Abstract
Micronutrient deficiencies in Crohn's disease (CD) patients are not uncommon and usually result in a combination of reduced dietary intake, disease-related malabsorption, and a catabolic state. Decreased serum thiamine levels are often reported in patients with CD. Wernicke's encephalopathy (WE) is a severe form of thiamine deficiency that can cause serious neurologic complications. Although WE is known to occur frequently in alcoholics, a number of non-alcoholic causes have also been reported. Here, we report two cases of non-alcoholic WE that developed in two severely malnourished CD patients who were supported by prolonged total parenteral nutrition without thiamine supplementation. These patients complained of sudden-onset ophthalmopathy, cerebellar dysfunction, and confusion. Magnetic resonance imaging allowed definitive diagnosis for WE despite poor sensitivity. The intravenous administration of thiamine alleviated the symptoms of WE dramatically. We emphasize the importance of thiamine supplementation for malnourished patients even if they are not alcoholics, especially in those with CD.
Keywords: Wernicke encephalopathy, Crohn disease, Thiamine
INTRODUCTION
Thiamine (vitamin B1) is a water-soluble vitamin absorbed in the duodenum and jejunum that functions as a coenzyme essential for glucose metabolism and neuronal activity.1,2,3 Thiamine deficiency can result in Wernicke's encephalopathy (WE), which is a serious neurologic disorder and characterized by the clinical triad of acute mental confusion, ataxia, and ophthalmoplegia.4 In developed countries, thiamine deficiency is typically associated with chronic alcoholism because alcohol affects thiamine uptake and utilization.4 However, WE may develop in non-alcoholic conditions such as prolonged starvation, gastrointestinal disease, vomiting due to hyperemesis gravidarum, and increased nutrient requirements such as in cases of trauma or septic shock.2,4 Because WE can be frequently undiagnosed and easily confused with other neurological problems, it may be more prevalent than commonly estimated.
Recently, the prevalence of WE in intravenously fed patients owing to a lack of thiamine supplementation has been increasing.3 The indications for total parenteral nutrition (TPN) are inadequate nutrient absorption resulting from short bowel syndrome, gastrointestinal fistula, bowel obstruction, and prolonged bowel rest. These conditions are frequently met in patients with CD.5 Here, we report the two cases of severely malnourished young patients with CD who developed WE after prolonged TPN.
CASE REPORTS
1. Case 1
A 22-year-old male patient diagnosed with CD was referred to our emergency room because of massive hematochezia and hypovolemic shock. He was treated for active CD involving the ileum and colon with a penetrating phenotype during a previous admission period. CT performed at another hospital revealed that the CD also involved multiple segments of the small bowel, including the jejunum, which is the main site of thiamine absorption (Fig. 1). He was also treated with intravenous infliximab. Initially, he was severely malnourished, with a BMI of 17.8 kg/m2. Initial laboratory findings were as follows: white blood cell (WBC) count 22,850/µL; hemoglobin level 8.8 g/dL; platelets 157,000/µL, CRP level 0.03 mg/dL; protein level 3.0 g/dL; albumin level 1.8 g/dL; triglyceride level 73 mg/dL; cholesterol level 119 mg/dL; BUN level 6 mg/dL; creatinine level 1.09 mg/d; and prothrombin time/international normalized ratio 20.6 s (1.78).
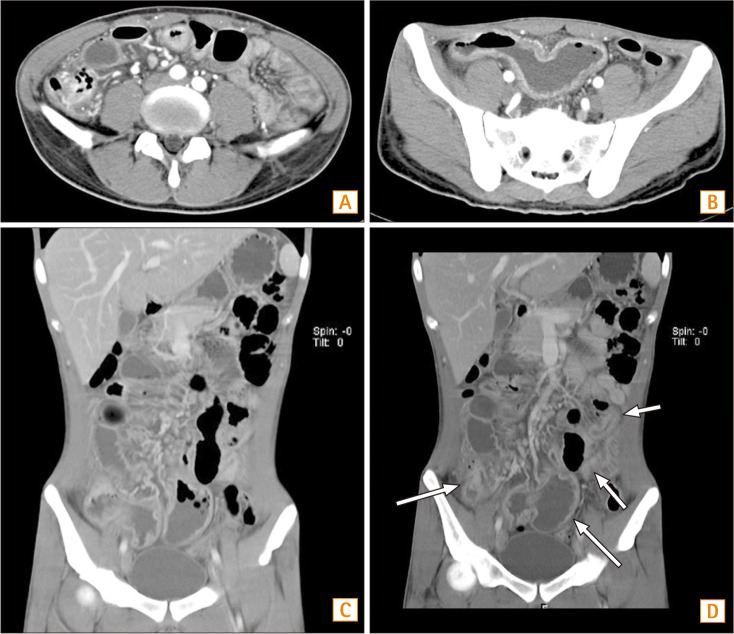
Fig. 1. Abdominal CT findings. (A) In the horizontal section, CD involvement of the ileocecal valve and terminal ileum is seen. (B) In the horizontal section, multisegmental small bowel wall thickening is found. (C) In the coronal view, a rim-enhancing low-density lesion in the right-side perianal area is seen. (D) In the coronal view, multifocal small bowel wall thickening is also noticed.
Emergent angiography revealed active bleeding from the ileal branch of the superior mesenteric artery, and the interventionist performed embolization using glue and lipiodol. In addition, abdominal CT suspected right-sided perianal abscess. He underwent incision and drainage with primary closure for the perianal abscess. After surgical intervention, the fasting period was continued for about 3 weeks, and peripheral TPN (Combiflex lipid peri® 1920 mL) without thiamine supplementation was administered during the fasting period. He started to consume a liquid diet, and 2 days later complained of progressive blurred vision, cognitive dysfunction, intermittent confused speech, and dizziness. He also experienced a brief course of generalized tonic-clonic seizure for 10 minutes. Neurologic examination and electroencephalography were not able to identify any abnormal neurologic signs except for slight latent nystagmus in the lateral gaze. MRI revealed a high signal-intensity lesion at the both right and left sides of the mammillary body and tectum, and the periaqueductal space on a T2-weighted image (T2WI, Fig. 2) which was compatible with WE. After intravenous thiamine replacement, his symptoms improved dramatically and thiamine supplementation was continued for 10 days. He was discharged without any neurologic sequelae, and an oral vitamin supplement was prescribed.
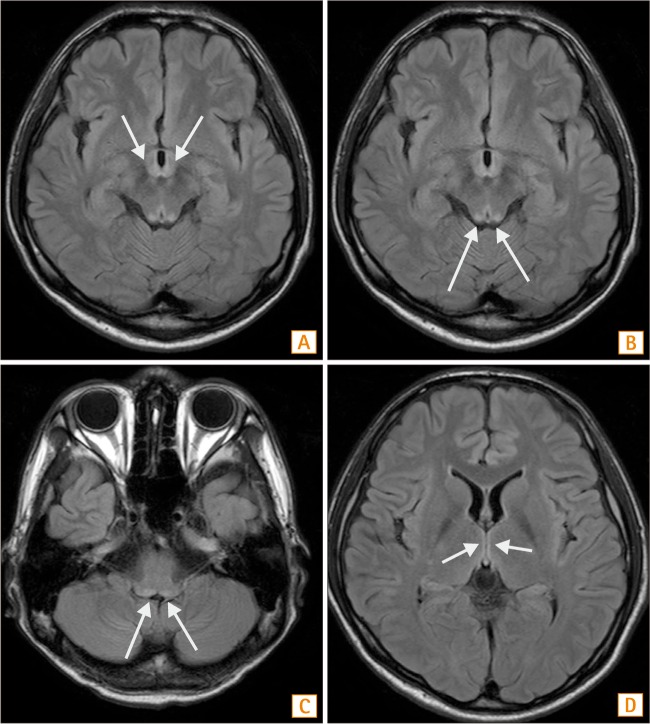
Fig. 2. Brain MRI findings. (A) In the horizontal section of a T2-weighed image (T2WI), a high signal intensity lesion is seen at both the right and left sides of the mammillary body. (B) In same scene of the T2WI, a high signal intensity lesion is present at both the right and left tectum. (C) In the horizontal section of the T2WI, the 4th ventricle floor showing a high signal intensity lesion. (D) At the medial thalamus, a high signal intensity lesion is noted.
2. Case 2
A 34-year-old female patient who was diagnosed with CD involving the small bowel including the distal ileum complained of abdominal cramping pain, postprandial vomiting, and diarrhea. Her symptoms were aggravated despite treatment with an anti-tumor necrosis factor-alpha agent. She was admitted to a general ward for workup. Initially, she was malnourished, with a BMI of 13 kg/m2. Initial laboratory findings were as follows: WBC count 22,850/µL; hemoglobin level 8.8 g/dL; platelet count 423,000/µL; CRP level 0.03 mg/ dL; protein level 3.0 g/dL; albumin level 1.8 g/dL; cholesterol level 182 mg/dL; BUN level 6 mg/dL; creatinine level 1.09 mg/dL; and prothrombin time/international normalized ratio 13.1 s (1.27).
An abdominal CT scan revealed enteromesenteric fistula, enteroenteric fistula, and intraabdominal abscess (Fig. 3), which were treated by fasting, high dose steroids, and antibiotics. She was also treated with peripheral TPN (Combiflex lipid peri® 1920 mL) without thiamine supplementation. After 17 days of fasting, she complained of sudden-onset dizziness and gait ataxia. The next day, her symptoms worsened and new symptoms including nausea, vomiting, and blurred vision developed. There was no organic abnormality apparent to explain her symptoms in ophthalmologic and otolaryngological examinations. Brain MRI revealed a high signalintensity lesion at the inferior colliculus of the midbrain and pontomedullary junction on a (Fig. 4), which was compatible with WE. After intravenous thiamine supplementation, her symptoms were resolved without any sequelae. Although she underwent ileocecectomy for fistula and intraabdominal abscess, her neurologic symptoms were fully resolved.
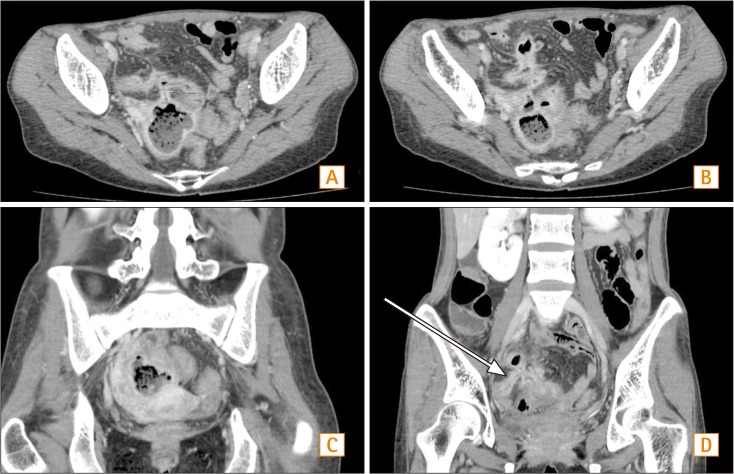
Fig. 3. Abdominal CT findings. (A) In the horizontal view, a lobulated abscess with an enteromesenteric fistula is noticed. (B) In the horizontal view, a multilobulated abscess in pelvic cavity is seen. (C) In the coronal view, abscess formation in the pelvic cavity is noticed. (D) In the coronal view, tubular tract between the distal ileum and abscess is noticed.
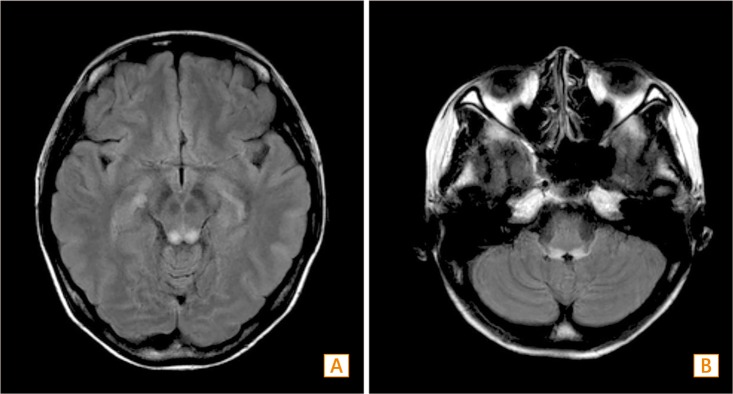
Fig. 4. Brain MRI findings. (A) In the horizontal section of a T2-weighted image (T2WI), a high signal intensity lesion is observed at the inferior colliculus. (B) In the horizontal section of the T2WI, a high signal intensity lesion is seen at the pontomedullary junction.
DISCUSSION
Micronutrient deficiencies in CD patients are not uncommon and usually result in a combination of reduced dietary intake, disease-related malabsorption, and a catabolic state.1,6 Decreased serum thiamine levels are often reported in patients with CD.7 Thiamine is an essential vitamin for glucose metabolism and energy production, and requirements increase during critical illness and chronically depleted states.4 The active form of thiamine (thiamine pyrophosphate) is also a cofactor in several biochemical pathways in the brain.8 Because thiamine-dependent enzymes play an important role in cerebral energy use, deficiencies may initiate tissue injury by inhibiting metabolism in brain regions with high metabolic requirements and high thiamine turnover.9 WE is a significantly disabling and potentially lethal condition that can be caused by thiamine deficiency and fully reversed with early thiamine replacement. Previously, only five cases of CD with WE have been reported in the literature. One was in a patient with clinically inactive CD,10 the second was in a young patient receiving TPN without vitamin supplementation,11 the third was in a middle-aged woman who suffered from chronic diarrhea and vomiting,12 the fourth was in patient receiving TPN during the postoperative period,1 and the last was in a Korean patient who was diagnosed with CD and an enterocutaneous fistula. This final patient was also supported by prolonged TPN.5
The patient in the current report had several risk factors for thiamine deficiency, such as an underlying malnourished state, active CD combined with infection, and postoperative state. Despite the relatively short period of fasting, the active inflammation caused by active CD, underlying malnourished state, infection, and surgery led to an increased thiamine requirement and the development of WE. In addition, refeeding syndrome, which is a life threatening shift of electrolyte and fluid levels caused by initial refeeding in malnourished patients, could be a co-existing factor. WE frequently occurs during early oral feeding periods.13 In the presence of excess glucose, low levels of thiamine could also potentially cause non-alcoholic WE.
WE is diagnosed clinically and has no characteristic and pathognomonic abnormalities in diagnostic studies. The only clinical clue for WE is the symptom triad including ophthalmopathy, cerebellar dysfunction, and confusion.3,4,8 Laboratory and radiographic tests remain important to exclude alternate or coexisting medical conditions. An assay for serum thiamine is not typically available for timely diagnostic purposes, and its sensitivity, specificity, accuracy, and doseresponse relationship have not yet been proven.9 However, thiamine levels may help clinicians to differentiate in ambiguous cases. In contrast, MRI allows definitive diagnosis antemortem despite poor sensitivity. Thiamine is a cofactor of several enzymes involved in glucose metabolism and cerebral energy utilization, so its depletion results in neuronal damage which can be seen on MRI, including T2 and fluidattenuated inversion recovery hyper-intense signaling in the mammillary bodies, periventricular thalamus, and periaqueductal gray matter, as well as diffusion-weighted imaging to differentiate vasogenic from cytotoxic edema.14,15,16
If patients present with symptoms consistent with WE, they should be empirically treated with a high dose of thiamine hydrochloride.8 However, there is no consensus on the optimal dose of thiamine, duration of treatment, or number of treatments. According to many case reports, treatment with either 100 or 200 mg of intravenous thiamine is curative in non-alcoholics. However, alcoholic patients with WE may need higher daily doses, and 500 mg three times daily has been recommended. Intravenous administration is also recommended. Thiamine should be continued until there is no further improvement in signs and symptoms.
The administration of thiamine alleviates the symptoms of WE to some degree in almost all cases. However, persistent neurologic dysfunction is common. Opthalmoplegia and mental confusion are typically resolved, but nystagmus and gait ataxia usually remain.9 It is important to remember that the early diagnosis and treatment of WE is crucial in order to avoid persistent brain damage.8
The development of acute encephalopathy in patients with malnutrition and inflammation should always raise the suspicion of thiamine deficiency. If in doubt, treatment should be followed, as the administration of thiamine is not potentially harmful. It should be emphasized that thiamine supplementation is crucial in all patients with a compromised gut.
The present case demonstrates to gastroenterologists that severe nutritional deficiencies such as those related to WE can develop acutely in malnourished CD patients within relatively short periods of fasting.
Footnotes
Financial support: None.
Conflict of interest: None.
References
- 1.Zeljko K, Darija VB, Dina LK, Marko B. Wernicke's encephalopathy during parenteral nutrition in a Crohn's disease patient. Nutrition. 2011;27:503–504. doi: 10.1016/j.nut.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Osiezagha K, Ali S, Freeman C, et al. Thiamine deficiency and delirium. Innov Clin Neurosci. 2013;10:26–32. [PMC free article] [PubMed] [Google Scholar]
- 3.Francini-Pesenti F, Brocadello F, Manara R, Santelli L, Laroni A, Caregaro L. Wernicke's syndrome during parenteral feeding: not an unusual complication. Nutrition. 2009;25:142–146. doi: 10.1016/j.nut.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Galvin R, Bårthen G, Ivashynka A, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17:1408–1418. doi: 10.1111/j.1468-1331.2010.03153.x. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Oh KW, Koh SH, et al. A case of Crohn's disease with optic neuritis and Wernicke's encephalopathy. J Korean Neurol Assoc. 2007;25:112–114. [Google Scholar]
- 6.Filippi J, Al-Jaouni R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohn's disease in remission. Inflamm Bowel Dis. 2006;12:185–191. doi: 10.1097/01.MIB.0000206541.15963.c3. [DOI] [PubMed] [Google Scholar]
- 7.Kuroki F, Iida M, Tominaga M, et al. Multiple vitamin status in Crohn's disease. Correlation with disease activity. Dig Dis Sci. 1993;38:1614–1618. doi: 10.1007/BF01303168. [DOI] [PubMed] [Google Scholar]
- 8.Busani S, Bonvecchio C, Gaspari A, et al. Wernicke's encephalopathy in a malnourished surgical patient: a difficult diagnosis. BMC Res Notes. 2014;7:718. doi: 10.1186/1756-0500-7-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnino MW, Vega J, Miller J, Walsh M. Myths and misconceptions of Wernicke's encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50:715–721. doi: 10.1016/j.annemergmed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Eggspühler AW, Bauerfeind P, Dorn T, Siegel AM. Wernicke encephalopathy - a severe neurological complication in a clinically inactive Crohn's disease. Eur Neurol. 2003;50:184–185. doi: 10.1159/000073064. [DOI] [PubMed] [Google Scholar]
- 11.Hahn JS, Berquist W, Alcorn DM, Chamberlain L, Bass D. Wernicke encephalopathy and beriberi during total parenteral nutrition attributable to multivitamin infusion shortage. Pediatrics. 1998;101:E10. doi: 10.1542/peds.101.1.e10. [DOI] [PubMed] [Google Scholar]
- 12.Larnaout A, El-Euch G, Kchir N, Filali A, Hamida MB, Hentati F. Wernicke's encephalopathy in a patient with Crohn's disease: a pathological study. J Neurol. 2001;248:57–60. doi: 10.1007/s004150170270. [DOI] [PubMed] [Google Scholar]
- 13.Boateng AA, Sriram K, Meguid MM, Crook M. Refeeding syndrome: treatment considerations based on collective analysis of literature case reports. Nutrition. 2010;26:156–167. doi: 10.1016/j.nut.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Kaneko A, Matsunaga T. MRI findings in nonalcoholic Wernicke's encephalopathy. Intern Med. 2011;50:2245–2246. doi: 10.2169/internalmedicine.50.5726. [DOI] [PubMed] [Google Scholar]
- 15.Antunez E, Estruch R, Cardenal C, Nicolas JM, Fernandez-Sola J, Urbano-Marquez A. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. AJR Am J Roentgenol. 1998;171:1131–1137. doi: 10.2214/ajr.171.4.9763009. [DOI] [PubMed] [Google Scholar]
- 16.Ha ND, Weon YC, Jang JC, Kang BS, Choi SH. Spectrum of MR imaging findings in Wernicke encephalopathy: are atypical areas of involvement only present in nonalcoholic patients? AJNR Am J Neuroradiol. 2012;33:1398–1402. doi: 10.3174/ajnr.A2979. [DOI] [PMC free article] [PubMed] [Google Scholar]