Graphical abstract
1. Introduction
Nanogels are three-dimensional hydrogel materials in the nanoscale size range formed by crosslinked swellable polymer networks with a high capacity to hold water, without actually dissolving into the aqueous medium. Nanogels can be composed of a variety of naturally occurring polymers, synthetic polymers or a combination thereof. Their characteristics such as size, charge, porosity, amphiphilicity, softness, and degradability can be fine-tuned by varying the chemical composition of the nanogels. They are mostly spherical particles but the current advancement in synthetic strategies allow for the fabrication of nanogels of different shapes [1,2]. They can be also designed to have either a core-shell or a core-shell-corona structure, with at least one of the layers crosslinked for structural integrity. Being mostly hydrophilic in nature, nanogels are highly biocompatible with a high loading capacity for guest molecules and their unique physical properties offer them distinct advantages over other types of nanomaterials for biomedical applications. Nanogels not only protect the cargo from degradation and elimination but also participate actively in the delivery process due to their characteristic properties like stimuli-responsive behavior, softness and swelling to help achieve a controlled, triggered response at the target site [3-9]. The versatility of their architecture allows for incorporation of a plethora of guest molecules ranging from inorganic nanoparticles to biomacromolecules like proteins and DNA with suitable modifications of the materials used for their construction, without compromising their gel-like behavior [10-16]. This multifunctionality and stability is hard to find in other types of nanoparticulate systems [17,18]; especially the ability to incorporate entities with very different physical properties within the same carrier. Inorganic nanomaterials have distinct material properties like optical activity, electrical conductivity and magnetic properties that make them suitable for in vivo diagnostic and imaging applications, but they suffer from limitations of poor colloidal stability, low aqueous solubility and rapid elimination by the mononuclear phagocytic system (MPS). Polymeric nanogels can be used as carriers for such imaging probes by imparting stability and increasing their utility. This lead to the evolution of a new class of agents termed ‘nanohybrids’ which are nanogels incorporating inorganic materials [19,20]. Such nanohybrids can contain a wide variety of diagnostic and imaging agents for different types of medical conditions. Nanogels prevent biomolecules like enzymes and genetic material from degradation while their own macromolecular properties help increase the circulation half-lives of small molecules, and serve as a highly convenient platform for combination delivery of therapeutic molecules. They can be targeted specifically to the site of interest by conjugation with a targeting ligand or due to the passive targeting that is a characteristic feature of their nanoscale size. Despite such diversity in their applications, nanogels are not yet a part of clinical use. Many comprehensive and more specialized review articles on synthesis and application of nanogels were recently published. For that reason, in the present paper we attempted to briefly consider characteristic features of nanogels and to demonstrate representative examples for major directions in their applications in the biomedical field. We also highlight some of the key hurdles that need to be overcome to make nanogels a part of routine clinical practice.
2. Nanogels: characteristic features
2.1 Synthesis of nanogels
Nanogels can be synthesized by a number of techniques. Since an in-depth discussion of all the available techniques is beyond the scope of this review, a brief overview of the techniques is given, along with reference to more detailed sources. Traditionally, nanogels have been classified based on the method of crosslinking as either physically or chemically (covalently) crosslinked nanogels. Chemical crosslinking involves formation of covalent bonds between the polymer chains during polymerization of low molecular weight monomers or crosslinking of polymer precursors. The most extensively employed methods for preparing chemically crosslinked nanogels utilize heterogeneous polymerization reactions in the presence of either bifunctional or multifunctional crosslinkers [21,22]. Conventional and controlled/living radical polymerization techniques allow for preparation of nanogels with different composition, dimensions, and architectures including core-shell and hollow nanogel particles [23,24]. The use of functional initiators and macroinitiators further allows the incorporation of functionalities in the interior or on the surface of nanogels, which facilitate multivalent bioconjugation [25]. A variety of other crosslinking approaches including click chemistry, Schiff-base reactions, thiol-disulfide exchange, amide crosslinking, photo-induced crosslinking, enzyme-mediated crosslinking etc., have been developed for the synthesis of nanogels from the polymer precursors. In addition, the crosslinking reactions carried out on preformed core-shell self-assemblies such as polymer micelles allow introducing a high degree of spatial organization into the nanogels [26,27]. For a comprehensive look into crosslinking strategies, the reader is directed to excellent review written by Haag and colleagues [28]. Recent advances in nanoscale fabrication methods provided further unique opportunities to fabricate well-defined nanogels with precise control over size, shape, deformability and surface chemistry in a high-throughput manner [29-31]. Physically crosslinked systems though formed under the mild conditions, tend to be more fragile than their covalently crosslinked counterparts since they are stabilized by relatively weak interactions between polymer chains such as hydrogen bonding, hydrophobic interactions or ionic interactions. The use of hydrophobically-modified polymers and other associating polymers for preparing functional nanogels has recently been reviewed by Sasaki and Akiyoshi [32]. One of the challenges in the formation of nanogels by such polymers is a control over the particle size, which requires fine-tuning of the polymer concentrations or environmental parameters such as temperature, pH, and ionic strength. A study by Nielsen and co-workers has demonstrated that these challenges can be addressed by utilizing a microfluidics-based approach [33]. Overall, the advances in polymer chemistry led to the exceptional diversity and control over the composition, architecture and functionality of crosslinked nanogels, which in turn provide more flexibility to tune their properties to comply with targeted biomedical applications.
2.2 Stimuli-responsive behavior
Stimuli-responsive behavior of nanogels is a sequence of events initiated by an external cue that comes either from the specific environment within the body like change in pH, temperature, redox conditions or enzyme concentration, or a stimulus that can be applied externally such as light, magnetic field, etc. This stimulus then causes a conformational or structural change in the nanogel, which is mediated by various factors including, but not limited to, transition in the temperature below or above its lower critical solution temperature (LCST) or the ionization of acidic or basic functional groups on the polymer chains. These changes also alter the hydrophilicity and/or hydrophobicity of the nanogels or, in other words, the extent of interaction of the system with water molecules [34-36] and is mostly manifested in the form of swelling or deswelling of the nanogel network, which in turn causes responses like release of the entrapped cargo [37,38]. One of the considerable advantages of nanogels over the macroscopic gels is their very rapid response to a change in environmental conditions [39]. The responsiveness of nanogels to the external physical or chemical signals can be tightly regulated by controlling the structure of the materials used for preparation of the nanogels [3,7]. For example, in the case of polyelectrolyte nanogels pH-dependent ionization of functional groups results in an increase of osmotic pressure inside the nanogel due to entrapped counterions and ultimately results in the swelling of the nanogels [7,40,41]. It is well recognized that a balance between the osmotic pressure and the polymer elasticity sets the physical dimensions of a hydrogel particle [42]. Thus, the extent of swelling also depends on structural characteristics of the nanogel like the chemical composition, hydrophilicity of crosslinkers, and the degree of crosslinking of the nanogel network, which controls the freedom of conformational mobility of the polymer chains [43,44]. Tan et al. studied pH-responsive polyampholyte nanogels with different fractions of methacrylic acid (MAA) and 2-diethylaminoethyl methacrylate (DEAM) units in the crosslinked polymer cores that were sterically stabilized by poly(ethylene glycol), PEG, shell [45]. The MAA segment in this nanogel is a weak acid and DEAM is a weak base (pKa = 5.4 for MAA and pKa = 7.3 for DEAM). The DEAM segments could be protonated at low pH, imparting a positive charge to this segment, whereas the MAA units are negatively charged at high pH. As a result, these nanogels exhibited marked swelling at both high and low pH values, but shrunk in the vicinity of isoelectric point due to overall charge neutralization. It was demonstrated that swelling-deswelling transition can be tuned by varying the composition of MAA and DEAM in the nanogels. The unique charge-switching properties of amphoteric nanogels based on poly(N-isopropylacrylamide) functionalized with aminophenylboronic acid (PBA) were explored by Hoare and Pelton to regulate the release of preloaded insulin in glucose-dependent manner [46]. The binding of glucose to the PBA residues shifts the boronic acid ionization equilibrium, increases the anionic charge density on the gel and drives a gel swelling response. These nanogels were designed to both swell and deswell in response to glucose according to the pH of the medium, the concentration of PBA groups grafted to the nanogel, and the relative concentrations of the cationic and anionic functional groups in the platform nanogel. Such PBA-nanogels had a high capacity for insulin uptake and selectively released insulin under physiological conditions in an “on-off” manner with fluctuating glucose level. Similar approach was adapted by Wu et al. for the design of a nanogel-based glucose sensor, which comprised of a core of Ag nanoparticle encapsulated within a glucose-recognizing crosslinked shell containing PBA residues. The swelling of the gel in response to glucose binding causes a change in the refractive index of the medium around Ag nanoparticle that leads to change in its fluorescence properties. The response rate is reported to be on the order of 100 ns, and the detection sensitivity for change in glucose concentration was reported to be ± 0.1 mM [47]. Thus, both sensitivity and rate of response of the nanogels can be programmed and fine-tuned by alterations in the structure of the building blocks [3,7]. Accordingly, the cargo (mostly drugs) can either be conjugated chemically to the nanogels or merely entrapped physically into its core [15], depending on the stimulus that is most feasible for utilization at the targeted site of disease.
Often multifunctional nanogels, which respond to a combination of more than one external signal, can also be fabricated for more site-specific response [48-51]. These stimuli are frequently disease- or organ-specific, since different pathological conditions are associated with changes in pH, temperature and redox balance or expression levels of certain biomolecules as compared to the normal physiological conditions [52]. The reversible crosslinks containing disulfide bonds that are stable in extracellular milieu but are cleaved in the reductive intracellular environments due to the differences in the reductive potential between the extracellular and intracellular compartments, are successfully utilized in design of redox-responsive nanogels [12,49,53,54]. The nanogels with disulfide links are stable during circulation in the blood but labile while internalized in cells, thus, facilitating the release of the drug. Matyjaszewski et al. have prepared biodegradable nanogels with disulfide-functionalized dimethacrylate crosslinkers that degraded into individual polymeric chains in the presence of glutathione and enabled both the release of encapsulated cargos and the removal of the carriers [55]. Thayumanavan and coworkers reported the preparation of redox-sensitive nanogels based on random amphiphilic copolymer that contains hydrophilic oligoethyleneglycol (OEG) and hydrophobic pyridyldisulfide (PDS) units as side-chain functionalities [56]. Self-assembled nanostructures formed by this polymer were crosslinked by initiating a thiol-disulfide exchange reaction among the PDS groups using dithiothreitol (DTT). The size of the resulting nanogels can be tuned by varying the copolymer molecular weight, composition and concentration, while the extent of crosslinking can be controlled by the amount of added DTT. The release of the encapsulated doxorubicin (DOX) was triggered by glutathione treatment and the release rate can be tuned by adjusting the crosslinking density.
The group of Wang took advantage of the activity of bacterial lipases, which are abundant in microbial flora, to construct a nanogel for the on-demand release of antibiotics [52]. In this approach, the triple-layered nanogel contains a hydrophobic lipase-sensitive poly(ε-caprolactone) (PCL) interlayer between the crosslinked polyphosphoester core and the PEG shell. Prior to reaching sites of bacterial infection, the antibiotics are protected inside the polyphosphoester core and are not released due to the compacted PCL molecular fence. However, rapid drug release was observed in the presence of lipase or lipase-secreting bacteria.
In another scenario, the changes in the properties of nanogels can be caused by external signals in the form of radiation or magnetic field depending on the accessibility of the targeted site in the body [57,58]. An elegant design of near-infrared (NIR) light-responsive core-shell nanogels was recently reported by Kang et al. [59]. Au-Ag-based nanorods were used as templates for the synthesis of double-stranded oligonucleotide crosslinked polyacrylamide shell. When exposed to NIR-radiation, the heating generated by Au-Ag rods through photothermal conversion induced efficient thermal dehybridization of the linker oligonucleotides from their complementary sequences and led to a rapid gel-to-sol transition of the gel shell and effective payload release.
2.3 In vivo behavior
Nanogels are macromolecular systems specifically designed to achieve long circulation half-lives of their cargo in vivo, along with their ability to deliver this cargo at the desired site (Figure 1). To realize this, a nanogel, or any nanoparticulate system has to overcome many barriers, especially when administered via routes other than intravenous, like oral, intradermal, pulmonary, intraocular, etc. Depending on the route of administration, nanogels are designed specifically to overcome associated barriers and reach the circulation intact. Nanogels prolong circulation half-life of their cargo by 1) preventing their fast clearance especially in the case of small molecules and 2) prevent quick degradation or metabolism which is more relevant for biomolecules. One of the most important obstacles to achieving prolonged circulation is opsonization of the nanogels followed by their clearance via organs of the MPS like liver and spleen, where they are taken up by the resident monocytes and macrophages [60]. PEGylation of the nanogel surface imparts them with ‘stealth’ properties by making the surface more hydrophilic, shielding a charge that the core might carry and establishing a steric hindrance for interaction with serum proteins, although this is highly dependent on the size of nanogel, its shape, molecular weight and surface density of the PEG used [61,62]. While PEGylation endows nanosystems with long circulation properties and reduces MPS uptake, eventually opsonization and macrophage clearance still occurs [63]. A number of studies have demonstrated that PEGylation of nanoparticles tends to shift their accumulation towards the spleen instead of the liver as compared to their non-PEGylated counterparts [64]. A unique feature that helps nanogels partially escape splenic filtration process is their softness and deformability. This is can be explained by an example in nature, namely erythrocytes, which in spite of having a size range in microns are easily able to pass through the splenic filtration bed that has a pore size of few hundred nanometers, due to their flexibility and deformability [62,65]. In fact, old RBCs are cleared from circulation mainly because they lose their flexibility. This biomimetic property of the nanogels can be highly advantageous for their in vivo application. For example, Lyon and colleagues have recently reported that soft spherical acrylamide-based nanogels deform and pass through membrane pores several times smaller than their hydrodynamic diameter under physiological pressures [66]. Also, varying the moduli of similar nanogels has been shown to affect their mechanism and rate of uptake in macrophages [67]. Merkel et al. reported that decreasing the modulus of microgel particles altered their biodistribution properties, allowing them to bypass several organs, such as the lung, that entrapped their more rigid counterparts, resulting in increasingly longer circulation times [68]. Convincing evidence for the prolonged circulation time of soft PEG-based hydrogel nanoparticles compared to hard nanogels of the same size was recently provided by Mitragori and coworkers [69]. The deformability of the nanogels can be modulated by varying the crosslink density within the particle matrix as well as by varying the size of the crosslinking moiety [70]. Incorporation of electrolyte moieties into the polymer network of hydrogel particles to increase the swelling ratio is another straightforward and quite efficient way to decrease the modulus [2]. However, the distribution of charged groups on the surface of a particle can accelerate the clearance of particle. To address this drawback, DeSimone's group has recently developed a strategy to generate highly-swollen polyelectrolyte gel particle with near-neutral charge while retaining charged group in the interior [71]. Fraction of nanogels that escapes clearance by the mechanisms discussed above is then distributed into various organs by the circulating blood. Nanogels are usually too big to pass through the tight junctions of the normal endothelium but can efficiently accumulate in solid tumors or inflamed tissues that have unique structural features such as defective leaky and loosely compacted vasculature and impaired lymphatic drainage leading to the well-characterized enhanced permeability and retention effect (EPR) [72,73]. In addition to EPR, bioconjugation of nanogels to targeting ligands allows directing them to specific receptors or molecules differentially overexpressed on the diseased cells/tissues thus improving their retention at the targeted site as well as facilitating their cellular uptake [74,75]. Various small molecules, peptides, aptamers, antibodies or antibody fragments have been explored for targeted delivery of nanogels and other nanomedicines in tissue- or cell-specific manner [76]. Ligand-mediated targeting influences the overall biodistribution profile of the nanogels as compared to their nontargeted counterparts and the bias of distribution is towards to those tissues that have a high expression of the receptor. This can help avoid excess accumulation of the nanogels at off target sites and reduce the associated side effects.
Figure 1.
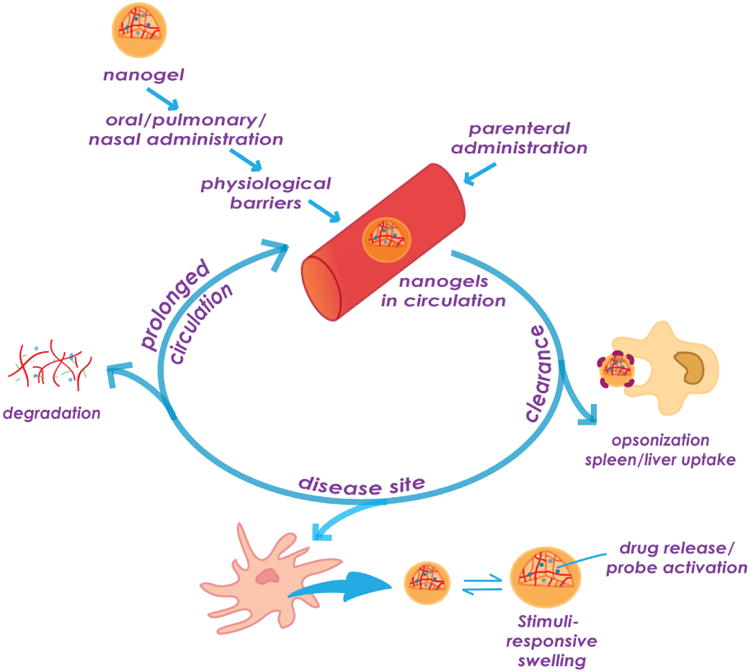
In vivo behavior of nanogels.
After extravasation from the blood compartment, nanogels have to diffuse through the tissue matrix in the interstitial space [77] and reach the targeted cells, where they are internalized by a number of different endocytotic mechanisms, depending on the size, shape, softness, charge and other surface properties of the nanogels and the type of cells and receptor being targeted. Internalization of the nanogels can occur via more than one pathway, making it a highly complex process. But in general, endocytosis eventually confines the particles into intracellular vesicles, from where they are trafficked into endosomes and ultimately lysosomes. At each of these stages, nanogels are exposed to varying pH of endosomal/lysosomal lumen, degrading enzymes or reducing environments, which are often utilized as stimuli for the release of cargo held within the nanogels. Nanogel carriers can also be designed to target specific intracellular organelles or escape them, depending on the type of cargo that they carry. For example, it is essential for the nanogels to undergo endosomal/lysosomal escape so that the encapsulated siRNA or oligonucleotides can be released in their active form in the cytosol where they are supposed to show their therapeutic effect [11]. This has brought growing interest in the use of nanogels made of bioresponsive polymers to promote escape by osmotic effects, membrane binding or membrane fusion or using pH-sensitive or reducible crosslinkers to facilitate nanogel destabilization following internalization and enhance delivery efficiency [78-81]. Degradability of the nanogels is also essential to minimize toxicities associated with the accumulation of the carrier in the body.
3. Nanogels as a therapeutic drug carrier
Nanogels are highly swollen and can incorporate 30% wt. or more of biological molecules and drugs through electrostatic, van der Waals and/or hydrophobic interactions or covalent bonding with the polymer chains. These loading capacities are unusually high and exceed those of liposomes and polymeric micelles [3,82]. As a result of drug loading, the nanogels collapse forming stable nanoparticles, in which biological agent becomes entrapped. Introducing dispersing hydrophilic polymers (e.g., PEG) in a nanogel structure can prevent their aggregation. During the collapse of the drug-nanogel complex hydrophilic polymer chains become exposed at the surface and form a protective layer around the nanogel. The control and versatility of polymer chemistry allows designing a broad range of drug formulations and inclusion of multiple therapeutic cargos within the same nanogel carrier [27,83]. Stimuli-responsive drug release via temperature or pH-induced volume collapse can also be very attractive for drug delivery applications. The functionalization of the nanogel surface can further facilitate their selective accumulation in the target tissue or cells [84-86]. Development of nanogels that can carry, protect, target and release therapeutic agents in spatially and temporally controlled manner is actively ongoing and their rational design can provide a platform for multiple applications.
3.1 Nanogels for small therapeutic molecule delivery
Over the past few years, significant progress has been achieved in application of nanogels as a delivery carrier for small biologically active molecules. Nanogels can be a versatile platform for the incorporation of various small drug molecules through the combination of electrostatic and hydrophobic interactions as well as hydrogen-bond formation [87]. The swelling of nanogels in an aqueous environment allows for easy permeation of the cargos. The rational design of the nanogels might be an effective tool to tune the drug release rates, to affect carrier-cell interactions, and achieve desirable therapeutic effect of the drugs. One of the most important features of weakly-crosslinked polyelectrolyte nanogels is their ability to incorporate molecules of the opposite charge. For example, cationic crosslinked PEG-polyethyleneimine (PEG-PEI) nanogels were explored for immobilization of negatively charged biologically active compounds such as retinoic acid, indomethacin or valproic acid [87,88]. These drug formulations formed stable colloidal dispersions at physiological pH and ionic strength, could be lyophilized and then redispersed. Similar nanogels and complexation strategy has been successfully utilized for incorporation of various nucleoside analog 5′-triphosphates [89,90]. It was reported that these drug-loaded nanogels could improve the delivery of the active triphosphates of therapeutic nucleoside analogs into cancer cells and inhibited tumor growth in the mammary carcinoma animal model [90]. Recently, the same group demonstrated the significant advantage of active 5′-triphosphates of nucleoside reverse transcriptase inhibitors encapsulated in cationic nanogels over free drugs in the antiviral therapy of HIV-1 infection in the central nervous system (CNS) [91]. Our group utilized a controlled template synthesis of nanogels by polyion complexation and crosslinking of doubly hydrophilic block ionomers, such as PEG-b-poly(methacrylic acid) (PEG-b-PMA) [21,40,92]. The resulting nanogels have swollen cores of a crosslinked PMA network surrounded by a shell composed of PEG chains. This synthetic approach allows versatile control of the macroscopic properties of nanogels (size, degree of swelling, drug loading) by changing the number and the chemical structure of the crosslinks [92]. Notably, in contrast to many nanoparticles these anionic nanogels exhibited very low nonspecific adhesion to nontargeted surfaces, which can minimize their off target effect and facilitate target-specific delivery [93]. Such core-shell nanogels can incorporate very large amounts (up to 50% wt.) of weakly basic drug DOX through electrostatic coupling with carboxylic groups in the cores. DOX-loaded nanogels were stable for a prolonged period of time, exhibited noticeable pH-sensitive behavior with accelerated release of DOX in acidic environment due to the protonation and swelling of the nanogel crosslinked cores, and demonstrated cytotoxic activities in cancer cell lines [94]. Introduction of reversible crosslinks with disulfide bonds in the PMA ionic cores (cystamine was used as a biodegradable crosslinker) allowed developing nanogels that are degradable in the presence of the reducing agent (glutathione, cysteine), which in turn facilitated the release of the incorporated DOX [94]. This difference in the release kinetics led to considerable increase of cytotoxicity: degradable DOX-loaded nanogels displayed nearly six-fold higher cytotoxic activity than non-degradable nanogels. Shi et al. used PEG-poly(L-glutamic acid-co-L-cystine) copolymers to prepare nanogels stabilized by disulfide bridges and also demonstrated that DOX release was accelerated in intracellular reductive and acidic conditions [95]. DOX was loaded into these polypeptide-based nanogels, and an accelerated release was observed in glutathione monoester pretreated HeLa cells.
Anionic PEG-b-PMA nanogels were further used to encapsulate hydrophilic drug cisplatin through coordination interactions with COOH functionalities [40]. Cisplatin-loaded nanogels displayed pH-sensitive release of Pt(II) species in sustained manner that can be effectively controlled by adjusting the degree of crosslinking of the crosslinked cores. The released platinum species retained their activity and were able to form Pt adducts with nuclear DNA in the cancer cells [40]. It was shown that loading of cisplatin into the nanogels greatly improved drug therapeutic index by improving PK, enhancing tumor delivery, increasing antitumor efficacy, and mitigating the cisplatin-mediated nephrotoxicity in a mouse model of ovarian cancer [96]. When the same cispaltin-loaded nanogels were also decorated with targeting ligands (e.g. folate or LHRH peptide), tumor growth inhibition could be even further enhanced [85,86,97]. Peng et al. described dual pH- and temperature-responsive nanogels based on N-isopropylacrylamide, MAA, and PEG methylether methacrylate (NIPAAm-MAA-PEGMA) for the entrapment and release of cisplatin. It was shown that cisplatin release can be accelerated at acidic pH, and could be additionally controlled by the temperature change due to the deswelling behavior of these nanogels at the body temperature [98].
Small molecules usually contain only limited number of ionic groups, which are able to interact with oppositely charged nanogels, and complementary hydrophobic, hydrogen or coordination bonding between drug molecules and nanogels can further stabilize the electrostatic pairing [87]. Recently, our laboratory demonstrated that microenvironment formed by the hydrophobic domains in the nanogel ionic cores influences solubilization capacity and release characteristics of the nanogels [99]. Diblock copolymer, PEG-b-poly(L-glutamic acid), hydrophobically-modified with L-phenylalanine methyl ester moieties was used for the synthesis of nanogels with small size (ca. 70nm in diameter) and narrow particle size distribution. Stable DOX-loaded hybrid nanogels were prepared at high DOX capacity (30% wt.). It was shown that the release rates of DOX from hydrophobically-modified carriers were substantially less compared to nonmodified nanogels: a burst release of over 85% of the incorporated drug within 8h for nonmodified nanogels was observed while only ∼20% of the incorporated DOX was released from hydrophobically-modified nanogels during the same period of time. These results suggested that intermolecular interactions in combination with more compact crosslinked core could account for the delayed and controlled release of DOX from hydrophobically-modified polyelectrolyte nanogels. It was also found that these DOX-loaded nanogels exhibited an improved antitumor efficacy compared to free DOX in an ovarian tumor xenograft mouse model.
Nanogels have also been explored as a carrier for poorly water-soluble drugs. Wang et al. introduced thermoresponsive nanogels based on chitosan–poly(NIPAAm-co-acrylamide) for the delivery of paclitaxel (PTX) [100]. Loading capacity of these nanogels for PTX was around 9% wt. and drug-nanogel formulation showed very good colloidal stability. This nanogel released PTX in a temperature-dependent manner wherein significantly faster drug release was achieved at higher temperature. Moreover, PTX-loaded nanogels demonstrated improved antitumor efficacy in mice bearing HT-29 colon carcinoma tumors after intravenous administration. In another study by Gref and coworkers, hydrophobic molecule benzophenone (widely used as sunscreen agent) was solubilized into nanogels formed spontaneously upon the association of a lauryl-modified dextran and a β-cyclodextrin polymer in aqueous media [101]. The highest benzophenone loadings (about 2.5 % wt.) were obtained by solubilizing it in both polymer solutions before mixing them to form nanogels. Such nanogel-based formulations present a compelling future opportunity for the application in the cosmetic field as sun screen carriers prepared by a simple “green” technology. The use of nanogels for the development of long-lasting formulations of local anesthetics has recently been reviewed by Tan et al [102]. Eckmann and colleagues explored biocompatible physically crosslinked hybrid nanogels consisting of a partially denaturated lysozyme cores and dextran shells for the local delivery of dexamethasone to alleviate acute pulmonary inflammation [103]. To target the pulmonary vasculature, nanogels were coated with antibodies directed to endothelial determinant, Intercellular Adhesion Molecule-1 (ICAM). The synthesized ICAM- targeted nanogels were loaded with dexamethasone at 5% wt. In vivo studies in animal model of LPS-induced lung injury showed that nanogels targeted to lungs succeeded in delivering encapsulated dexamethasone, as was indicated by drastic reduction in pulmonary vasculature inflammation to levels found in naïve mice. In other work, similar dextran-lysozyme nanogels were utilized as scaffolds for the in situ synthesis of silver nanoparticles [104,105]. Such hybrid nanogels exhibited bactericidal properties towards E. coli and bacteriostatic properties towards S. aureus, and their antibacterial activity can be tuned by varying lysozyme content. The tunability of the hybrid nanogels makes it possible to optimize release of the bactericidal agent for specific clinical use and type of infection. Nagasaki's group used the protonated crosslinked poly(2-[N,N-diethylamino]ethyl methacrylate) (PEAMA) cores of the stimuli-responsive PEGylated nanogels for the synthesis of gold nanoparticles without any reducing agents (“gold nanogels”) [106]. It was shown that the tertiary amino groups in the PEAMA gel core play a crucial role in the reduction of the Au(III) ions (nanoreactor) as well as the immobilization of the resulting gold nanoparticles (nanomatrix) to attain large payloads of gold nanoparticles. The resulting gold-containing nanogels showed a remarkable photothermal efficacy in response to laser-irradiation resulting in selective cytotoxicity in cancer cells. In other studies, a potential of the similar constructs to enhance cancer cell radiosensitivity was reported [107].
3.2 Nanogels for oligonucleotide delivery
Therapeutic oligonucleotides (ONs) including antisense oligodeoxynucleotides (ODNs), small interfering RNAs (siRNAs), and the more recently discovered micro RNAs (miRNAs) designed for targeted inhibition of specific mRNA sequences are of emerging interest for the treatment and diagnosis of cancer [108-110], neurodegenerative disorders [111-113] and lethal viral infections [114-116]. Some oligonucleotide-based therapies have already achieved significant success in clinical trials [117,118]. However, the delivery of ONs into targeted cells remains a key challenge to realizing their full therapeutic potential because ONs are negatively charged, hydrophilic molecules that are unable to penetrate cell membranes on their own, can be degraded by endogenous nucleases and can stimulate innate immune system. Thus, ONs require a delivery vehicle to bring them to site of action without adverse effects. Cationic nanogels have emerged as promising new class of nanomaterials to address the challenges of in vivo ONs delivery [84]. Composed of weakly crosslinked hydrophilic polymer chains, nanogels have a high degree of porosity that permits the effective encapsulation of macromolecular therapeutics, which usually cannot be achieved with conventional nanocarriers.
In one of the first reports, Vinogradov et al. exploited cationic PEG-PEI nanogels as potential carriers for antisense phosphorothioate ODN specific to human mdr1 gene [119]. Significant enhancement of antisense inhibition of Pgp as a result of incorporation of ODN in the nanogel was observed. Modification of ODN-loaded nanogels with transferrin targeting moieties additionally increased the mdr1-inhibitory effects and was shown to facilitate transport of the loaded nanogels across the blood-brain barrier [84]. Subsequently, DeSimone and coworkers used inverse miniemulsion polymerization of 2-acryloxyethyltrimethylammonium chloride and 2-hydroxyethylacrylate with PEG-diacrylate crosslinker to synthesize biocompatible nanogels with controlled size, morphology, and composition capable of forming stable ODN complexes and enhancing cellular delivery of ODNs in vitro [16]. Nowadays, a variety of cationic nanogel particles have been actively adapted to deliver siRNA molecule [81,120-128]. For example, Anderson and coworkers synthesized a library of 1,536 structurally distinct core-shell nanogels for siRNA complexation and delivery with great variability in the chemical nature of the protonizable amine-based core and a shell with variation in polymer length and chemical properties [129]. The evaluation of this library revealed that internalization and/or complexation alone is not sufficient for silencing and that certain chemical functionalities with potential buffering capacity may be advantageous for nanogel-based delivery. Chemical modifications of siRNA may also be used to enhance the transfection efficiency of these nanocarriers and to impart greater stability to their nucleic acid cargoes. The recent report by Zentel group [78] also emphasized that size-dependent uptake and intracellular distribution mechanism of siRNA-loaded cationic nanogels may be essential for tuning their knockdown potential. The authors used two sets of well-defined nanogels based on amphiphilic block copolymers of pentafluorophenyl methacrylate and tri(ethylene glycol) methyl ether methacrylate crosslinked by spermine with diameters of 40 nm and 100 nm. Both formulations show similar physicochemical properties, loading efficiencies and release capabilities of siRNA. However, only the small sized anti-luciferase siRNA-loaded nanogels with diameters of about 40 nm were able to avoid acidic compartments of endolysosomal uptake pathway and induce gene knockdown, while the 100 nm-sized nanogels did not affect the gene expression of luciferase at all. The reader is referred to more detailed review articles that summarize the key properties of the cationic nanogels that enable successful in vivo delivery of various nucleic acids [130].
3.3 Nanogels for delivery of protein therapeutics
Owing to the ability of nanogels to encapsulate high amounts of biomacromolecules and prevent them from degradation, they have been also widely explored for the delivery of proteins and peptides. In their pioneering work, Akiyoshi et al. reported that the nanogel of self-assembled cholesterol-modified pullulan (CHP) forms a complex with various kinds of proteins spontaneously, primarily through hydrophobic interactions [131,132]. The amount of protein complexed by such nanogels depends on the molecular weight and hydrophobicity of the protein. Complexation drastically suppressed the thermal denaturation and subsequent aggregation of proteins as well as protected them from enzymatic degradation. This platform was further extended by cationization of the polysaccharide-based nanogels to utilize both hydrophobic and electrostatic interactions for effective protein trapping and to enhance cellular internalization of the protein-loaded carriers [10]. However, one of the drawbacks of such delivery systems is the destabilization of the nanogel–protein complex in vivo in the presence of high protein concentrations. To preserve the long-term stability of such complexes, the same group developed the raspberry-like assembly of nanogels with narrow size distribution (40-120 nm size) by crosslinking acrylate-group-modified CHP with thiol-modified tetra-armed PEG [133]. These nanogel assemblies showed high encapsulation efficiency for interleukin-12 (IL-12) and were able to maintain steady plasma IL-12 level in mice up to 72 h following subcutaneous administration. Nagahama et al. demonstrated that hybrid self-assembled nanogels based on poly-(L-lactide)-grafted dextran enable sustained release of the entrapped lysozyme in its active form for a week without initial burst release at physiological conditions [134]. Thienen et al. developed biodegradable lipid-coated dextran nanogels by UV polymerization of dextran derivatized with 2-hydroxyethyl methacrylate moieties using liposomes as a nanoscaled reactor [135]. Proteins can be loaded into the nanogel cores with high efficiency during the polymerization process and their release can be controlled by crosslinking density of the gel matrix. The application of polysaccharide-based nanogels for delivering proteins and other macromolecular therapeutics has recently been summarized in a review article [136]. Matyjaszewski and coworkers demonstrated that application of atom transfer radical polymerization (ATRP) enabled the synthesis of functionalized nanogels with a uniform network that is capable of efficient encapsulation of proteins in situ [137]. Similarly, the addition of cytochrome C during the inverse emulsion reversible addition fragmentation chain transfer (RAFT) polymerization of N-(2-hydroxypropyl)methacrylamide and N,N′-bis(acryloyl)cystamine also showed high loading efficiency (73 % wt.) of the protein in the resulting nanogels, which were shown to release their payload under reductive conditions [138]. In another interesting work reported by Chen et al. RAFT polymerization was used to prepare disulfide crosslinked nanogels based on PEG-b-poly(2-(hydroxyethyl) methacrylate-co-acryloyl carbonate) for loading (∼50 % wt.) and triggered intracellular release of proteins [139]. The in vitro release studies showed that release of fluorescently-labeled cytochrome C was minimal under physiological conditions while complete release of the protein from nanogel was observed in the presence of 10 mM dithiothreitol over 22 h. Cytochrome C-loaded reduction-sensitive nanogels demonstrated apparently better apoptotic activity than free cytochrome C and reduction-insensitive controls. Shi et al. developed acid-labile nanogels via inverse emulsion polymerization of N-vinylformamide in the presence of a ketal-containing crosslinker. The loading capacity of these nanogels for lysozyme was ∼60% wt. and approximately 95% of lysozyme encapsulated in nanogels released over 3 hours at pH 5.8 compared to only ∼15% released at pH 7.4. Notably, released lysozyme retained about 50% of the original activity. Although the better loading efficiency is a significant advantage of the aforementioned in situ loading technologies, the retention of activity, structural identity, and stability of protein after encapsulation are basic concerns in development of biopharmaceutical products.
Polymeric nanogels have also found an application in the formulation of a new generation of therapeutic and preventive vaccines. Intrinsic properties of polymeric nanogels, such as material chemistry, size and shape, surface charge, and hydrophobicity or hydrophilicity, may be determining factors in designing the induced immune response. These materials can thus work as synthetic adjuvants, which can also be conjugated with immunostimulants. Furthermore, nanogels having a size of 50 nm or less were found to be quite effective in delivery across the hydrogel-like network of mucus [140] and it has been reported that in the case of nanogels for vaccine delivery, the type and intensity of immune response can be modulated by altering the size of the nanogels [141-143]. In a very interesting report published by Akiyoshi and Kiyono group, cationic cholesterol-bearing pullulan (cCHP) nanogels were explored as protein-delivery system for adjuvant-free intranasal vaccines [14]. As shown in Figure 2, they demonstrated that intranasally administered cCHP nanogel loaded with a non-toxic subunit fragment of Clostridium botulinum type-A neurotoxin BoHc/A (cCHP–BoHc/A) adhered to the nasal epithelium and BoHc/A was effectively taken up by mucosal dendritic cells after its release from the cCHP nanogel. Importantly, intranasally-administered cCHP–BoHc/A did not accumulate in the olfactory bulbs or brain, diminishing safety concerns about the potential dissemination of vaccine antigens to the CNS. Moreover, intranasally immunized tetanus toxoid with cCHP nanogel induced strong tetanus-toxoid-specific systemic and mucosal immune responses. The CHP platform has also been tested in Phase 1 clinical trials as carrier for HER-2 antigen in patients with therapy-refractory HER-2 positive cancers [144] as well as for delivery of NY-ESO-1 antigen in esophageal cancer and malignant melanoma patients [145,146]. Collectively, the results from several clinical trials performed using the CHP-based antitumor vaccines demonstrated the safety of the vaccines after repeated subcutaneous administration as well as document their success in inducing both antigen-specific CD4+ and CD8+ T-cell response along with humoral immunity. According to a more recent report, a MAGE-A4-specific humoral immune response was observed in four patients out of fifteen vaccinated with MAGE-4A protein encapsulated in CHP nanogel [147]. These patients showed prolonged overall survival, significantly longer than that of patients without a MAGE-A4 antibody response after vaccination. We encourage the readers to refer to a review by Ferreira et al. [141] and the latest review by Akiyoshi [148] for the details about the application of nanogels as vaccine carriers.
Figure 2.
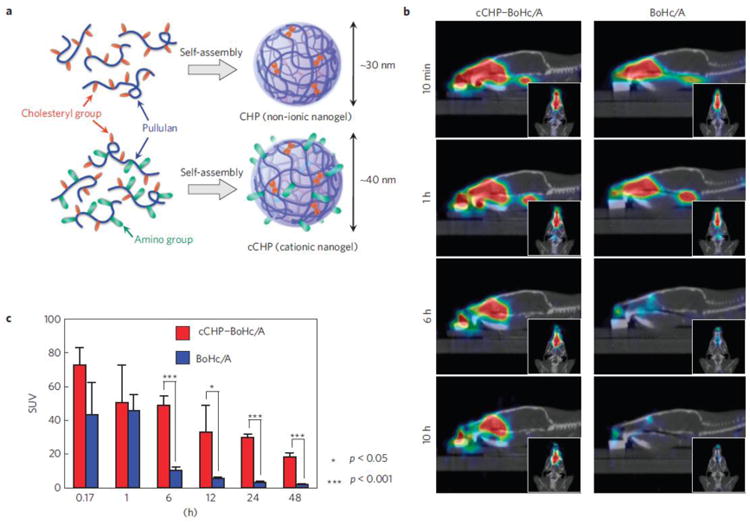
Use of cCHP nanogel as a new antigen-delivery vehicle for intranasal vaccination. A) Generation of cCHP nanogel from a cationic type of cholesterol-group-bearing pullulan. B) PET images showing that intranasally administered cCHP nanogels carrying [18F]-labelled BoHc/A were effectively delivered to the nasal mucosa. C) Direct quantitative study with [111In]-labelled BoHc/A further demonstrated that BoHc/A was retained in the nasal tissues for more than two days after intranasal immunization with cCHP nanogel. In contrast, most naked BoHc/A disappeared from the nasal cavity within 6 h after administration. Adopted with permission from [14].
3.4 Nanogels for combination drug delivery
Drug combinations directed simultaneously at multiple pharmacological targets have the potential to dramatically improve the response to treatment and are already a standard clinical practice in the treatment of cancer and infectious diseases [149]. However, the difference in pharmacokinetic and pharmacodynamic profiles among diverse drug molecules makes dosing and scheduling optimization very challenging. Combining drugs in one delivery carrier is a well-suited strategy for controlling the pharmacokinetics and co-delivery of the desired drug ratio in vivo, and a variety of nanoscale carriers, including nanogels, have been investigated in terms of their ability to deliver multiple drugs [27,83,150,151]. As highlighted in previous sections, the nanogel structure can be readily adjusted to integrate features of different materials and, thus, offer advantages for combinatorial encapsulation of drugs with varying physicochemical properties such as small molecules, proteins and nucleic acids. Fahmy and coworkers developed liposomal nanogels of drug-complexed cyclodextrins and cytokine-encapsulating biodegradable polymers that can deliver small hydrophobic molecular TGF-β inhibitor and water-soluble protein cytokine (IL-2) in a sustained fashion to the tumor microenvironment [150]. They demonstrated that synergistic effects of simultaneously delivered IL-2 and TGF-β inhibitor on activation of the innate arm of the immune system led to delayed tumor growth and enhanced survival of melanoma tumor-bearing mice after systemic administration. In line with the challenge of delivering drugs with different properties, our group developed multi-compartment core-shell nanogels based on hybrid triblock copolymers, PEG- poly(L-glutamic acid)- poly(L-phenylalanine) (PEG–PGlu–PPhe). Such nanogels have a central hydrophobic core formed by PPhe chains, a crosslinked anionic layer of PGlu chains, and an outer shell composed of PEG chains [27]. These hybrid biodegradable nanogels can entrap considerable amount of drugs with very different physical properties such as hydrophilic cisplatin (15 % wt. loading) and hydrophobic paclitaxel (9% wt.). Binary drug combination in nanogels exhibited synergistic cytotoxicity against human ovarian A2780 cancer cells and exerted a superior antitumor activity in cancer xenograft models in vivo as compared to individual drug-loaded nanogels or free drugs. The benefits of synchronized co-delivery of the platinum-taxane drug combination via single carrier can be further enhanced by targeting nanogels to folate receptor, which are overexpressed in most ovarian cancers [97]. In another study, we reported an efficient co-encapsulation of DOX and 17-allylaminodemethoxygeldanamycin (17-AAG) into PEG-PGlu nanogels with multiple hydrophobic domains that are located within the crosslinked polyion PGlu core [83]. Dual drug-loaded nanogels displayed potent cytotoxicity in a breast cancer cell panel and exerted selective synergistic anticancer activity against ErbB2-overexpressing breast cancer cell lines. This synergistic effect was attributed to the action of 17-AAG, HSP90 inhibitor, which induces degradation of many of the proteins required for DNA- damage response as well as attenuates hyperactive ErbB2 downstream oncogenic signaling, thereby rendering cancer cells more vulnerable to the cytotoxic effects of DOX. Consistent with the in vitro findings, combination treatment with nanogels exhibited superior antitumor efficacy, both in terms of tumor inhibition and survival, in an ErbB2-driven xenograft model compared to the cocktail of free drugs at equivalent drug concentrations.
Akiyoshi and colleagues evaluated nanogels based on acrylate group–modified CHP for co-delivery of prostaglandin E2 receptor–specific agonist EP4A (small molecule) in combination with bone morphogenic proteins (BMP-2) for bone regeneration [151]. In this study, EP4A-containing nanogels and BMP-2-containing nanogels were crosslinked with thiol-bearing PEG to obtain disc-like scaffolds for the implantation into large bone defects. Combination treatment with EP4A and low-dose BMP-2 efficiently activated bone cells to regenerate calvarial bone by forming both outer and inner cortical plates as well as bone marrow tissue and resulted in the formation of enough bone to cover the defects in calvarial bone. In another study, cationic physically-crosslinked nanogels composed of a hexadecyl group-bearing cycloamylose and spermine-modified cycloamylose to complex and co-deliver plasmid DNA along with phospholipaseA2 (PLA2) [152]. As a lipolytic enzyme, PLA2 catalyzes hydrolysis of a variety of different phospholipids. Thus, being delivered into endosomes by nanogel it could disrupt the lipid membrane and subsequently trigger the release of the co-encapsulated DNA into the cytoplasm. Transfection experiments confirmed that DNA expression level was enhanced when complexed with PLA2. Similarly, pDNA and proteins were successfully co-encapsulated using pH- and temperature-sensitive carbohydrate-based nanogels [79]. The nanogels had a core-shell structure with a crosslinked hydrophobic core that could be loaded with proteins and the shell contained carbohydrate residues that allowed for the complexation of DNA. These nanogels were capable of loading larger-than-normal amounts of cargo by using a heating and cooling cycle. Altogether, these studies may open new avenues for the development of the carrier-mediated combination therapies.
4. Nanogels in diagnostics and imaging
Structural versatility of nanogels, high water content, fluid-like transport properties and biocompatibility make them ideal carriers for various imaging probes and contrast agents. Introduction of multiple functional groups either in the interior or on the surface of nanogels allows for incorporation/conjugation of multiple dyes, reporter molecules or inorganic nanoparticles. Encapsulation of magnetic nanoparticles such as iron oxide into crosslinked nanogels has been shown to confer both colloidal stability and better sensitivity than when these agents are administered as non-encapsulated entities [153,154]. Nanogels allow for the encapsulation of a large cargo of the magnetic nanoparticles, which can lead to generation of much stronger local magnetic fields due to the cluster effect [154-157]. Moreover, the hydrogel coating further increases the relaxivities by lowering the diffusion coefficient of water near the particles and prolonging the interaction between the water protons and the high magnetic fields at the surface of the particle. The extent of reduction in the diffusivity of the water molecules depends on the thickness of the gel coating around the magnetic particle [156-159]. This could allow for partial control over the relaxation times by manipulating the swelling/deswelling transition of the nanogel matrix [157]. Interestingly, Okada et al. reported that nanogels consisting of crosslinked PMA can function as magnetic resonance imaging (MRI) pH-sensors without the need of any inorganic paramagnetic material [160]. The shrinking of pH-sensitive nanogel at acidic pH induces more rigid configurations and slower rotational motions of the polymer chains than in the swollen state thereby leading to shorter transverse relaxation time because the mobility of bound water molecules is highly restricted. Another factor that influences the relaxation time of contrast agents like Gd chelates is the freedom of tumbling motion [155]. One of the best methods to restrict tumbling is by conjugation of the contrast agent to a macromolecular system, and nanogels serve as the perfect hydrophilic platform with flexible loading capacity for entrapment for such agents, which was shown to result in a further relaxivity enhancement. [156,161,162]. Gd3+ ion is toxic and it is clinically administered in the form of chelates. However, even the chelates have the potential to show toxicity due to transmetallation reactions, which involve the displacement of the chelated metal ion by another competing ion. It has been reported that diethylenetriaminepentaacetic acid (DTPA)-based nanogels are more inert to transmetallation reactions than free chelates [163]. Encapsulation into nanogels also helps in reducing the toxicity associated with inorganic nanoparticles [164-168]. Furthermore, it has been shown that the electronic and optical properties of gold and silver nanoparticles are highly dependent on their size and shape [169-171] and encapsulation of such nanoparticles into nanogels becomes essential not only for their colloidal stability but also for consistency of the imaging purpose. As in the case of contrast agents, the swelling/deswelling of the surrounding nanogel network in response to changes in the microenvironment can reversibly alter the interparticle distance between gold nanoparticles leading to a corresponding change in their optical properties. This can impart tissue specificity to the imaging function to a certain extent [171,172].
4.1 Nanogels as MR contrast agents
Small molecule MR contrast agents based on gadolinium (Gd) and manganese (Mn) are all rapidly cleared from the body and suffer from toxicity issues. [173,174]. To overcome these challenges, Soleimani et al. prepared a nanogel by copolymerization of PEGMA, N-(2-aminoethyl)methacrylamide hydrochloride, and the crosslinker ethylene glycol dimethacrylate under free radical conditions [156]. The reaction conditions were optimized to obtain nanogels with a size on the order of 10 nm. An isothiocyanate derivative of the chelator DTPA was then conjugated to nanogel for the insertion of Gd (III). The nanogel contrast agent exhibited ∼ 5-fold enhancement in relaxivity compared to clinically used Gd(III)–DTPA (Magenvist). Signal enhancement, however in lesser extent, was also observed for non-crosslinked polymer constructs containing similar amount of Gd-chelates. The modeling of nuclear magnetic resonance dispersion data suggested that the enhancement in relaxivity for nanogel vs linear polymer arise from the constraint on polymer chain motion imparted by the crosslinking that leads to additional slowing of the chelates' molecular tumbling rate. Alternatively to post polymerization functionalization of nanogel with contrast agents, Gd-chelates can be incorporated into nanogels within the crosslinking moieties or Gd3+ ions can be used as metal crosslinkers themselves. The later strategy was utilized by Kim and coworkers to prepare Gd-coordinated nanogels by crosslinking of branched PEI with Gd3+ ions in inverse microemulsion followed by surface functionalization with PEG chains in order to increase the blood circulation time [175]. It was shown that polydentate chelation by PEI is robust enough to minimize the liberation of toxic Gd3+ ions under the physiological conditions. Interestingly, the determined longitudinal T1 relaxivity values for Gd-PEI nanogels were lower than that for Gd-DTPA chelates. The reduced relaxivity was attributed to the large nanogel core size with a small surface-to-volume ratio, which hinders the direct Gd3+-water contact that is prerequisite for influencing the longitudinal relaxation. In contrast to other T1-enhancing Gd complexes, Gd-PEI nanogels showed the capability of enhancing negative T2 contrast, which was assigned by high density of the Gd3+ ions in the cores of the nanogels. Almutairi's group reported Gd-chelating polyacrylamide nanogels prepared by radical polymerization of acrylamide with metal chelating crosslinking agents in an inverse emulsion [163]. The resulting crosslinked nanogels had a size in the range of 50 – 85 nm, were stable against transmetallation, and exhibited a substantial (3 to 4-fold) increase in relaxivity. To further improve nanogels biocompatibility, the same group utilized nanogels formulated from cholesterol- and acryloyl-modified pullulan (CHPOA). The surface acryloyl groups of CHPOA were chemically crosslinked with Gd-chelating 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) crosslinker. At a magnetic field of 1.41 T, T1 relaxivity of these Gd-chelating pullulan nanogels was measured to be 24.1 ± 0.6 mM-1 s-1, which corresponds to 6-fold enhancement as compared to low-molecular weight chelates, Magnevist and Dotarem. The reported value was also the highest relaxivity among all other nanogel-based contrast agents. MRI of tumor-bearing mice showed that Gd-CHPOA provides great contrast in tumors with exceptionally high signal-to-background ratio. This allowed for discriminative and accurate tumor identification along with determination of the surgical margin. Importantly, the long-term accumulation of Gd-CHPOA in organs of the MPS like liver and spleen did not cause any damage or toxicity up to three months after injection.
Superparamagnetic iron oxide (SPIO) nanoparticles function as T2-weighted MR contrast agents and have exerted a large impact on the field of molecular and bioimaging. Their nonspecific uptake by MPS cells found clinical applications for imaging liver tumors [176] and lymph nodes [177]. SPIO nanoparticles' magnetic properties can be manipulated by changes in size and surface chemistry. Specifically, the local T2 proton relaxivity of SPIO nanoparticles can be modified via interfacial surface chemistry to induce aggregation or dispersion in the colloid, thus providing control of the ultimate nanoparticle aggregate size and magnetic properties [178]. Katagiri et al. reported a synthetic approach for the in situ formation and immobilization of SPIO nanoparticle aggregates in nanogels [153]. Iron ions were complexed within the pre-heated network of physically crosslinked CHP nanogels and oxidized by addition of ammonia water solutions. This strategy minimized homogeneous nucleation outside of nanogels, and the resulting hybrid nanogels had a size similar to that of the empty nanogels (∼ 30 nm). Essentially, the empty nanogels acted as nanoreactors wherein the volume in which the reaction could occur was confined by the nanogel network. However, the crystallinity of iron oxide particles synthesized in nanogels was relatively low, and will not translate into high saturation magnetization; therefore, these hybrid nanogels are not suitable for use as MR contrast agent. To overcome this drawback, the preformed oleate-coated iron oxide nanoparticles with size of 12 nm and high crystallinity were complexed with nanogels through hydrophobic interactions with cholesterol groups in the nanogels [179]. The resulting hybrid showed high colloidal stability and displayed higher relaxivity than existing contrast agent Resovist. In addition, the hybrid nanogel generated heat following irradiation with alternating magnetic field, which indicates that these materials can also be suitable for magnetic hyperthermia therapy.
Recently, Wang et al. described a nanogel-based system co-loaded with both T1 and T2 relaxivity contrast agents, manganese oxide and SPIO nanoparticles, since the use of dual contrast agents can potentially allow for higher accuracy of imaging. These agents cannot be simply mixed in a single system, since in close proximity they would lead to mutual nullification of the signal, as the water molecules can interact freely with both the agents. This problem, however, was solved by the use of stimuli-responsive chitosan-based nanogels, which release Mn2+ ions only in the acidic pH inside the tumor cells while at physiological pH in normal cells the system remains silenced, thus improving specificity of imaging. This technique can be potentially useful for detection of small tumors with better accuracy than the existing single agent probes [180].
Nagasaki's group developed a unique tumor specific nanogel-based probe for 19F magnetic resonance spectroscopic imaging (19F MRS/I) [181]. 19F MRS/I has been recognized as a powerful methodology due to high MR sensitivity of 19F and low background noise which is attributed to the absence of endogenous 19F in the body. The 19F MRS/I of tumors can be further improved using a probe that attenuates the 19F MR signal outside of the tumor and switch on the signal inside the tumor. To this end, a pH-responsive nanogel consisting of a crosslinked PEAMA-co-poly(2,2,2-trifluoroethyl methacrylate) gel core and PEG shells was synthesized. These nanogels showed remarkable activation of 19F MR signals upon change of pH from 7.4 to pH 6.5, which corresponds to the extracellular pH of tumor tissues (7.0 – 6.5). Such enhancement was attributed to the volume phase transition of the gel core at acidic pH that leads to an increase in molecular motion of the 19F atoms and longer T2 relaxation times.
4.2 Nanogels for PET imaging
Polyacrylamide-based nanogel crosslinked with polydentate chelating ligands that were developed by Allmutairi and coworkers can be also used as a scaffold for metal radionuclides to obtain PET radiotracers [182]. Various crosslinkers based on DTPA, DOTA or 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) were synthesized to optimize the chelation stability of nanogels. Experiments in mouse serum indicated that NOTA-based nanogels retained 64Cu most stably, with very little transchelation in comparison with the other two crosslinkers. 64Cu-DOTA-crosslinked nanogels showed high accumulation in the tumor as well as lower signal in the liver and spleen compared to DOTA-based nanogels. In some cases, the accumulation of 64Cu-DOTA in metastases was even higher than in the primary subcutaneous tumor. These promising data suggest that nanogels incorporating metal-chelating crosslinks can be useful as PET agents in cancer diagnosis and therapy monitoring. An advantage of such systems is that the radioisotope can be easily incorporated into preformed nanogels immediately before their clinical application.
Singh et al. designed reduction-sensitive crosslinked nanogels containing chelating groups for 68Ga and other trivalent metals [183]. Self-assembly of amphiphilic, partially thiolated star-shaped poly(ethylene oxide-stat-polypropylene oxide) copolymers was used for the synthesis of the nanogels. The thiol chemistry was used for both crosslinking and derivatization for radiolabeling. It was shown that 68Ga can be chelated very efficiently with high radiochemical yields. However, the size of the resulting nanogels (290 ± 50 nm) may not provide optimal circulation time and tumor uptake. In vivo PET imaging of 68Ga-labeled nanogels has not yet been reported.
4.3 Nanogels for optical imaging
In vivo fluorescence-based optical imaging is most effective by using agents that emit in the NIR region (> 700 nm) due to minimal auto-fluorescence from the tissues in this wavelength range, and deep tissue penetration of excitation light [184,185]. Among available NIR probes, only indocyanin green (ICG) has been approved for clinical imaging applications. ICG, however, suffers from several limitations such as low quantum yield, relatively short circulation half-life, degradation in aqueous media and self-quenching above a certain concentration, and nonspecific interaction with various proteins in blood plasma, which alter its fluorescence emission properties [186].
Nanogels have been employed to overcome the above shortcomings of the dye in order to extend its potential as a NIR imaging agent. In fact, some of these limitations were used as advantages to design systems that would be activated specifically in the targeted cell population. Park et al. designed cancer cell-targeted nanogels containing ICG as the reporter molecule that were non-fluorescent in the normal cells. A pH sensitive polymer, poly(β-amino ester) (PBAE) and CD44 receptor-targeting hyaluronic acid were used to form the nanogels which entrapped ICG. Under normal physiological conditions, the ICG fluorescence was quenched in the intact nanogels. Once ICG-loaded nanogel is internalized by receptor-mediated endocytosis, PBAE is degraded in the acidic environment of endosomes and lysosomes, gradually releasing ICG, thus generating a fluorescence signal [187]. In a similar vein, hyaluronic acid was used to prepare nanogels that could be used for monitoring hyaluronidase activity in vivo, as it is associated with tumor metastasis and angiogenesis. Shell crosslinked nanogels were prepared from hyaluronic acid via a reducible covalent linkage, which could incorporate ICG derivatives [188]. In the absence of the enzyme, HA-based nanogels had negligible fluorescence as compared to free ICG due to self-quenching effect. However, in the presence of the enzyme, the nanogel structure was disturbed and a strong fluorescence signal was generated. This was illustrated both in vitro and in vivo after intradermal injection of the nanogels in the forepaw of mice. Nanogels showed a strong fluorescence signal 5 mins after injection of hyaluronidase into the forepaw, indicating that this system could be used as hyaluronidase-specific probe. NIR imaging is also useful for detecting the cancer metastasis to regional lymph nodes, particularly the sentinel lymph node (SLN). Biopsy of SLN is usually done during tumor resection surgeries to determine whether metastasis has occurred, for which visualization of these lymph nodes is necessary during the surgical procedure. Small molecule dyes used clinically for this purpose suffer from the drawbacks of rapid lymphatic clearance and spreading to the entire lymphatic system and can therefore give false-positive results. Dextran-poly(acrylic acid) nanogels (DNG) were synthesized via self-assembly assisted method which involved hydrogen bonding between hydroxyl groups of dextran and carboxyl groups of poly(acrylic acid) [189]. The additional free carboxyl groups of poly(acrylic acid) served as a platform for the conjugation of amine-containing dyes. As a model agent, green 5-aminofluorescein was conjugated to obtain fluorescent DNG, which were found to drain via the lymphatic vessels and into SLN within a minute of being injected intradermally, and the fluorescence signal peaked around 12 h and lasted for 60 hours. This extended imaging window is well-suited for surgical applications wherein the visualization of lymph nodes is required. Another nanogel-based system designed for the same application has been described by Noh et al. [190]. IRDye800 containing nanogels composed of cholesterol-modified pullulan (NIR-CHP) had hydrodynamic diameters of about 30 nm, which is considered optimal for lymph node uptake (Figure 3). NIR-CHP probes exhibited enhanced photostability, and could accumulate and retain in the SLN after intradermal injection. The NIR-CHP were present at qualitatively consistent levels in the lymph node even at 48 h post injection, whereas the IRDye800 was not visibly present in the lymph nodes at this time point. In another study, the utility of NIR-CHP probes for SLN mapping was evaluated in large animal models [191]. The NIR-CHP signal intensity was relatively small at the injection site and limited to only one sentinel node with no spreading to consecutive distal lymph nodes. These characteristics of NIR-CHP make them useful as imaging contrast agents in sentinel node navigation surgery.
Figure 3.
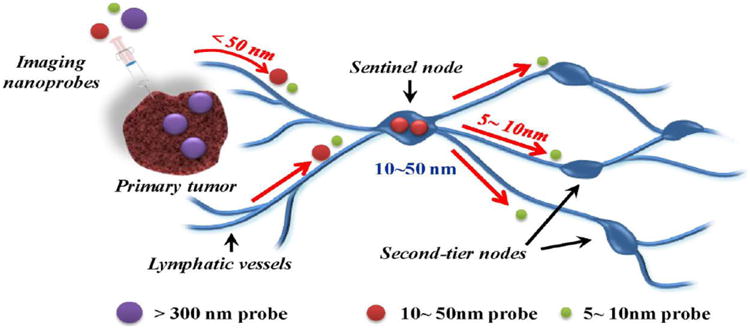
Schematic illustration of sentinel lymph node (SLN) mapping using a nanoscale imaging probe. Imaging probes on 5 – 10 nm size scale can flow through the SLN into adjacent nodes in the chain; nanoprobes > 300 nm in size rarely leave the injection site, while those with a size range of 10 – 50 nm exhibit rapid uptake into SLN and do not leave. Adapted with permission from [190].
In recent years, there has also been a considerable interest in the development of optical probes based on nanogels and inorganic nanoparticles such as quantum dots and gold nanoparticles. The reader is referred to some excellent review articles on the design of such hybrid nanogels and their application for optical sensing and bioimaging [165,192].
4.4 Nanogels for multimodal imaging agents
While a number of imaging techniques can be employed to obtain detailed information regarding various organs and tissues of the body, each imaging modality has limitations of either sensitivity or resolution. Therefore, the integration of several imaging agents with different properties into multifunctional nanoparticles may provide precise information about the existing pathological conditions through synergetic multimodal imaging. [193]. Due to their unique multifunctionality, large surface area, and structural diversity, nanogels are capable of loading more than one imaging/contrast agent within the same carrier that can help realize this objective.
For example, some of optical imaging complications associated with poor spatial resolution and lack of anatomical reference can possibly be mitigated when combined with other high resolution anatomical imaging modalities such as MRI. This combination was employed to define the glioma margins using targeted pH/temperature sensitive nanogels based on poly(NIPAAm-co-acrylic acid). Magnetic SPIO nanoparticles were incorporated into nanogel network during emulsion polymerization followed by conjugation with Cy5.5-labled lactoferrin as an optical probe and an effective targeting ligand for glioma [194,195]. The LCST of the nanogels at physiological pH of 7.4 was 40°C, making the nanogels swollen and hydrophilic, which could prolong the blood circulation time. In the acidic environment of tumor tissues (pH 6.8), the LCST is lowered to 34 °C leading to collapse of the nanogels, which could in turn potentiate their accumulation in tumor tissue and internalization by tumor cells. The authors reported very accurate signal localization and correlation of in vivo results to ex vivo images of excised brain (Figure 4) which was attributed to active targeting ability of the lactoferrin and passive targeting ability enhancement caused by the pH/temperature sensitivities of the nanogels [196].
Figure 4.
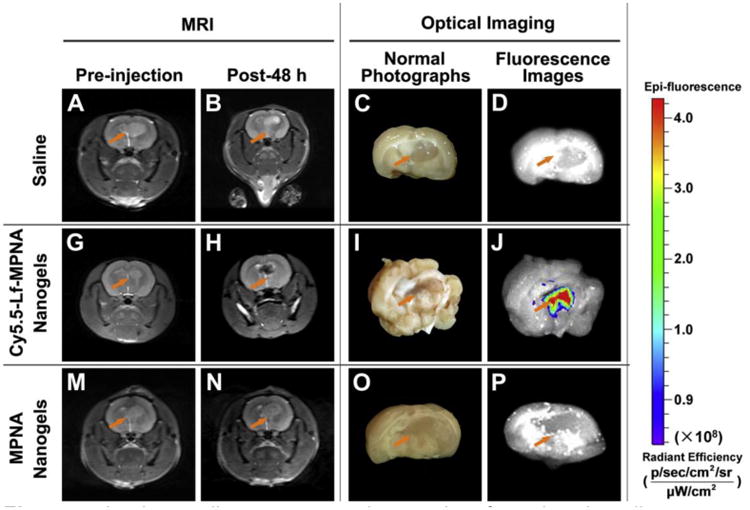
In vivo studies: representative results of rats bearing gliomas treated with saline as a control (upper row), Cy5.5-Lf-MPNA nanogels (middle row), and MPNA nanogels (lower row), respectively (n = 9 in each group). T2-weighted MR Images of gliomas before injection (A, G, M) and at 48 h post-injection (B, H, N); normal photographs (C, I, O) and ex vivo fluorescence images (D, J, P) of gliomas at 48 h post-injection; Adapted with permission from [196].
Another multi-imaging study using nanogel-based dual MRI and optical imaging probe was reported by Lim et al. Core-shell nanogels containing Gd+3-coordinated PEI core and PEG shell were functionalized with a NIR dye, Cy5.5 and evaluated in SCC7 tumor-bearing mice [175]. Although no targeting group was attached to the nanogels, tumors displayed strong NIR fluorescence signals up to 1 day post injection. Notably, the amount of injected nanogels corresponded to a much lower dose of contrast agent (38 μmol Gd/kg) than the standard dose of Gd-DTPA (100 μmol Gd/kg) and no notable contrast enhancement was observed in T1-weighted images. However, a significant signal darkening was seen in certain regions of the tumor at 2 h post-injection, visualizing the intratumor structure at high spatial resolution, indicating that these nanogels could act as negative contrast enhancers (see section 4.1) for tumor imaging with the added advantage of fluorescence imaging [175].
Nanogels can also be designed to have self-fluorescence properties, as demonstrated by Kim et al. [197] using oppositely charged polyelectrolytes to form the gel network within which MnFe2O4 nanoparticles were encapsulated. Negatively charged poly(γ-glutamic acid) was condensed with polylysine (PLL) in the presence of MnFe2O4. The free amino groups of PLL were then crosslinked with glutaraldehyde, which also involved formation of a self-fluorescent carbon–nitrogen double bond (C=N) and carbon-carbon double bond (C=C). These nanogels were finally surface-grafted with PEG. These dual-modality nanoprobes showed high performance in the labeling and monitoring of therapeutic immune cells both in vitro and in vivo. Nanogels can also be fabricated to have a core-shell-corona structure in which a shell of inorganic material like silica protects the core of magnetic nanoparticles while its permeability to water allows for the interaction of the water molecules with the magnetic core that is essential for its imaging function. Ruhland et al. reported such nanogels that had a temperature-sensitive corona of crosslinked PNIPAAm. High magnetization and/or bright luminescence was achieved due to a core of maghemite and CdSe(ZnS) while the silica shell provided a strong protection to the core from degradation in conditions of extreme pH, thus giving these nanogels a potential for oral administration [198].
Recently, Wang et al. developed a multifunctional nanogel-based probe for pathological responsive ultrasound and MR imaging [199]. This was achieved by co-loading of two enzymes, catalase (CAT) and superoxide dismutase (SOD) along with SPIO nanoparticles into glycol chitosan-based pH-sensitive nanogels. Most pathological sites have a high concentration of reactive oxygen species, which are converted into molecular oxygen (O2) by SOD and CAT. O2 is released in the form of microbubbles that act as a contrast medium for ultrasound imaging. Encapsulation of enzymes within nanogels protects them from inactivation in circulation while the porous and hydrophilic nature of the gel network allows the substrate molecules to diffuse freely within the matrix thus allowing the enzymes to perform their catalytic activity [200,201]. Moreover, the density of cationic charges on the gel surface increases in the acidic pathological microenvironment leading to improved accumulation of nanogels in the target area. In addition, encapsulation of SPIO within the nanogel significantly enhances transverse relaxation rates and generates strong MR signals. The proof-of-concept in vivo studies indicated that signals obtained from ultrasound imaging from tumors 1 h after injection are enhanced approximately 7-fold. In the case of MR imaging, significantly darkened tumor signals with approximately 16% enhancement were detected after the injection of nanoprobes.
5. Obstacles to clinical translation and strategies being developed to overcome them
Nanogels, since the time of their discovery, have come a long way and found applications in almost all biomedical fields, ranging from drug therapy to imaging and diagnostics. While a few nanogel-based formulations have reached clinical trials [144-147] for subcutaneous delivery of vaccine antigens, the clinical translation of nanogels for all other diverse applications that they are suitable for remains to be realized. A number of parameters affect the efficacy of this delivery system and requires further optimization. Similar to many other nanomedicines, one of the major drawbacks is that no more than 5-10 % of the injected dose actually reaches the target site, with maximum dose reaching organs involved in clearance like kidney, liver, spleen, etc. [202,203]. The tissue distribution of nanogels is governed by a number of parameters like their size, shape, charge, composition, surface properties and the cargo that they carry. We have attempted to summarize some of the key factors that are proving to be roadblocks in this journey, along with the alternative approaches being explored to overcome them. However, it must be realized that the final efficacy of the nanogel-based delivery system depends on a complex interplay of all of these factors and an ideal system would be the one with a perfect balance between all of them.
Rapid clearance
Spleen is one of the important organs responsible for the filtration of foreign substances from blood. Splenic filtration is lower for softer particles like nanogels having a size less than 200 nm, that can squeeze through the splenic filtration bed [204,205], while extremely small nanogels (< 20 nm) are usually excreted via renal filtration [70,206], indicating that the size and flexibility of the nanogels have to be well-defined. Shape of the nanogels is also an important parameter that defines their circulation half-life, and filamentous or rod-shaped particles are known to have a considerably longer circulation time than their spherical counterparts [207]. Apart from the size, opsonization or coating by serum proteins also plays a key role in the clearance of the particles. Adsorption of plasma proteins leads to their uptake by the organs of MPS, such as liver and spleen, and can be partially prevented by PEGylation [205,208]. However, PEG is not completely inert, and PEG-specific IgM antibodies are known to be generated after administration of a single dose of PEGylated nanoparticles, resulting in their accelerated blood clearance. This compromises their efficacy on subsequent dosing [209-211]. Furthermore, this response is known to be dependent on the hydrophobicity of the core of carrier to which PEG is conjugated, as well as the type of cargo [209,212]. N-substituted polypeptides or polypeptoids have been recently explored as antifouling agents. Poly (N-methyl glycine) or polysarcosine has the simplest structure in class and possesses a flexible backbone [213,214]. A comparative study of block copolymer micelles, differing only in their hydrophilic block as being either PEG or polysarcosine, revealed considerable overlap between the two systems in terms of their physical properties, solution and assembly behavior, indicating the potential of peptoids like polysarcosine in serving as alternatives to PEG in nanogel systems of the next generation [215].
Charge
The presence of surface charge can alter the opsonization profile of the nanogel, its recognition by cells in the organs of the MPS and its plasma circulation profile. Gel particles having a close to neutral surface charge have been demonstrated to have longer circulation times [216,217]. However, the most important feature of nanogels, their stimuli-responsive behavior, is usually attributable to the charged groups incorporated into network. Moreover, the presence of such groups is often desired to facilitate the binding of drug cargo. Thus it is very difficult to achieve this delicate balance between charge-related responsive behavior and avoidance of the nonspecific interactions of the nanogels with other in vivo components. Cationic polymers like PEI are commonly used in the preparation of nanogel carriers for the delivery of nucleic acids. Complexation with negatively charged DNA minimizes the charge-related toxicity of the complexes in circulation; additionally PEG can be conjugated to the nanogels to provide further charge shielding. However, once nucleic acid is released from the carrier intracellularly, the polymer returns back to its native cationic state and is free to show toxicity like shrinkage of the cells, formation of vacuoles and disturbance in the cell cycle [218]. This toxicity was found to be dependent on the molecular weight of PEI used as well as its dose. To reduce toxicity, polymers based on low molecular weight PEI conjugated via labile linkers were used to prepare the gene carriers [210,219], but it is hard to eliminate the charge effect completely.
Challenges pertaining to biomolecule delivery
The most effective carriers for the delivery of genes are viral vectors; however, due to the limited loading capacity and risk of immune reactions, non-viral delivery systems like those based on nanogels have gained popularity [220,221]. A major limitation encountered by these systems is the low efficiency relative to viral vectors. This is mainly due to the degradation of the nucleic acids in the endosomes and late lysosomes and it is a great challenge to design a delivery system that can facilitate effective endosomal release of the carrier [222,223]. Majority of the synthetic carriers used for the delivery of nucleic acids are cationic in nature in order to form stable complex with the negatively charged nucleic acid to protect them from degradation and to improve circulation time. It has been shown that many of such systems do not remain intact in circulation and are disrupted at the glomerular basement membrane that also carries a negative charge due to the presence of proteoglycans. Thus, the purpose of prolonging the half-life of such agents is defeated [224,225], and the therapeutic agents are cleared by the kidney quite quickly after the disruption of the carrier system [118]. Moreover, the high positive charge density of the carriers increase the propensity of hemolysis and opsonization in circulation, especially if the system is found to be unstable in circulation which can expose the charged residues to serum components. Therapeutic proteins can also be incorporated into nanogels for their delivery. Activity of proteins is highly dependent on their conformational flexibility that makes proteins quite sensitive to various steps involved in formulation development, both physical processes like shear processes experienced during vortexing, and chemical conjugation reactions like crosslinking of the nanogel networks after protein incorporation [226-228]. Careful selection of the process parameters is therefore essential to preserve protein function.
Targeted delivery
In the case of nanogel carriers designed to deliver chemotherapeutics to tumors, the probability of nanogels reaching the core of the tumors, even if they are small enough (30-50 nm) and deformable, is limited which, among other factors, is due to the hampered interstitial transport and the high interstitial fluid pressure [229,230]. In many hypoxic tumors, the vasculature is poorly developed, and almost all large tumors have a tendency to develop a hypoxic and necrotic core, thus making it inaccessible for nanogels via the EPR effect [231]. This approach of passive targeting is therefore useful only for well-vascularized, small tumors or can be potentiated by extravasation/penetration-enhancing pretreatment [77,232,233]. Active targeting of the nanogels by their conjugation to receptor-specific ligands, although can improve the binding of the carrier to specific cells after extravasation, has its own set of challenges. It is often hard to find receptors that are exclusively expressed only on the tissue of interest. For example, folate receptor is overexpressed in a large number of malignancies, but also has a moderate to high level of expression on other normal organs like small intestine, placenta and kidneys [234,235]. Moreover, the expression of receptors on all the cells of the malignant tissue is seldom homogenous, which eventually results in non-uniform accumulation of the delivery system in that tissue [236-238]. Active targeting approach is also challenging to execute from the perspective of nanogel design. Despite of the recent advances in nanoscale fabrication methods that allow better control over the size and particle size distribution of the nanogels, in majority of cases nanogel samples are heterogeneous, and it is difficult to control the stoichiometry of functional biomolecules on their surface. Biomolecules like antibodies or their fragments, or even peptides are known to be very sensitive to bioconjugation reactions, and can easily lose their binding affinity due to the attachment to nanocarrier [239]. Reproducibility of the nanogel-antibody conjugates in a consistent manner from batch-to-batch is difficult to achieve, since majority of the conjugation reactions use free amines on the lysine side-chain or the cysteine residues obtained from reduction of disulfide bridges in the hinge region of antibodies, which not only results in a variable stoichiometry of conjugation but can also affect the binding affinity of the antibody [240-245]. In recent years, various bioorthogonal chemistries have been successfully developed to achieve site-specific conjugation, but these strategies also required introduction of artificial chemical moieties to the ligand structure and may be less viable for proteins produced through recombinant or bioengineering strategies [246]. In all cases, the introduction of targeting ligands on the surface of the nanogels can also have detrimental effects on their surface characteristics like charge and hydrophobicity, which may lead to increased opsonization, aggregation and clearance by the MPS in vivo [247].
Degradation
Because the molecular weight of nanogels is far above the renal threshold (∼40 KDa for copolymers), they cannot be removed from the body via the kidneys and if the polymers are nondegradable, so they would tend to accumulate in the body. Even if the nanogels are designed to be degraded into smaller polymer fragments to activate the release of their cargo and eventually facilitate their renal elimination, there is a risk of cellular accumulation of polymer chains by sequestration in the lysosomal compartments. Thus, the use of biodegradable polymers, both natural and synthetic, is most preferable. However, it should be noted that chemical functionalization can alter the pattern and rate of polymer degradation or even render natural polymer nonbiodegradable [248]. Therefore, further investigation of the metabolism and elimination profile of the polymeric nanogels is warranted before they can be proposed for long-term clinical use.
Drug release
Nanogels are designed to be stimuli-responsive for the release of their cargo. At the same time, biodegradable systems are intended to undergo degradation at the target site. Thus, it is difficult to control the rate of both stimuli-responsive drug release and degradation kinetics along with stability in circulation, and it is always a trade-off between these properties that decides the ultimate performance of the delivery system. This may lead to a pattern of release very different from the one usually observed in the in vitro experimental settings. Nanogels can also demonstrate burst release for their hydrophilic as well as hydrophobic cargoes which can result in substantial loss of the drug in circulation upon intravenous administration and leaving very little of the drug to be delivered to the target site through the nanogel carrier, while at the same time exposing healthy organs to toxic drugs [249]. These limitations can be addressed partially by altering the composition of the polymers used for the synthesis of nanogels, but add another layer of complexity to an already intricate system [250,251].
6. Conclusions and future perspectives
As a carrier system, nanogels have evolved over time to be able to encapsulate different types of guest molecules. This is a direct result of the advancement in their synthesis techniques as well as a deeper understanding of their material properties like softness and swelling behavior. This understanding allows us to explore their applications in diverse biomedical fields along with the possibility of fine-tuning these properties to our advantage. Progress in analytical techniques also gives us a better insight about their behavior in vivo that can give a direction to efforts being made to improve their pharmacokinetic and degradation profiles to design nanogels of the future.
Nanogels can be designed to be compatible with small molecules like drugs and fluorophores, proteins, peptides, nucleic acids and even inorganic nanoparticles composed of gold, silver, or iron oxide. These tiny carriers can also hold a combination of two or more agents depending on the purpose, and recent years have witnessed the evolution of nanogels as multi-drug carriers and multi-modal imaging agents. They can be surface-functionalized to present targeting ligands to a receptor of interest to home them at the desired site. Nanogels respond to environmental or external stimuli by undergoing volume phase transitions that are manifested in the form of swelling of their crosslinked network. This allows for spatial as well as temporal control of drug release and/or activation of reporter molecules, which in turn can generate signals for the purpose of imaging and diagnosis. These properties allow nanogels to surpass other nanoparticulate systems in terms of their applicability.
Despite the progress made in the field of nanogel design so far, very few nanogels have been explored in the clinical studies. The complexity of the system and intricate structural properties demand careful engineering of the nanogel in order to achieve the desired effect. The scalable production and batch-to batch reproducibility can also be hurdles that need to be addressed. Challenges exist in terms of delivery of the cargo to the desired site as well as efficient clearance of the nanogels once they have accomplished their mission in vivo. Although many studies tested the efficacy of nanogel formulations and their safety, reports on their long-term accumulation and degradation profiles are few and far between. Improvements in the design along with detailed investigations regarding the in vivo behavior of the nanogels will help in eventually taking them from bench to bedside.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant P20GM103480.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 2.Kersey FR, Merkel TJ, Perry JL, Napier ME, DeSimone JM. Effect of aspect ratio and deformability on nanoparticle extravasation through nanopores. Langmuir. 2012;28:8773–8781. doi: 10.1021/la301279v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kabanov AV, Vinogradov SV. Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zha L, Banik B, Alexis F. Stimulus responsive nanogels for drug delivery. Soft Matter. 2011;7:5908–5916. [Google Scholar]
- 6.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 7.Motornov M, Roiter Y, Tokarev I, Minko S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog Polym Sci. 2010;35:174–211. [Google Scholar]
- 8.Stuart MAC, Huck WT, Genzer J, Müller M, Ober C, Stamm M, et al. Emerging applications of stimuli-responsive polymer materials. Nat Mater. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- 9.Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog Polym Sci. 2008;33:448–477. [Google Scholar]
- 10.Ayame H, Morimoto N, Akiyoshi K. Self-assembled cationic nanogels for intracellular protein delivery. Bioconjug Chem. 2008;19:882–890. doi: 10.1021/bc700422s. [DOI] [PubMed] [Google Scholar]
- 11.Raemdonck K, Demeester J, De Smedt S. Advanced nanogel engineering for drug delivery. Soft Matter. 2009;5:707–715. [Google Scholar]
- 12.Qiao Z, Zhang R, Du F, Liang D, Li Z. Multi-responsive nanogels containing motifs of ortho ester, oligo (ethylene glycol) and disulfide linkage as carriers of hydrophobic anti-cancer drugs. J Control Release. 2011;152:57–66. doi: 10.1016/j.jconrel.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Oh JK, Lee DI, Park JM. Biopolymer-based microgels/nanogels for drug delivery applications. Prog Polym Sci. 2009;34:1261–1282. [Google Scholar]
- 14.Nochi T, Yuki Y, Takahashi H, Sawada S, Mejima M, Kohda T, et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater. 2010;9:572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 15.Chacko RT, Ventura J, Zhuang J, Thayumanavan S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv Drug Deliv Rev. 2012;64:836–851. doi: 10.1016/j.addr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAllister K, Sazani P, Adam M, Cho MJ, Rubinstein M, Samulski RJ, et al. Polymeric nanogels produced via inverse microemulsion polymerization as potential gene and antisense delivery agents. J Am Chem Soc. 2002;124:15198–15207. doi: 10.1021/ja027759q. [DOI] [PubMed] [Google Scholar]
- 17.Malmsten M. Soft drug delivery systems. Soft Matter. 2006;2:760–769. doi: 10.1039/b608348j. [DOI] [PubMed] [Google Scholar]
- 18.Napier ME, DeSimone JM. Nanoparticle drug delivery platform. J Macromolecular Sci, Part C: Polym Rev. 2007;47:321–327. [Google Scholar]
- 19.Beija M, Marty J, Destarac M. RAFT/MADIX polymers for the preparation of polymer/inorganic nanohybrids. Prog Polym Sci. 2011;36:845–886. [Google Scholar]
- 20.Siegwart DJ, Oh JK, Matyjaszewski K. ATRP in the design of functional materials for biomedical applications. Prog Polym Sci. 2012;37:18–37. doi: 10.1016/j.progpolymsci.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bronich TK, Keifer PA, Shlyakhtenko LS, Kabanov AV. Polymer micelle with cross-linked ionic core. J Am Chem Soc. 2005;127:8236–8237. doi: 10.1021/ja043042m. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly RK, Hawker CJ, Wooley KL. Cross-linked block copolymer micelles: functional nanostructures of great potential and versatility. Chem Soc Rev. 2006;35:1068–1083. doi: 10.1039/b514858h. [DOI] [PubMed] [Google Scholar]
- 23.Oishi M, Nagasaki Y. Stimuli-responsive smart nanogels for cancer diagnostics and therapy. Nanomedicine. 2010;5:451–468. doi: 10.2217/nnm.10.18. [DOI] [PubMed] [Google Scholar]
- 24.Chiang W, Ho VT, Huang W, Huang Y, Chern C, Chiu H. Dual stimuli-responsive polymeric hollow nanogels designed as carriers for intracellular triggered drug release. Langmuir. 2012;28:15056–15064. doi: 10.1021/la302903v. [DOI] [PubMed] [Google Scholar]
- 25.Sanson N, Rieger J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym Chem. 2010;1:965–977. [Google Scholar]
- 26.Elsabahy M, Heo GS, Lim S, Sun G, Wooley KL. Polymeric Nanostructures for Imaging and Therapy. Chem Rev. 2015 doi: 10.1021/acs.chemrev.5b00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desale SS, Cohen SM, Zhao Y, Kabanov AV, Bronich TK. Biodegradable hybrid polymer micelles for combination drug therapy in ovarian cancer. J Control Release. 2013;171:339–348. doi: 10.1016/j.jconrel.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Malhotra S, Molina M, Haag R. Micro-and nanogels with labile crosslinks– from synthesis to biomedical applications. Chem Soc Rev. 2015;44:1948–1973. doi: 10.1039/c4cs00341a. [DOI] [PubMed] [Google Scholar]
- 29.Gratton SE, Pohlhaus PD, Lee J, Guo J, Cho MJ, DeSimone JM. Nanofabricated particles for engineered drug therapies: A preliminary biodistribution study of PRINT™ nanoparticles. J Control Release. 2007;121:10–18. doi: 10.1016/j.jconrel.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry JL, Herlihy KP, Napier ME, DeSimone JM. PRINT: a novel platform toward shape and size specific nanoparticle theranostics. Acc Chem Res. 2011;44:990–998. doi: 10.1021/ar2000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glangchai LC, Caldorera-Moore M, Shi L, Roy K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J Control Release. 2008;125:263–272. doi: 10.1016/j.jconrel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki Y, Akiyoshi K. Nanogel engineering for new nanobiomaterials: from chaperoning engineering to biomedical applications. Chem Rec. 2010;10:366–376. doi: 10.1002/tcr.201000008. [DOI] [PubMed] [Google Scholar]
- 33.Water JJ, Kim Y, Maltesen MJ, Franzyk H, Foged C, Nielsen HM. Hyaluronic acid-based nanogels produced by microfluidics-facilitated self-assembly improves the safety profile of the cationic host defense peptide novicidin. Pharm Res. 2015;32:2727–2735. doi: 10.1007/s11095-015-1658-6. [DOI] [PubMed] [Google Scholar]
- 34.Lai H, Wu P. A infrared spectroscopic study on the mechanism of temperature-induced phase transition of concentrated aqueous solutions of poly(N-isopropylacrylamide) and N-isopropylpropionamide. Polymer. 2010;51:1404–1412. [Google Scholar]
- 35.Jochum FD, Theato P. Temperature-and light-responsive smart polymer materials. Chem Soc Rev. 2013;42:7468–7483. doi: 10.1039/c2cs35191a. [DOI] [PubMed] [Google Scholar]
- 36.Mok H, Jeong H, Kim S, Chung BH. Indocyanine green encapsulated nanogels for hyaluronidase activatable and selective near infrared imaging of tumors and lymph nodes. Chem Commun. 2012;48:8628–8630. doi: 10.1039/c2cc33555g. [DOI] [PubMed] [Google Scholar]
- 37.Canal T, Peppas NA. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J Biomed Mater Res. 1989;23:1183–1193. doi: 10.1002/jbm.820231007. [DOI] [PubMed] [Google Scholar]
- 38.Lustig SR, Peppas NA. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. J Appl Polym Sci. 1988;36:735–747. [Google Scholar]
- 39.Eichenbaum GM, Kiser PF, Simon SA, Needham D. pH and ion-triggered volume response of anionic hydrogel microspheres. Macromolecules. 1998;31:5084–5093. doi: 10.1021/ma970897t. [DOI] [PubMed] [Google Scholar]
- 40.Oberoi HS, Laquer FC, Marky LA, Kabanov AV, Bronich TK. Core cross-linked block ionomer micelles as pH-responsive carriers for cis-diamminedichloroplatinum(II) J Control Release. 2011;153:64–72. doi: 10.1016/j.jconrel.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura G, Shinohara Y, Tamura A, Sanada Y, Oishi M, Akiba I, et al. Dependence of the swelling behavior of a pH-responsive PEG-modified nanogel on the cross-link density. Polym J. 2012;44:240–244. [Google Scholar]
- 42.Ricka J, Tanaka T. Swelling of ionic gels: quantitative performance of the Donnan theory. Macromolecules. 1984;17:2916–2921. [Google Scholar]
- 43.Pikabea A, Aguirre G, Miranda JI, Ramos J, Forcada J. Understanding of nanogels swelling behavior through a deep insight into their morphology. Journal of Polymer Science Part A: Polym Chem. 2015;53:2017–2025. [Google Scholar]
- 44.Eichenbaum GM, Kiser PF, Dobrynin AV, Simon SA, Needham D. Investigation of the swelling response and loading of ionic microgels with drugs and proteins: The dependence on cross-link density. Macromolecules. 1999;32:4867–4878. [Google Scholar]
- 45.Tan BH, Ravi P, Tam KC. Synthesis and Characterization of Novel pH-Responsive Polyampholyte Microgels. Macromol Rapid Commun. 2006;27:522–528. [Google Scholar]
- 46.Hoare T, Pelton R. Charge-switching, amphoteric glucose-responsive microgels with physiological swelling activity. Biomacromolecules. 2008;9:733–740. doi: 10.1021/bm701203r. [DOI] [PubMed] [Google Scholar]
- 47.Wu W, Mitra N, Yan EC, Zhou S. Multifunctional hybrid nanogel for integration of optical glucose sensing and self-regulated insulin release at physiological pH. ACS Nano. 2010;4:4831–4839. doi: 10.1021/nn1008319. [DOI] [PubMed] [Google Scholar]
- 48.Cheng R, Meng F, Deng C, Klok H, Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34:3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 49.Morimoto N, Qiu X, Winnik FM, Akiyoshi K. Dual stimuli-responsive nanogels by self-assembly of polysaccharides lightly grafted with thiol-terminated poly (N-isopropylacrylamide) chains. Macromolecules. 2008;41:5985–5987. [Google Scholar]
- 50.Pan Y, Chen Y, Wang D, Wei C, Guo J, Lu D, et al. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials. 2012;33:6570–6579. doi: 10.1016/j.biomaterials.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 51.Xing Z, Wang C, Yan J, Zhang L, Li L, Zha L. Dual stimuli responsive hollow nanogels with IPN structure for temperature controlling drug loading and pH triggering drug release. Soft Matter. 2011;7:7992–7997. [Google Scholar]
- 52.Xiong M, Bao Y, Yang X, Wang Y, Sun B, Wang J. Lipase-sensitive polymeric triple-layered nanogel for “on-demand” drug delivery. J Am Chem Soc. 2012;134:4355–4362. doi: 10.1021/ja211279u. [DOI] [PubMed] [Google Scholar]
- 53.Maciel D, Figueira P, Xiao S, Hu D, Shi X, Rodrigues J, et al. Redox-responsive alginate nanogels with enhanced anticancer cytotoxicity. Biomacromolecules. 2013;14:3140–3146. doi: 10.1021/bm400768m. [DOI] [PubMed] [Google Scholar]
- 54.Li M, Tang Z, Sun H, Ding J, Song W, Chen X. pH and reduction dual-responsive nanogel cross-linked by quaternization reaction for enhanced cellular internalization and intracellular drug delivery. Polym Chem. 2013;4:1199–1207. [Google Scholar]
- 55.Oh JK, Siegwart DJ, Lee H, Sherwood G, Peteanu L, Hollinger JO, et al. Biodegradable nanogels prepared by atom transfer radical polymerization as potential drug delivery carriers: synthesis, biodegradation, in vitro release, and bioconjugation. J Am Chem Soc. 2007;129:5939–5945. doi: 10.1021/ja069150l. [DOI] [PubMed] [Google Scholar]
- 56.Ryu J, Chacko RT, Jiwpanich S, Bickerton S, Babu RP, Thayumanavan S. Self-cross-linked polymer nanogels: a versatile nanoscopic drug delivery platform. J Am Chem Soc. 2010;132:17227–17235. doi: 10.1021/ja1069932. [DOI] [PubMed] [Google Scholar]
- 57.Maya S, Sarmento B, Nair A, Rejinold NS, Nair SV, Jayakumar R. Smart stimuli sensitive nanogels in cancer drug delivery and imaging: a review. Curr Pharm Des. 2013;19:7203–7218. doi: 10.2174/138161281941131219124142. [DOI] [PubMed] [Google Scholar]
- 58.Thornton PD, Mart RJ, Ulijn RV. Enzyme-Responsive Polymer Hydrogel Particles for Controlled Release. Adv Mater. 2007;19:1252–1256. [Google Scholar]
- 59.Kang H, Trondoli AC, Zhu G, Chen Y, Chang Y, Liu H, et al. Near-infrared light-responsive core–shell nanogels for targeted drug delivery. ACS Nano. 2011;5:5094–5099. doi: 10.1021/nn201171r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Owens DE, III, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 61.Jeon SI, Lee JH, Andrade JD, De Gennes PG. Protein-surface interactions in the presence of polyethylene oxide. I. Simplified theory. J Colloid Interface Sci. 1991;142:149–158. [Google Scholar]
- 62.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peracchia MT, Fattal E, Desmaële D, Besnard M, Noël JP, Gomis JM, et al. Stealth(®) PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J Control Release. 1999;60:121–128. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 65.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 66.Hendrickson GR, Lyon LA. Microgel translocation through pores under confinement. Angew Chem Int Ed. 2010;49:2193–2197. doi: 10.1002/anie.200906606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Banquy X, Suarez F, Argaw A, Rabanel J, Grutter P, Bouchard J, et al. Effect of mechanical properties of hydrogel nanoparticles on macrophage cell uptake. Soft Matter. 2009;5:3984–3991. [Google Scholar]
- 68.Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M, et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci U S A. 2011;108:586–591. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anselmo AC, Zhang M, Kumar S, Vogus DR, Menegatti S, Helgeson ME, et al. Elasticity of Nanoparticles Influences Their Blood Circulation, Phagocytosis, Endocytosis, and Targeting. ACS Nano. 2015;9:3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L, Cao Z, Li Y, Ella-Menye JR, Bai T, Jiang S. Softer zwitterionic nanogels for longer circulation and lower splenic accumulation. ACS Nano. 2012;6:6681–6686. doi: 10.1021/nn301159a. [DOI] [PubMed] [Google Scholar]
- 71.Chen K, Xu J, Luft JC, Tian S, Raval JS, DeSimone JM. Design of Asymmetric Particles Containing a Charged Interior and a Neutral Surface Charge: Comparative Study on in Vivo Circulation of Polyelectrolyte Microgels. J Am Chem Soc. 2014;136:9947–9952. doi: 10.1021/ja503939n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 74.Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Holl MMB. The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chem Biol. 2007;14:107–115. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 75.Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 76.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Nuhn L, Tomcin S, Miyata K, Mailänder V, Landfester K, Kataoka K, Zentel R. Size-Dependent Knockdown Potential of siRNA-Loaded Cationic Nanohydrogel Particles. Biomacromolecules. 2014;15:4111–4121. doi: 10.1021/bm501148y. [DOI] [PubMed] [Google Scholar]
- 79.Ahmed M, Narain R. Intracellular delivery of DNA and enzyme in active form using degradable carbohydrate-based nanogels. Mol Pharm. 2012;9:3160–3170. doi: 10.1021/mp300255p. [DOI] [PubMed] [Google Scholar]
- 80.Nuhn L, Braun L, Overhoff I, Kelsch A, Schaeffel D, Koynov K, et al. Degradable Cationic Nanohydrogel Particles for Stimuli-Responsive Release of siRNA. Macromol Rapid Commun. 2014;35:2057–2064. doi: 10.1002/marc.201400458. [DOI] [PubMed] [Google Scholar]
- 81.Averick SE, Paredes E, Irastorza A, Shrivats AR, Srinivasan A, Siegwart DJ, et al. Preparation of cationic nanogels for nucleic acid delivery. Biomacromolecules. 2012;13:3445–3449. doi: 10.1021/bm301166s. [DOI] [PubMed] [Google Scholar]
- 82.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2012;64:302–315. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 83.Desale SS, Raja SM, Kim JO, Mohapatra B, Soni KS, Luan H, et al. Polypeptide-based nanogels co-encapsulating a synergistic combination of Doxorubicin with 17-AAG show potent anti-tumor activity in ErbB2-driven breast cancer models. J Control Release. 2015;208:59–66. doi: 10.1016/j.jconrel.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vinogradov SV, Batrakova EV, Kabanov AV. Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem. 2004;15:50–60. doi: 10.1021/bc034164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nukolova NV, Oberoi HS, Cohen SM, Kabanov AV, Bronich TK. Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials. 2011;32:5417–5426. doi: 10.1016/j.biomaterials.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nukolova NV, Oberoi HS, Zhao Y, Chekhonin VP, Kabanov AV, Bronich TK. LHRH-targeted nanogels as a delivery system for cisplatin to ovarian cancer. Mol Pharm. 2013;10:3913–3921. doi: 10.1021/mp4003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vinogradov SV. Colloidal microgels in drug delivery applications. Curr Pharm Des. 2006;12:4703–4712. doi: 10.2174/138161206779026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bronich TK, Vinogradov SV, Kabanov AV. Interaction of nanosized copolymer networks with oppositely charged amphiphilic molecules. Nano Letters. 2001;1:535–540. [Google Scholar]
- 89.Kohli E, Han H, Zeman AD, Vinogradov SV. Formulations of biodegradable Nanogel carriers with 5′-triphosphates of nucleoside analogs that display a reduced cytotoxicity and enhanced drug activity. J Control Release. 2007;121:19–27. doi: 10.1016/j.jconrel.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galmarini CM, Warren G, Kohli E, Zeman A, Mitin A, Vinogradov SV. Polymeric nanogels containing the triphosphate form of cytotoxic nucleoside analogues show antitumor activity against breast and colorectal cancer cell lines. Mol Cancer Ther. 2008;7:3373–3380. doi: 10.1158/1535-7163.MCT-08-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerson T, Makarov E, Senanayake TH, Gorantla S, Poluektova LY, Vinogradov SV. Nano-NRTIs demonstrate low neurotoxicity and high antiviral activity against HIV infection in the brain. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:177–185. doi: 10.1016/j.nano.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim JO, Nukolova NV, Oberoi HS, Kabanov AV, Bronich TK. Block Ionomer Complex Micelles with Cross-Linked Cores for Drug Delivery. Polym Sci Ser A Chem Phys. 2009;51:708–718. doi: 10.1134/S0965545X09060169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sahay G, Kim JO, Kabanov AV, Bronich TK. The exploitation of differential endocytic pathways in normal and tumor cells in the selective targeting of nanoparticulate chemotherapeutic agents. Biomaterials. 2010;31:923–933. doi: 10.1016/j.biomaterials.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim JO, Sahay G, Kabanov AV, Bronich TK. Polymeric micelles with ionic cores containing biodegradable cross-links for delivery of chemotherapeutic agents. Biomacromolecules. 2010;11:919–926. doi: 10.1021/bm9013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shi F, Ding J, Xiao C, Zhuang X, He C, Chen L, et al. Intracellular microenvironment responsive PEGylated polypeptide nanogels with ionizable cores for efficient doxorubicin loading and triggered release. J Mater Chem. 2012;22:14168–14179. [Google Scholar]
- 96.Oberoi HS, Nukolova NV, Laquer FC, Poluektova LY, Huang J, Alnouti Y, et al. Cisplatin-loaded core cross-linked micelles: comparative pharmacokinetics, antitumor activity, and toxicity in mice. Int J Nanomedicine. 2012;7:2557. doi: 10.2147/IJN.S29145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Desale SS, Soni KS, Romanova S, Cohen SM, Bronich TK. Targeted delivery of platinum-taxane combination therapy in ovarian cancer. J Control Release. 2015 doi: 10.1016/j.jconrel.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peng J, Qi T, Liao J, Chu B, Yang Q, Li W, et al. Controlled release of cisplatin from pH-thermal dual responsive nanogels. Biomaterials. 2013;34:8726–8740. doi: 10.1016/j.biomaterials.2013.07.092. [DOI] [PubMed] [Google Scholar]
- 99.Kim JO, Oberoi HS, Desale S, Kabanov AV, Bronich TK. Polypeptide nanogels with hydrophobic moieties in the cross-linked ionic cores: synthesis, characterization and implications for anticancer drug delivery. J Drug Target. 2013;21:981–993. doi: 10.3109/1061186X.2013.831421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Xu H, Wang J, Ge L, Zhu J. Development of a Thermally Responsive Nanogel Based on Chitosan–Poly (N-Isopropylacrylamide-co-Acrylamide) for Paclitaxel Delivery. J Pharm Sci. 2014;103:2012–2021. doi: 10.1002/jps.23995. [DOI] [PubMed] [Google Scholar]
- 101.Daoud-Mahammed S, Couvreur P, Bouchemal K, Chéron M, Lebas G, Amiel C, et al. Cyclodextrin and polysaccharide-based nanogels: entrapment of two hydrophobic molecules, benzophenone and tamoxifen. Biomacromolecules. 2009;10:547–554. doi: 10.1021/bm801206f. [DOI] [PubMed] [Google Scholar]
- 102.Tan JP, Tan MB, Tam MK. Application of nanogel systems in the administration of local anesthetics. Local Reg Anesth. 2010;3:93. doi: 10.2147/LRA.S7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ferrer MCC, Shuvaev VV, Zern BJ, Composto RJ, Muzykantov VR, Eckmann DM. Icam-1 targeted nanogels loaded with dexamethasone alleviate pulmonary inflammation. Plos One. 2014 doi: 10.1371/journal.pone.0102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ferrer MCC, Ferrier RC, Jr, Eckmann DM, Composto RJ. A facile route to synthesize nanogels doped with silver nanoparticles. J Nanopart Res. 2013;15:1–7. doi: 10.1007/s11051-012-1323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferrer MCC, Dastgheyb S, Hickok NJ, Eckmann DM, Composto RJ. Designing nanogel carriers for antibacterial applications. Acta Biomater. 2014;10:2105–2111. doi: 10.1016/j.actbio.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakamura T, Tamura A, Murotani H, Oishi M, Jinji Y, Matsuishi K, et al. Large payloads of gold nanoparticles into the polyamine network core of stimuli-responsive PEGylated nanogels for selective and noninvasive cancer photothermal therapy. Nanoscale. 2010;2:739–746. doi: 10.1039/b9nr00329k. [DOI] [PubMed] [Google Scholar]
- 107.Yasui H, Takeuchi R, Nagane M, Meike S, Nakamura Y, Yamamori T, et al. Radiosensitization of tumor cells through endoplasmic reticulum stress induced by PEGylated nanogel containing gold nanoparticles. Cancer Lett. 2014;347:151–158. doi: 10.1016/j.canlet.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 108.Dias N, Stein C. Potential roles of antisense oligonucleotides in cancer therapy. The example of Bcl-2 antisense oligonucleotides. Eur J Pharm Biopharm. 2002;54:263–269. doi: 10.1016/s0939-6411(02)00060-7. [DOI] [PubMed] [Google Scholar]
- 109.Leong PL, Andrews GA, Johnson DE, Dyer KF, Xi S, Mai JC, et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci U S A. 2003;100:4138–4143. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Olie RA, Simoes-Wust AP, Baumann B, Leech SH, Fabbro D, Stahel RA, et al. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000;60:2805–2809. [PubMed] [Google Scholar]
- 111.Seidman S, Eckstein F, Grifman M, Soreq H. Antisense technologies have a future fighting neurodegenerative diseases. Antisense Nucleic Acid Drug Dev. 1999;9:333–340. doi: 10.1089/oli.1.1999.9.333. [DOI] [PubMed] [Google Scholar]
- 112.Ho SP, Hartig PR. Antisense oligonucleotides for target validation in the CNS. Curr Opin Mol Ther. 1999;1:336–343. [PubMed] [Google Scholar]
- 113.McCarthy MM, Auger AP, Mong JA, Sickel MJ, Davis AM. Antisense oligodeoxynucleotides as a tool in developmental neuroendocrinology. Methods. 2000;22:239–248. doi: 10.1006/meth.2000.1075. [DOI] [PubMed] [Google Scholar]
- 114.Agrawal S, Ikeuchi T, Sun D, Sarin PS, Konopka A, Maizel J, et al. Inhibition of human immunodeficiency virus in early infected and chronically infected cells by antisense oligodeoxynucleotides and their phosphorothioate analogues. Proc Natl Acad Sci U S A. 1989;86:7790–7794. doi: 10.1073/pnas.86.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu GY, Wu CH. Specific inhibition of hepatitis B viral gene expression in vitro by targeted antisense oligonucleotides. J Biol Chem. 1992;267:12436–12439. [PubMed] [Google Scholar]
- 116.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 117.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8:173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 118.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 119.Vinogradov S, Batrakova E, Kabanov A. Poly (ethylene glycol)–polyethyleneimine NanoGel™ particles: novel drug delivery systems for antisense oligonucleotides. Colloids Surf B Biointerfaces. 1999;16:291–304. [Google Scholar]
- 120.Tamura A, Oishi M, Nagasaki Y. Enhanced cytoplasmic delivery of siRNA using a stabilized polyion complex based on PEGylated nanogels with a cross-linked polyamine structure. Biomacromolecules. 2009;10:1818–1827. doi: 10.1021/bm900252d. [DOI] [PubMed] [Google Scholar]
- 121.Mimi H, Ho KM, Siu YS, Wu A, Li P. Polyethyleneimine-based core-shell nanogels: a promising siRNA carrier for argininosuccinate synthetase mRNA knockdown in HeLa cells. J Control Release. 2012;158:123–130. doi: 10.1016/j.jconrel.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 122.Raemdonck K, Naeye B, Buyens K, Vandenbroucke RE, Høgset A, Demeester J, et al. Biodegradable dextran nanogels for RNA interference: focusing on endosomal escape and intracellular siRNA delivery. Adv Functl Mater. 2009;19:1406–1415. [Google Scholar]
- 123.Ahmed M, Wattanaarsakit P, Narain R. Cationic glyco-nanogels for epidermal growth factor receptor (EGFR) specific siRNA delivery in ovarian cancer cells. Polym Chem. 2013;4:3829–3836. [Google Scholar]
- 124.De Backer L, Braeckmans K, Stuart MC, Demeester J, De Smedt SC, Raemdonck K. Bio-inspired pulmonary surfactant-modified nanogels: A promising siRNA delivery system. J Control Release. 2015;206:177–186. doi: 10.1016/j.jconrel.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 125.Pereira P, Morgado D, Crepet A, David L, Gama FM. Glycol Chitosan-Based Nanogel as a Potential Targetable Carrier for siRNA. Macromol Biosci. 2013;13:1369–1378. doi: 10.1002/mabi.201300123. [DOI] [PubMed] [Google Scholar]
- 126.Smith MH, Lyon LA. Multifunctional nanogels for siRNA delivery. Acc Chem Res. 2011;45:985–993. doi: 10.1021/ar200216f. [DOI] [PubMed] [Google Scholar]
- 127.Naeye B, Raemdonck K, Remaut K, Sproat B, Demeester J, De Smedt S. PEGylation of biodegradable dextran nanogels for siRNA delivery. Eur J Pharm Sci. 2010;40:342–351. doi: 10.1016/j.ejps.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 128.Fujii H, Shin-Ya M, Takeda S, Hashimoto Y, Mukai S, Sawada S, et al. Cycloamylose-nanogel drug delivery system-mediated intratumor silencing of the vascular endothelial growth factor regulates neovascularization in tumor microenvironment. Cancer Sci. 2014;105:1616–1625. doi: 10.1111/cas.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siegwart DJ, Whitehead KA, Nuhn L, Sahay G, Cheng H, Jiang S, et al. Combinatorial synthesis of chemically diverse core-shell nanoparticles for intracellular delivery. Proc Natl Acad Sci U S A. 2011;108:12996–13001. doi: 10.1073/pnas.1106379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramos J, Forcada J, Hidalgo-Alvarez R. Cationic polymer nanoparticles and nanogels: from synthesis to biotechnological applications. Chem Rev. 2013;114:367–428. doi: 10.1021/cr3002643. [DOI] [PubMed] [Google Scholar]
- 131.Nishikawa T, Akiyoshi K, Sunamoto J. Supramolecular assembly between nanoparticles of hydrophobized polysaccharide and soluble protein complexation between the self-aggregate of cholesterol-bearing pullulan and. alpha.-chymotrypsin. Macromolecules. 1994;27:7654–7659. [Google Scholar]
- 132.Akiyoshi K, Kobayashi S, Shichibe S, Mix D, Baudys M, Kim SW, et al. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. J Control Release. 1998;54:313–320. doi: 10.1016/s0168-3659(98)00017-0. [DOI] [PubMed] [Google Scholar]
- 133.Hasegawa U, Sawada S, Shimizu T, Kishida T, Otsuji E, Mazda O, et al. Raspberry-like assembly of cross-linked nanogels for protein delivery. J Control Release. 2009;140:312–317. doi: 10.1016/j.jconrel.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 134.Nagahama K, Ouchi T, Ohya Y. Biodegradable Nanogels Prepared by Self-Assembly of Poly (L-lactide)-Grafted Dextran: Entrapment and Release of Proteins. Macromol Biosci. 2008;8:1044–1052. doi: 10.1002/mabi.200800175. [DOI] [PubMed] [Google Scholar]
- 135.Van Thienen T, Raemdonck K, Demeester J, De Smedt S. Protein release from biodegradable dextran nanogels. Langmuir. 2007;23:9794–9801. doi: 10.1021/la700736v. [DOI] [PubMed] [Google Scholar]
- 136.Ganguly K, Chaturvedi K, More UA, Nadagouda MN, Aminabhavi TM. Polysaccharide-based micro/nanohydrogels for delivering macromolecular therapeutics. J Control Release. 2014;193:162–173. doi: 10.1016/j.jconrel.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 137.Siegwart DJ, Srinivasan A, Bencherif SA, Karunanidhi A, Oh JK, Vaidya S, et al. Cellular uptake of functional nanogels prepared by inverse miniemulsion ATRP with encapsulated proteins, carbohydrates, and gold nanoparticles. Biomacromolecules. 2009;10:2300–2309. doi: 10.1021/bm9004904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wutzel H, Richter FH, Li Y, Sheiko SS, Klok H. Poly [N-(2-hydroxypropyl) methacrylamide] nanogels by RAFT polymerization in inverse emulsion. Polym Chem. 2014;5:1711–1719. [Google Scholar]
- 139.Chen W, Zheng M, Meng F, Cheng R, Deng C, Feijen J, et al. In situ forming reduction-sensitive degradable nanogels for facile loading and triggered intracellular release of proteins. Biomacromolecules. 2013;14:1214–1222. doi: 10.1021/bm400206m. [DOI] [PubMed] [Google Scholar]
- 140.Amidi M, Romeijn SG, Borchard G, Junginger HE, Hennink WE, Jiskoot W. Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J Control Release. 2006;111:107–116. doi: 10.1016/j.jconrel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 141.Ferreira SA, Gama FM, Vilanova M. Polymeric nanogels as vaccine delivery systems. Nanomedicine. 2013;9:159–173. doi: 10.1016/j.nano.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 142.Gutierro I, Hernández RM, Igartua M, Gascón AR, Pedraz JL. Size dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheres. Vaccine. 2002;21:67–77. doi: 10.1016/s0264-410x(02)00435-8. [DOI] [PubMed] [Google Scholar]
- 143.Oyewumi MO, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev Vaccines. 2010;9:1095–1107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kitano S, Kageyama S, Nagata Y, Miyahara Y, Hiasa A, Naota H, et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Cancer Res. 2006;12:7397–7405. doi: 10.1158/1078-0432.CCR-06-1546. [DOI] [PubMed] [Google Scholar]
- 145.Uenaka A, Wada H, Isobe M, Saika T, Tsuji K, Sato E, et al. T cell immunomonitoring and tumor responses in patients immunized with a complex of cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein. Cancer Immun. 2007;7:9. [PMC free article] [PubMed] [Google Scholar]
- 146.Kageyama S, Wada H, Muro K, Niwa Y, Ueda S, Miyata H, et al. Dose-dependent effects of NY-ESO-1 protein vaccine complexed with cholesteryl pullulan (CHP-NY-ESO-1) on immune responses and survival benefits of esophageal cancer patients. J Transl Med. 2013;11:246. doi: 10.1186/1479-5876-11-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saito T, Wada H, Yamasaki M, Miyata H, Nishikawa H, Sato E, et al. High expression of MAGE-A4 and MHC class I antigens in tumor cells and induction of MAGE-A4 immune responses are prognostic markers of CHP-MAGE-A4 cancer vaccine. Vaccine. 2014;32:5901–5907. doi: 10.1016/j.vaccine.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 148.Tahara Y, Akiyoshi K. Current advances in self-assembled nanogels for immunotherapy. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 149.Woodcock J, Griffin JP, Behrman RE. Development of novel combination therapies. N Engl J Med. 2011;364:985–987. doi: 10.1056/NEJMp1101548. [DOI] [PubMed] [Google Scholar]
- 150.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kamolratanakul P, Hayata T, Ezura Y, Kawamata A, Hayashi C, Yamamoto Y, et al. Nanogel-based scaffold delivery of prostaglandin E2 receptor-specific agonist in combination with a low dose of growth factor heals critical-size bone defects in mice. Arthritis Rheum. 2011;63:1021–1033. doi: 10.1002/art.30151. [DOI] [PubMed] [Google Scholar]
- 152.Toita S, Sawada S, Akiyoshi K. Polysaccharide nanogel gene delivery system with endosome-escaping function: co-delivery of plasmid DNA and phospholipase A2. J Control Release. 2011;155:54–59. doi: 10.1016/j.jconrel.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 153.Katagiri K, Ohta K, Koumoto K, Kurosu K, Sasaki Y, Akiyoshi K. Templated nucleation of hybrid iron oxide nanoparticles on polysaccharide nanogels. Colloid Polym Sci. 2013;291:1375–1380. [Google Scholar]
- 154.Choo ESG, Tang X, Sheng Y, Shuter B, Xue J. Controlled loading of superparamagnetic nanoparticles in fluorescent nanogels as effective T 2-weighted MRI contrast agents. J Mater Chem. 2011;21:2310–2319. [Google Scholar]
- 155.Terreno E, Castelli DD, Viale A, Aime S. Challenges for molecular magnetic resonance imaging. Chem Rev. 2010;110:3019–3042. doi: 10.1021/cr100025t. [DOI] [PubMed] [Google Scholar]
- 156.Soleimani A, Martínez F, Economopoulos V, Foster PJ, Scholl TJ, Gillies ER. Polymer cross-linking: a nanogel approach to enhancing the relaxivity of MRI contrast agents. J Mater Chem B. 2013;1:1027–1034. doi: 10.1039/c2tb00352j. [DOI] [PubMed] [Google Scholar]
- 157.Paquet C, de Haan HW, Leek DM, Lin H, Xiang B, Tian G, et al. Clusters of superparamagnetic iron oxide nanoparticles encapsulated in a hydrogel: a particle architecture generating a synergistic enhancement of the T2 relaxation. ACS Nano. 2011;5:3104–3112. doi: 10.1021/nn2002272. [DOI] [PubMed] [Google Scholar]
- 158.Bloembergen N, Purcell EM, Pound RV. Relaxation effects in nuclear magnetic resonance absorption. Phys Rev. 1948;73:679. [Google Scholar]
- 159.Shapiro YE. Structure and dynamics of hydrogels and organogels: An NMR spectroscopy approach. Prog Polym Sci. 2011;36:1184–1253. [Google Scholar]
- 160.Okada S, Mizukami S, Matsumura Y, Yoshioka Y, Kikuchi K. A nanospherical polymer as an MRI sensor without paramagnetic or superparamagnetic species. Dalton Trans. 2013;42:15864–15867. doi: 10.1039/c3dt50378j. [DOI] [PubMed] [Google Scholar]
- 161.Nicolle GM, Tóth É, Schmitt-Willich H, Radüchel B, Merbach AE. The impact of rigidity and water exchange on the relaxivity of a dendritic MRI contrast agent. Chem Eur J. 2002;8:1040–1048. doi: 10.1002/1521-3765(20020301)8:5<1040::aid-chem1040>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 162.Rudovský J, Hermann P, Botta M, Aime S, Lukeš I. Dendrimeric Gd (III) complex of a monophosphinated DOTA analogue: optimizing relaxivity by reducing internal motion. Chem Commun. 2005:2390–2392. doi: 10.1039/b418712a. [DOI] [PubMed] [Google Scholar]
- 163.Lux J, Chan M, Vander Elst L, Schopf E, Mahmoud E, Laurent S, et al. Metal chelating crosslinkers form nanogels with high chelation stability. J Mater Chem B. 2013;1:6359–6364. doi: 10.1039/C3TB21104E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lewinski N, Colvin V, Drezek R. Cytotoxicity of Nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 165.Wu W, Zhou S. Hybrid micro-/nanogels for optical sensing and intracellular imaging. Nano Rev. 2010;1 doi: 10.3402/nano.v1i0.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu W, Aiello M, Zhou T, Berliner A, Banerjee P, Zhou S. In-situ immobilization of quantum dots in polysaccharide-based nanogels for integration of optical pH-sensing, tumor cell imaging, and drug delivery. Biomaterials. 2010;31:3023–3031. doi: 10.1016/j.biomaterials.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 167.Chen Y, Zheng X, Wang X, Wang C, Ding Y, Jiang X. Near-Infrared Emitting Gold Cluster–Poly (acrylic acid) Hybrid Nanogels. ACS Macro Lett. 2013;3:74–76. doi: 10.1021/mz4005748. [DOI] [PubMed] [Google Scholar]
- 168.Kim J, Piao Y, Hyeon T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem Soc Rev. 2009;38:372–390. doi: 10.1039/b709883a. [DOI] [PubMed] [Google Scholar]
- 169.Wiley B, Sun Y, Xia Y. Synthesis of silver nanostructures with controlled shapes and properties. Acc Chem Res. 2007;40:1067–1076. doi: 10.1021/ar7000974. [DOI] [PubMed] [Google Scholar]
- 170.Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- 171.Lim SI, Zhong CJ. Molecularly mediated processing and assembly of nanoparticles: exploring the interparticle interactions and structures. Acc Chem Res. 2009;42:798–808. doi: 10.1021/ar8002688. [DOI] [PubMed] [Google Scholar]
- 172.Qian X, Li J, Nie S. Stimuli-responsive SERS nanoparticles: conformational control of plasmonic coupling and surface Raman enhancement. J Am Chem Soc. 2009;131:7540–7541. doi: 10.1021/ja902226z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xiao J, Tian X, Yang C, Liu P, Luo N, Liang Y, et al. Ultrahigh relaxivity and safe probes of manganese oxide nanoparticles for in vivo imaging. Sci Rep. 2013;3:3424. doi: 10.1038/srep03424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259–1267. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lim C, Singh A, Heo J, Kim D, Lee KE, Jeon H, et al. Gadolinium-coordinated elastic nanogels for in vivo tumor targeting and imaging. Biomaterials. 2013;34:6846–6852. doi: 10.1016/j.biomaterials.2013.05.069. [DOI] [PubMed] [Google Scholar]
- 176.Stark DD, Weissleder R, Elizondo G, Hahn PF, Saini S, Todd LE, et al. Superparamagnetic iron oxide: clinical application as a contrast agent for MR imaging of the liver. Radiology. 1988;168:297–301. doi: 10.1148/radiology.168.2.3393649. [DOI] [PubMed] [Google Scholar]
- 177.Weissleder R, Elizondo G, Wittenberg J, Lee AS, Josephson L, Brady TJ. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology. 1990;175:494–498. doi: 10.1148/radiology.175.2.2326475. [DOI] [PubMed] [Google Scholar]
- 178.Jun Y, Huh Y, Choi J, Lee J, Song H, Kim S, et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J Am Chem Soc. 2005;127:5732–5733. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]
- 179.Katagiri K, Ohta K, Sako K, Inumaru K, Hayashi K, Sasaki Y, et al. Development and Potential Theranostic Applications of a Self-Assembled Hybrid of Magnetic Nanoparticle Clusters with Polysaccharide Nanogels. ChemPlusChem. 2014;79:1631–1637. [Google Scholar]
- 180.Wang X, Niu D, Wu Q, Bao S, Su T, Liu X, et al. Iron oxide/manganese oxide co-loaded hybrid nanogels as pH-responsive magnetic resonance contrast agents. Biomaterials. 2015;53:349–357. doi: 10.1016/j.biomaterials.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 181.Oishi M, Sumitani S, Bronich TK, Kabanov AV, Boska MD, Nagasaki Y. Novel 19F MRS/I nanoprobe based on pH-responsive PEGylated nanogel: pH-dependent 19F magnetic resonance studies. Chem Lett. 2009;38:128–129. [Google Scholar]
- 182.Lux J, White AG, Chan M, Anderson CJ, Almutairi A. Nanogels from Metal-Chelating Crosslinkers as Versatile Platforms Applied to Copper-64 PET Imaging of Tumors and Metastases. Theranostics. 2015;5:277. doi: 10.7150/thno.10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Singh S, Bingöl B, Morgenroth A, Mottaghy FM, Möller M, Schmaljohann J. Radiolabeled nanogels for nuclear molecular imaging. Macromol Rapid Commun. 2013;34:562–567. doi: 10.1002/marc.201200744. [DOI] [PubMed] [Google Scholar]
- 184.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–316. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 186.Yu J, Javier D, Yaseen MA, Nitin N, Richards-Kortum R, Anvari B, et al. Self-assembly synthesis, tumor cell targeting, and photothermal capabilities of antibody-coated indocyanine green nanocapsules. J Am Chem Soc. 2010;132:1929–1938. doi: 10.1021/ja908139y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park HS, Lee JE, Cho MY, Hong JH, Cho SH, Lim YT. Hyaluronic Acid/Poly (β-Amino Ester) Polymer Nanogels for Cancer-Cell-Specific NIR Fluorescence Switch. Macromol Rapid Commun. 2012;33:1549–1555. doi: 10.1002/marc.201200246. [DOI] [PubMed] [Google Scholar]
- 188.Kim J, Chong Y, Mok H. Shell-Crosslinked Hyaluronic Acid Nanogels for Live Monitoring of Hyaluronidase Activity In Vivo. Macromol Biosci. 2014;14:881–888. doi: 10.1002/mabi.201300511. [DOI] [PubMed] [Google Scholar]
- 189.Dai T, Zhou S, Yin C, Li S, Cao W, Liu W, et al. Dextran-based fluorescent nanoprobes for sentinel lymph node mapping. Biomaterials. 2014;35:8227–8235. doi: 10.1016/j.biomaterials.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 190.Noh Y, Kong S, Choi D, Park HS, Yang H, Lee H, et al. Near-infrared emitting polymer nanogels for efficient sentinel lymph node mapping. ACS Nano. 2012;6:7820–7831. doi: 10.1021/nn301949y. [DOI] [PubMed] [Google Scholar]
- 191.Kong S, Noh Y, Suh Y, Park HS, Lee H, Kang KW, et al. Evaluation of the novel near-infrared fluorescence tracers pullulan polymer nanogel and indocyanine green/γ-glutamic acid complex for sentinel lymph node navigation surgery in large animal models. Gastric Cancer. 2015;18:55–64. doi: 10.1007/s10120-014-0345-3. [DOI] [PubMed] [Google Scholar]
- 192.Molina M, Asadian-Birjand M, Balach J, Bergueiro J, Miceli E, Calderón M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem Soc Rev. 2015;44:6161–6186. doi: 10.1039/c5cs00199d. [DOI] [PubMed] [Google Scholar]
- 193.Cherry SR. Multimodality in vivo imaging systems: twice the power or double the trouble? Annu Rev Biomed Eng. 2006;8:35–62. doi: 10.1146/annurev.bioeng.8.061505.095728. [DOI] [PubMed] [Google Scholar]
- 194.Huang R, Ke W, Liu Y, Jiang C, Pei Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials. 2008;29:238–246. doi: 10.1016/j.biomaterials.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 195.Xie H, Zhu Y, Jiang W, Zhou Q, Yang H, Gu N, et al. Lactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials. 2011;32:495–502. doi: 10.1016/j.biomaterials.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 196.Jiang L, Zhou Q, Mu K, Xie H, Zhu Y, Zhu W, et al. pH/temperature sensitive magnetic nanogels conjugated with Cy5. 5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials. 2013;34:7418–7428. doi: 10.1016/j.biomaterials.2013.05.078. [DOI] [PubMed] [Google Scholar]
- 197.Kim HM, Noh Y, Park HS, Cho MY, Hong KS, Lee H, et al. Self-Fluorescence of Chemically Crosslinked MRI Nanoprobes to Enable Multimodal Imaging of Therapeutic Cells. Small. 2012;8:666–670. doi: 10.1002/smll.201102361. [DOI] [PubMed] [Google Scholar]
- 198.Ruhland TM, Reichstein PM, Majewski AP, Walther A, Müller AHE. Superparamagnetic and fluorescent thermo-responsive core-shell-corona hybrid nanogels with a protective silica shell. J Colloid Interface Sci. 2012;374:45–53. doi: 10.1016/j.jcis.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 199.Wang X, Niu D, Li P, Wu Q, Bo X, Liu B, et al. A Dual-Enzyme Loaded Multifunctional Hybrid Nanogel System for Pathological Responsive Ultrasound Imaging and T2-Weighted Magnetic Resonance Imaging. ACS Nano. 2015;9:5646–5656. doi: 10.1021/nn5068094. [DOI] [PubMed] [Google Scholar]
- 200.Thornton PD, Mart RJ, Webb SJ, Ulijn RV. Enzyme-responsive hydrogel particles for the controlled release of proteins: designing peptide actuators to match payload. Soft Matter. 2008;4:821–827. doi: 10.1039/b714750c. [DOI] [PubMed] [Google Scholar]
- 201.Wang Q, Yang Z, Zhang X, Xiao X, Chang C, Xu B. A Supramolecular-Hydrogel-Encapsulated Hemin as an Artificial Enzyme to Mimic Peroxidase. Angew Chem Int Ed. 2007;46:4285–4289. doi: 10.1002/anie.200700404. [DOI] [PubMed] [Google Scholar]
- 202.Vinogradov SV. Nanogels in the race for drug delivery. Nanomedicine. 2010;5:165. doi: 10.2217/nnm.09.103. [DOI] [PubMed] [Google Scholar]
- 203.Li Y, Xiao K, Zhu W, Deng W, Lam KS. Stimuli-responsive cross-linked micelles for on-demand drug delivery against cancers. Adv Drug Deliv Rev. 2014;66:58–73. doi: 10.1016/j.addr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Moghimi SM, Porter CJH, Muir IS, Illum L, Davis SS. Non-phagocytic uptake of intravenously injected microspheres in rat spleen: Influence of particle size and hydrophilic coating. Biochem Biophys Res Commun. 1991;177:861–866. doi: 10.1016/0006-291x(91)91869-e. [DOI] [PubMed] [Google Scholar]
- 205.Gref R, Domb A, Quellec P, Blunk T, Müller RH, Verbavatz JM, et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Deliv Rev. 2012;64(Supplement):316–326. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tamura M, Ichinohe S, Tamura A, Ikeda Y, Nagasaki Y. In vitro and in vivo characteristics of core–shell type nanogel particles: Optimization of core cross-linking density and surface poly(ethylene glycol) density in PEGylated nanogels. Acta Biomater. 2011;7:3354–3361. doi: 10.1016/j.actbio.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 209.Shiraishi K, Hamano M, Ma H, Kawano K, Maitani Y, Aoshi T, et al. Hydrophobic blocks of PEG-conjugates play a significant role in the accelerated blood clearance (ABC) phenomenon. J Control Release. 2013;165:183–190. doi: 10.1016/j.jconrel.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 210.Ishihara T, Takeda M, Sakamoto H, Kimoto A, Kobayashi C, Takasaki N, et al. Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm Res. 2009;26:2270–2279. doi: 10.1007/s11095-009-9943-x. [DOI] [PubMed] [Google Scholar]
- 211.Ishida T, Harada M, Wang XY, Ichihara M, Irimura K, Kiwada H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: Effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J Control Release. 2005;105:305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 212.Saadati R, Dadashzadeh S, Abbasian Z, Soleimanjahi H. Accelerated blood clearance of PEGylated PLGA nanoparticles following repeated injections: effects of polymer dose, PEG coating, and encapsulated anticancer drug. Pharm Res. 2013;30:985–995. doi: 10.1007/s11095-012-0934-y. [DOI] [PubMed] [Google Scholar]
- 213.Lau KH, Ren C, Sileika TS, Park SH, Szleifer I, Messersmith PB. Surface-grafted polysarcosine as a peptoid antifouling polymer brush. Langmuir. 2012;28:16099–16107. doi: 10.1021/la302131n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hara E, Ueda M, Kim CJ, Makino A, Hara I, Ozeki E, et al. Suppressive immune response of poly-(sarcosine) chains in peptide-nanosheets in contrast to polymeric micelles. J Pept Sci. 2014;20:570–577. doi: 10.1002/psc.2655. [DOI] [PubMed] [Google Scholar]
- 215.Huesmann D, Sevenich A, Weber B, Barz M. A head-to-head comparison of poly (sarcosine) and poly (ethylene glycol) in peptidic, amphiphilic block copolymers. Polymer. 2015;67:240–248. [Google Scholar]
- 216.Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, et al. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002;62:6831–6836. [PubMed] [Google Scholar]
- 217.Nakamura M, Davila-Zavala P, Tokuda H, Takakura Y, Hashida M. Uptake and gene expression of naked plasmid DNA in cultured brain microvessel endothelial cells. Biochem Biophys Res Commun. 1998;245:235–239. doi: 10.1006/bbrc.1998.8334. [DOI] [PubMed] [Google Scholar]
- 218.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 219.Kim YH, Park JH, Lee M, Kim Y, Park TG, Kim SW. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release. 2005;103:209–219. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 220.Fischer A, Hacein-Bey-Abina S, Lagresle C, Garrigue A, Cavazana-Calvo M. Gene therapy of severe combined immunodeficiency disease: proof of principle of efficiency and safety issues. Gene therapy, primary immunodeficiencies, retrovirus, lentivirus, genome, Bulletin-Academie Nationale de Medecine. 2005;189:779. [PubMed] [Google Scholar]
- 221.Itaka K, Kataoka K. Recent development of nonviral gene delivery systems with virus-like structures and mechanisms. Eur J Pharm Biopharm. 2009;71:475–483. doi: 10.1016/j.ejpb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 222.Reischl D, Zimmer A. Drug delivery of siRNA therapeutics: potentials and limits of nanosystems. Nanomedicine. 2009;5:8–20. doi: 10.1016/j.nano.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 223.Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: barriers and carriers. The AAPS journal. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zuckerman JE, Choi CH, Han H, Davis ME. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc Natl Acad Sci U S A. 2012;109:3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Naeye B, Deschout H, Caveliers V, Descamps B, Braeckmans K, Vanhove C, et al. In vivo disassembly of IV administered siRNA matrix nanoparticles at the renal filtration barrier. Biomaterials. 2013;34:2350–2358. doi: 10.1016/j.biomaterials.2012.11.058. [DOI] [PubMed] [Google Scholar]
- 226.Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 227.Krishnamurthy R, Manning MC. The stability factor: importance in formulation development. Curr Pharm Biotechnol. 2002;3:361–371. doi: 10.2174/1389201023378229. [DOI] [PubMed] [Google Scholar]
- 228.Kamerzell TJ, Esfandiary R, Joshi SB, Middaugh CR, Volkin DB. Protein–excipient interactions: Mechanisms and biophysical characterization applied to protein formulation development. Adv Drug Deliv Rev. 2011;63:1118–1159. doi: 10.1016/j.addr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 229.Heldin C, Rubin K, Pietras K, Östman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 230.Singh A, Talekar M, Tran T, Samanta A, Sundaram R, Amiji M. Combinatorial approach in the design of multifunctional polymeric nano-delivery systems for cancer therapy. J Mater Chem B. 2014 doi: 10.1039/c4tb01083c. [DOI] [PubMed] [Google Scholar]
- 231.Maki S, Konno T, Maeda H. Image enhancement in computerized tomography for sensitive diagnosis of liver cancer and semiquantitation of tumor selective drug targeting with oily contrast medium. Cancer. 1985;56:751–757. doi: 10.1002/1097-0142(19850815)56:4<751::aid-cncr2820560409>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 232.Seynhaeve AL, Hoving S, Schipper D, Vermeulen CE, de Wiel-Ambagtsheer G, van Tiel ST, et al. Tumor necrosis factor alpha mediates homogeneous distribution of liposomes in murine melanoma that contributes to a better tumor response. Cancer Res. 2007;67:9455–9462. doi: 10.1158/0008-5472.CAN-07-1599. [DOI] [PubMed] [Google Scholar]
- 233.Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M, et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci U S A. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2001;1513:49–54. doi: 10.1016/s0005-2736(01)00340-6. [DOI] [PubMed] [Google Scholar]
- 235.Desmoulin SK, Hou Z, Gangjee A, Matherly LH. The human proton-coupled folate transporter: Biology and therapeutic applications to cancer. Cancer Biol Ther. 2012;13:1355–1373. doi: 10.4161/cbt.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994;73:2432–2443. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 237.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 238.Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Jr, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992;52:3396–3401. [PubMed] [Google Scholar]
- 239.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 240.Michaelsen TE, Brekke OH, Aase A, Sandin RH, Bremnes B, Sandlie I. One disulfide bond in front of the second heavy chain constant region is necessary and sufficient for effector functions of human IgG3 without a genetic hinge. Proc Natl Acad Sci U S A. 1994;91:9243–9247. doi: 10.1073/pnas.91.20.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Seegan GW, Smith CA, Schumaker VN. Changes in quaternary structure of IgG upon reduction of the interheavy-chain disulfide bond. Proc Natl Acad Sci U S A. 1979;76:907–911. doi: 10.1073/pnas.76.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26:925–932. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 243.Wang L, Amphlett G, Blättler WA, Lambert JM, Zhang W. Structural characterization of the maytansinoid–monoclonal antibody immunoconjugate, huN901– DM1, by mass spectrometry. Protein Sci. 2005;14:2436–2446. doi: 10.1110/ps.051478705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Stimmel JB, Merrill BM, Kuyper LF, Moxham CP, Hutchins JT, Fling ME, et al. Site-specific conjugation on serine right-arrow cysteine variant monoclonal antibodies. J Biol Chem. 2000;275:30445–30450. doi: 10.1074/jbc.M001672200. [DOI] [PubMed] [Google Scholar]
- 245.McDonagh CF, Turcott E, Westendorf L, Webster JB, Alley SC, Kim K, et al. Engineered antibody-drug conjugates with defined sites and stoichiometries of drug attachment. Protein Eng Des Sel. 2006;19:299–307. doi: 10.1093/protein/gzl013. [DOI] [PubMed] [Google Scholar]
- 246.Shi M, Lu J, Shoichet MS. Organic nanoscale drug carriers coupled with ligands for targeted drug delivery in cancer. J Mater Chem. 2009;19:5485–5498. [Google Scholar]
- 247.Phillips MA, Gran ML, Peppas NA. Targeted nanodelivery of drugs and diagnostics. Nano Today. 2010;5:143–159. doi: 10.1016/j.nantod.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vercauteren R, Bruneel D, Schacht E, Duncan R. Effect of the chemical modification of dextran on the degradation by dextranase. J Bioact Compatible Polym. 1990;5:4–15. [Google Scholar]
- 249.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 250.Arifin DY, Lee LY, Wang C. Mathematical modeling and simulation of drug release from microspheres: Implications to drug delivery systems. Adv Drug Deliv Rev. 2006;58:1274–1325. doi: 10.1016/j.addr.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 251.Tan JPK, Wang Q, Tam KC. Control of burst release from nanogels via layer by layer assembly. J Control Release. 2008;128:248–254. doi: 10.1016/j.jconrel.2008.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


