Abstract
Individual differences in cognitive efficiency, particularly in relation to working memory (WM), have been associated both with personality dimensions that reflect enduring regularities in brain configuration, and with short‐term neural plasticity, that reflects task‐related changes in brain connectivity. To elucidate the relationship of these two divergent mechanisms, we tested the hypothesis that personality dimensions, which reflect enduring aspects of brain configuration, inform about the neurobiological framework within which short‐term, task‐related plasticity, as measured by effective connectivity, can be facilitated or constrained. As WM consistently engages the dorsolateral prefrontal (DLPFC), parietal (PAR), and anterior cingulate cortex (ACC), we specified a WM network model with bidirectional, ipsilateral, and contralateral connections between these regions from a functional magnetic resonance imaging dataset obtained from 40 healthy adults while performing the 3‐back WM task. Task‐related effective connectivity changes within this network were estimated using Dynamic Causal Modelling. Personality was evaluated along the major dimensions of Neuroticism, Extraversion, Openness to Experience, Agreeableness, and Conscientiousness. Only two dimensions were relevant to task‐dependent effective connectivity. Neuroticism and Conscientiousness respectively constrained and facilitated neuroplastic responses within the WM network. These results suggest individual differences in cognitive efficiency arise from the interplay between enduring and short‐term plasticity in brain configuration. Hum Brain Mapp 36:4158–4163, 2015. © 2015 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: dynamic causal modelling, effective connectivity, personality, working memory
INTRODUCTION
Working memory (WM) is a fundamental cognitive function that involves the maintenance of representations of task‐related rules and information over delay periods in the absence of external cues [Barch and Smith, 2008]. Numerous functional magnetic resonance imaging (fMRI) studies have confirmed that WM tasks are associated with increased blood‐oxygen‐level‐dependent (BOLD) signal in the dorsolateral prefrontal (DLPFC) and parietal cortices (PAR) and the dorsal anterior cingulate cortex (ACC) [Rottschy et al., 2012; Wager and Smith, 2003]. Accumulating evidence provides robust support linking personality traits of neuroticism (propensity for negative emotionality) and conscientiousness (goal‐directed self‐regulation) to individual differences in WM task‐performance and to the macro‐configuration of the WM neural circuitry. Individuals with high levels of neuroticism perform worse on WM tasks [Smith et al., 2011], have lower DLPFC and ACC volumes [DeYoung et al., 2010; Kapogiannis et al., 2013; Wright et al., 2006, 2007], show reduced fronto‐parietal anatomical connectivity [Bjørnebekk et al., 2013], and attenuated DLPFC activation [Kumari et al., 2004]. Conversely, higher levels of conscientiousness have been linked to better WM task performance [Smith et al., 2011] and greater DLPFC volume [DeYoung et al., 2010; Kapogiannis et al., 2013; Wright et al., 2006, 2007].
At the same time, it is well established that WM is critically dependent on neuronal computations at the micro‐scale level of synaptic‐ and neural network‐level plasticity. Successful maintenance of task‐related representations is causally linked to persistent firing of prefrontal neurons [Fuster, 2001; Goldman‐Rakic, 1995; Goto et al., 2010] that are organized into dynamic assemblies based on Hebbian principles [Fujisawa et al., 2008]. These neural assemblies become sequentially active throughout the WM task with each receding assembly passing its representational “content” to further assemblies. The integrated effect generates local field potentials [Okun et al., 2010] which are tightly linked to the fMRI‐BOLD signal [Logothetis, 2008].
At present there are no mechanistic insights as to how aspects of personality, that are associated with the macro‐organization of the brain, might relate to neuronal computations at the micro‐scale level. Dynamic Causal Modelling [DCM; Friston et al., 2003] is a powerful tool for the investigation of this relationship in living humans. DCM models the directed influence of one neuronal group over another (effective connectivity), which provides an indirect measure of network‐level neuroplasticity [Stephan et al., 2007]. We have already demonstrated that high WM load increases inter‐regional effective connectivity within the WM network [Dima et al., 2014]. Here, we focus on exploring the relationship between DCM‐inferred network‐level plasticity during WM and personality dimensions. We use our previous data (Dima et al., 2014) to examine the relationship between parameter estimates of WM modulation and personality measures derived from the five factor model [Costa and McCrae, 1992]. Based on the available literature, we expected neuroticism and conscientiousness to have opposite effects, respectively negative and positive, on inter‐regional connectivity within the WM circuitry.
MATERIAL AND METHODS
Participants
Forty healthy participants were included in the study if they (i) had no personal lifetime history of mental disorder or substance use as assessed following personal interview using the Structured Interview for DSM‐IV‐TR Axis I Disorders, non‐patient edition [First et al., 2002], (ii) had no history of medical disorders or head injury, and (iii) did not take any prescribed medication. All participants were right‐handed, based on self‐report. Their intellectual quotient (IQ) was assessed using the Wechsler Adult Intelligence Scale—Revised [WAIS‐R; Wechsler, 1981]. Personality was assessed using the Neuroticism‐Extraversion‐Openness Personality Inventory‐Revised (NEO‐PI‐R) [Costa and McCrae, 1992].
Details of the study sample are shown in Table 1. The study was approved by the Ethics Committee of the Institute of Psychiatry and the South London and Maudsley National Health Service Trust. Written informed consent was obtained from all subjects.
Table 1.
Characteristics of study sample (n = 40)
| Demographic data | |
| Sex (male:female) | 20:20 |
| Age (years) | 31.5 (10.4) |
| WAIS‐R IQ | 115.5 (15.9) |
| NEO‐PI‐R scores | |
| Agreeableness | 113 (21.8) |
| Conscientiousness | 119.8 (32) |
| Extraversion | 110.8 (22.3) |
| Neuroticism | 84.8 (34) |
| Openness/Intellect | 112.3 (13.7) |
| Behavioural Performance on the n‐back task | |
| Response time to 3‐back (ms) | 748 (224) |
| Accuracy for 3‐back (% correct) | 72.8 (16.1) |
Continuous variables are presented as mean and standard deviation; WAIS‐R = Wechsler Adult Intelligence Scale‐Revised; IQ = Intelligence Quotient; ms = milliseconds.
Working Memory Functional Imaging Task
The n‐back task was employed in a block design incorporating alternating experimental and sensorimotor control conditions. A series of letters in yellow font were displayed on a blue screen for 2 s each. Participants were instructed to indicate by a button press whether the letter currently displayed matched the letter from the preceding n trials. In the sensorimotor control (0‐back), the letter “X” was the designated target. In the experimental conditions (1, 2, 3‐back), the target letter was defined as any letter that was identical to the one presented in the preceding one, two, or three trials. There were 18 epochs in all, each lasting 30 s, comprising 14 letters with a ratio of target to non‐target letters ranging from 2:12 to 4:10 per epoch. The entire experiment lasted 9 min and included a total of 49 target and 203 non‐target stimuli. To avoid any systematic order effects the conditions were pseudo‐randomised. Performance was evaluated in terms of reaction time to target letters and accuracy (% correct responses). The task was explained to participants prior to scanning but there was no training.
Image Acquisition
Gradient echo planar magnetic resonance (MR) images were acquired using a 1.5‐Tesla GE Neuro‐optimised Signa MR system (General Electric, Milwaukee, WI) fitted with 40 mT/m highspeed gradients, at the Maudsley Hospital, London. Foam padding and a forehead strap were used to limit head motion. A quadrature birdcage head coil was used for radio frequency (RF) transmission and reception. A total of 180 T2*‐weighted MR brain volumes depicting blood‐oxygenation level‐dependent (BOLD) contrast were acquired at each of 36 near‐axial planes parallel to the inter‐commissural (AC‐PC) plane; repetition time (TR) = 3,000 ms, echo time (TE) = 40 ms, slice thickness = 3 mm, voxel dimensions = 3.75 × 3.75 × 3.30 mm, interslice gap = 0.3 mm, matrix size = 64 × 64, flip angle = 90°. Prior to each acquisition sequence, four dummy data acquisition scans were performed to allow the scanner to reach a steady state in T1 contrast. During the same session, a high‐resolution T1‐weighted structural image was acquired in the axial plane (inversion recovery prepared, spoiled gradient‐echo sequence; TR = 18 ms, TE = 5.1 ms, TI = 450 ms, slice thickness = 1.5 mm, voxel dimensions = 0.9375 × 0.9375 × 1.5 mm, matrix size 256 × 192, field of view = 240 × 180 mm, flip angle = 20°, number of excitations = 1) for subsequent co‐registration.
Functional Image Processing
Conventional and DCM analyses were implemented using Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and the corresponding DCM analysis software (DCM8). fMRI images were realigned, normalized, and smoothed using an 8 mm full‐width‐half‐maximum Gaussian kernel. The smoothed single‐participant images were analyzed via multiple regressions using the linear convolution model, with vectors of onset representing the experimental conditions (1, 2, and 3 back) and the 0‐back condition as sensorimotor control. Six movement parameters were also entered as nuisance covariates. Serial correlations were removed using an AR(1) model. A high pass filter (128 s) was applied to remove low‐frequency noise. Contrast images of each memory load condition versus baseline were produced for each participant.
Conventional fMRI Analysis
Group‐level analyses were based on random‐effects analyses of the single‐participant contrast images using the summary statistic approach. Regions showing significant task effect across all participants were identified using one‐sample t tests against zero. The statistical threshold was set to P < 0.05 with familywise error (FWE) correction on a voxelwise basis and minimum cluster size 20 voxels. For all analyses, results are reported in Montreal Neurological Institute (MNI) space. Using the above parameters, we identified the functional network engaged by the n‐back task (Supporting Information Table S1).
Dynamic Causal Modeling
Selection of volumes of interest
Volumes of interest (VOIs) were defined bilaterally in the PAR, ACC, and DLPFC based on our results and supported by previous studies demonstrating robust and consistent involvement of these regions in WM [Rottschy et al., 2012; Wager and Smith, 2003]. The coordinates for the VOIs were based on the group maxima from the contrast of 1‐, 2‐, and 3‐back minus 0‐back condition after conjunction analysis. The co‐ordinates of the group maxima within the DLPFC [Left: x = −48, y = 36, z = 30; Right: x = 48, y = 38, z = 30], PAR [Left: x = −38, y = −56, z = 42; Right: x = 36, y = −52, z = 44], and ACC [Left: x = −10, y = 26, z = 28; Right: x = 12, y = 24, z = 28]. For each participant, VOIs of 5 mm radius were defined centered on participant‐specific maxima in these regions that were (i) within 4 mm from the group maxima, (ii) within the same anatomical area, as defined by the PickAtlas toolbox (http://www.fil.ion.ucl.ac.uk/spm/ext/#WFU_PickAtlas), and (iii) adjusted using the effect of interest F‐contrast. Regional time series were summarized with the first eigenvariate of all activated (at P < 0.01) voxels within the participant‐specific VOIs.
Model specification
As we have previously described [Dima et al., 2014], we used the VOIs defined above in the left and right PAR, left and right ACC, and left and right DLPFC to specify a six‐area DCM in all participants for each experimental condition (1, 2, and 3 back). Within each hemisphere, we defined bidirectional connections between these regions. Bidirectional connections were also specified between homologous regions in each hemisphere. For each experimental condition, 18 endogenous connections were specified in total with the main effect of memory as the driving input entering the PAR bilaterally. For each experimental condition, this base model was elaborated systematically to produce alternative variants to test how WM load could modulate the 18 connections.
From this point on, all analyses were restricted to the high memory load condition (3‐back) versus sensorimotor control (0‐back) contrast because differences in cognitive and neural efficiency are more apparent at high WM load [Gevins and Smith, 2000]. (Analyses relevant to the 2‐back versus 0‐back contrast may be found in the Supporting Information Material).
Model comparison
As we have previously described [Dima et al., 2014], we used random‐effects Bayesian Model Selection (BMS) [Stephan et al., 2009] in DCM8 in order to compare all plausible models of effective connectivity. For the 3‐back vs. 0‐back condition, 18 models were compared in terms of their exceedance and posterior probabilities in order to identify the optimal model.
Connectivity parameter estimation
We then used random effects Bayesian Model Averaging (BMA) to obtain average connectivity estimates across all models for each participant [Penny et al., 2010] as BMA accommodates uncertainty about models when estimating the consistency and strength of connections.
Relationship Between Connectivity Estimates and NEO‐PI‐R Domains Scores
BMA connectivity estimates of the connections modulated by WM within the optimal DCM model were examined in terms of their relationship with all NEO‐PI‐R domain scores using a forced regression model into which age [Stanley et al., in press], sex [Hill et al., 2014], and IQ [Nisbett et al., 2012] were also considered as these factors are known to influence WM.
RESULTS
NEO‐PI‐R Domains
As shown in Table 1, the means of the NEO‐PI‐R scores of the study sample were within their respective normative range. Cronbach's alpha coefficients for the five personality dimensions in our sample were high (Neuroticism = 0.90, Extraversion = 0.92, Agreeableness = 0.88, Openness/Intellect = 0.87, and Conscientiousness = 0.91).
Identification of Optimal DCM
DCM analysis identified a right sided, forward WM model with an exceedance probability of 96%. In this optimal model, the WM load at 3‐back significantly increased effective connectivity in the forward connection from the right PAR to the right DLPFC (P < 0.002). The strength of the WM modulation of the effective connectivity from the right PAR to the right DLPFC was associated with improved response time (r = −0.33, P = 0.04), without change in performance accuracy.
Regression Analysis
The BMA connectivity estimate of the forward connection from the right PAR to the right DLPFC was entered as a dependent variable in a forced regression model with age, sex, IQ, and NEO‐PI‐R scores of all five personality domains as factors. We found that higher neuroticism scores were associated with reduced WM modulation of the connection from the right PAR to the right DLPFC (β = −0.86, P = 0.001), while the opposite was the case for conscientiousness (β = 0.74, P = 0.001). Agreeableness (β = −0.11, P > 0.2), Extraversion (β = 0.21, P > 0.2), Openness to Experience (Β = 0.13, P > 0.2), age (β = −0.07, P > 0.2), sex (β = −0.02, P > 0.2), and IQ (β = 0.17, P > 0.2), were not significant. The overall model fit was R 2 = 0.46 (Fig. 1).
Figure 1.
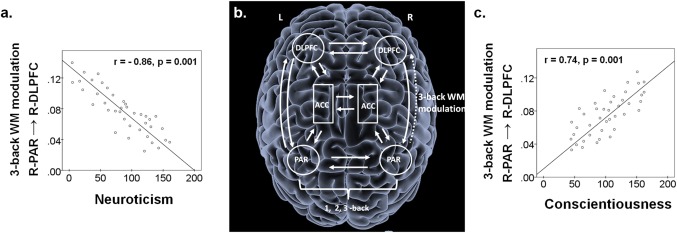
(a) Neuroticism was negatively correlated with the WM modulation of the forward connection from the R‐PAR to the R‐DLPFC; (b) Random‐effects Bayesian Model Selection identified one model with an exceedance probability of 96%. Post hoc t‐tests on Bayesian Model Averages of connectivity confirmed that WM load significantly modulated the forward connection from the R‐PAR to the R‐DLPFC in all participants; (c) Conscientiousness was positively correlated with WM modulation of the forward connection from the R‐PAR to the R‐DLPFC. Abbreviations: L = left; R = right; ACC = anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; PAR = parietal cortex; WM = working memory.
DISCUSSION
In healthy individuals, neuroticism, characterized by negative affect, has been associated with disadvantageous changes in cognition, brain structure, anatomical connectivity [Bjørnebekk et al., 2013; DeYoung et al., 2010; Kapogiannis et al., 2013; Kumari et al., 2004; Wright et al., 2006, 2007], and resting state connectivity [Kruschwitz et al., 2015]. Conversely, conscientiousness, characterized by adherence to rules and goals, seems to have a protective role [DeYoung et al., 2010; Forbes et al., 2014; Kapogiannis et al., 2013; Wright et al., 2006, 2007].
The novel finding in this study is the identification of a mechanistic link between personality dimensions and cognitive efficiency during a WM task through changes in right frontoparietal effective connectivity. Effective connectivity is considered an index of short‐term neural‐network level plasticity [Friston, 1994; Friston et al., 2003; Stephan et al., 2010]. This short‐term plasticity represents a fundamental mechanism by which the brain alters or contextualizes its connectivity and function in response to external or internal cues [Salinas and Sejnowski, 2001]. Two independent lines of evidence support the crucial role of forward connections from the parietal to the frontal cortex and their relationship to WM. Electrophysiological studies have found that tasks with high WM load place greater demands on parietal regions, expressed as persistently high EEG alpha rhythm [Gevins and Smith, 2000] and increased parietal involvement improves response time without affecting accuracy. Similarly, repeated transcranial magnetic stimulation has been shown to increase parieto‐frontal functional connectivity and improve response time in WM tasks [Esslinger et al., 2014]. The parieto‐frontal changes in connectivity were context sensitive (task‐related) and not present in resting state data [Esslinger et al., 2014]. Together, this evidence suggests that during WM, high‐load conditions transiently increase parieto‐frontal coupling. We have shown here that WM modulation of the right parieto‐frontal effective connectivity seems to be influenced by personality traits of neuroticism and conscientiousness. Higher neuroticism scores were associated with reduced WM modulation while higher conscientiousness scores were associated with increased WM modulation.
This study presents a powerful approach for identifying mechanistic links between cognitive neuroscience constructs and domains of psychopathology. Deficits in WM of medium to large effect size are present in most if not all neuropsychiatric disorders [recently reviewed by Snyder et al., 2015] although their diagnostic and prognostic significance has been studied most extensively in psychotic disorders, namely schizophrenia and bipolar disorder [Frangou, 2014; Schmidt et al., 2015]. It is also the case that abnormalities in fronto‐parietal connectivity in psychotic disorders have also been robustly associated both with WM dysfunction and the clinical symptom expression [Frangou, 2014; Schmidt et al., 2015].
There are several methodological considerations in interpreting our results. The NEO‐PI‐R is a self‐report instrument. Although there is much evidence in support of its validity and reliability, particularly in healthy individuals [Young and Schinka, 2001], self‐report measures inevitably reflect subjective judgement. Furthermore, our sample is relatively small for the study of individual differences. Although the association between personality dimensions and effective connectivity is robust, confirmation of our findings in a larger, independent sample would be desirable. Last, DCM is not a direct measure of short‐term neuroplasticity. In fMRI studies, neuroplastic responses can only be inferred from task‐related changes in BOLD signal. The advantage of DCM is that it does not rely simply on changes in the hemodynamic response [Friston et al., 2003]. It informs about changes in neuronal states and therefore provides a better model for the underlying neuroplastic changes [Friston et al., 2003].
Our results demonstrate that neural network plasticity, as measured by changes in effective connectivity, links individual differences in behaviour and cognitive efficiency.
Supporting information
Supporting Information
REFERENCES
- Barch DM, Smith E (2008): The cognitive neuroscience of working memory: Relevance to CNTRICS and schizophrenia. Biol Psychiatry, 64:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnebekk A, Fjell AM, Walhovd KB, Grydeland H, Torgersen S, Westlye LT (2013): Neuronal correlates of the five factor model (FFM) of human personality: Multimodal imaging in a large healthy sample. Neuroimage 65:194–208. [DOI] [PubMed] [Google Scholar]
- Costa PT Jr, McCrae RR (1992): NEO PI‐R Professional Manual. Odessa (FL): Psychological Assessment Resources, Inc. [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR (2010): Testing predictions from personality neuroscience. Brain structure and the big five. Psychol Sci 21:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D, Jogia J, Frangou S (2014): Dynamic Causal Modeling of load‐dependent modulation of effective connectivity within the verbal working memory network. Hum Brain Mapp 35:3025–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Schuler N, Sauer C, Gass D, Mier D, Braun U, Ochs E, Schulze TG, Rietschel M, Kirsch P, Meyer‐Lindenberg A (2014): Induction and quantification of prefrontal cortical network plasticity using 5 Hz rTMS and fMRI. Hum Brain Mapp 35:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002): Structured Clinical Interview for DSM‐IV Axis I Disorders, Research Version, Non‐patient Edition (SCID‐I/NP). New York (NY): Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Forbes CE, Poore JC, Krueger F, Barbey AK, Solomon J, Grafman J (2014): The role of executive function and the dorsolateral prefrontal cortex in the expression of neuroticism and conscientiousness. Soc Neurosci 9:139–151. [DOI] [PubMed] [Google Scholar]
- Frangou S (2014): A systems neuroscience perspective of schizophrenia and bipolar disorder. Schizophr Bull 40:523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ (1994): Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Mapp 2:56–78. [Google Scholar]
- Friston KJ, Harrison L, Penny W (2003): Dynamic causal modelling. Neuroimage 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsáki G (2008): Behavior‐dependent short‐term assembly dynamics in the medial prefrontal cortex. Nat Neurosci 11:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM (2001): The prefrontal cortex: An update: Time is of the essence. Neuron 30:319–333. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME (2000): Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb Cortex 10:829–839. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS (1995): Cellular basis of working memory. Neuron 14:477–485. [DOI] [PubMed] [Google Scholar]
- Goto Y, Yang CR, Otani S (2010): Functional and dysfunctional synaptic plasticity in prefrontal cortex: Roles in psychiatric disorders. Biol Psychiatry 67:199–207. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Sutin A, Davatzikos C, Costa P Jr, Resnick S (2013): The five factors of personality and regional cortical variability in the baltimore longitudinal study of aging. Hum Brain Mapp 34:2829–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AC, Laird AR, Robinson JL (2014): Gender differences in working memory networks: A BrainMap meta‐analysis. Biol Psychol 102:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, ffytche DH, Williams SC, Gray JA (2004): Personality predicts brain responses to cognitive demands. J Neurosci 24:10636–10641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschwitz JD, Walter M, Varikuti D, Jensen J, Plichta MM, Haddad L, Grimm O, Mohnke S, Pöhland L, Schott B, Wold A, Mühleisen TW, Heinz A, Erk S, Romanczuk‐Seiferth N, Witt SH, Nöthen MM, Rietschel M, Meyer‐Lindenberg A, Walter H (2015): 5‐HTTLPR/rs25531 polymorphism and neuroticism are linked by resting state functional connectivity of amygdala and fusiform gyrus. Brain Struct Funct 220:2373–2385. [DOI] [PubMed] [Google Scholar]
- Logothetis NK (2008): What we can do and what we cannot do with fMRI. Nature 453:869–878. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Aronson J, Blair C, Dickens W, Flynn J, Halpern DF, Turkheimer E (2012): Intelligence: New findings and theoretical developments. Am Psychol 67:130–159. [DOI] [PubMed] [Google Scholar]
- Okun M, Naim A, Lampl I (2010): The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats. J Neurosci 30:4440–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Daunizeau J, Rosa MJ, Friston KJ, Schofield TM, Leff AP (2010): Comparing families of dynamic causal models. PLoS Comput Biol 6(3):e1000709 10.1371/journal.pcbi.1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB (2012): Modelling neural correlates of working memory: A coordinate‐based meta‐analysis. Neuroimage 60:830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ (2001): Gain Modulation in the Central Nervous System: Where Behavior, Neurophysiology, and Computation Meet. Neuroscientist 7:430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Diwadkar VA, Smieskova R, Harrisberger F, Lang UE, McGuire P, Fusar‐Poli P, Borgwardt S (2015): Approaching a network connectivity‐driven classification of the psychosis continuum: A selective review and suggestions for future research. Front Hum Neurosci 8:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Persyn D, Butler P (2011): Prospective memory, personality, and working memory: A Formal modeling approach. Z Psychol 219:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, Hankin BL (2015): Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Front Psychol 6:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley ML, Simpson SL, Dagenbach D, Lyday RG, Burdette, JH , Laurienti PJ (2015): Changes in brain network efficiency and working memory performance in aging. PLoS One 10:e0123950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Harrison LM, Kiebel SJ, David O, Penny WD, Friston KJ (2007): Dynamic causal models of neural system dynamics: Current state and future extensions. J Biosci 32:129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ (2009): Bayesian model selection for group studies. Neuroimage 46:1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HEM, Daunizeau J, Friston KJ (2010): Ten simple rules for dynamic causal modelling. Neuroimage 49:3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE (2003): Neuroimaging studies of working memory: A meta‐analysis. Cogn Affect Behav Neurosci 3:255–274. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1981). Manual for the Wechsler Adult Intelligence Scale—Revised. New York (NY): Psychological Corporation. [Google Scholar]
- Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, Wedig MM (2006): Neuroanatomical correlates of extraversion and neuroticism. Cereb Cortex 16:1809–1819. [DOI] [PubMed] [Google Scholar]
- Wright CI, Feczko E, Dickerson BC, Williams D (2007): Neuroanatomical correlates of personality in the elderly. Neuroimage 35:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MS, Schinka JA (2001): Research Validity Scales for the NEO‐PI‐R: Additional evidence for reliability and validity. J Pers Assess 76:412–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


