Abstract
Background
This study investigated whether a selective serotonin reuptake inhibitor (SSRI), citalopram, downregulates the expression of HIV receptor (CD4) and coreceptors (CCR5 and CXCR4) on peripheral blood mononuclear cells (PBMC) and macrophages ex vivo as a potential mechanism of reducing susceptibility to HIV infection.
Methods
The sample included 150 participants ages 18–58 (59% women, 65% African American, 61% depressed). Monocyte depleted PBMC were treated with phytohemagglutinin (PHA) for 72-hours and then cultured in the presence of IL-2 with vehicle control or SSRI (10−6 M) for 2-hours. To generate monocyte derived macrophages (MDM), monocytes were cultured for 7-days, after which either vehicle control or SSRI (10−6 M) was added for 2-hours. RNA was then collected from both cell types, and CD4, CCR5 and CXCR4 mRNA expression was measured by real-time PCR.
Results
In PBMC, SSRI treatment decreased expression of CD4 (p=.009), CCR5 (p=.008), and CXCR4 (p<.0001). In MDM, SSRI decreased expression of CD4 (p<.0001) and CXCR4 (p=.0003), but not CCR5 (p=.71). The suppressive effects of SSRI on receptor expression did not differ as a function of depression diagnosis or depressive symptom severity.
Conclusions
SSRI treatment at a physiologic dose decreased CD4, CCR5 and CXCR4 expression on PBMC and macrophages ex vivo. These findings suggest that SSRI treatment, independent of depression status, downregulates HIV receptor and coreceptor expression and thus may reduce susceptibility of immune cells to HIV infection and decrease inflammation. If clinical trials confirm the present findings, ultimately there may be a role for using SSRI treatment adjunctively in HIV/AIDS.
Keywords: CD4, CCR5, CXCR4, depression, HIV, SSRI
INTRODUCTION
Cell surface receptor expression on immune cells is implicated in the pathogenesis of human immunodeficiency virus (HIV) and a number of other medical conditions (1). Cluster of differentiation 4 (CD4), for example, is a transmembrane glycoprotein found on the surface of several immune cells, including T helper cells, monocytes, macrophages, and dendritic cells (2, 3). A member of the immunoglobulin superfamily, CD4 interacts with major histocompatibility complex (MHC) class II receptor involved in antigen presentation, signal transduction, and T-cell activation in response to viral infection (2). In the context of HIV, the CD4 receptor and the chemokine receptor type 4 (CXCR4) function as coreceptors for viral entry into T helper cells – the primary mechanism leading to immune suppression, and, ultimately, acquired immune deficiency syndrome (AIDS) (4). Chemokine receptors, including CXCR4 and CCR5, play a broad role in viral pathogenesis, in part through the regulation of immune cell trafficking and effector functions (5). Whereas acute CXCR4 and CCR5 mediated responses to infection are adaptive, chronically elevated expression of these receptors on lymphocytes and macrophages may contribute to persistent immune activation and comorbid conditions, such as cardiovascular disease (5, 6).
With chronic HIV infection, the widespread activation of T cells and monocytes/macrophages is associated with proinflammatory cytokine production, increased viral replication, and cellular immune activation, which, over time, leads to suppressed innate and adaptive immune function and susceptibility to opportunistic infections and cancers (4). Moreover, persistent immune activation can promote proinflammatory processes involved in atherosclerotic cardiovascular disease and neurocognitive impairment, both of which are highly prevalent in HIV/AIDS (4, 7). Thus, although CD4, CCR5, and CXCR4 receptors normally function to help immune cells identify and eradicate viruses, these receptors may be related to the pathogenesis of disease characterized by chronic immune activation and inflammation, including HIV (8–11). By extension, drugs that downregulate CD4, CCR5, and CXCR4 receptors could potentially modify HIV entry and replication, as well as chronic immune activation and systemic inflammation.
Serotonin (5-hydroxytryptamine, or 5-HT) receptors and the 5-HT transporter are widely distributed on monocytes, macrophages, T cells, and possibly natural killer (NK) cells (12). Thus, serotonin and serotonin-modulating agents may have a direct effect on both innate and cellular immunity, which are critical pathways in host defense again viral pathogens, including HIV. Serotonin, for example, enhances the cytolytic activity of NK cells, possibly through 5-HT1A receptors on monocytes (13, 14), and may protect the function of NK cells (13). Serotonin also activates 5-HT receptors on T cells, suggesting potential effects on cellular activation, signal transduction, and cell surface receptor expression (15). Additional studies (16–18) have also suggested similar serotonin up-regulation of T-cell function in HIV infection. Thus, drugs affecting the serotonin system, including selective serotonin reuptake inhibitors (SSRIs), may regulate both innate and adaptive immune function (19, 20).
We have previously shown that SSRI treatment ex vivo exerts a number of immune effects pertinent to the control of HIV infection, including: (a) enhanced NK cytolytic activity that is involved in directly killing HIV-infected cells (21); (b) reduced infection of macrophages (22); and (c) decreased HIV replication in latently infected T-cell and macrophage cell lines (22). Across these previous studies, SSRI effects were independent of depression status, suggesting a direct immunomodulatory effect of blocking serotonin reuptake irrespective of depression diagnosis or depressive symptom severity. Because depression has been associated with both immune dysregulation (23–33) and accelerated HIV disease progression (34–48), SSRIs could conceivably enhance mood and help restore innate and cell-mediated immunity simultaneously for patients with comorbid depression and HIV infection. It remains unknown, however, whether SSRIs could also have an antiviral effect via blocking the expression of HIV receptor (CD4) and coreceptors (CCR5 and CXCR4), which are needed for cell entry.
To our knowledge, this is the first study to assess the effect of a SSRI on the expression of cell surface receptors (CD4, CCR5, CXCR4) ex vivo. Based on our prior work and a review of coreceptor biology, we hypothesized that SSRI would downregulate receptor expression across two types of immune cells implicated in HIV pathogenesis – T-lymphocytes and monocyte derived macrophages (MDM).
METHODS AND MATERIALS
Subjects
In order to achieve a representative sample of depressed and nondepressed adults, subjects were recruited from organizations focusing on depression treatment in the University of Pennsylvania Health System and the surrounding Philadelphia community, via community outreach presentations, clinician referrals, response to flyers, and word of mouth. Subjects were included if they were 18–65 years old, male or female, of any race or ethnicity and able to communicate in English. Depressed subjects had current depressive symptoms (17-item Hamilton Rating Scale for Depression score ≥ 8) and a diagnosis of either major depression or non-major depression, which included dysthymia or adjustment disorder with depressed mood. Subjects were excluded if they had: 1) HIV infection; 2) Acute suicidal ideation and intent; 3) Met diagnostic criteria for active substance dependence or abuse within the 12 months prior to enrollment; 4) Use of a medication known to alter immune function within 4 weeks prior to enrollment, or history of immunomodulatory therapy; 5) Presence of psychotic symptoms; 6) Currently taking pharmacotherapy for any psychiatric disorder, including any antipsychotic, antidepressant, antimanic, or anxiolytic medication within 4 weeks prior to evaluation. For the antidepressant fluoxetine, subjects were excluded if fluoxetine had been used within 8 weeks prior to study enrollment; 7) Pregnant or nursing; 8) Significant chronic systemic illness; 9) Hemoglobin ≤ 12.5 g/dl and/or Hematocrit ≤ 38% (American Red Cross blood donor criteria). The same inclusion and exclusion criteria were used for nondepressed control subjects, except that they did not have a SCID diagnosis of depression and they had a HAMD 17 score ≤ 7.
Procedures
Each subject received a comprehensive medical and psychiatric assessment, including medical history, review of systems, and a physical examination including a standardized neurological examination. Current and lifetime DSM-IV Axis I diagnoses were assessed by a research psychiatric clinician with a modified Structured Clinical Interview for DSM-IV (49). Consensus diagnoses were evaluated and determined at diagnostic conferences. Depression symptom severity was evaluated with the 17-item Hamilton Depression Rating Scale (50).
In order to control for potential circadian effects on immunity, subjects were studied at the same time of day, as in our previous studies (21, 22). Subjects were placed in a recumbent position, an intravenous line was started at approximately 8:30 AM, and blood was drawn 30 minutes later following an acclimation period (27).
Receptor and Coreceptor Assessment
Peripheral whole blood was drawn from each subject. Within one hour of collection PBMC were isolated by standard Ficoll-Paque separation technique (GE Healthcare, Piscataway, New Jersey). Monocytes were isolated from total peripheral blood mononuclear cells (PBMC) by our well established adherence to plastic technique (51).
Monocytes were incubated for 7 days to generate monocyte-derived macrophages (MDM) using 48-well plates, 250,000 cells per well in DMEM, 10% fetal bovine serum (FBS). After 7 days one subset of MDM received active SSRI (10−6 mol/L, a physiologic concentration) (52, 53) while the other subset was treated with the nonactive SSRI diluent (pure sterile H2O). After 2 hours of incubation with SSRI or diluent, total RNA was extracted using an RNeasy kit (Qiagen, Valencia, CA).
Monocyte depleted PBMC were incubated with phytohemagglutinin (PHA 7.5 ug/ml) for 3 days then washed and cultured using well-established techniques (54–56) using 48-well plates, 1,000,000 cells per well, in RPMI, 10% FBS supplemented with IL-2 (29 units/ml). One subset of PBMC received active SSRI (10−6 mol/L) while the other received nonactive SSRI diluent (pure sterile H2O). After 2 hours of incubation with SSRI or diluent, total RNA was extracted using an RNeasy kit (Qiagen, Valencia, CA).
The expression of HIV receptor (CD4) and chemokine coreceptors (CCR5 and CXCR4) was determined using real-time PCR (57). Total RNA was treated with RNAse free DNAse (Ambion/Life Technologies, Grand Island, NY) and reverse transcribed (1μg) using an AffinityScript qPCR cDNA synthesis kit (Agilent, Santa Clara, CA) with random primer, all as instructed by the manufacturer. One-tenth of the resulting cDNA was used as a template for real-time PCR amplification using MyiQ iCycler system (Bio-Rad Laboratories, Inc., Hercules, CA). The sequences of the primers and probes used in this study are listed (57). All primers and probes were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). PCR fragments were cloned into pGEM-T vector (Promega, Madison, WI) and used to generate standard curves for corresponding genes. The expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used for normalization of gene expression (58). All amplification reactions were performed in duplicate and an average mRNA quantity is expressed as copy number per 1,000 copies of GAPDH. The specificity of the amplification with SYBR Green was confirmed by dye dissociation curve.
Statistical Analysis
The CD4, CCR5, and CXCR4 receptor mRNA expression was measured under a control condition and under a physiological dose of SSRI, for each of two cell types (PBMC/T-cells and MDM). For each response within each cell type, we used linear mixed models (LMM) to accommodate within-subject correlations of these repeated responses across drug treatment conditions. We used log10 transformation of the coreceptor expression responses to reduce levels of skewness to appropriate levels for LMM analyses. Each statistical model analyzed the main effect of SSRI, the main effect of depression diagnosis, and the interaction between SSRI treatment and depression diagnosis as predictors of HIV receptor and coreceptor expression, within each cell type. To adjust for multiple comparisons across three dependent variables (CD4, CCR5, and CXCR4), we used an adjusted critical alpha level of p=.017.
The sample included 150 participants. The age range was 18–58 years (mean = 32.09, SD = 10.78). The majority was female (59%), African American (65%), and depressed (61%). The depressed group was comprised of individuals with major depression (n=86), and non-major depression (n=6), including dysthymia (n=2) and adjustment disorder with depressed mood (n=4). The depressed and non-depressed groups did not differ on mean (SD) age (depressed group: 33 (11); non-depressed group: 34 (12); p=1.00), sex (depressed group: 40% male; non-depressed group: 43% male; p=0.73), and race (depressed group: 64% African-American; non-depressed group: 66% African-American; p=0.86). The 17-item HAMD score ranged from 0 to 32 in the full sample, with a mean of 12.55 (SD=9.24). By design, depression symptom severity was higher for the depressed group (HAMD = 18.39, SD = 4.64) than the non-depressed group (HAMD = 1.40, SD = 1.62; p<.0001). Because some subjects did not provide a sufficient volume of blood for the ex vivo experiments, sample sizes in the statistical models ranged from 139 to 149.
RESULTS
Effect of SSRI (citalopram) on HIV Receptor and Coreceptor Expression in PBMC
As shown in Figure 1, there was a significant suppressive effect of SSRI treatment on receptor and coreceptor expression in PBMC. For CD4 expression, the mean log10 responses were 2.47 and 2.44 for control and SSRI, respectively [F(1,146)=7.05, p=.0088]. For CCR5 expression, the mean log10 responses were 1.68 and 1.64 for control and SSRI, respectively [F(1,146)=7.35, p=.0075]. For CXCR4 expression, the mean log10 responses were 2.79 and 2.75 for control and SSRI, respectively [F(1,148)=20.19, p<.0001].
Figure 1.
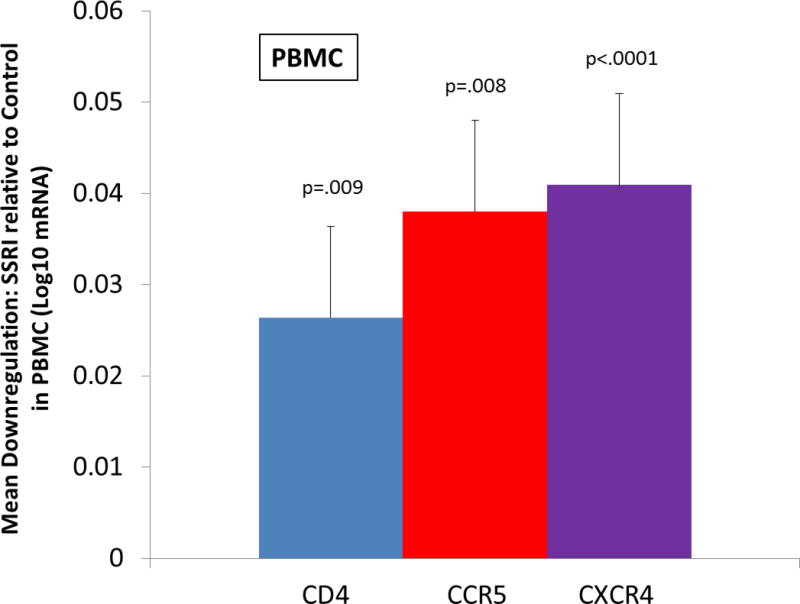
Ex vivo effect of selective serotonin reuptake inhibitor (SSRI) on CD4, CCR5, and CXCR4 receptor expression on peripheral blood mononuclear cells (PBMC) from depressed and non-depressed adults. Monocyte depleted PBMC were immediately incubated with phytohemagglutinin (PHA) for 3 days then washed and cultured in the presence of IL-2. The vertical bars represent the distribution of within-subject differences in mRNA receptor expression (log10 scale) measured using RT-PCR after 2-hours of incubation with SSRI at a physiologic concentration (10−6 mol/L) compared to diluent (pure sterile H2O).
Effect of SSRI (citalopram) on HIV Receptor and Coreceptor Expression in MDM
A similar pattern of HIV receptor and coreceptor expression findings emerged for MDM (see Figure 2). For CD4 expression, the mean log10 responses were 3.24 and 3.13 for control and SSRI, respectively [F(1,138)=48.26, p<.0001]. For CCR5 expression, the mean log10 responses were 2.62 and 2.62 for control and SSRI, respectively [F(1,139)=0.13, p=.71]. For CXCR4 expression, the mean log10 responses were 2.62 and 2.57 for control and SSRI, respectively [F(1,140)=14.06, p=.0003].
Figure 2.
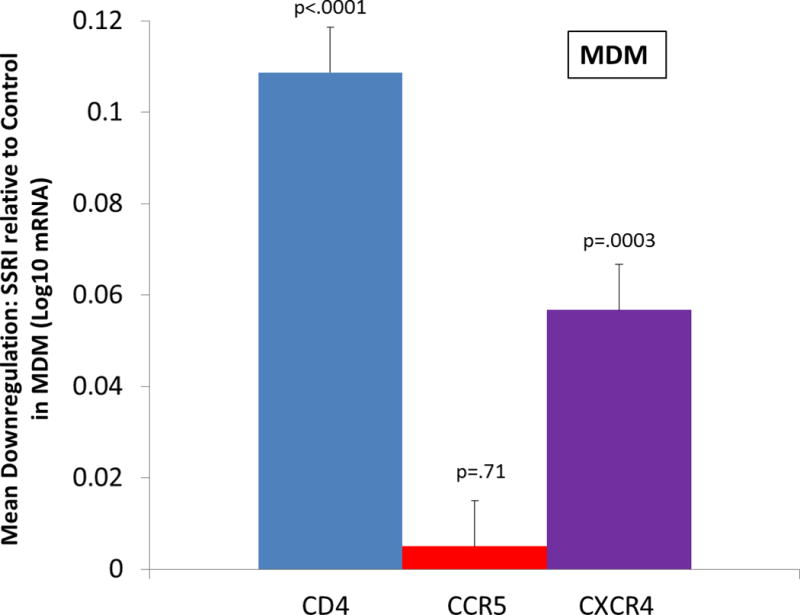
Ex vivo effect of selective serotonin reuptake inhibitor (SSRI) on CD4, CCR5, and CXCR4 receptor expression on monocyte derived macrophages (MDM) from depressed and non-depressed adults. The vertical bars represent the distribution of within-subject differences in mRNA receptor expression (log10 scale) measured using RT-PCR after 2-hours of incubation with SSRI at a physiologic concentration (10−6 mol/L) compared to diluent (pure sterile H2O)
Effects of Depression on HIV Receptor and Coreceptor Expression in PBMC and MDM
There were no significant interactions between SSRI treatment condition and depression status for any of the three receptor expression variables across the two cell types. Thus, depression status was tested as a main effect. In PBMC, depression diagnosis was independently associated with lower expression of CD4 (log10 response = 2.39 for depressed vs. 2.53 for non-depressed, t=2.56, df=146, p=.011) and CXCR4 (log10 response = 2.70 for depressed vs. 2.84 for non-depressed, t=2.97, df=148, p=.003). The effect of depression diagnosis on CCR5 in PBMC, however, was not statistically significant. In MDM, the main effect of depression diagnosis was non-significant for all three receptors. There were no significant interactions between depression severity (HAMD) and SSRI treatment in the depressed group. Correlation values between HAMD scores and expression level of the three receptors were all negative, regardless of cell type or SSRI treatment or control. None of the correlations between HAMD score and CD4 expression were significant (all p-values > .20). The correlations between HAMD score and CCR5 expression were significant for both SSRI treatment (r = −.24, p=.003) and control (r = −.31, p=.022) conditions for PBMC, and non-significant in the MDM, regardless of treatment condition (p-values > .10). The correlations between HAMD score and CXCR4 were significant (−.29 < r −.35, p-values <.01) regardless of treatment condition and cell type.
Covariate Analyses: Effects of Potentially Confounding Demographic Variables
Adjustment for age, sex, and race had no effect on the analyses of SSRI treatment, depression diagnosis, and treatment by diagnosis interaction effects. Non-significant effects of demographic covariates were found across both cell types, and across all three coreceptors. Specifically, there were no significant interactions between SSRI treatment and depression diagnosis, age, sex, or race. For PBMC, interaction p-values exceeded 0.17, 0.22, and 0.37 for CD4, CCR5, and CXCR4, respectively. For MDM, interaction p-values exceeded 0.16, 0.32, and 0.16 for CD4, CCR5, and CXCR4, respectively. Thus, effects of SSRI on receptor and coreceptor expression were independent of demographic variables.
DISCUSSION
These findings are the first to our knowledge that show a SSRI can suppress expression of CD4, CCR5 and CXCR4 receptors on immune cells in an ex vivo model. Specifically, the SSRI citalopram at a physiologic dose (52, 53) significantly decreased mRNA expression of all three cell surface receptors studied in PBMC, and in two out of three cell surface receptors in MDM (CD4, CXCR4). These findings support the hypothesis that SSRI treatment may exert a direct biologic effect on receptor expression, thereby suggesting a potentially novel biological pathway through which SSRI could reduce HIV entry into T-cells and macrophages and thus possibly limit viral infectivity.
Our findings are consistent with a prior in vitro study which found that 5-HT decreased acute HIV replication by down-regulating the CCR5 receptor as well as by increasing the secretion of the HIV suppressive chemokine MIP-1 (59). Those in vitro findings on the direct effects of serotonin are consistent with our previous finding of the direct effects of the SSRI citalopram on decreasing HIV infectivity of human macrophages in an acute ex vivo model as well as a chronic model of both a T cell line and a macrophage cell line (22). Furthermore, there is clinical evidence of antiviral activity of SSRIs in the central nervous system. Letendre et al. (60) reported that individuals taking SSRIs (citalopram, sertraline or trazodone) were more likely to have lower HIV viral loads in cerebrospinal fluid (CSF) and better neuropsychological performance. Moreover, two clinical studies by Irwin and colleagues (61, 62) have demonstrated that SSRI treatment boosts baseline and vaccine stimulated varicella zoster virus specific immunity in depressed patients, further supporting the potential effect of antidepressant medication on clinically relevant markers of immune function. In addition, recent studies have also demonstrated anti-inflammatory effects of SSRIs in microglia (63, 64). Relatedly, significant decreases in serotonin concentrations have been found in the CSF of HIV seropositive individuals (65). Thus, serotonin and SSRIs may regulate central and peripheral immune system function, potentially benefitting individuals with HIV (19, 20). Taken together, the present study, in the context of prior work, is consistent with the possibility that SSRI treatment may exert an immunoprotective effect, involving a combination of innate immunity and direct antiviral activity at the cellular level.
We speculate that SSRIs may inhibit HIV entry and replication in T-cells and monocytes/macrophages by binding to the serotonin transporter, resulting in increased extracellular 5HT concentrations. The change in 5HT concentrations, in turn, may downregulate expression of CD4 receptor and CXCR4 and CCR5 coreceptors, reducing HIV entry. In addition, 5HT may augment the release of chemokines (CCL3, CCL4, and CCL5) from NK/CD8+ cells, monocytes/macrophages, and T-cells. These anti-HIV chemokines block CD4, CCR5, and CXCR4 receptors, further limiting HIV infectivity (66). The possibility of such direct, molecular biological effects of SSRI on immune function awaits further investigation.
Consistent with our previous ex vivo studies (21, 22), the immune effects of SSRI in this study were observed in both depressed and non-depressed subjects. Specifically, there was no interaction between SSRI treatment of cells and participants’ depression diagnostic status. Moreover, after adjusting for depression diagnosis, the effects of SSRI on receptor expression remained significant. Additionally, no significant effect of depression diagnosis on CD4, CCR5, or CXCR4 expression in MDM or CCR5 expression in PBMC was found. Depression diagnosis was associated with greater suppression of CD4 and CXCR4 in PBMC. However, PBMC obtained from depressed individuals are known to have reduced lymphocyte proliferation in response to mitogen stimulation (32, 33, 67). Therefore, it is likely that the lower levels of receptors observed in PBMC of depressed individuals were a consequence of decreased response to PHA stimulation and therefore reduction of lymphocyte cell numbers. In contrast, MDM differentiation does not require PHA stimulation and we did not observe any depression diagnosis effects in MDMs. Just as there were no interactions between SSRI effects and depression diagnosis, there were no interactions between depression severity and SSRI effects in the depressed subjects. Although no effects of depression diagnosis on MDM receptors were observed, depression severity was associated with a decrease in CXCR4 receptor expression. Unlike PBMC this was not related to PHA stimulation. Rather, it is well known that depression is associated with increased proinflammatory cytokines (30, 33, 68, 69). Proinflammatory cytokines promote M1 polarization of macrophages, and M1 polarization is associated with reduced production of CXCR4 receptors (70). Thus, this mechanism could account for the depression severity effect seen in the CXCR4 receptor in MDM.
This study had a number of strengths, including a large sample size, a carefully controlled ex vivo model for SSRI effects on cell surface receptor expression, and the analysis of two key cell types directly involved in HIV pathogenesis (T-cells and macrophages). In addition, our immune assessment was standardized by performing all blood draws at the same time of day, following 30-minutes of recumbency, to avoid diurnal effects on immunity and possible non-specific methodological factors (71–73). Subjects were also excluded for medical comorbidities, current substance abuse, or recent use of psychotropic medications or other immunomodulatory drugs that could affect immune function. Notably, the study used a physiological dose of SSRI (10−6 M) to produce a similar exposure that would occur in vivo. The SSRI dose used in the present study was the same dose that significantly enhanced natural killer cell innate immunity in our previous ex vivo work with HIV-seropositive individuals (21).
There were also a number of possible limitations. First, because the majority of our sample was African-American, it remains uncertain whether the effects of SSRI on receptor expression will generalize to all racial subgroups. Second, the experimental method involving PHA mitogen stimulated proliferation of PBMC to generate T-cells, may have resulted in less cell surface receptor expression for depressed compared to non-depressed individuals. This limitation does not apply to MDM, and as noted the SSRI effects were independent of depression. Third, because this study used an ex vivo design with medically healthy adults, future studies are required to test whether the effects observed here can be demonstrated in vivo, among both HIV seronegative and HIV seropositive individuals, with and without depression. Our prior work in depressed and nondepressed HIV seropositive women found that SSRI treatment ex vivo exerted a number of immunomodulatory effects, including enhanced innate immunity (NK cytotoxicity), enhanced suppression of HIV replication in latently infected T-cells by killer lymphocytes (NK/CD8), and decreased HIV infection of macrophages (21, 22), suggesting that the effects of SSRI on HIV receptor and coreceptor expression in HIV seropositive individuals is plausible. Because mRNA, and not actual protein level, was used to measure receptor and coreceptor expression in this study, future studies should directly measure protein levels of cell surface receptor expression, using flow cytometry, for example. Finally, future in vivo studies are also needed to investigate the role of various other SSRIs on cell surface receptor expression and on other measures of innate and adaptive immune function.
In summary, this study tested the effect of a SSRI (citalopram) on HIV receptor and coreceptor expression ex vivo, using two cell types (PBMC and MDM). The results support a novel biological pathway through which SSRI treatment could conceivably inhibit HIV cell entry and replication, namely through downregulating CD4 expression and chemokine receptor expression (CCR5, CXCR4). Furthermore, given the role of CD4, CCR5, and CXCR4 receptor expression in a number of other diseases characterized by immune activation and inflammation, including atherosclerosis (5, 6), the present data suggest the additional possibility that SSRI treatment could modify other inflammatory-related diseases, an area for future research. Taken together, the current data with our prior ex vivo investigations of HIV infection support the direct effect of SSRIs on immune regulation and viral control. Future studies, such as randomized clinical trials of SSRI effects on immune modulation, are needed to determine whether SSRI treatment decreases mRNA and protein levels of CD4, CCR5 and CXCR4 on immune cells in a sustained manner in vivo. If so, there may be a role for the clinical use of SSRI adjunctively for immune restoration in HIV/AIDS, in both depressed and nondepressed individuals.
Acknowledgments
This research was directly supported by a grant from the National Institutes of Health (NIH): R01MH082670 (Principal Investigator: D.L. Evans). This publication was made possible through support from the Penn Mental Health AIDS Research Center (PMHARC), a NIH funded program: P30MH097488 (Principal Investigator: D.L. Evans). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. Portions of the data herein were presented as a poster at the 70th Annual Meeting of the Society of Biological Psychiatry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest associated with this research.
References
- 1.Male DK, Brostoff J, Roth D, Roitt I. Immunology. 8th. United States: Elsevier/Saunders; 2013. [Google Scholar]
- 2.Janeway CA., Jr The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645–674. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 3.Gibbings D, Befus AD. CD4 and CD8: an inside-out coreceptor model for innate immune cells. Journal of leukocyte biology. 2009;86:251–259. doi: 10.1189/jlb.0109040. [DOI] [PubMed] [Google Scholar]
- 4.Lucas S, Nelson AM. HIV and the spectrum of human disease. J Pathol. 2015;235:229–241. doi: 10.1002/path.4449. [DOI] [PubMed] [Google Scholar]
- 5.Jones KL, Maguire JJ, Davenport AP. Chemokine receptor CCR5: from AIDS to atherosclerosis. British journal of pharmacology. 2011;162:1453–1469. doi: 10.1111/j.1476-5381.2010.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doring Y, Pawig L, Weber C, Noels H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol. 2014;5:212. doi: 10.3389/fphys.2014.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain, behavior, and immunity. 2015;45:1–12. doi: 10.1016/j.bbi.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: Roles in viral entry, tropism, and disease. Annual Review of Immunology. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 9.Alkhatib G, Berger EA. HIV coreceptors: from discovery and designation to new paradigms and promise. Eur J Med Res. 2007;12:375–384. [PubMed] [Google Scholar]
- 10.d’Ettorre G, Paiardini M, Ceccarelli G, Silvestri G, Vullo V. HIV-associated immune activation: from bench to bedside. AIDS research and human retroviruses. 2011;27:355–364. doi: 10.1089/aid.2010.0342. [DOI] [PubMed] [Google Scholar]
- 11.Rajasuriar R, Khoury G, Kamarulzaman A, French MA, Cameron PU, Lewin SR. Persistent immune activation in chronic HIV infection: do any interventions work? AIDS. 2013;27:1199–1208. doi: 10.1097/QAD.0b013e32835ecb8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mossner R, Lesch KP. Role of serotonin in the immune system and in neuroimmune interactions. Brain, behavior, and immunity. 1998;12:249–271. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- 13.Hellstrand K, Hermodsson S. Role of serotonin in the regulation of human natural killer cell cytotoxicity. Journal of immunology. 1987;139:869–875. [PubMed] [Google Scholar]
- 14.Hellstrand K, Hermodsson S. Enhancement of human natural killer cell cytotoxicity by serotonin: role of non-T/CD16+ NK cells, accessory monocytes, and 5-HT1A receptors. Cellular immunology. 1990;127:199–214. doi: 10.1016/0008-8749(90)90125-b. [DOI] [PubMed] [Google Scholar]
- 15.Aune TM, Golden HW, McGrath KM. Inhibitors of serotonin synthesis and antagonists of serotonin 1A receptors inhibit T lymphocyte function in vitro and cell-mediated immunity in vivo. Journal of immunology. 1994;153:489–498. [PubMed] [Google Scholar]
- 16.Hofmann B, Nishanian P, Nguyen T, Liu M, Fahey JL. Restoration of T-cell function in HIV infection by reduction of intracellular cAMP levels with adenosine analogues. AIDS. 1993;7:659–664. doi: 10.1097/00002030-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann B, Afzelius P, Iversen J, Kronborg G, Aabech P, Benfield T, et al. Buspirone, a serotonin receptor agonist, increases CD4 T-cell counts and modulates the immune system in HIV-seropositive subjects. AIDS. 1996;10:1339–1347. doi: 10.1097/00002030-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Eugen-Olsen J, Afzelius P, Andresen L, Iversen J, Kronborg G, Aabech P, et al. Serotonin modulates immune function in T cells from HIV-seropositive subjects. Clinical immunology and immunopathology. 1997;84:115–121. doi: 10.1006/clin.1997.4384. [DOI] [PubMed] [Google Scholar]
- 19.Gordon J, Barnes NM. Lymphocytes transport serotonin and dopamine: agony or ecstasy? Trends in immunology. 2003;24:438–443. doi: 10.1016/s1471-4906(03)00176-5. [DOI] [PubMed] [Google Scholar]
- 20.O’Connell PJ, Wang X, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107:1010–1017. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans DL, Lynch KG, Benton T, Dube B, Gettes DR, Tustin NB, et al. Selective serotonin reuptake inhibitor and substance P antagonist enhancement of natural killer cell innate immunity in human immunodeficiency virus/acquired immunodeficiency syndrome. Biological psychiatry. 2008;63:899–905. doi: 10.1016/j.biopsych.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benton T, Lynch K, Dube B, Gettes DR, Tustin NB, Ping Lai J, et al. Selective serotonin reuptake inhibitor suppression of HIV infectivity and replication. Psychosomatic medicine. 2010;72:925–932. doi: 10.1097/PSY.0b013e3181f883ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin M, Smith TL, Gillin JC. Low Natural-Killer Cytotoxicity in Major Depression. Life sciences. 1987;41:2127–2133. doi: 10.1016/0024-3205(87)90531-5. [DOI] [PubMed] [Google Scholar]
- 24.Schleifer SJ, Keller SE, Bond RN, Cohen J, Stein M. Major depressive disorder and immunity. Role of age, sex, severity, and hospitalization. Archives of general psychiatry. 1989;46:81–87. doi: 10.1001/archpsyc.1989.01810010083011. [DOI] [PubMed] [Google Scholar]
- 25.Irwin M, Patterson T, Smith TL, Caldwell C, Brown SA, Gillin JC, et al. Reduction of Immune Function in Life Stress and Depression. Biological psychiatry. 1990;27:22–30. doi: 10.1016/0006-3223(90)90016-u. [DOI] [PubMed] [Google Scholar]
- 26.Irwin M, Caldwell C, Smith TL, Brown S, Schuckit MA, Gillin JC. Major Depressive Disorder, Alcoholism, and Reduced Natural-Killer-Cell Cytotoxicity – Role of Severity of Depressive Symptoms and Alcohol-Consumption. Archives of general psychiatry. 1990;47:713–719. doi: 10.1001/archpsyc.1990.01810200021003. [DOI] [PubMed] [Google Scholar]
- 27.Evans DL, Folds JD, Petitto JM, Golden RN, Pedersen CA, Corrigan M, et al. Circulating natural killer cell phenotypes in men and women with major depression. Relation to cytotoxic activity and severity of depression. Archives of general psychiatry. 1992;49:388–395. doi: 10.1001/archpsyc.1992.01820050052009. [DOI] [PubMed] [Google Scholar]
- 28.Raison CL, Miller AH. The neuroimmunology of stress and depression. Seminars in clinical neuropsychiatry. 2001;6:277–294. doi: 10.1053/scnp.2001.0060277. [DOI] [PubMed] [Google Scholar]
- 29.Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. Journal of psychosomatic research. 2002;53:873–876. doi: 10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- 30.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sephton SE, Kraemer HC, Neri E, Stites DP, Weissbecker I, Spiegel D. Improving methods of assessing natural killer cell cytotoxicity. International journal of methods in psychiatric research. 2006;15:12–21. doi: 10.1002/mpr.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain, behavior, and immunity. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain, behavior, and immunity. 2011;25:221–229. doi: 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA : the journal of the American Medical Association. 1993;270:2568–2573. [PubMed] [Google Scholar]
- 35.Patterson TL, Shaw WS, Semple SJ, Cherner M, McCutchan JA, Atkinson JH, et al. Relationship of psychosocial factors to HIV disease progression. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 1996;18:30–39. doi: 10.1007/BF02903937. [DOI] [PubMed] [Google Scholar]
- 36.Page-Shafer K, Delorenze GN, Satariano WA, Winkelstein W., Jr Comorbidity and survival in HIV-infected men in the San Francisco Men’s Health Survey. Annals of epidemiology. 1996;6:420–430. doi: 10.1016/s1047-2797(96)00064-6. [DOI] [PubMed] [Google Scholar]
- 37.Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ. Depressive affect and survival among gay and bisexual men infected with HIV. Archives of internal medicine. 1996;156:2233–2238. [PubMed] [Google Scholar]
- 38.Lyketsos CG, Hoover DR, Guccione M, Dew MA, Wesch JE, Bing EG, et al. Changes in depressive symptoms as AIDS develops. The Multicenter AIDS Cohort Study. The American journal of psychiatry. 1996;153:1430–1437. doi: 10.1176/ajp.153.11.1430. [DOI] [PubMed] [Google Scholar]
- 39.Leserman J, Jackson ED, Petitto JM, Golden RN, Silva SG, Perkins DO, et al. Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosomatic medicine. 1999;61:397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Leserman J, Petitto JM, Golden RN, Gaynes BN, Gu H, Perkins DO, et al. Impact of stressful life events, depression, social support, coping, and cortisol on progression to AIDS. The American journal of psychiatry. 2000;157:1221–1228. doi: 10.1176/appi.ajp.157.8.1221. [DOI] [PubMed] [Google Scholar]
- 41.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA : the journal of the American Medical Association. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 42.Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, et al. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychological medicine. 2002;32:1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 43.Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. The American journal of psychiatry. 2002;159:1752–1759. doi: 10.1176/appi.ajp.159.10.1752. [DOI] [PubMed] [Google Scholar]
- 44.Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biological psychiatry. 2003;54:295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 45.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, et al. Mood disorders in the medically ill: scientific review and recommendations. Biological psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosomatic medicine. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 47.Temoshok LR, Wald RL, Synowski S, Garzino-Demo A. Coping as a multisystem construct associated with pathways mediating HIV-relevant immune function and disease progression. Psychosomatic medicine. 2008;70:555–561. doi: 10.1097/PSY.0b013e318177354f. [DOI] [PubMed] [Google Scholar]
- 48.Ironson G, O’Cleirigh C, Kumar M, Kaplan L, Balbin E, Kelsch CB, et al. Psychosocial and Neurohormonal Predictors of HIV Disease Progression (CD4 Cells and Viral Load): A 4 Year Prospective Study. AIDS and behavior. 2014 doi: 10.1007/s10461-014-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perkins DO, Stern RA, Golden RN, Murphy C, Naftolowitz D, Evans DL. Mood disorders in HIV infection: prevalence and risk factors in a nonepicenter of the AIDS epidemic. The American journal of psychiatry. 1994;151:233–236. doi: 10.1176/ajp.151.2.233. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 51.Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- 52.Gutierrez M, Abramowitz W. Steady-state pharmacokinetics of citalopram in young and elderly subjects. Pharmacotherapy. 2000;20:1441–1447. doi: 10.1592/phco.20.19.1441.34851. [DOI] [PubMed] [Google Scholar]
- 53.Bareggi SR, Bianchi L, Cavallaro R, Gervasoni M, Siliprandi F, Bellodi L. Citalopram concentrations and response in obsessive-compulsive disorder. Preliminary results. CNS Drugs. 2004;18:329–335. doi: 10.2165/00023210-200418050-00004. [DOI] [PubMed] [Google Scholar]
- 54.Castro BA, Weiss CD, Wiviott LD, Levy JA. Optimal conditions for recovery of the human immunodeficiency virus from peripheral blood mononuclear cells. Journal of clinical microbiology. 1988;26:2371–2376. doi: 10.1128/jcm.26.11.2371-2376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manak MM, Moshkoff DA, Nguyen LT, Meshki J, Tebas P, Tuluc F, et al. Anti-HIV-1 activity of the neurokinin-1 receptor antagonist aprepitant and synergistic interactions with other antiretrovirals. AIDS. 2010;24:2789–2796. doi: 10.1097/QAD.0b013e3283405c33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spitsin S, Tustin NB, Riedel E, Tustin R, 3rd, Murray JB, Peck LM, et al. Programmed death 1 receptor changes ex vivo in HIV-infected adults following initiation of highly active antiretroviral therapy. Clin Vaccine Immunol. 2012;19:752–756. doi: 10.1128/CVI.00093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spitsin S, Stevens KE, Douglas SD. Expression of substance P, neurokinin-1 receptor and immune markers in the brains of individuals with HIV-associated neuropathology. Journal of the neurological sciences. 2013;334:18–23. doi: 10.1016/j.jns.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chege D, Chai Y, Huibner S, McKinnon L, Wachihi C, Kimani M, et al. Evaluation of a quantitative real-time PCR assay to measure HIV-specific mucosal CD8+ T cell responses in the cervix. PLoS One. 2010;5:e13077. doi: 10.1371/journal.pone.0013077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maneglier B, Guillemin GJ, Clayette P, Rogez-Kreuz C, Brew BJ, Dormont D, et al. Serotonin decreases HIV-1 replication in primary cultures of human macrophages through 5-HT(1A) receptors. British journal of pharmacology. 2008;154:174–182. doi: 10.1038/bjp.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Letendre SL, Marquie-Beck J, Ellis RJ, Woods SP, Best B, Clifford DB, et al. The role of cohort studies in drug development: clinical evidence of antiviral activity of serotonin reuptake inhibitors and HMG-CoA reductase inhibitors in the central nervous system. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2007;2:120–127. doi: 10.1007/s11481-006-9054-y. [DOI] [PubMed] [Google Scholar]
- 61.Irwin MR, Levin MJ, Laudenslager ML, Olmstead R, Lucko A, Lang N, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;56:1085–1093. doi: 10.1093/cid/cis1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irwin MR, Levin MJ, Carrillo C, Olmstead R, Lucko A, Lang N, et al. Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain, behavior, and immunity. 2011;25:759–766. doi: 10.1016/j.bbi.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu D, Wang Z, Liu S, Wang F, Zhao S, Hao A. Anti-inflammatory effects of fluoxetine in lipopolysaccharide(LPS)-stimulated microglial cells. Neuropharmacology. 2011;61:592–599. doi: 10.1016/j.neuropharm.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 64.Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain, behavior, and immunity. 2012;26:469–479. doi: 10.1016/j.bbi.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 65.Kumar AM, Fernandez JB, Borodowsky I, Gonzalez L, Kumar M. HIV-1 Infection and Central Monoamine Neurotransmitters. Am J Infect Dis. 2007;3:177–183. [Google Scholar]
- 66.Ansari AW, Heiken H, Moenkemeyer M, Schmidt RE. Dichotomous effects of C-C chemokines in HIV-1 pathogenesis. Immunology letters. 2007;110:1–5. doi: 10.1016/j.imlet.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain, behavior, and immunity. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 68.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depression and anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cassol E, Cassetta L, Rizzi C, Alfano M, Poli G. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. Journal of immunology. 2009;182:6237–6246. doi: 10.4049/jimmunol.0803447. [DOI] [PubMed] [Google Scholar]
- 71.Petitto JM, Folds JD, Ozer H, Quade D, Evans DL. Abnormal diurnal variation in circulating natural killer cell phenotypes and cytotoxic activity in major depression. The American journal of psychiatry. 1992;149:694–696. doi: 10.1176/ajp.149.5.694. [DOI] [PubMed] [Google Scholar]
- 72.Evans DL, Leserman J, Perkins DO, Stern RA, Murphy C, Tamul K, et al. Stress-associated reductions of cytotoxic T lymphocytes and natural killer cells in asymptomatic HIV infection. The American journal of psychiatry. 1995;152:543–550. doi: 10.1176/ajp.152.4.543. [DOI] [PubMed] [Google Scholar]
- 73.Leserman J, Petitto JM, Perkins DO, Folds JD, Golden RN, Evans DL. Severe stress, depressive symptoms, and changes in lymphocyte subsets in human immunodeficiency virus-infected men. A 2-year follow-up study. Archives of general psychiatry. 1997;54:279–285. doi: 10.1001/archpsyc.1997.01830150105015. [DOI] [PubMed] [Google Scholar]


