Abstract
Depression is associated with alterations in corticostriatal reward circuitry. One pathophysiological pathway that may drive these changes is inflammation. Biomarkers of inflammation (for example, cytokines and C-reactive protein (CRP)) are reliably elevated in depressed patients. Moreover, administration of inflammatory stimuli reduces neural activity and dopamine release in reward-related brain regions in association with reduced motivation and anhedonia. Accordingly, we examined whether increased inflammation in depression affects corticostriatal reward circuitry to lead to deficits in motivation and goal-directed motor behavior. Resting-state functional magnetic resonance imaging was conducted on 48 medically stable, unmedicated outpatients with major depression. Whole-brain, voxel-wise functional connectivity was examined as a function of CRP using seeds for subdivisions of the ventral and dorsal striatum associated with motivation and motor control. Increased CRP was associated with decreased connectivity between ventral striatum and ventromedial prefrontal cortex (vmPFC) (corrected P<0.05), which in turn correlated with increased anhedonia (R=−0.47, P=0.001). Increased CRP similarly predicted decreased dorsal striatal to vmPFC and presupplementary motor area connectivity, which correlated with decreased motor speed (R=0.31 to 0.45, P<0.05) and increased psychomotor slowing (R=−0.35, P=0.015). Of note, mediation analyses revealed that these effects of CRP on connectivity mediated significant relationships between CRP and anhedonia and motor slowing. Finally, connectivity between striatum and vmPFC was associated with increased plasma interleukin (IL)-6, IL-1beta and IL-1 receptor antagonist (R=−0.33 to −0.36, P<0.05). These findings suggest that decreased corticostriatal connectivity may serve as a target for anti-inflammatory or pro-dopaminergic treatment strategies to improve motivational and motor deficits in patients with increased inflammation, including depression.
Introduction
Patients with major depression exhibit altered functional connectivity between ventral striatal and prefrontal cortical circuits that regulate motivation.1, 2, 3, 4 These alterations in functional connectivity have been associated with anhedonia, a core symptom of depression that reflects decreased motivation.5 One pathway that may contribute to alterations in corticostriatal reward circuitry and anhedonia within depression is inflammation.
Neuroimaging work by our group and others has revealed that exogenous administration of inflammatory cytokines or cytokine inducers (for example, endotoxin or vaccination) alters activation of reward-related brain regions,6, 7, 8, 9, 10 including reduced responses of the ventral striatum to hedonic reward.6, 10 Moreover, our preclinical data in non-human primates suggest that the effects of inflammation on reward circuitry are mediated by cytokine-induced reductions in striatal dopamine release, as measured by in vivo microdialysis, which can be reversed by administration of the dopamine precursor levodopa.11, 12 These cytokine-induced alterations in activation of reward circuitry and reductions in dopamine in humans and monkeys have in turn been associated with decreased motivation and anhedonia.6, 10
A significant proportion of patients with depression exhibit increased inflammation, as measured by peripheral inflammatory cytokines and acute-phase reactants, such as C-reactive protein (CRP).13, 14 However, whether increased inflammation alters reward circuitry leading to anhedonia and other depressive symptoms in patients with depression has yet to be examined.2, 15, 16 Herein we conducted a whole-brain analysis using blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) to determine whether increased inflammation (plasma CRP) in depressed patients is associated with altered functional connectivity of subdivisions of the ventral and dorsal striatum with other subcortical or cortical brain regions that subserve reward processing and other goal-directed behaviors, such as motor control.17, 18 This technique has been shown to reveal a distinct pattern of functional connectivity with whole-brain for each striatal sub-region,3, 17 and corticostriatal functional connectivity assessed by this method has been found to be sensitive to pharmacological manipulation of the dopamine system.19, 20, 21 We hypothesized that increased inflammation in depression would disrupt functional connectivity within reward-relevant corticostriatal neurocircuitry in association with reduced motivation and motor slowing.
METHODS AND MATERIALS
Participants
Forty-eight participants (18–65 years) with a primary diagnosis of major depressive disorder or bipolar disorder, current episode depressed as determined by Structured Clinical Interview for Diagnostic and Statistical Manual-IV-TR were enrolled.22 Subjects were free of psychotropic medications (for example, antidepressants, mood stabilizers, antipsychotics, stimulants, sedative hypnotics and benzodiazepines) for at least 4 weeks (8 weeks for fluoxetine). Participants were also free of medications known to affect the immune system, including nonsteroidal or steroidal anti-inflammatories, statins or angiotensin 2 receptor inhibitors, and were tested for drugs of abuse at screening and on the day of the scan. Medications for other medical conditions were allowed as dictated by the patients' treating physicians, although patients were required to be medically stable as determined by medical history, physical exam and laboratory testing (see Supplementary Information). CRP was assessed over 2–4 screening visits spaced 1–4 weeks apart to ensure stable levels of inflammatory markers (values within 25% of each other on two occasions). Participants with evidence of active infections were excluded until medically stable. Subjects were recruited from a parent study on phenotyping depressed patients with increased inflammation (ClinicalTrials.gov NCT01426997). All procedures were approved a priori by the Institutional Review Board of Emory University. All participants provided written informed consent.
BOLD fMRI data acquisition and preprocessing
Data was acquired on a 3 T Magnetom Trio scanner (Siemens, Malvern, PA, USA) with a 20-channel head coil at the Emory-GaTech BME Biomedical Imaging Technology Center in the afternoon (1500 hours±2 h). Anatomic images were obtained using a T1-weighted, magnetization prepared rapid gradient echo sequence.23 Wakeful resting-state fMRI images were acquired using a Z-saga pulse sequence for recovering ventral–frontal signal losses regularly seen in gradient-echo BOLD fMRI24 (See Supplementary Information). Analysis of functional connectivity was conducted with AFNI (http://afni.nimh.nih.gov/). Preprocessing25, 26 included slice timing correction, volume alignment, anatomy-to-EPI co-registration,27 nuisance signal (head motion, cerebral spinal fluid and white matter) regression, band pass filtering (0.009<f<0.08 Hz) and 5 mm full-width half-maximum spatial smoothing. No participant had movement >3.4 mm degree−1 in translation/rotation, consistent with previous studies28, 29 (see Supplementary Information). Individual's 4D fMRI data were spatially normalized into a standard stereotaxic space, Montreal Neurological Institute (MNI) template with 1 mm3 resolution.
BOLD fMRI data analysis
Seed-to-whole-brain connectivity analysis
Whole-brain resting-state connectivity was examined using 3-mm radius spherical seeds centered on ventral and dorsal striatal regions of interest (ROIs) (Supplementary Table S1). Inferior ventral striatum (iVS) (including nucleus accumbens) was defined from coordinates demonstrating maximal decreases in response to hedonic reward (gambling task) in subjects administered the inflammatory cytokine interferon-alpha.10 Additionally, ventral rostral putamen (vrP) and two dorsal striatal ROIs encompassing dorsal caudate (dC) and dorsal caudal putamen (dcP) were examined. These seeds are consistent with previously determined functional subregions of striatum3, 10, 17, 19 and allowed identification of distinct patterns of functional connectivity for each ROI that parallel motivational and motor striatal subdivisions.18, 30 Hemispheres were assessed separately consistent with previous studies using similar methods and because inflammation has been reported to have greater effects on neural activation and glucose or neurotransmitter metabolism in left striatum.6, 17, 19, 31, 32 Voxel-wise whole-brain correlations were computed as a function of plasma CRP (mg l−1) to identify brain regions for which functional connectivity with each bilateral striatal ROI was associated with inflammation. To correct for multiple comparisons, resulting statistical maps were cluster corrected (P<0.01 per voxel plus 1392 mm3 cluster, corrected P<0.05) using AFNI's 3dClustSim. Subject-level functional connectivity correlations for identified ROIs were Fisher's Z-transformed {Z(R)=0.5ln[(1+R)/(1−R)]} for use in regression models. For illustrative purposes, Z-score maps were generated to visualize patterns of positive functional connectivity with iVS separately for patients with ‘low' versus ‘high' inflammation (plasma CRP concentrations, <1 and >3 mg l−1, respectively, as defined by the American Heart Association).33 For correlations with inflammatory cytokines and their receptors, the mean time series from all eight striatal ROIs was calculated (as the overall striatal seed), and Z-scores from brain regions that showed altered connectivity with this ‘overall striatal seed' as a function of CRP were extracted.
Targeted connectivity analysis with ventromedial prefrontal cortex (vmPFC)
To further validate our findings, and to protect against inflation of regression coefficients that may result from extracting functional connectivity Z-scores from vmPFC voxels identified by correlation with CRP in whole-brain analysis, we extracted Z-scores for subject-level connectivity correlations of ventral and dorsal striatal ROIs with a vmPFC region (MNI coordinates x=0, y=44, z=−8 and cluster size=1408 mm3) previously reported to be associated with neural activation in response to receipt of reward versus loss in a meta-analysis of neuroimaging studies.34 These Z-scores were then correlated with inflammatory markers and behavior.
Behavioral assessments
Depression severity was assessed using the 17-item Hamilton Rating Scale for Depression (HAM-D).35 Anhedonia was assessed using the Snaith-Hamilton Pleasure Scale (SHAPS)36 and an anhedonia subscale of the Inventory of Depressive Symptomatology- Self-Report (IDS-SR).37 The IDS-SR anhedonia subscale consists of three items and has been previously demonstrated to highly correlate with the self-administered and clinician-administered versions of the SHAPS,38 which was replicated in our sample (R=0.63, P<0.001). Motor speed was determined as the number of taps/trial using the Finger Tapping Test,39, 40 and psychomotor performance was assessed as the time to compete Trail Making Test Part A41,42,43,44 (see Supplement Information).
Inflammatory biomarkers
Blood was collected for batched analysis of plasma CRP and the inflammatory cytokines interleukin (IL)-6, IL-1beta and tumor necrosis factor and their soluble receptors, which have been found to be elevated in patients with major depression.45, 46 The immunoturbidometric method was used to measure high-sensitivity CRP concentrations with a Beckman AU480 chemistry analyzer (Beckman Coulter, Brea, CA, USA) and Ultra WR CRP Kit (Sekisui Diagnostics, San Diego, CA, USA) as described.47 Concentrations of cytokines and their soluble receptors were assessed in duplicate using multiplex bead-based assays (R&D Systems, Minneapolis, MN, USA) and analyzed on a MAGPIX CCD imager (Luminex, Austin, TX, USA).48, 49 Mean inter- and intra-assay coefficients of variation were <10% (Supplementary Table S2). Consistent with previous analyses, cytokine and cytokine receptor values were natural log transformed to achieve normality for statistical modeling.50, 51, 52 See Supplementary Information for details of sample collection and cytokine assays.
Statistical analysis
Characteristics of the study sample were summarized using mean and s.d. for continuous variables and percentage for categorical variables. Subject-level connectivity Z-scores for relationships between ventral or dorsal striatal ROIs (or overall average striatal activity) with brain regions identified by fMRI connectivity analysis were entered into linear regression models (as the dependent variable) to assess relationships with inflammatory cytokines and their receptors and (as the independent variables) to assess relationships between functional connectivity and behavior. Relationships between ventral and dorsal striatal functional connectivity and symptoms of depression related to motivation and motor function, respectively, were assessed separately. Significant relationships between connectivity Z-scores with inflammatory biomarkers and behaviors were assessed in linear models using backward (significance level stay=0.05) and forward (significance level entry=0.05) selection with covariates that may contribute to inflammation and/or influence neural circuitry and behavior,52, 53, 54, 55 including age, sex, race, smoking status and body mass index (BMI). To identify specific relationships between functional connectivity and inflammatory markers independent of depression severity, we also controlled for HAM-D scores. To assess relationships between functional connectivity and behavior independent of inflammation, we also controlled for CRP. Furthermore, mediation analyses using Sobel tests56, 57, 58 were conducted to examine whether functional connectivity mediated the significant relationships observed between CRP and anhedonia and CRP and motor slowing reported in Table 1 (see Supplementary Information). Tests of significance were two-tailed, α<0.05, conducted in SAS (Cary, NC, USA) and IBM SPSS Statistics 23.0 (New York, NY, USA).
Table 1. Demographic, clinical and inflammatory variables of the study sample and their relationship to the primary variable of inflammation (plasma CRP).
| Variable | Mean (s.d.) (n=48) | Association with CRP (R) | P-valuea |
|---|---|---|---|
| Demographic | |||
| Age (years) | 38.3 (10.9) | −0.12 | 0.400 |
| Sex, Male (n, %) | 14 (29.2) | 0.17 | 0.236 |
| Race | |||
| Caucasian (n, %) | 18 (37.5) | −0.10 | 0.482 |
| African American (n, %) | 30 (62.5) | ||
| Smoking status, yes (n, %) | 8 (16.7) | −0.06 | 0.641 |
| BMI (kg m−2) | 31.2 (7.6) | 0.64 | <0.001*** |
| Clinical | |||
| HAM-D Depression Score | 23.2 (3.2) | 0.25 | 0.091 |
| IDS-SR Anhedonia Score | 4.8 (1.9) | 0.34 | 0.020* |
| SHAPS Score | 5.6 (3.0) | 0.26 | 0.073 |
| Finger Tapping Test, Dominant Hand (mean number taps/10 s) | 40.0 (9.9) | −0.29 | 0.049* |
| Trail Making Test A (s) | 32.6 (14.5) | 0.27 | 0.066 |
| Inflammatory | |||
| CRP (mg l−1) | 2.2 (2.4) | NA | NA |
| IL-6 (pg ml−1) | 1.61 (1.07) | 0.55 | <0.001*** |
| TNF (pg ml−1) | 5.52 (2.23) | 0.06 | 0.708 |
| IL-1beta (pg ml−1) | 0.42 (0.23) | 0.17 | 0.249 |
| IL-6sr (ng ml−1) | 15.75 (3.45) | 0.16 | 0.268 |
| sTNFR2 (ng ml−1) | 2.50 (0.89) | 0.09 | 0.554 |
| IL-1ra (ng ml−1) | 1.61 (2.22) | 0.41 | 0.004** |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; HAM-D, Hamilton Depression Rating Scale; IDS-SR, Inventory of Depressive Symptomatology, Self-Report; IL, interleukin; IL-1ra, IL-1 receptor antagonist; IL-6sr, IL-6 soluble receptor; NA, not applicable; SHAPS, Snaith–Hamilton Pleasure Scale; sTNFR2, soluble tumor necrosis factor receptor 2. *P<0.05, **P<0.01, ***P<0.001.
P-value for the correlation with CRP.
Results
Patient characteristics
Characteristic of the study sample and their relationship to plasma CRP are summarized in Table 1. BMI was significantly correlated with CRP (R=0.64, P<0.001) and was therefore included as a covariate in statistical models along with other indicated covariates. In addition, plasma CRP was correlated with IL-6 (R=0.55, P<0.001) and IL-1 receptor antagonist (IL-1ra) (R=0.41, P=0.004). Finally, CRP was significantly associated with anhedonia (R=0.34, P=0.020), as measured by the subscale of the IDS-SR, and motor slowing (R=−0.29, P=0.049), as measured by the Finger Tapping Test, whereas trends were observed for SHAPS and Trail Making Test (R=0.26–0.27, P<0.075).
Association between plasma CRP and functional connectivity: whole-brain analysis
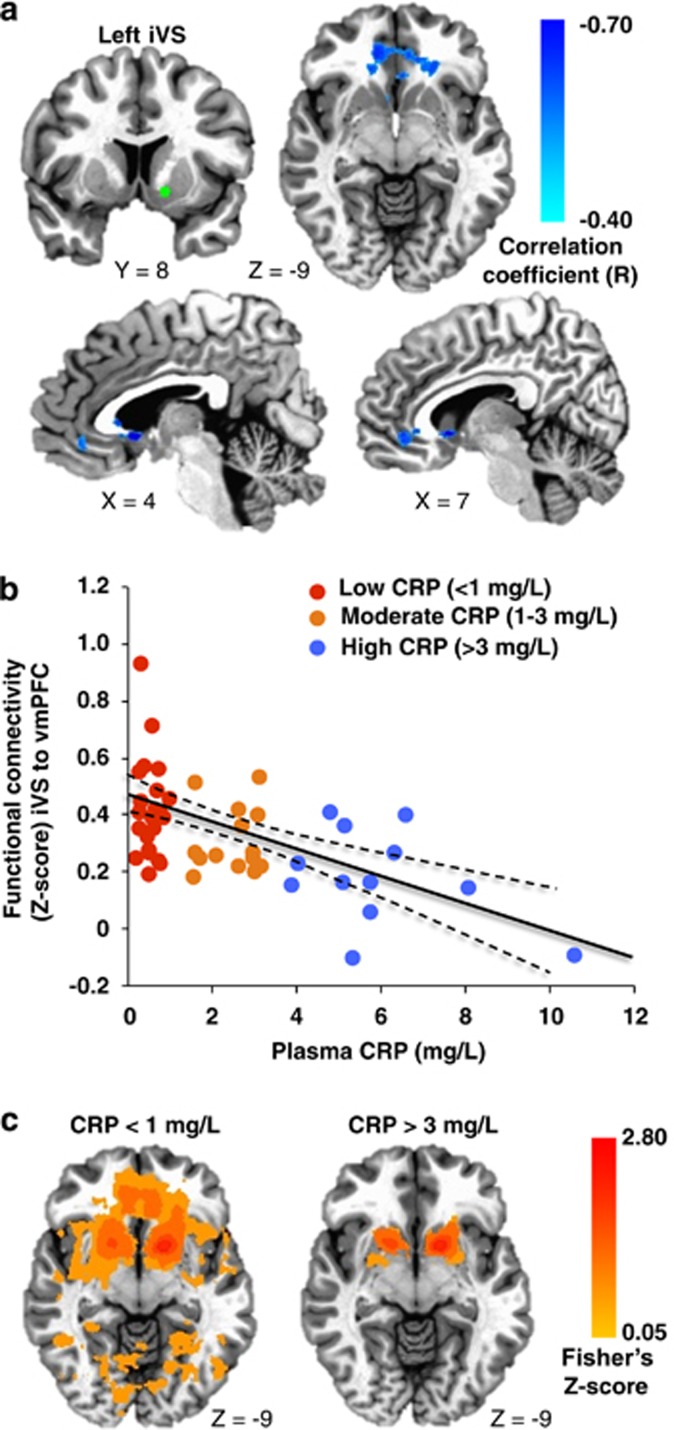
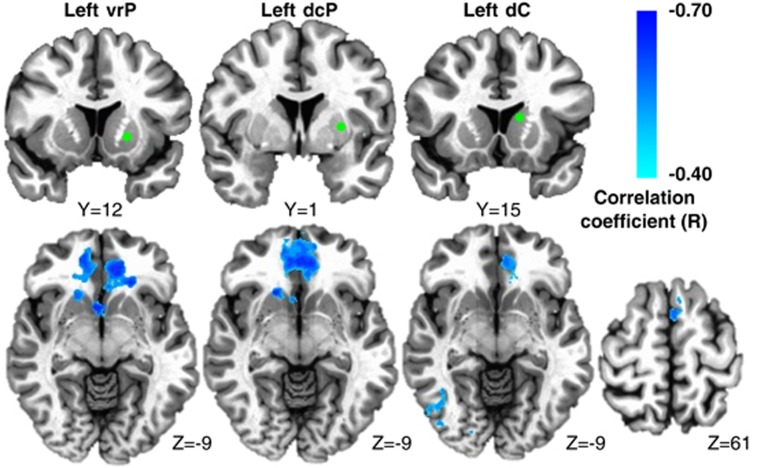
Increasing plasma CRP (mg l−1) was associated with reduced connectivity between left iVS and a cluster in vmPFC (cluster centroid BA32, R=−0.56, cluster=4327 mm3; Figures 1a and b; Table 2). Consistent with this relationship, Z-score maps demonstrated that patients with high inflammation (CRP>3 mg l−1) exhibited no significant connectivity between left iVS and vmPFC, whereas subjects with low inflammation (CRP<1 mg l−1) exhibited robust connectivity between these regions (as well as other cortical and subcortical regions; Figure 1c). This pattern of corticostriatal connectivity observed in patients with low inflammation is consistent with patterns of ventral striatal functional connectivity observed in healthy controls in numerous studies assessing voxel-wise whole-brain correlations with sub-regions of the striatum.3, 17, 19, 59 Negative correlations were also observed between CRP and functional connectivity of the left and right vrP, dcP and dC with clusters in vmPFC (all BA11; R=−0.53 to −0.62, cluster=3279–16 332 mm3); left and right dC with right fusiform gyrus (BA37; R=−0.59 and −0.53, cluster=4487 and 1429 mm3); and left dC with left superior frontal gyrus/presupplementary motor area (pre-SMA) (BA6; R=−0.53, cluster=1403 mm3; Figure 2; Table 2). Plasma CRP continued to account for significant variability in these corticostriatal connectivity relationships after controlling for possible effects of age, race, sex, smoking status, BMI and depression severity (adjusted R=−0.32 to −0.49; all P<0.05). Of note, the cluster threshold was re-estimated using the most recent release of 3dClustSim (May 2015). The updated cluster threshold of 1485 versus 1392 mm3 did not affect the reported results except for association between CRP and connectivity of left dC to pre-SMA and connectivity of right dC to fusiform gyrus (both P<0.07).
Figure 1.
Plasma C-reactive protein (CRP) was negatively associated with functional connectivity between left inferior ventral striatum (iVS) (green seed) and ventromedial prefrontal cortex (vmPFC; BA32, x=−2, y=33, z=−6), with increasing CRP predicting decreasing connectivity (cyan-blue intensity, R=−0.40 to −0.70) in patients with depression (a and b). Z-score maps demonstrated that, whereas patients with high inflammation (CRP>3 mg l−1) exhibited no significant connectivity between left iVS and vmPFC, the subjects with low inflammation (CRP<1 mg l−1) exhibited robust positive connectivity (yellow-red intensity) between these brain regions (c). Clusters are overlaid onto canonical structural brain images in the axial (z=−9: a and c) and sagittal (x=4 and 7: a) planes, corrected P<0.05.
Table 2. Ventral and dorsal striatal connectivity was negatively associated with inflammation (plasma CRP) in depression.
| Seed ROI | Brain region | mm3 |
Cluster centroid (MNI) |
BA | R-valuea | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| iVS | |||||||
| Left | vmPFC/rACC | 4327 | −2 | 32 | −6 | 32 | −0.56 |
| vrP | |||||||
| Left | vmPFC/mOFC | 11 696 | 1 | 29 | −11 | 11 | −0.56 |
| Right | vmPFC/mOFC | 10 005 | 2 | 27 | −12 | 11 | −0.57 |
| dcP | |||||||
| Left | vmPFC/mOFC | 12 913 | 1 | 35 | −14 | 11 | −0.59 |
| Right | vmPFC/mOFC | 16 332 | 2 | 36 | −13 | 11 | −0.62 |
| dC | |||||||
| Left | vmPFC/mOFC | 4190 | 0 | 33 | −19 | 11 | −0.55 |
| Right fusiform gyrus | 4487 | 38 | −61 | −14 | 37 | −0.59 | |
| Left superior frontal gyrus/pre-SMA | 1403 | −5 | 17 | 59 | 6 | −0.53 | |
| Right | Left vmPFC/mOFC | 3279 | −6 | 35 | −16 | 11 | −0.53 |
| Right fusiform gyrus | 1429 | 35 | −56 | −18 | 37 | −0.53 | |
Abbreviations: BA, Brodmann area; CRP, C-reactive protein; dC, dorsal caudate; dcP, dorsal caudal putamen; iVS, inferior ventral striatum; MNI, Montreal Neurological Institute and Hospital coordinate system; mOFC, medial orbitofrontal cortex; rACC, rostral anterior cingulate cortex; ROI, region of interest; SMA, supplementary motor area; vmPFC, ventromedial prefrontal cortex; vrP, ventral rostral putamen. Functional connectivity between ventral and dorsal striatum and cortical brain regions that was negatively associated with plasma CRP as determined by whole-brain analysis, voxel-wise P<0.01 plus cluster >1392 mm3: P<0.05 corrected.
All R values remained significant (P<0.05) after adjusting for covariates, including sex, age, race, smoking status, body mass index and depression severity (Hamilton Depression Rating Scale scores).
Figure 2.
Plasma C-reactive protein (CRP) was negatively associated with functional connectivity between ventral rostral putamen (vrP), dorsal caudal putamen (dcP) and dorsal caudate (dC) subdivisions of the striatum and other cortical brain regions in depressed subjects. Examples from the left hemisphere of functional connectivity between vrP, dcP and dC regions of interest (green seeds) and cortical brain regions that were negatively correlated with CRP (cyan-blue intensity; R=−0.40 to −0.70). Clusters in ventromedial prefrontal cortex (BA11; x=−6 to 2, y=27 to 36, z=−11 to −19; R=−0.62 to −0.53), right fusiform gyrus (BA37; x=37 and 35, y=−61 and −56, z=−14 and −16) and left superior frontal gyrus/supplementary motor area (BA6; x=−5, y=17, z=59; R=−0.51 to −0.53) are overlaid onto canonical structural brain images in the axial plane (z=−9 and 61), corrected P<0.05.
Correlations between connectivity and depressive symptoms: whole-brain analyses
Ventral striatal connectivity and anhedonia
Decreased connectivity (Fisher's Z-scores) between left iVS and the vmPFC, as well as left and right vrP to vmPFC, was negatively correlated with increased anhedonia, as measured by the subscale of IDS-SR (R=−0.29 to −0.48, all P<0.05). Decreased connectivity between right vrP and vmPFC was significantly correlated with increased SHAPS scores (R=−0.30, P=0.04), whereas a trend was observed for left iVS to vmPFC connectivity and SHAPS scores (R=−0.28, P=0.051). Forward and backward linear regression models, including age, sex, race, smoking status, BMI and CRP, demonstrated that functional connectivity between left iVS and vmPFC was the most significant predictor of the anhedonia subscale of IDS-SR (R=−0.47, P=0.001; Table 3), and right vrP to vmPFC connectivity was a significant predictor of SHAPS scores using forward selection only (R=−0.30, P=0.039). Of note, mediation analysis confirmed that connectivity between left iVS and vmPFC significantly mediated the relationship between CRP and anhedonia as reported in Table 1 (Z=2.26, s.e.=0.09, P=0.024; Supplementary Figure S1 and Supplementary Table S3).
Table 3. Connectivity between ventral and dorsal striatum and vmPFC predicted anhedonia, motor and psychomotor processing speed.
| Behavioral assessment | Functional connectivity (Z-scores) | Adjusted R | Adjusted P-value |
|---|---|---|---|
| Anhedonia: Subscale from IDS-SR | Left iVS to vmPFC | −0.47 | 0.001** |
| Anhedonia: SHAPS scores | Right vrP to vmPFC | −0.30 | 0.039* |
| Motor speed: Finger Tapping Test, Dominant Hand (mean taps per trial) | Right dcP to vmPFC | 0.45 | 0.002** |
| Psychomotor processing time: Trail Making Test A (s) | Right dC to vmPFC | −0.35 | 0.015* |
Abbreviations: dC, dorsal caudate; dcP, dorsal caudal putamen; IDS-SR, Inventory of Depressive Symptomatology, Self-Report; iVS, inferior ventral striatum; SHAPS, Snaith–Hamilton Pleasure Scale; vmPFC, ventromedial prefrontal cortex; vrP, ventral rostral putamen. Functional connectivity between the indicated brain regions significantly predicted motivational and motor behavior in linear regression models that included age, sex, race, body mass index, smoking status and C-reactive protein. *P<0.05; **P<0.01.
Dorsal striatal connectivity and psychomotor slowing
Decreased dcP and dC to vmPFC, and dC to pre-SMA, connectivity was correlated with reduced motor speed as assessed by the Finger Tapping Test (mean number of taps per timed trial; R=0.31 to 0.45, all P<0.05). Only decreased connectivity between left and right dC and vmPFC were associated with reduced psychomotor performance (time to complete Trail Making Test A; R=−0.33 and −0.36, P<0.05). Forward and backward linear regression models controlling for age, sex, race, smoking status, BMI and CRP demonstrated that dcP to vmPFC connectivity was the most significant positive predictor of motor speed (mean taps per trial; R=0.45, P=0.002) and right dC to vmPFC connectivity was the most significant negative predictor of psychomotor performance (completion time; R=−0.35, P=0.015) (Table 3). Of note, connectivity between left and right dC and fusiform gyrus was not significantly correlated with psychomotor behavior. Mediation analysis confirmed that connectivity between right dcP and vmPFC significantly mediated the relationship between CRP and motor slowing as reported in Table 1 (Z=2.27, s.e.=0.51, P=0.023; Supplementary Figure S1 and Supplementary Table S3).
Relationships of inflammatory cytokines and their soluble receptors with functional connectivity
Because CRP was associated with decreased connectivity between all ventral and dorsal striatal ROIs and vmPFC, and to reduce the number of comparisons with multiple other inflammatory markers, whole-brain functional connectivity with an overall striatal seed (that is, the mean time series from all eight striatal ROIs) as a function of CRP was assessed. The resulting Z-scores representing the overall striatal connectivity with vmPFC as a function of CRP were then correlated with inflammatory cytokines and their receptors. Increased plasma IL-6, IL-1beta and IL-1ra predicted decreased connectivity with vmPFC (R=−0.33 to 0.36, all P<0.05). IL-1ra was found to be the strongest predictor after controlling for age, sex, race, smoking status and BMI (adjusted R=−0.30, P=0.042). A significant relationship between CRP and overall striatal connectivity with fusiform gyrus was also observed but did not correlate with inflammatory markers when controlling for covariates (P>0.25).
Corticostriatal connectivity, inflammation and depressive symptoms: targeted analysis
To further validate our findings, functional connectivity between the striatal ROIs (and average overall striatal activity) and a vmPFC cluster previously identified in neuroimaging meta-analyses as being activated by a variety of rewarding stimuli34, 60, 61 was assessed and correlated with inflammatory markers and behavior in exploratory analyses. Decreased connectivity between the left iVS, left and right vrP, left and right dcP and left and right dC and this a priori defined vmPFC region were all associated with increased CRP, consistent with findings from whole-brain analysis (R=−0.29 to −0.56, all P<0.05). Finally, overall striatal connectivity with this vmPFC region was associated with CRP (adjusted R=−0.42, P=0.003) and IL-1beta after controlling for covariates, including depression severity (adjusted R=−0.33, P=0.024). Left iVS, right dcP and right dC connectivity with this a priori vmPFC region also significantly predicted anhedonia (adjusted R=−0.48, P=0.001), motor speed (adjusted R=0.41, P=0.004) and psychomotor processing time (adjusted R=−0.36, P=0.011), respectively, when controlling for covariates, including CRP. Ventral striatal connectivity did not predict motor speed or psychomotor performance, and dorsal striatal connectivity did not predict anhedonia (all adjusted P>0.25), indicating that distinct functional striatal subdivisions were related to motivation or motor behavior. Moreover, associations between iVS to vmPFC connectivity and anhedonia remained significant when controlling for right dC and right dcP to vmPFC connectivity (R=−0.57, P<0.001). Similar significant relationships were observed between dorsal striatum and motor speed and motor performance when controlling for iVS to vmPFC connectivity (R=0.35, P=0.017; and R=−0.39, P=0.006; respectively).
Discussion
Increased inflammation as reflected by plasma CRP and inflammatory cytokines was associated with decreased connectivity within reward-related brain regions using a whole-brain analysis. Interestingly, we observed that decreased connectivity between ventral striatum and vmPFC was in turn correlated with symptoms of anhedonia, whereas decreased connectivity between dorsal striatum and vmPFC correlated with psychomotor slowing. Although CRP was correlated with both anhedonia and motor slowing, mediation analysis revealed that the effects of CRP on corticostriatal connectivity mediated these relationships between CRP and behavior. The vmPFC regions identified by whole-brain analysis corresponded closely to a region recently identified as predicting non-response to transcranial magnetic stimulation in patients with depression who exhibited increased anhedonia.60 Furthermore, this vmPFC region is consistent with that previously described in meta-analysis of neuroimaging studies as being activated by a variety of rewarding stimuli.34 Targeted analysis of ventral and dorsal striatal connectivity with this reward-related vmPFC region identified in neuroimaging meta-analysis revealed correlations with anhedonia and psychomotor slowing, respectively, as well as with CRP and inflammatory cytokines. Therefore, decreased functional connectivity between striatum and vmPFC assessed using seeds described in the literature that did not depend on ROI identification by whole-brain analysis was associated with increased inflammation in depression. Based on these data, this unbiased method for assessing reward-related connectivity using a priori defined regions from the literature may be used as an objective and efficient way to assess inflammation effects on neurocircuitry across a number of psychiatric or medical illnesses associated with increased inflammation. Moreover, this strategy may be applied to studies investigating pharmacological strategies to reverse inflammation effects on reward neurocircuitry and behavior.
Potential mechanisms of inflammation-related decreases in corticostriatal connectivity
Consistent with decreased activation of ventral striatum to hedonic reward,10 our non-human primate work has demonstrated that chronic administration of inflammatory cytokines decreases striatal dopamine release, as measured by translational neuroimaging and in vivo microdialysis,12 in a monkey model of cytokine-induced depression.62, 63 Decreased dopamine release, as measured by in vivo microdialysis, was correlated with reduced effort-based sucrose consumption, a measure of reward sensitivity and anhedonia in monkeys.64 Of note, similar decreases in effort-based responses for sucrose reward in the absence of a decrease in consumption of freely available sucrose have been observed in rats administered peripheral IL-1β and decreased motivation for, but not sensitivity to, reward has been reported in mice following lipopolysaccharide administration.65, 66 Moreover, anhedonia-related reductions in striatal dopamine release in monkeys were restored by the dopamine precursor, levodopa, administered via reverse microdialysis.11 The primary findings of this study indicate that increased inflammation in depression disrupts connectivity between both ventral and dorsal striatum and vmPFC, a region which, similar to ventral striatum, receives significant mesocorticolimbic dopamine innervation.67 Accordingly, inflammation-related decreases in corticostriatal connectivity with vmPFC may involve cytokine-induced decreases in dopamine and have potential for reversal with pharmacological strategies that increase dopamine availability.62
Strengths, limitations and future directions
One strength of the study was the use of a targeted, hypothesis-driven ROI approach based on known functional sub-regions of the striatum that have been associated with specific depressive symptoms as well as inflammation.6, 10, 29, 31 Indeed, this approach identified that inflammation-associated connectivity between ventral striatum and vmPFC correlated with anhedonia, whereas inflammation-related connectivity between dorsal striatum and vmPFC correlated with motor slowing, highlighting the specificity of our findings. The striatal seed-to-whole brain method has been used extensively in studies examining functional connectivity with the striatum3, 19, 20, 59 and has been shown to be sensitive to increasing brain dopamine, for instance with levodopa.19, 20 Future studies will include pharmacological challenge paradigms to further understand the mechanisms by which inflammation disrupts connectivity in reward-related brain regions to lead to anhedonia in depression. An important limitation of the study is the lack of a healthy control group. However, patients with low inflammation (CRP<1 mg l−1) exhibited robust connectivity between ventral striatum and vmPFC that is consistent with patterns of ventral striatal functional connectivity with the whole brain in healthy control subjects reported in numerous studies.3, 17, 19, 59 Another limitation was the cross-sectional nature of the study. Longitudinal work will be required to determine causal links between inflammatory markers and alterations in corticostriatal connectivity in depression using anti-inflammatory challenge strategies. It should also be noted that, although similar results were observed for the two measurements of anhedonia (which were highly correlated with each other—see Methods section), there was some fluctuation in the statistical significance in the relationship of these scales to corticostriatal connectivity. These findings may represent nuanced differences in the aspects of anhedonia measured by these scales, for instance SHAPS focuses exclusively on pleasure (liking), whereas the IDS subscale probes interest in people and activities (wanting) in addition to pleasure. Alternatively, these differences may be secondary to a lack of power. Nevertheless, the sample size was sufficient to test the primary outcome of interest (the impact of inflammation on corticostriatal connectivity) with medium-to-large effect sizes and power >80%. Finally, depression is only one of several psychiatric illnesses that have been associated with increased inflammation, and it is unknown whether these findings in depression are generalizable to other psychiatric illnesses such as anxiety disorders and schizophrenia where high inflammation and motivational and motor deficits are prevalent.68, 69, 70 Of note, increased inflammation has been associated with non-response to standard antidepressant treatments71, 72 but is a positive predictor of antidepressant response to therapies that reduce inflammation, such as cytokine antagonists or polyunsaturated fatty acids.47, 73 Therefore, inflammation-related reductions in corticostriatal connectivity may serve as a potential predictor and target of response for a number of novel therapeutic strategies that inhibit inflammation or reverse its effects on dopamine or other neurotransmitter systems.
Acknowledgments
We thank Evanthia Wommack and Robert Smith for technical assistance with laboratory assays and MRI data acquisition. This work was supported by funds from the National Institute of Mental Health to AHM (R01MH087604) and EH (K23MH091254) and by the Brain and Behavioral Research Foundation to JCF (BBRF22296). In addition, the study was supported in part by PHS Grants UL1TR000454 and KL2TR000455 from the Clinical and Translational Science Award program and by the NIH/NCI under award number P30CA138292.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Treadway MT, Pizzagalli DA. Imaging the pathophysiology of major depressive disorder - from localist models to circuit-based analysis. Biol Mood Anxiety Disord 2014; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 2015; 72: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biol Mood Anxiety Disord 2011; 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry 2011; 16: 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Demyttenaere K, Bruffaerts R, Hermans D, Pizzagalli DA, Sienaert P et al. Dimensions in major depressive disorder and their relevance for treatment outcome. J Affect Disord 2014; 155: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 2010; 68: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry 2008; 63: 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 2009; 66: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Cercignani M, Voon V, Critchley HD. Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology 2015; 40: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 2012; 69: 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Hernandez CR, Miller AH. Levodopa reverses cytokine-induced reductions in striatal dopamine release. Int J Neuropsychopharmacol 2015; 18http://dx.doi.org/10.1093/ijnp/pyu084; advance online publication, 31 January 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR et al. Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 2013; 38: 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci 2013; 14: 135–151. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol 2014; 10: 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 2011; 35: 537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex 2008; 18: 2735–2747. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF et al. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci 2009; 29: 7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Peltier S, Bohnen NI, Muller ML, Dayalu P, Seidler RD. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front Syst Neurosci 2010; 4: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry 2013; 70: 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry 1988; 45: 742–747. [DOI] [PubMed] [Google Scholar]
- Mugler JP 3rd, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 1990; 15: 152–157. [DOI] [PubMed] [Google Scholar]
- Heberlein KA, Hu X. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magn Reson Med 2004; 51: 212–216. [DOI] [PubMed] [Google Scholar]
- Li Z, Moore AB, Tyner C, Hu X. Asymmetric connectivity reduction and its relationship to "HAROLD" in aging brain. Brain Res 2009; 1295: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Lynch ME, Hamann S, Peltier S et al. Increased "default mode" activity in adolescents prenatally exposed to cocaine. Hum Brain Mapp 2011; 32: 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage 2009; 44: 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oathes DJ, Patenaude B, Schatzberg AF, Etkin A. Neurobiological signatures of anxiety and depression in resting-state functional magnetic resonance imaging. Biol Psychiatry 2015; 77: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 2009; 46: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 2006; 16: 1508–1521. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 2007; 32: 2384–2392. [DOI] [PubMed] [Google Scholar]
- Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR et al. IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology 2014; 39: 1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003; 107: 363–369. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 2012; 50: 1252–1266. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 1995; 167: 99–103. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996; 26: 477–486. [DOI] [PubMed] [Google Scholar]
- Ameli R, Luckenbaugh DA, Gould NF, Holmes MK, Lally N, Ballard ED et al. SHAPS-C: the Snaith-Hamilton pleasure scale modified for clinician administration. PeerJ 2014; 2: e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. Oxford University Press: New York, USA, 1991. [Google Scholar]
- Aparicio P, Diedrichsen J, Ivry RB. Effects of focal basal ganglia lesions on timing and force control. Brain Cogn 2005; 58: 62–74. [DOI] [PubMed] [Google Scholar]
- Shindo A, Terada S, Sato S, Ikeda C, Nagao S, Oshima E et al. Trail making test part a and brain perfusion imaging in mild Alzheimer's disease. Dement Geriatr Cogn Dis Extra 2013; 3: 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Paradiso S. Cognitive and neurological impairment in mood disorders. Psychiatr Clin North Am 2004; 27: 19–36, vii-viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, Halldin C et al. Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry 2000; 157: 635–637. [DOI] [PubMed] [Google Scholar]
- Demakis GJ. Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, stroop test, and trail-making test. J Clin Exp Neuropsychol 2004; 26: 441–450. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71: 171–186. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67: 446–457. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology 2015; 40: 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I et al. The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain Behav Immun 2015; 44: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry 2009; 65: 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Pace TW, Liu T, Felger JC, Mister D, Doho GH et al. Predictors of depression in breast cancer patients treated with radiation: role of prior chemotherapy and nuclear factor kappa B. Cancer 2013; 119: 1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Felger JC, Woolwine BJ, Chen X, Parekh S, Spivey JR et al. Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: Preliminary findings. Brain Behav Immun 2014; 46: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpson NJ, Nordestgaard BG, Harbord RM, Zacho J, Frayling TM, Tybjaerg-Hansen A et al. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int J Obes (Lond) 2011; 35: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson ES, Freedson PS, Osganian SK, Matthews CE, Reed G, Ockene IS. Body mass index, but not physical activity, is associated with C-reactive protein. Med Sci Sports Exerc 2003; 35: 1160–1166. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage 2009; 47: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51: 1173–1182. [DOI] [PubMed] [Google Scholar]
- Sobel ME, Becker MP. Asymptotic confidence intervals for indirect effects in structural equation model. In: Sociological Methodology. American Sociological Association: Washington, DC, USA, 1982, pp 290–312.
- Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci 2007; 18: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KA, Cohen AL, Power JD, Nelson SM, Dosenbach YB, Miezin FM et al. Identifying basal ganglia divisions in individuals using resting-state functional connectivity MRI. Front Syst Neurosci 2010; 4: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry 2014; 76: 176–185. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev 2008; 59: 164–184. [DOI] [PubMed] [Google Scholar]
- Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol 2012; 33: 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM et al. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry 2007; 62: 1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Mun J, Kimmel H, Nye JA, Drake DF, Hernandez CR et al. Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in non-human primates. Neuropsychopharmacology 2013; 38: 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Estrada A, Epling B, Hart EE, Lee CA et al. Effort-related motivational effects of the pro-inflammatory cytokine interleukin 1-beta: studies with the concurrent fixed ratio 5/ chow feeding choice task. Psychopharmacology (Berl) 2014; 231: 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichaya EG, Hunt SC, Dantzer R. Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology 2014; 39: 2884–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci 2013; 14: 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz J, Mata I, Barrera J, Perez-Iglesias R, Varela I, Arranz MJ et al. Inflammatory and immune response genes have significantly altered expression in schizophrenia. Mol Psychiatry 2013; 18: 1056–1057. [DOI] [PubMed] [Google Scholar]
- Meyer U, Schwarz MJ, Muller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther 2011; 132: 96–110. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Corwin E, Bradley B, Ressler KJ, Jovanovic T. Psychophysiology and posttraumatic stress disorder symptom profile in pregnant African-American women with trauma exposure. Arch Womens Ment Health 2014; 18: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 445–450. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology 2013; 38: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry; advance online publication, 24 March 2015; doi: 10.1038/mp.2015.22 [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.