74.2% (23/31) and 71% (22/31) as compared to microscopy and nested PCR respectively. Of the four P. falciparum cases missed by the BinaxNOW RDT, only one sample was previously frozen at −80°C before performing RDT while the other three samples were fresh blood. LAMP with NINA and thermo-cycler successfully identified all these cases. As the BinaxNOW test was carried out on fresh samples in the case of all non-falciparum RDT testing, low sensitivity is not attributable to the long storage time of the samples. The inability of the BinaxNOW RDT to detect non-falciparum (aldolase based detection) cases has already been demonstrated. Particularly, groups from Japan and France have reported poor performance in detecting P. ovale (6, 39). This lack of sensitivity can perhaps be attributed to lower production of aldolase by P.ovale (6, 7). In the present study, BinaxNOW RDT could detect only 80% of P. ovale cases. Overall (pan-specific) sensitivity of the BinaxNOW malaria RDT (85.5% and 85.9%) approximate the most recent evaluation in a US hospital (84.2%) and a previous study conducted in Toronto (83.7%) (9, 15). However, high sensitivity and specificity (97.2% and 93.6%) was reported from Ghana (27). We have observed excellent specificity of both the BinaxNOW RDT test and the Loopamp Malaria Pan/Pf assay. False positive cases used in the calculation came from the failure of standard methods (microscopy and nested PCR) to detect specific cases. False positive results for both the BinaxNOW and Loopamp have been reported previously (22, 35). Throughout the present study, LAMP positivity was determined by consensus visualization of turbidity in the reaction tube by naked eye for three independent readers. Only one instance of discrepant visual readout (only 2 of 3 readers agreed) was observed out of all 140 tests performed either with the NINA or thermo-cycler. Calcein fluorescence could not be utilized because samples were collected in EDTA coated collection tubes which quench the Calcein fluorescence as per manufacturer guidelines.
We observed results similar to that observed previously in a similar traveler’s clinic at London (31). Similarly sensitivity of LAMP were demonstrated in field settings in Uganda (14) and Gondar, Ethiopia (35) by using the same Loopamp Malaria Pan/Pf kit from Eiken Chemical Company, Japan. Additionally, the high sensitivity and specificity of non-falciparum diagnosis observed in the current study is similar with previous controlled laboratory studies conducted in Calgary and London (31, 35). A major benefit of LAMP over RDT is the identification of non-falciparum cases which is critical for regions approaching elimination in Southeast Asia and Latin America (30). We have demonstrated equal efficacy of LAMP-based pathogen infection detection utilizing the NINA electricity-free platform heaters and a programmable PCR thermo-cycler for consistent maintenance of temperature throughout the assay. The single-use Mg-Fe fuel pack and saline buffer and the semi-reusable phase change material (PCM) required for NINA operation are very inexpensive costing approximately $0.11/test (10 samples). Currently, the PCM for this particular application can be used for at least 15 cycles. To our knowledge, the NINA platform heater is the least expensive DNA amplification system for point-of-care use at US$13 for the device start up cost. In comparison, a real time turbidity meter currently costs approximately US$28,000, a fluorescent scanner costs approximately US$6300, and a thermo-cycler can cost US$3000–8000. Other less expensive battery powered heaters do exist (Diagenetix, Hawaii, USA), yet even a very simple heat block starts at US$200 (30). The Eiken Loopamp Malaria Pan/Pf kit is produced in a 48-sample pack. The potential for cross contamination of samples exists. We have seen compromised specificity in the field (84.3% and 81.2%) due to contamination (35) also observed by others in Uganda (84.9%), although a specific reason has not been given in the latter study (14). As a result, we suggest an alternate individual use “blister” packaging of the kit, similar to RDT kits, to minimize the risk of contamination.
We are still dependent on electrical centrifugation and heat block for the proscribed boil and spin DNA extraction protocol. New extraction approaches suitable for coupling with the NINA-LAMP are necessary. Several improvements to the current NINA platform are likely required. The NINA used in this study was 12-well however greater throughput may be required for mass screening in elimination campaigns. Finally, an integrated sample-to-results closed system would be optimal to reduce the risk of contamination. We have not studied the effect of external temperature and humidity on the efficacy of NINA-LAMP in the present study.
Highlights.
LAMP can be applied as a point of care test for malaria in returning travelers
LAMP is more sensitive than standard RDTs especially for non-falciparum malaria
LAMP is cost effective and requires minimal capital equipment investment
Acknowledgments
We thank the expert technical assistance of medical laboratory technologists and assistants at Calgary Laboratory Services (Calgary, Canada).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdul-Ghani R, Al-Mekhlafi AM, Karanis P. Loop-mediated isothermal amplification (LAMP) for malarial parasites of humans: would it come to clinical reality as a point-of-care test? Acta Trop. 2012;122:233–40. doi: 10.1016/j.actatropica.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Alam MS, Mohon AN, Mustafa S, Khan WA, Islam N, Karim MJ, Khanum H, Sullivan DJ, Jr, Haque R. Real-time PCR assay and rapid diagnostic tests for the diagnosis of clinically suspected malaria patients in Bangladesh. Malar J. 2011;10:175. doi: 10.1186/1475-2875-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B. Spread of artemisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacaner N, Stauffer B, Boulware DR, Walker PF, Keystone JS. Travel medicine considerations for North American immigrants visiting friends and relatives. JAMA. 2004;291:2856–64. doi: 10.1001/jama.291.23.2856. [DOI] [PubMed] [Google Scholar]
- 5.Behrens RH, Alexander N. Malaria knowledge and utilization of chemoprophylaxis in the UK population and in UK passengers departing to malaria-endemic areas. Malar J. 2013;12:461. doi: 10.1186/1475-2875-12-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigaillon C, Fontan Eo, Cavallo J-D, Hernandez E, Spiegel A. Ineffectiveness of the Binax NOW malaria test for diagnosis of Plasmodium ovale malaria. Journal of clinical microbiology. 2005;43:1011–1011. doi: 10.1128/JCM.43.2.1011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho CH, Nam MH, Kim JS, Han ET, Lee WJ, Oh JS, An SSA, Lim CS. Genetic variability in Plasmodium vivax aldolase gene in Korean isolates and the sensitivity of the Binax Now malaria test. Tropical Medicine & International Health. 2011;16:223–226. doi: 10.1111/j.1365-3156.2010.02691.x. [DOI] [PubMed] [Google Scholar]
- 8.Cullen KA, Arguin PM, D. Division of Parasitic, C. f. G. H. C. f. D. C. Malaria, and Prevention Malaria surveillance–United States, 2011. MMWR Surveill Summ. 2013;62:1–17. [PubMed] [Google Scholar]
- 9.DiMaio MA, Pereira IT, George TI, Banaei N. Performance of BinaxNOW for diagnosis of malaria in a US hospital. Journal of clinical microbiology. 2012;50:2877–2880. doi: 10.1128/JCM.01013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenner L, Weber R, Steffen R, Schlagenhauf P. Imported infectious disease and purpose of travel, Switzerland. Emerg Infect Dis. 2007;13:217–22. doi: 10.3201/eid1302.060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geduld JBM, Straight-Caron T. Presented at the 12th Conference of the International Society of Travel Medicine; Boston, Massachusetts, USA. 2011. [Google Scholar]
- 12.Global Health Group, M. A. P. Presented at the Atlas of malaria eliminating countries. San Francisco: University of California; 2011. [Google Scholar]
- 13.Han ET, Watanabe R, Sattabongkot J, Khuntirat B, Sirichaisinthop J, Iriko H, Jin L, Takeo S, Tsuboi T. Detection of four Plasmodium species by genus-and species-specific loop-mediated isothermal amplification for clinical diagnosis. Journal of clinical microbiology. 2007;45:2521–2528. doi: 10.1128/JCM.02117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins H, Gonzalez IJ, Polley SD, Angutoko P, Ategeka J, Asiimwe C, Agaba B, Kyabayinze DJ, Sutherland CJ, Perkins MD, Bell D. Highly Sensitive Detection of Malaria Parasitemia in a Malaria-Endemic Setting: Performance of a New Loop-Mediated Isothermal Amplification Kit in a Remote Clinic in Uganda. J Infect Dis. 2013 doi: 10.1093/infdis/jit184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khairnar K, Martin D, Lau R, Ralevski F, Pillai DR. Multiplex real-time quantitative PCR, microscopy and rapid diagnostic immuno-chromatographic tests for the detection of Plasmodium spp: performance, limit of detection analysis and quality assurance. Malar J. 2009;8:10.1186. doi: 10.1186/1475-2875-8-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaBarre P, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Weigl B. Presented at the Engineering in Medicine and Biology Society (EMBC) 2010 Annual International Conference of the IEEE. 2010 doi: 10.1109/IEMBS.2010.5627346. [DOI] [PubMed] [Google Scholar]
- 17.LaBarre P, Hawkins KR, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Boyle D, Weigl B. A simple, inexpensive device for nucleic acid amplification without electricity— toward instrument-free molecular diagnostics in low-resource settings. PloS one. 2011;6:e19738. doi: 10.1371/journal.pone.0019738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leder K, Tong S, Weld L, Kain KC, Wilder-Smith A, von Sonnenburg F, Black J, Brown GV, Torresi J, N. GeoSentinel Surveillance Illness in travelers visiting friends and relatives: a review of the GeoSentinel Surveillance Network. Clin Infect Dis. 2006;43:1185–93. doi: 10.1086/507893. [DOI] [PubMed] [Google Scholar]
- 19.Lee CS, Gregson DB, Church D, Laupland KB, Eckhardt R, Ross T, Chan W, Pillai DR. Population-based laboratory surveillance of imported malaria in metropolitan Calgary, 2000–2011. PLoS One. 2013;8:e60751. doi: 10.1371/journal.pone.0060751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malhotra RK, Indrayan A. A simple nomogram for sample size for estimating sensitivity and specificity of medical tests. Indian journal of ophthalmology. 2010;58:519. doi: 10.4103/0301-4738.71699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy AE, Morgan C, Prematunge C, Geduld J. Severe malaria in Canada, 2001–2013. Malar J. 2015;14:151. doi: 10.1186/s12936-015-0638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meatherall B, Preston K, Pillai DR. False positive malaria rapid diagnostic test in returning traveler with typhoid fever. BMC infectious diseases. 2014;14:377. doi: 10.1186/1471-2334-14-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohon AN, Alam MS, Bayih AG, Folefoc A, Shahinas D, Haque R, Pillai DR. Mutations in Plasmodium falciparum K13 propeller gene from Bangladesh (2009–2013) Malaria journal. 2014;13:431. doi: 10.1186/1475-2875-13-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohon AN, Elahi R, Khan WA, Haque R, Sullivan DJ, Alam MS. A new visually improved and sensitive loop mediated isothermal amplification (LAMP) for diagnosis of symptomatic falciparum malaria. Acta tropica. 2014;134:52–57. doi: 10.1016/j.actatropica.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohon AN, Elahi R, Podder MP, Mohiuddin K, Hossain MS, Khan WA, Haque R, Alam MS. Evaluation of the OnSite (Pf/Pan) rapid diagnostic test for diagnosis of clinical malaria. Malar J. 2012;11:415. doi: 10.1186/1475-2875-11-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman RD, Parise ME, Barber AM, Steketee RW. Malaria-related deaths among U.S. travelers, 1963–2001. Ann Intern Med. 2004;141:547–55. doi: 10.7326/0003-4819-141-7-200410050-00012. [DOI] [PubMed] [Google Scholar]
- 27.Nkrumah B, Acquah SEK, Ibrahim L, May J, Brattig N, Tannich E, Nguah SB, Adu-Sarkodie Y, Huenger F. Comparative evaluation of two rapid field tests for malaria diagnosis: Partec Rapid Malaria Test® and Binax Now® malaria rapid diagnostic test. BMC infectious diseases. 2011;11:143. doi: 10.1186/1471-2334-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odolini S, Gautret P, Parola P. Epidemiology of imported malaria in the mediterranean region. Mediterr J Hematol Infect Dis. 2012;4:e2012031. doi: 10.4084/MJHID.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel JC, Lucchi NW, Srivastava P, Lin JT, Sug-aram R, Aruncharus S, Bharti PK, Shukla MM, Congpuong K, Satimai W. Field evaluation of a real-time fluorescence loop mediated isothermal amplification (RealAmp) assay for the diagnosis of malaria in Thailand and India. Journal of Infectious Diseases:jiu252. 2014 doi: 10.1093/infdis/jiu252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polley SD, Gonzalez IJ, Mohamed D, Daly R, Bowers K, Watson J, Mewse E, Armstrong M, Gray C, Perkins MD, Bell D, Kanda H, Tomita N, Kubota Y, Mori Y, Chiodini PL, Sutherland CJ. Clinical Evaluation of a Loop-Mediated Amplification Kit for Diagnosis of Imported Malaria. J Infect Dis. 2013 doi: 10.1093/infdis/jit183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polley SD, Mori Y, Watson J, Perkins MD, González IJ, Notomi T, Chiodini PL, Sutherland CJ. Mitochondrial DNA targets increase sensitivity of malaria detection using loop-mediated isothermal amplification. Journal of clinical microbiology. 2010;48:2866–2871. doi: 10.1128/JCM.00355-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poon LLM, Wong BWY, Ma EHT, Chan KH, Chow LMC, Abeyewickreme W, Tangpukdee N, Yuen KY, Guan Y, Looareesuwan S. Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clinical chemistry. 2006;52:303–306. doi: 10.1373/clinchem.2005.057901. [DOI] [PubMed] [Google Scholar]
- 34.Reyburn H, Ruanda J, Mwerinde O, Drakeley C. The contribution of microscopy to targeting antimalarial treatment in a low transmission area of Tanzania. Malar J. 2006;5 doi: 10.1186/1475-2875-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sema M, Alemu A, Bayih AG, Getie S, Getnet G, Guelig D, Burton R, LaBarre P, Pillai DR. Evaluation of non-instrumented nucleic acid amplification by loop-mediated isothermal amplification (NINA-LAMP) for the diagnosis of malaria in Northwest Ethiopia. Malaria Journal. 2015;1:20. doi: 10.1186/s12936-015-0559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singleton J, Osborn JL, Lillis L, Hawkins K, Guelig D, Price W, Johns R, Ebels K, Boyle D, Weigl B, LaBarre P. Electricity-free amplification and detection for molecular point-of-care diagnosis of HIV-1. PLoS One. 2014 Nov 26;9(11) doi: 10.1371/journal.pone.0113693. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singleton J, Zentner C, Buser J, Yager P, LaBarre P, Weigl BH. Presented at the SPIE MOEMS-MEMS. 2013 doi: 10.1117/12.2005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Molecular and biochemical parasitology. 1993;61:315. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 39.Tanizaki R, Kato Y, Iwagami M, Kutsuna S, Ujiie M, Takeshita N, Hayakawa K, Kanagawa S, Kano S, Ohmagari N. Performance of Rapid Diagnostic Tests for Plasmodium ovale Malaria in Japanese Travellers. Tropical medicine and health. 2014;42:149. doi: 10.2149/tmh.2014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao ZY, Zhou HY, Xia H, Xu S, Zhu HW, Culleton RL, Han ET, Lu F, Fang Q, Gu YP. Adaptation of a visualized loop-mediated isothermal amplification technique for field detection of Plasmodium vivax infection. Parasites & vectors. 4:1–8. doi: 10.1186/1756-3305-4-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nature protocols. 2008;3:877–882. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- 42.WHO. World Malaria Report. 2014. [Google Scholar]


