Abstract
Phosphoinositides (PIs) are minor components of cell membranes, but play key roles in cell function. Recent refinements in techniques for their detection, together with imaging methods to study their distribution and changes, have greatly facilitated the study of these lipids. Such methods have been complemented by the parallel development of techniques for the acute manipulation of their levels, which in turn allow bypassing the long-term adaptive changes implicit in genetic perturbations. Collectively, these advancements have helped elucidate the role of PIs in physiology and the impact of the dysfunction of their metabolism in disease. Combining methods for detection and manipulation enables the identification of specific roles played by each of the PIs and may eventually lead to the complete deconstruction of the PI signaling network. Here, we review current techniques used for the study and manipulation of cellular PIs and also discuss advantages and disadvantages associated with the various methods. This article is part of a Special Issue entitled Phosphoinositides.
Keywords: Lipid binding domain, Rapamycin, Cryptochrome, Optogenetics, Optical manipulation, Phosphatidylinositol, PI 3-kinase
1. Introduction
Reversible phosphorylation of phosphatidylinositol at the 3, 4 and 5 positions of its inositol head group by phosphatidylinositol kinases and phosphatases gives rise to the seven different phosphoinositides (PIs) and the heterogeneous distribution of these lipids contributes to cellular membrane identity (Fig. 1). PIs are versatile signaling molecules important for diverse cellular functions such as signal transduction, transport across membranes, membrane trafficking, regulation of the cytoskeleton, cell migration and proliferation [1,2]. Consistent with the fundamental roles of these lipids, the network of enzymes responsible for their synthesis and degradation are largely conserved from yeast to mammals, although the genes encoding several of these enzymes have undergone duplications during evolution. Mutations in PI-metabolizing enzymes are associated with the development of diseases, including psychiatric and neurological disorders, cancer, diabetes and allergy [3] (De Matteis, this volume). This has spurred the development of techniques both for the detection and for the manipulation of these lipids. Biochemical detection techniques now allow quantification of all seven PIs, and the use of fluorescently tagged PI-binding domains enables real-time visualization of most of them in intact cells [4,5] (see Balla, this volume). Together, these and other methods now allow us to examine the dynamics of the seven PIs at different levels, from global changes in cells and tissues down to changes in a specific PI in a cellular subcompartment. Parallel to the development of detection techniques, new techniques for the chronic or acute, cell-wide or spatially localized manipulation of PIs have been developed. In this Review we summarize and discuss available methodology for the analysis and manipulation of PIs, compare the strengths and weaknesses of different methods and also suggest future directions for this field of PI biology.
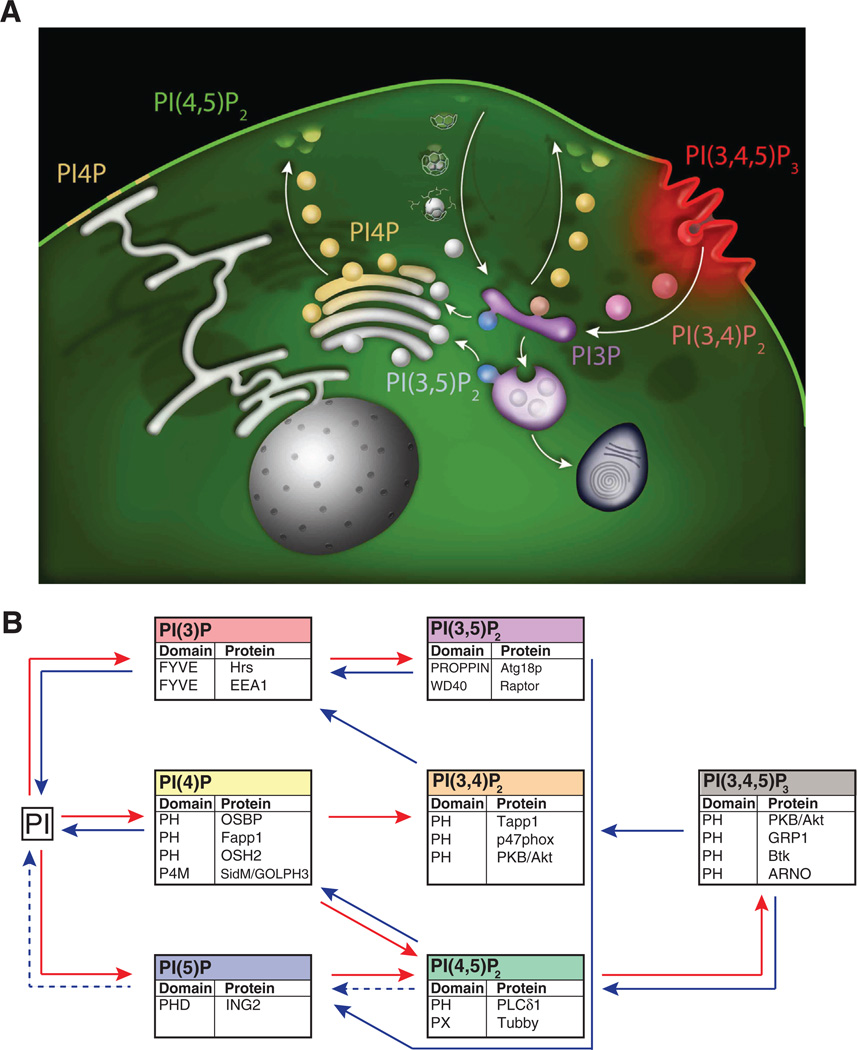
Fig. 1.
The PI network. A. Heterogeneous distribution of PIs in subcellular membranes. The cartoon depicts the predominant localizations of different PI species. It should be noted that small, but physiologically important, PI pools that do not fit this simplified view (for example 3-phosphorylated PIs at sites of clathrin-mediated endocytosis [91]) also occur in cells. Arrows indicate membrane traffic directions thus illustrating the coupling of membrane transport reactions to PI conversion. B. The illustration shows the seven PIs and the enzymatic steps involved in their synthesis (red) or dephosphorylation (blue). Below each PI are indicated protein modules typically used for the detection of that PI, as well as the proteins from which they are derived. Metabolic reactions that have not been well characterized are indicated with dashed arrows.
2. Measuring PI levels
Several excellent reviews on techniques for PI detection have been previously published [5–8] (see also Balla in this volume). Briefly, these techniques can be divided into biochemistry- and microscopy-based methods.
2.1. Biochemical detection of PIs
PIs present in tissue and cell lipid extracts are typically identified and quantified by thin layer chromatography (TLC) or by ion-exchange HPLC separation of their glycerophosphoinositol moieties following deacylation [6,7]. As PIs represent minor species in cellular lipid extracts, their detection requires previous metabolic labeling (optimally equilibrium labeling) with [3H]inositol or [32P]inorganic phosphate. Nonradioactive detection of HPLC separated PIs is also possible using HPLC followed by suppressed conductivity measurements [9,10]. This method efficiently detects only PIP and PIP2 without discriminating between the phosphorylated positions on the inositol ring. However, since PI4P and PI(4,5)P2 are the predominant PIs in cells of high eukaryotes, levels of PIP and PIP2 roughly reflect the levels of these two PIs. Mass spectrometry methods can also be used. Mass spectrometry has great sensitivity and also allows identification of the fatty acid chains and not just of the head group. Combining chromatographic separation with mass spectrometry improves both sensitivity and specificity of detection without need for radiolabeling [4,11,12].
A limitation inherent to biochemical detection is the poor temporal resolution, as it provides a snapshot of the PI composition of cells but does not give information about dynamic changes in PI levels. Moreover, the metabolic labeling required to detect minor PI species precludes experiments in whole organisms due to the problems associated with the use of radioactive tracers. Another limitation of biochemical detection is the lack of information about the distribution of PIs within cells, as subcellular fractionation prior to lipid extraction results, at least to a large extent, in their dephosphorylation. An important part of the signaling power embedded in the phosphoinositide code comes from spatial segregation of different PI species inside cells [2] (see Fig. 1A). Recent developments in imaging mass spectrometry are pushing the resolution of this technique into the micrometer range, which may allow biochemical detection of individual PI species with subcellular resolution in the near future [13]. A major advantage with biochemical detection techniques is that they allow the simultaneous detection of all PIs, which in turn enables the identification of compensatory changes in lipid levels associated with the manipulation of the intricate PI network.
2.2. Microscopy-based detection of PIs
Microscopic detection of PIs utilizes the specific interaction of different PIs with protein domains or antibodies that can be labeled with fluorescent probes. This method allows determining the intracellular location of specific PIs and their relative levels in different membranes. Importantly, light microscopy-based live imaging of cells expressing PI binding modules fused to fluorescent protein permits us to monitor changes in PI levels or distribution in response to physiological or experimental perturbations. Most commonly, the detection is done using a regular wide-field epifluorescence microscope or a confocal microscope, which provides high-resolution images of optical sections of the specimen. In some cases, the changes in PI(s) of interest take place in the plasma membrane, allowing the use of total internal reflection fluorescence (TIRF) microscopy. This technique provides selective imaging of fluorescence in the thin volume of the cell directly adjacent to the coverslip which comprises the plasma membrane [14]. These microscopy techniques typically have x-y resolution of 0.2 µm under optimal conditions, which does not allow discriminating between closely adjacent small membrane compartments or membrane domains. To overcome this problem, super-resolution microscopy techniques that have resolution in the range of 10’s of nm can be employed [15]. So far, these techniques have been primarily limited to the detection of PIs in fixed cells or membrane sheets [16,17], but progress in the field is now making possible super-resolution video microscopy. For example, a super-resolution imaging technique was recently used to detect diffraction-limited clusters of PI(3,4,5)P3 at sites of synaptic vesicle exocytosis in live Drosophila neurons [18]. Even higher resolution has been achieved with electron microscopic detection of PIs using PI binding modules, although fixation and post-fixation tissue manipulations affect and complicate the interpretation of results obtained by this technique [19,20].
2.3. Protein domains for the detection of PIs
The use of fluorescent proteins fused to protein modules with specific PI-binding properties has become a most valuable tool in the study of PIs in cells, including living cells. This methodology is extensively reviewed by Balla in this issue and is only briefly summarized here. Analysis of fluorescent reporter protein localization and stimulus-induced translocation provides information about the intracellular distribution and changes in relative levels of a particular lipid. A large number of protein domains have been identified that are useful to monitor distribution and changes in most PIs (Fig. 1). 3- and 4-monophosphorylated PIs are detected using FYVE (PI3P) [19,21–23] or PH/P4M (PI4P) [24–26] domains whereas no well-characterized lipid binding domain for PI5P exists, although the PHD domain from ING2 has been used [27]. Among the bisphosphorylated PIs, PI(4,5)P2 can be readily detected by the PH domain from PLCδ1 or the PX domain from Tubby [28–30]. PI(3,4)P2 can be detected using the PH-domains from Tapp1 and p47phox [31,32], whereas the PROPPIN domain from Atg18p and the WD40 domain from Raptor has been used as a biosensor for PI(3,5)P2 [33,34]. Several PH-domains have been characterized as specific binding partners for the tris-phosphorylated PI, PI(3,4,5)P3, including those of Akt1, GRP1, Btk and ARNO [35–38].
These tools have greatly advanced our knowledge of PIs biology, but care must be taken when interpreting the results. The overexpression of PI-binding proteins may prevent endogenous proteins from interacting with their cognate lipid, thereby interfering with downstream signaling and cell functions. For example, overexpression of the PI(4,5)P2-binding PH-domain from PLCδ1 has been used as a tool to buffer this lipid in living cells [39]. Another limitation with some of the protein-based PI-sensors is that their interaction with a given PI must synergize with other interactions in order to yield sufficient affinity for membrane binding (dual key mechanisms or coincidence detection) [2]. For example, large pools of PI4P are present both in Golgi complex membranes and in the plasma membrane. However, most of the commonly used PI4P–binding protein domains (Fapp1-PH, OSBP-PH, OSH1-PH) only recognize the Golgi complex pool, whereas others (OSH2-PH) only recognize the plasma membrane pool [40,41]. Moreover, some domains recognize more than one PI species. For example, the PH-domain from Akt1 is widely used as a biosensor for both PI(3,4)P2 and PI(3,4,5)P3 [35,42]. This, together with differences in PI-affinity that may preclude detection of low concentrations of the lipid, warrants the use of multiple, overlapping biosensors to confirm the presence of a specific PI.
In addition to their use as direct reporters of PI localization and levels based on their subcellular localizations and stimulus-dependent trans-location [43,44] (Fig. 2A), fluorescent PI binding modules can be used in settings where lipid binding can be detected as altered fluorescence resonance energy transfer (FRET) between their fluorophore and that of another fluorophore. In one approach, the other fluorophore can be bound to a fluorescent protein localized in the same membrane as the target PI. In another FRET-based strategy, versions of the same PI-binding domain tagged with two different fluorophores (for example CFP and YFP) are co-expressed (Fig. 2B). Colocalization of both tagged domains at the membrane containing the target PI allows FRET to occur between CFP and YFP, and dissociation from the membrane upon reduction in PI levels results in reduced FRET [45]. FRET-based methodology, as opposed to directly monitoring translocation of a biosensor, has the advantage of simplified image analysis, since whole cell signals can be detected and used as a readout for changes in PI levels. In addition, it also allows detection of biosensor redistribution even in small cell compartments, where translocation cannot be easily appreciated by traditional fluorescence microscopy.
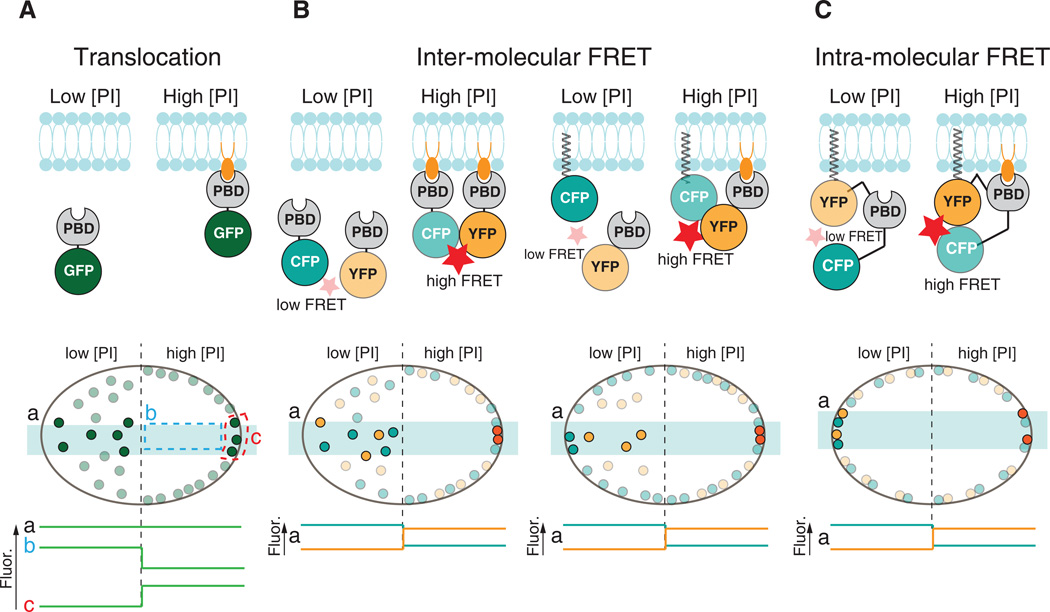
Fig. 2.
Detection of PIs by protein modules. Schematic illustration of the three major types of protein-based biosensors used for the microscopic detection of PIs. A. A translocation biosensor comprising a PI-binding module (PBD) fused to a fluorescent protein (GFP) binds to a specific PI in cellular membranes. The subcellular distribution of the biosensor reflects an equilibrium between the free pool in the cytosolic and the lipid-bound pool at the membrane. Changes in lipid levels at the membrane can be monitored by e.g. confocal microscopy as a redistribution of fluorescence. The global fluorescence signal in the cell (a) is constant even if lipid levels change, but analysis of either cytosolic (b) or membrane (c) fluorescence can be used to estimate relative changes in lipid levels. B. Biosensors based on inter-molecular FRET are identical to the translocation biosensors, but require coexpression of an additional fluorescence-tagged module that allows for FRET to occur between the two fluorophores. The other component of the FRET pair can either be permanently anchored to a membrane of interest (right)or consist of the same lipid binding domain fused to a compatible fluorophore (left). When not bound to a membrane, the biosensor molecules are far apart from their FRET partners and FRET between the donor and acceptor fluorophore is low. Upon recruitment to the membrane by an increase in the appropriate PI, FRET increases and can be detected with e.g. a confocal microscope as reduced donor fluorescence and increased acceptor fluorescence. Off-line analysis is simplified compared to the translocation biosensors since whole cell fluorescence (a) can be used to estimate protein sensor redistribution and thus relative changes in lipid levels. C. The principle for biosensors based on intra-molecular FRET is similar to that of inter-molecular FRET (see B). A major advantage is that these biosensors can be anchored to a specific cellular membrane to monitor changes in PI levels specifically at that membrane. Analysis of these changes is simplified compared to the translocation biosensors since it can be done by collecting fluorescence from the whole cell (a). In all bottom fields, the light blue horizontal rectangles indicate the cell volume illuminated by a confocal microscope. Biosensors contained within this volume are shown in darker color to reflect the fluorescence produced by their excitation and/or altered FRET.
Another type of FRET sensors relies on changes in intra-molecular FRET occurring upon PI-binding. These sensors are permanently anchored to a subcellular membrane, for example by a lipidated motif, and specifically detect changes in PI levels at that membrane as a result of a conformational change that couples PI binding to FRET changes (Fig. 2C). For example, the PH-domain of GRP1 fused at each terminus to CFP and YFP, respectively, has been used to report changes in PI(3,4,5)P3 levels on intracellular membranes [46] and at synaptic vesicle release sites [18]. Similar to FRET sensors, Yang and colleagues recently reported the use of split luciferase to detect changes in plasma membrane PI(3,4,5)P3 levels through bioluminescence [47]. Although FRET-based or other split-protein sensors in principle can be designed for measurements of most PIs, proper detection of FRET can be technically challenging and is often associated with poor signal-to-noise ratio due to inefficient energy transfer between the fluorophores. However, this methodology is rapidly advancing with the development on improved FRET sensors and fluorescent FRET pairs [48].
PI binding modules can also be produced as recombinant proteins and used to detect PIs in fixed samples or permeabilized cells, similar to antibodies and with the same limitations (see below).
2.4. Antibody-based detection of PIs
Commercial antibodies are available against all major PIs except PI5P and can be used to detect PIs by immunocytochemistry, primarily immunofluorescence. Due to the need of specimen fixation, antibody detection of PIs only provides a snapshot of the cell at a given time point and cannot be used to detect transient changes [49]. This method can be useful for detecting PI localization when the use of genetically encoded fluorescently tagged lipid binding modules is precluded, such as in tissue samples. However, antibody-based methods are at best semi-quantitative and influenced by sample preparation procedures, as samples cannot be treated with standard fixatives or detergents since this may result in extraction of the lipids [6,50]. Moreover, the masking of PIs by bound proteins may complicate the interpretation of results obtained in fixed cells, when instead of detecting PIs in living cells by expression of a fluorescently labeled PI-binding protein, equilibrium occurs between endogenous ligands and the fluorescent probe that allows detection of the PI levels. However, precisely because of this equilibrium, one should also consider that the expression of the probe might affect physiological processes.
3. Manipulating phosphoinositide levels
Techniques that enable the manipulation of the cellular levels of specific PIs have been of great importance in the exploration of the cellular function of these lipids. There are many different approaches to affect PI levels exist, including perturbations of PI metabolizing enzymes by genetic or pharmacological methods, use of PI analogs and chemical-or light-inducible manipulations.
3.1. Genetic perturbations
A powerful approach to studying PIs is the analysis of the effects produced by manipulation of the genes responsible for the synthesis, metabolism and degradation of these lipids. This can be achieved in organisms and in cultured cells by gene Knock-Out (KO), Knock-Down (KD) or Knock-In (KI) techniques, as well as by gene overexpression (e.g. [51–57]). Conversely, useful information can be obtained by the analysis of the phenotypic manifestations of spontaneous mutations of genes encoding PI metabolizing enzymes in model organisms or human patients (e.g. [58]) (see also chapter by De Matteis in this volume for a review of human diseases involving defects in PI metabolism). The use of these strategies, however, is associated with several caveats. First, in studies at the organismal level involving germline mutations, assessment of PI changes on specific physiological processes can be complicated by effects on development, including, in some cases, embryonic lethality. Second, even when these problems can be bypassed by conditional gene disruption (or overexpression) or by studies in cultured cells, compensatory adaptive changes (such as up- or down-regulation of other PI metabolizing enzymes) can affect the results, as the effects of these manipulations occur in the range of hours to days. Third, genetic redundancy, where more than one enzyme can catalyze the same step in the PI metabolic network (see Fig. 1), also complicates the use of these approaches. These shortcomings can be overcome by techniques that allow acute manipulation of specific PIs as discussed below.
3.2. Pharmacological manipulation
3.2.1. PI 3-kinase
Potent pan-PI 3-kinase inhibitors like wortmannin and LY294002 have been around for 20 years [59,60]. Both of them are competitive inhibitors targeting the ATP-binding pocket in the p110 catalytic subunit of class I PI 3-kinase. These drugs have been powerful tools to investigate the role of PI 3-kinase and its lipid products PI(3,4)P2 and PI(3,4,5)P3 in cell regulation. However, one should consider that these compounds also inhibit class III PI 3-kinase [61], the PI 3-kinase regulator mTOR [62] and some PI 4-kinase isoforms (see below) when used at high concentrations [63–65].
More specific drugs affecting PI(3,4,5)P3 signaling have been developed as attractive candidates for cancer treatment, since dysregulation of this signaling pathway is a major contributing factor to the development of many forms of cancer and immune-mediated pathologies. Genetic studies suggest that the PI 3-kinase pathway is the most frequently altered pathway in human tumors, with the gene encoding the PI 3-kinase catalytic p110α subunit (PIK3CA) being the second most frequently mutated oncogene and PTEN being among the most frequently mutated tumor suppressor genes [66]. Drugs targeting this pathway are therefore attractive candidates for cancer treatment and several pan-PI3-kinase and isoform-selective inhibitors, such as BAY806946 (pan-PI 3-kinase), NVP-BYL719 (p110a) and GSK2636771 (p110β), are currently in clinical trials (reviewed elsewhere) [67,68]. The most impressive results have been achieved with the p110δ-selective inhibitor GS-1101 (idelalisib) in the treatment of chronic lymphocytic leukemia and other B-cell malignancies [69,70]. There are also a number of isoform selective PI 3-kinase inhibitors used in experimental settings. These include YM024 (p110α), TGX221 (p110β) and AS252424 (p110δ) [71–73]. Inhibitors of the lipid 3-phosphatase PTEN, like VO-OHpic, cause increases in PI(3,4,5)P3 levels and have been used to study the effects of this lipid [74,75]. An inhibitor of PIP kinase type IIβ (a PI5P 4-kinase) has been recently described [76].
3.2.2. PI 5-phosphatases
Concerning 5-phosphatases, there are inhibitors (Pelorol, AS1949490, 3AC) and activators (AQX-MN100, AQX-1125) of the 5-phosphatases SHIP1/2. Some of these are currently in clinical trials for the treatment of cancer and inflammatory diseases like asthma (reviewed in [77]). An inhibitor of the 5-phosphatase OCRL, YU142670, which when administered in cells caused elevation of cellular PI(4,5)P2, was recently described [78].
3.2.3. PI 4-kinase
The thiol-targeting agent phenylarseneoxide (PAO) has traditionally been used as a PI 4-kinase inhibitor. While this compound preferentially targets PI4KIIIα (PIK4CA) among the four PI 4-kinase isoforms, i.e. the enzyme that produces the bulk of the plasma membrane PI4P pool [79,80], it also has many other cellular targets and is therefore quite non-specific. High doses of the PI 3-kinase inhibitor wortmannin (see above) also inhibit PI4KIIIα. Novel PI4KIIIα inhibitors based on the 4-anilino quinazoline and benzimidazole scaffolds have recently been developed. These inhibitors show selectivity for PI4KIIIα over both other PI 4-kinase isoforms and the class I PI 3-kinases and cause a specific reduction in cellular PI4P levels at the plasma membrane in cell-based assays [81,82]. PI4KIIIα (PIK4CB), which acts at the Golgi complex and has been implicated in infection by several classes of viruses that utilize this organelle as part of their life cycle [83], can be specifically pharmacologically targeted by the inhibitor PIK93 [84].
3.2.4. 3.2.3 PI3P 5-kinase
A single PI3P 5-kinase is encoded by mammalian genomes, called PIKfyve (PIP5K3). The PIKfyve inhibitor YM201636 blocks PI(3,5)P2 production and endomembrane trafficking as well as the formation of PI5P [85]. It is not clear if the effect on PI5P levels is due to a direct role of PIKfyve in the synthesis of PI5P or indirectly through inhibition of PI(3,5)P2 production, which can serve as a substrate for MTMR3 to produce PI5P. A recent paper suggests that YM201636 selectively blocks PI5P synthesis at low (nM) concentrations and both PI5P and PI(3,5) P2 synthesis at higher (µM) concentrations [86]. PIKfyve is also potently inhibited by apilimod, an immunosuppressant used experimentally to treat Crohn’s disease and other autoimmune diseases. In cell based assays, apilimod at nanomolar concentrations resulted in increased levels of PI3P and reduced level of PI(3,5)P2 [87].
A general problem with pharmacological manipulations of PIs is that drugs are only specific within a very narrow concentration range and can have prominent off-target effects. While some drugs are highly effective on purified proteins, their action in cell based or in vivo settings are affected by permeability problems that will influence the effective concentration of the drug.
3.3. PI analogs
Another approach to manipulate intracellular levels of PIs is direct addition of membrane permeable PI analogs. One note of caution when using these lipids is that they could in principle insert into all lipid bilayers and may thus not faithfully reproduce effects caused by the heterogeneous subcellular distribution typically observed for endogenous PIs. The major challenge in the synthesis of these lipids is that all negative charges on the inositol head-group must be neutralized in order to allow diffusion over cellular membranes [88]. However, membrane permeable analogs of several PIs have been synthesized and successfully used. Using these compounds it was proven that PI(3,4)P2 and PI(3,4,5)P3 directly stimulate the formation of cell protrusions, tyrosine kinase receptor recycling and clathrin-mediated endocytosis [89–91]. More recently, these lipid analogs have been employed to show a role of PIs in actomyosin contraction in the developing Drosophila larvae, proving their applicability in in vivo settings [92]. The somewhat long time required to observe effects (20 min) is due to the enzymatic ester hydrolysis required to make the lipids biologically active. To circumvent this step, photolysable analogs [PI(3,4)P2, PI(3,4,5) P3 and PI3P] were recently synthesized [89,93,94]. These lipid precursors are loaded into cells in an inactive form and made active by brief UV-light illumination, giving rise to observable effects within 30 s. This method also could allow control of lipid levels in a subcellular microenvironment by the use of a focused UV-light source.
3.4. Inducible acute manipulations
A common feature of this approach is that they allow rapid (milliseconds to minutes) and controlled manipulation of specific PI pools within cells. They are based on the exogenous expression of PI-metabolizing enzymes whose activity can be triggered by changes in membrane potential (electrical genetic), or engineered in such a way that the addition of a small compound (chemical genetic) or illumination (optogenetic) can trigger their activity (see Fig. 3).
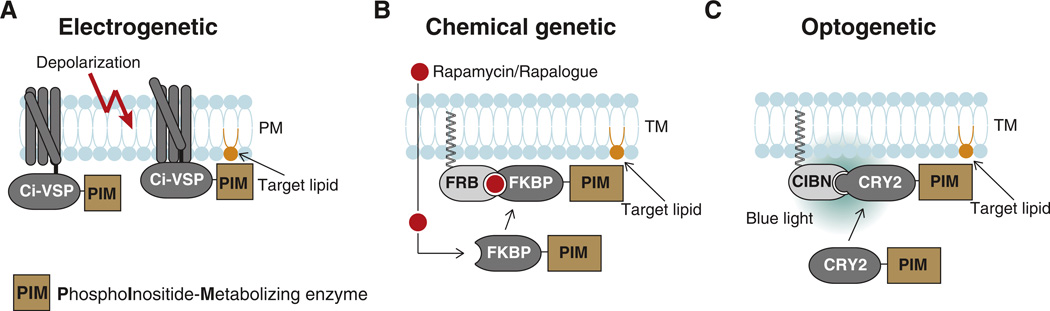
Fig. 3.
Genetic tools used to acutely manipulate PI levels in live cells. A. Electrogenetic manipulation is based on the ectopic expression of a voltage-sensing domain (VSP) fused to a PI-metabolizing enzyme (PIM). This fusion protein is inserted into the plasma membrane (PM) and enzymatic activity is stimulated by plasma membrane depolarization. B. Chemical genetic manipulation requires the expression of FRB anchored to a target membrane (TM) and FKBP12 fused to a PI-metabolizing enzyme (PIM) or vice versa. Addition of rapamycin (or a rapalog) promotes FRB-FKBP dimerization and brings the enzyme to the target membrane where it will act on its PI(s) targets. C. Optogenetic manipulation is based on blue light-induced dimerization between a membrane-anchored protein (CIBN) and its binding partner (CRY2) fused to a PI-metabolizing enzyme (PIM). PhyB and PIF6 can be used as alternative light-sensitive dimerization pairs.
3.4.1. Electrogenetic manipulations
Voltage-dependent control of PIs has been achieved by the heterologous expression of the plasma membrane localized and voltage sensitive 5-phosphatase from the sea squirt Ciona intestinalis (Ci-VSP). This is a protein comprising a transmembrane portion (the voltage sensor), coupled to the phosphatase module. Changes in the membrane potential from the negative resting voltage to slightly positive voltages causes a conformational change that allows the 5-phosphatase domain to access its lipid substrates, PI(4,5)P2 and PI(3,4,5)P3 [95,96] (Fig. 3). Intriguingly, a recent paper proposes that increasing the voltage even further induces a shift in substrate specificity with a preference for the 3-phosphate of PI(3,4)P2 [97]. This method has powerful applications for the study of ion channels. For example, depolarization of Ci-VSP expressing cells results in rapid loss of plasma membrane PI(4,5)P2 with resulting closure of neuronal KCNQ2/3 channels [98]. Recently, a genetically engineered Ci-VSP where the native phosphatase domain was replaced by that of PTEN was generated and used to selectively de-phosphorylate plasma membrane PI(3,4,5)P3 [99]. A limitation of this approach, besides the substrate selectivity of Ci-VSP, is that it can only be used to manipulate PIs in the plasma membrane, where Ci-VSP is localized and where its activity can be acutely manipulated by a change in voltage.
3.4.2. Chemical-genetic manipulations
These methods are based on the inducible acute translocation of PI metabolizing enzymes, or their catalytic modules, from the cytosol to specific membranes, thus drastically increasing their action at these membranes. The first developed and best-characterized system for inducible translocation is based on the chemically induced hetero-dimerization of FKBP12 and the FRB domain of mTOR [100]. Heterologous expression of fusion proteins consisting of the enzyme of interest fused to FRB and a membrane-targeting sequence fused to FKBP12 (or vice versa) allows translocation of the enzyme to the target membrane upon addition of rapamycin or analogs of this drug (rapalogs) (Fig. 3). Complex formation occurs within a minute and is irreversible. This approach has been successfully applied to the study of different PIs in different membranes. The first example was the recruitment of 5-phosphatase modules (from INPP5E and from Inp54p) to deplete PI(4,5)P2 at the plasma membrane with resulting block of receptor internalization, clathrin-mediated endocytosis and K+ channel (KCNQ) permeability [101–103]. In similar experiments, recruitment of PI4P 5-kinase to the plasma membrane to elevate PI(4,5)P2 levels showed roles of this lipid in actin nucleation [39]. Recruitment of the endogenous p110α subunits of PI3-kinase using its p85 regulatory subunit fused to FKBP12 as bait has been used to elucidate the mechanisms by which PI(3,4,5)P3 controls membrane identity [104] whereas the recruitment of MTM1 to deplete PI3P on early endosomes demonstrated the importance of this lipid for the normal progression of cargo through the endo-lysosomal system [105,106]. Using this inducible strategy to recruit the 4-phosphatase domain from Sac1, it was also recently demonstrated that Golgi PI4P is required for exit of cargo from this organelle [107] and that plasma membrane PI4P act as a regulator of ion channels [49,108].
As is obvious from the large number of studies that have already utilized the FKBP12-FRB heterodimerization system, this technique has many appealing features. It has the advantage of modularity, where the use of different enzyme domains and membrane targeting sequences will allow control of different PIs on different membranes. The recent development of photolysable rapamycin also allows spatial control of dimerization with subcellular precision [109]. As with all techniques, it also has some drawbacks. For example, dimerization requires the addition of a compound (rapamycin or rapalog) in the medium, which complicates in vivo experiments, although this technique was successfully used almost 20 years ago to control gene transcription from genetically engineered cells transplanted to mice [110] and more recently used to control PI(3,4,5)P3 levels in live Drosophila larvae [18]. Moreover, the use of rapamycin may interfere with the PI 3-kinase pathway through inhibition of mTOR, which may affect interpretations of the effect of changes in PI levels [66]. This obstacle can be overcome by the use of rapalogs that can induce dimerization without activating endogenous enzymes. Another problem is that the ternary complex formed upon rapamycin addition is irreversible, thus precluding multiple rounds of PI perturbation in the same biological sample. This obstacle was addressed in a recent study, where the authors were able to reverse dimerization by the addition of a competing ligand [111].
3.4.3. Optogenetic manipulations
Recently, tools for light-induced dimerization were developed and also adapted to the manipulation of PIs [112,113]. The principle is the same as for the chemical-genetic dimerization system, but the interaction is instead reversibly controlled by light of specific wavelengths. Two main systems have been used: CRY2-CIBN and PhyB-PIF6 [114,115]. The CRY2-CIBN interaction is induced by brief (ms to s) blue-light illumination and is spontaneously reversible (within minutes) upon interruption of illumination [114] (Fig. 4A, B). Fusion of a 5-phosphatase domain or a PI3-kinase module to CRY2 and a membrane targeting sequence to CIBN, followed by expression of these tools in cells, allowed optic control of PI(4,5)P2 and PI(3,4,5)P3 [112] (Fig. 4). Changes in lipid levels were observed seconds after illumination and focal illumination allowed control of PI levels within specific subcellular compartments. Such local changes in lipid levels were found to control cell polarity, where local loss of PI(4,5)P2 led to cell retraction, whereas local PI(3,4,5)P3 synthesis drove actin polymerization and formation of cellular protrusions with an impact on directed migration (see Fig. 4D,E). Optogenetic depletion of PI(4,5)P2 was also used to identify this lipid as the plasma membrane receptor for the ER-anchored Extended-Synaptotagmins (E-Syts). The E-Syts bind the plasma membrane in trans and thus expand ER-plasma membrane appositions. Loss of PI(4,5)P2 resulted in dissociation of the E-Syts from the plasma membrane with accompanying reduction of plasma membrane-ER contacts [116].
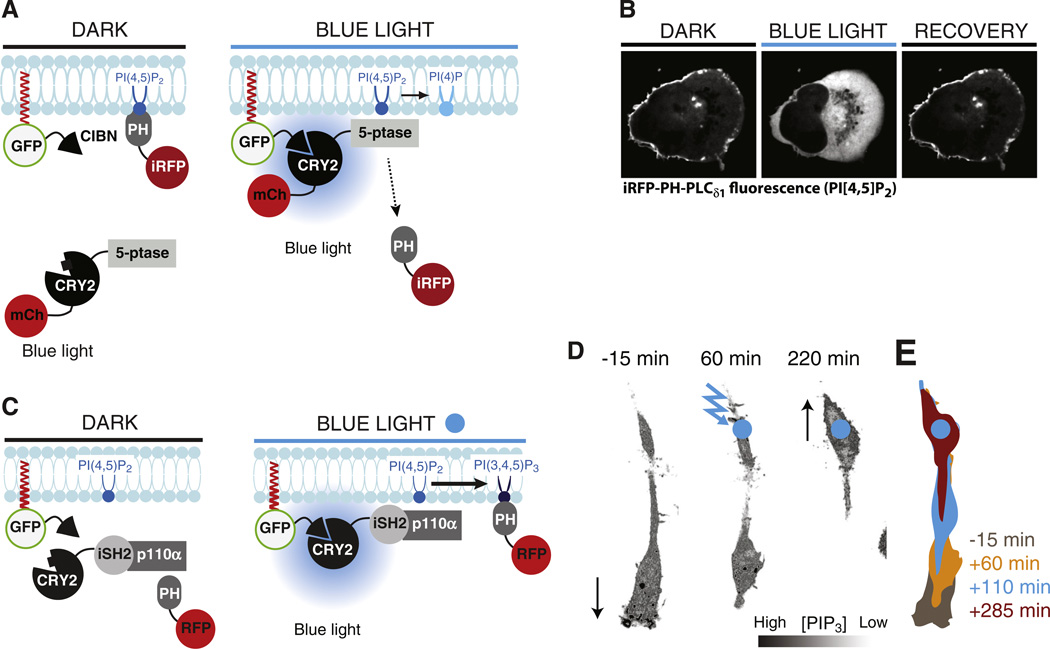
Fig. 4.
Manipulation of PIs by the blue-light-induced CRY2-CIBN heterodimerization system. A. Schematic drawing showing the principle underlying blue-light-induced PI(4,5)P2 dephosphorylation. CIBN is targeted to the plasma membrane while CRY2 is fused to a 5-phosphatase (5-ptase) domain and carries an mCherry-tag (mCh) for microscopic detection. mCh-CRY2-5-ptase is cytosolic under dark conditions but binds CIBN, and thus is recruited at the target membrane, upon blue-light illumination. This translocation results in dephosphorylation of PI(4,5)P2 and this reaction can be detected as loss of iRFP-PH-PLCδ1 [a PI(4,5)P2 binding module] from the plasma membrane. B. Confocal microscopy images of a HeLa cell showing loss of iRFP-PH-PLCδ1 from the plasma membrane upon blue-light-induced recruitment of mCh-CRY2-5-ptase to this cellular compartment. Pictures were taken 10 s before, 10 s after and 15 min after a 200 ms pulse of blue-light illumination [112]. C. Schematic drawing showing the principle underlying blue-light-induced PI(3,4,5)P3 synthesis via the recruitment of the endogenous p110α subunit of type I PI 3-kinase to a CRY2 fusion of the iSH2-region of its regulatory subunit p85 [101,112]. PI(3,4,5)P3 is detected by the RFP-tagged PH-domain from Akt. D. Confocal microscopy images of a migrating RAW264.7 macrophage expressing the fusion proteins shown in C. Continuous focal illumination (blue circle) results in local PI(3,4,5)P3 synthesis and reverses the direction of cell movement occurring before illumination by inducing local ruffling (arrows). E. Illustrations showing the changes in cell shape and position induced by local PI(3,4,5)P3 synthesis.
The PhyB-PIF6 modules have also been used to locally control the synthesis of PI(3,4,5)P3 [113]. The interaction of these two modules is stimulated by red light and their dissociation is accelerated by far-red light but requires a cofactor in mammalian cells [115]. This system could prove to be particularly useful since it allows clamping of a given PI at a specific concentration using feedback control of the two light inputs, thus potentially avoiding compensatory or non-specific reactions from occurring [113,117].
A third light-regulated system, based on the light-oxygen-voltage (LOV) domain from A. sativa, also exist and has been used to spatially control e.g. MAP-kinase signaling [118]. Although not yet used to manipulate PIs, these domains are attractive modules due to their relatively small size (approximately 13 kDa), blue-light activation and reversibility.
As in the case of methods that rely on chemically induced dimerization (see above), all three optogenetic systems have the advantage of modularity, where a PI-metabolizing enzyme of choice can be targeted to any cellular membrane of interest. An alternative way to achieve optogenetic control of PI levels is via the use of engineered light-sensitive receptors. Chimeras of GPCRs and opsins have been generated which activate endogenous enzymes in response to light of specific wavelengths. This approach has been used to show that local PI(3,4,5)P3 synthesis can induce neurite initiation and extension and also to steer migrating macrophages [119,120]. In another receptor-based approach, control of PI3-kinase was obtained using blue-light induced homo-dimerization of CRY2 coupled to the fibroblast growth factor receptor [121]. An advantage with these receptor-based techniques, relative to the optically-induced membrane recruitment of an enzyme, is that they do not involve the overexpression of PI metabolizing enzymes, which may exhibit some catalytic activity towards PIs even in the absence of activating illumination. On the other hand, they involve triggering of endogenous signaling pathways that may affect cellular processes other than those directly regulated by PIs. Additionally, they are restricted to actions at the plasma membrane, thus precluding manipulation of PIs in other cellular compartments.
Advantages of optogenetic methods include speed, reversibility, potential for subcellular precision and non-invasiveness. A problem with these techniques is that they limit the number of fluorescent proteins that can be used to report PI levels, or the cellular effects of a change in their levels, since a portion of the visual spectrum must be dedicated to the activation of the light-regulated modules. All these optogenetic techniques, due to their non-invasiveness, are well suited for in vivo experiments. The impressive success of techniques for the optogenetic manipulation of neuronal excitability speaks to the feasibility of optical manipulation in vivo [122]. A recent study expressed the CRY2-CIBN-based system in live C. elegans to show an involvement of PI3-kinase in behavioral learning [123].
4. Future developments
The last decade has seen an explosion in techniques for both the detection and the manipulation of PIs (see Fig. 5 for a summary). Studies that have capitalized on these developments have greatly enhanced our understanding of the signaling role of these lipids in cell physiology and the impact of their dysfunction in disease. Still, there is a need for tool refinement, new tool designs and other methodological advancements.
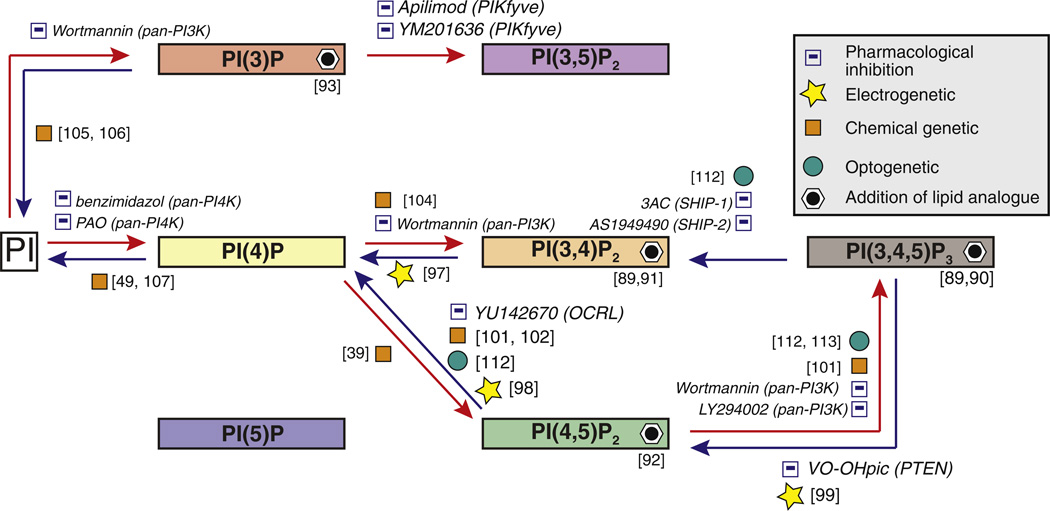
Fig. 5.
Strategies for the acute manipulation of the PI network. Summary of the tools that have already been used for the acute manipulation of PIs. These tools include pharmacological inhibitors, lipid analogs as well as genetically encoded proteins and protein modules that allow PI perturbation in specific membranes by voltage, chemical compounds or light. The modular nature of the chemical genetic and optogenetic methods make them broadly applicable to all PI metabolizing reactions. Strategies aimed at long-term manipulation of PIs, such as transient knockdown or knockout of PI-metabolizing, are not shown.
On the detection side, an important issue to be addressed for a precise interpretation of the subcellular localization of PI binding modules, is the identification of mechanisms explaining their preferential labeling of specific pools of a given PI. While the occurrence of membrane co-receptors besides the PI itself has been hypothesized, only in a few cases (e.g. [124]) has such co-receptor been identified. Additionally, protein modules that recognize reliably PI(5)P and PI(3,5)P2 are still missing. Model predictions, like those used to identify novel PI(3,4,5)P3 interacting proteins, could help design such modules [125]. The expansion of the fluorescent protein palette available will improve the possibility to monitor simultaneously different PIs and to combine detection and optical manipulation of different PIs. Advances in super-resolution video microscopy will increase the spatial and temporal resolution of these analyses. Finally, further developments of mass spectrometry methods will make possible detailed profiling of the full PI spectrum even when the amount of material is very limited and will allow this approach to be applied beyond highly specialized labs.
Concerning PI manipulation, genetic techniques are rapidly advancing and the recent introduction of CRISPR/Cas9 methodology [126] for gene editing promises to have a revolutionary impact. Beyond facilitating gene KO studies (including simultaneous multiple gene KOs), this methodology facilitates the generation of tagged proteins expressed by their endogenous loci and thus expressed at physiological levels. Improved and new drugs for the control specific enzymes need to be developed, in particular compounds that affect enzymes of medical relevance. Despite decades of research, only a few drugs are available for experimental use and fewer still are in clinical trials despite their therapeutic potential [66,71]. Given the rapid advances of structural studies, the design of pharmacological modulators based on crystal structures and molecular dynamics simulations are helping in overcoming this obstacle [66]. In spite of the caveats discussed in Section 3.3, new PI analogs that can be added to the medium and penetrate cells will be powerful tools for specific applications. Finally, a particularly promising technique, open to major further developments, is optogenetics [112,113], a technique which is also suitable for in vivo studies [123]. New light sensitive heterodimerization pairs will expand the range of the visible light spectrum compatible with these methods, while advances in super-resolution video-microscopy will improve detection of physiological effects. Optogenetic tools could also be combined with chemically inducible systems to enable simultaneous control of two signaling pathways by combined illumination and addition of rapamycin. Such multiplex manipulations, in combination with simultaneous detection of multiple PIs, would open new doors for studying cross talk between signaling pathways.
Footnotes
This article is part of a Special Issue entitled Phosphoinositides.
References
- 1.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 3.McCrea HJ, De Camilli P. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology. 2009;24:8–16. doi: 10.1152/physiol.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakelam MJ. The uses and limitations of the analysis of cellular phosphoinositides by lipidomic and imaging methodologies. Biochim. Biophys. Acta. 2014 doi: 10.1016/j.bbalip.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Varnai P, Balla T. Live cell imaging of phosphoinositide dynamics with fluorescent protein domains. Biochim. Biophys. Acta. 2006;1761:957–967. doi: 10.1016/j.bbalip.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Rusten TE, Stenmark H. Analyzing phosphoinositides and their interacting proteins. Nat. Methods. 2006;3:251–258. doi: 10.1038/nmeth867. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Shanta SR, Zhou LH, Kim KP. Mass spectrometry based cellular phosphoinositides profiling and phospholipid analysis: a brief review. Exp. Mol. Med. 2010;42:1–11. doi: 10.3858/emm.2010.42.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurley JH, Meyer T. Subcellular targeting by membrane lipids. Curr. Opin. Cell Biol. 2001;13:146–152. doi: 10.1016/s0955-0674(00)00191-5. [DOI] [PubMed] [Google Scholar]
- 9.Voronov SV, Frere SG, Giovedi S, Pollina EA, Borel C, Zhang H, Schmidt C, Akeson EC, Wenk MR, Cimasoni L, Arancio O, Davisson MT, Antonarakis SE, Gardiner K, De Camilli P, Di Paolo G. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down’s syndrome. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9415–9420. doi: 10.1073/pnas.0803756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasuhoglu C, Feng S, Mao J, Yamamoto M, Yin HL, Earnest S, Barylko B, Albanesi JP, Hilgemann DW. Nonradioactive analysis of phosphatidylinositides and other anionic phospholipids by anion-exchange high-performance liquid chromatography with suppressed conductivity detection. Anal. Biochem. 2002;301:243–254. doi: 10.1006/abio.2001.5489. [DOI] [PubMed] [Google Scholar]
- 11.Milne SB, Ivanova PT, DeCamp D, Hsueh RC, Brown HA. A targeted mass spectrometric analysis of phosphatidylinositol phosphate species. J. Lipid Res. 2005;46:1796–1802. doi: 10.1194/jlr.D500010-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Wenk MR, Lucast L, Di Paolo G, Romanelli AJ, Suchy SF, Nussbaum RL, Cline GW, Shulman GI, McMurray W, De Camilli P. Phosphoinositide profiling in complex lipid mixtures using electrospray ionization mass spectrometry. Nat. Biotechnol. 2003;21:813–817. doi: 10.1038/nbt837. [DOI] [PubMed] [Google Scholar]
- 13.Kraft ML, Klitzing HA. Imaging lipids with secondary ion mass spectrometry. Biochim. Biophys. Acta. 2014 doi: 10.1016/j.bbalip.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat. Rev. Mol. Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- 15.Toomre D, Bewersdorf J. A new wave of cellular imaging. Annu. Rev. Cell Dev. Biol. 2010;26:285–314. doi: 10.1146/annurev-cellbio-100109-104048. [DOI] [PubMed] [Google Scholar]
- 16.Honigmann A, van den Bogaart G, Iraheta E, Risselada HJ, Milovanovic D, Mueller V, Mullar S, Diederichsen U, Fasshauer D, Grubmuller H, Hell SW, Eggeling C, Kuhnel K, Jahn R. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat. Struct. Mol. Biol. 2013;20:679–686. doi: 10.1038/nsmb.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, Dier M, Hell SW, Grubmuller H, Diederichsen U, Jahn R. Membrane protein sequestering by ionic protein-lipid interactions. Nature. 2011;479:552–555. doi: 10.1038/nature10545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khuong TM, Habets RL, Kuenen S, Witkowska A, Kasprowicz J, Swerts J, Jahn R, van den Bogaart G, Verstreken P. Synaptic PI(3,4,5)P3 is required for Syntaxin1A clustering and neurotransmitter release. Neuron. 2013;77:1097–1108. doi: 10.1016/j.neuron.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 19.Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T. A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9256–9261. doi: 10.1073/pnas.0900216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patki V, Lawe DC, Corvera S, Virbasius JV, Chawla A. A functional PtdIns(3)P-binding motif. Nature. 1998;394:433–434. doi: 10.1038/28771. [DOI] [PubMed] [Google Scholar]
- 22.Gaullier JM, Simonsen A, D’Arrigo A, Bremnes B, Stenmark H, Aasland R. FYVE fingers bind PtdIns(3)P. Nature. 1998;394:432–433. doi: 10.1038/28767. [DOI] [PubMed] [Google Scholar]
- 23.Burd CG, Emr SD. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell. 1998;2:157–162. doi: 10.1016/s1097-2765(00)80125-2. [DOI] [PubMed] [Google Scholar]
- 24.Levine TP, Munro S. The pleckstrin homology domain of oxysterol-binding protein recognises a determinant specific to Golgi membranes. Curr. Biol. 1998;8:729–739. doi: 10.1016/s0960-9822(98)70296-9. [DOI] [PubMed] [Google Scholar]
- 25.Dippold HC, Ng MM, Farber-Katz SE, Lee SK, Kerr ML, Peterman MC, Sim R, Wiharto PA, Galbraith KA, Madhavarapu S, Fuchs GJ, Meerloo T, Farquhar MG, Zhou H, Field SJ. GOLPH3 bridges phosphatidylinositol-4-phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139:337–351. doi: 10.1016/j.cell.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood CS, Schmitz KR, Bessman NJ, Setty TG, Ferguson KM, Burd CG. PtdIns4P recognition by Vps74/GOLPH3 links PtdIns 4-kinase signaling to retrograde Golgi trafficking. J. Cell Biol. 2009;187:967–975. doi: 10.1083/jcb.200909063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gozani O, Karuman P, Jones DR, Ivanov D, Cha J, Lugovskoy AA, Baird CL, Zhu H, Field SJ, Lessnick SL, Villasenor J, Mehrotra B, Chen J, Rao VR, Brugge JS, Ferguson CG, Payrastre B, Myszka DG, Cantley LC, Wagner G, Divecha N, Prestwich GD, Yuan J. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell. 2003;114:99–111. doi: 10.1016/s0092-8674(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 28.Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr. Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 29.Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L. G-protein signaling through tubby proteins. Science. 2001;292:2041–2050. doi: 10.1126/science.1061233. [DOI] [PubMed] [Google Scholar]
- 30.Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowler S, Currie RA, Campbell DG, Deak M, Kular G, Downes CP, Alessi DR. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 2000;351:19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karathanassis D, Stahelin RV, Bravo J, Perisic O, Pacold CM, Cho W, Williams RL. Binding of the PX domain of p47(phox) to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 2002;21:5057–5068. doi: 10.1093/emboj/cdf519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dove SK, Piper RC, McEwen RK, Yu JW, King MC, Hughes DC, Thuring J, Holmes AB, Cooke FT, Michell RH, Parker PJ, Lemmon MA. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 2004;23:1922–1933. doi: 10.1038/sj.emboj.7600203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bridges D, Ma JT, Park S, Inoki K, Weisman LS, Saltiel AR. Phos-phatidylinositol 3,5-bisphosphate plays a role in the activation and subcellular localization of mechanistic target of rapamycin 1. Mol. Biol. Cell. 2012;23:2955–2962. doi: 10.1091/mbc.E11-12-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-tris-phosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 36.Klarlund JK, Rameh LE, Cantley LC, Buxton JM, Holik JJ, Sakelis C, Patki V, Corvera S, Czech MP. Regulation of GRP1-catalyzed ADP ribosylation factor guanine nucleotide exchange by phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:1859–1862. doi: 10.1074/jbc.273.4.1859. [DOI] [PubMed] [Google Scholar]
- 37.Varnai P, Rother KI, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton’s tyrosine kinase pleckstrin homology domain visualized in single living cells. J. Biol. Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]
- 38.Oatey PB, Venkateswarlu K, Williams AG, Fletcher LM, Foulstone EJ, Cullen PJ, Tavare JM. Confocal imaging of the subcellular distribution of phosphatidylinositol 3,4,5-trisphosphate in insulin- and PDGF-stimulated 3T3-L1 adipocytes. Biochem. J. 1999;344(Pt 2):511–518. [PMC free article] [PubMed] [Google Scholar]
- 39.Ueno T, Falkenburger BH, Pohlmeyer C, Inoue T. Triggering actin comets versus membrane ruffles: distinctive effects of phosphoinositides on actin reorganization. Sci. Signal. 2011;4:ra87. doi: 10.1126/scisignal.2002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine TP, Munro S. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 2002;12:695–704. doi: 10.1016/s0960-9822(02)00779-0. [DOI] [PubMed] [Google Scholar]
- 41.Roy A, Levine TP. Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. J. Biol. Chem. 2004;279:44683–44689. doi: 10.1074/jbc.M401583200. [DOI] [PubMed] [Google Scholar]
- 42.Gray A, Van Der Kaay J, Downes CP. The pleckstrin homology domains of protein kinase B and GRP1 (general receptor for phosphoinositides-1) are sensitive and selective probes for the cellular detection of phosphatidylinositol 3,4-bisphosphate and/or phosphatidylinositol 3,4,5-trisphosphate in vivo. Biochem. J. 1999;344(Pt 3):929–936. [PMC free article] [PubMed] [Google Scholar]
- 43.Balla T, Varnai P. Visualization of cellular phosphoinositide pools with GFP-fused protein-domains. In: Bonifacino Juan S, et al., editors. Current Protocols in Cell Biology. 2009. (Chapter 24, Unit 24 24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wuttke A, Idevall-Hagren O, Tengholm A. Imaging phosphoinositide dynamics in living cells. Methods Mol. Biol. 2010;645:219–235. doi: 10.1007/978-1-60327-175-2_14. [DOI] [PubMed] [Google Scholar]
- 45.Hertel F, Switalski A, Mintert-Jancke E, Karavassilidou K, Bender K, Pott L, Kienitz MC. A genetically encoded tool kit for manipulating and monitoring membrane phosphatidylinositol 4,5-bisphosphate in intact cells. PLoS One. 2011;6:e20855. doi: 10.1371/journal.pone.0020855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato M, Ueda Y, Takagi T, Umezawa Y. Productionof PtdInsP3atendomembranes is triggered by receptor endocytosis. Nat. Cell Biol. 2003;5:1016–1022. doi: 10.1038/ncb1054. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Nasu Y, Hattori M, Yoshimura H, Kanno A, Ozawa T. Bioluminescent probes to analyze ligand-induced phosphatidylinositol 3,4,5-trisphosphate production with split luciferase complementation. Anal. Chem. 2013;85:11352–11359. doi: 10.1021/ac402278f. [DOI] [PubMed] [Google Scholar]
- 48.Lindenburg L, Merkx M. Engineering genetically encoded FRET sensors. Sensors. 2014;14:11691–11713. doi: 10.3390/s140711691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science. 2012;337:727–730. doi: 10.1126/science.1222483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammond GR, Schiavo G, Irvine RF. Immunocytochemical techniques reveal multiple distinct cellular pools of PtdIns4P and PtdIns(4,5)P(2) Biochem. J. 2009;422:23–35. doi: 10.1042/BJ20090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- 52.Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 53.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 54.Vaccari I, Dina G, Tronchere H, Kaufman E, Chicanne G, Cerri F, Wrabetz L, Payrastre B, Quattrini A, Weisman LS, Meisler MH, Bolino A. Genetic interaction between MTMR2 and FIG4 phospholipid phosphatases involved in Charcot- Marie-Tooth neuropathies. PLoS Genet. 2011;7:e1002319. doi: 10.1371/journal.pgen.1002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang YJ, Li WH, Wang J, Xu K, Dong P, Luo X, Yin HL. Critical role of PIP5KI {gamma}87 in InsP3-mediated Ca(2+) signaling. J. Cell Biol. 2004;167:1005–1010. doi: 10.1083/jcb.200408008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milosevic I, Sorensen JB, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J. Neurosci. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tengholm A. T. Meyer, A PI3-kinase signaling code for insulin-triggered insertion of glucose transporters into the plasma membrane. Curr. Biol. 2002;12:1871–1876. doi: 10.1016/s0960-9822(02)01223-x. [DOI] [PubMed] [Google Scholar]
- 58.Nandez R, Balkin DM, Messa M, Liang L, Paradise S, Czapla H, Hein MY, Duncan JS, Mann M, De Camilli P. A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of Lowe syndrome cells. eLife. 2014:e02975. doi: 10.7554/eLife.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J. 1993;296(Pt 2):297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 61.Devereaux K, Dall’Armi C, Alcazar-Roman A, Ogasawara Y, Zhou X, Wang F, Yamamoto A, De Camilli P, Di Paolo G. Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PLoS One. 2013;8:e76405. doi: 10.1371/journal.pone.0076405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 63.Meyers R, Cantley LC. Cloning and characterization of a wortmannin-sensitive human phosphatidylinositol 4-kinase. J. Biol. Chem. 1997;272:4384–4390. doi: 10.1074/jbc.272.7.4384. [DOI] [PubMed] [Google Scholar]
- 64.Balla T, Downing GJ, Jaffe H, Kim S, Zolyomi A, Catt KJ. Isolation and molecular cloning of wortmannin-sensitive bovine type III phosphatidylinositol 4-kinases. J. Biol. Chem. 1997;272:18358–18366. doi: 10.1074/jbc.272.29.18358. [DOI] [PubMed] [Google Scholar]
- 65.Downing GJ, Kim S, Nakanishi S, Catt KJ, Balla T. Characterization of a soluble adrenal phosphatidylinositol 4-kinase reveals wortmannin sensitivity of type III phosphatidylinositol kinases. Biochemistry. 1996;35:3587–3594. doi: 10.1021/bi9517493. [DOI] [PubMed] [Google Scholar]
- 66.Fruman DA, Rommel C. PI3K, cancer: lessons challenges and opportunities. Nat. Rev. Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, Fracasso PR, Wilkie DP, Hentemann M, Wilhelm SM, Scott WJ, Mumberg D, Ziegelbauer K. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol. Cancer Ther. 2013;12:2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 68.Furet P, Guagnano V, Fairhurst RA, Imbach-Weese P, Bruce I, Knapp M, Fritsch C, Blasco F, Blanz J, Aichholz R, Hamon J, Fabbro D, Caravatti G. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg. Med. Chem. Lett. 2013;23:3741–3748. doi: 10.1016/j.bmcl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Macias-Perez IM, Flinn IW. GS-1101: a delta-specific PI3K inhibitor in chronic lymphocytic leukemia. Curr. Hematol Malig Rep. 2013;8:22–27. doi: 10.1007/s11899-012-0142-1. [DOI] [PubMed] [Google Scholar]
- 70.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A, Blum KA, Goy AH, Davies AJ, Zinzani PL, Dreyling M, Johnson D, Miller LL, Holes L, Li D, Dansey RD, Godfrey WR, Salles GA. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frazzetto M, Suphioglu C, Zhu J, Schmidt-Kittler O, Jennings IG, Cranmer SL, Jackson SP, Kinzler KW, Vogelstein B, Thompson PE. Dissecting isoform selectivity of PI3K inhibitors: the role of non-conserved residues in the catalytic pocket. Biochem. J. 2008;414:383–390. doi: 10.1042/BJ20080512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pomel V, Klicic J, Covini D, Church DD, Shaw JP, Roulin K, Burgat-Charvillon F, Valognes D, Camps M, Chabert C, Gillieron C, Francon B, Perrin D, Leroy D, Gretener D, Nichols A, Vitte PA, Carboni S, Rommel C, Schwarz MK, Ruckle T. Furan-2-ylmethylene thiazolidinediones as novel potent and selective inhibitors of phosphoinositide 3-kinase gamma. J. Med. Chem. 2006;49:3857–3871. doi: 10.1021/jm0601598. [DOI] [PubMed] [Google Scholar]
- 73.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim. Biophys. Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Mak LH, Vilar R, Woscholski R. Characterisation of the PTEN inhibitor VO-OHpic. J. Chem. Biol. 2010;3:157–163. doi: 10.1007/s12154-010-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G, Rosivatz E, Woscholski R, Cognetti F, Scher HI, Pandolfi PP. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J. Clin. Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voss MD, Czechtizky W, Li Z, Rudolph C, Petry S, Brummerhop H, Langer T, Schiffer A, Schaefer HL. Discovery pharmacological characterization of a novel small molecule inhibitor of phosphatidylinositol-5-phosphate 4-kinase, type II beta. Biochem. Biophys. Res. Commun. 2014;449:327–331. doi: 10.1016/j.bbrc.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 77.Blunt MD, Ward SG. Targeting PI3K isoforms and SHIP in the immune system: new therapeutics for inflammation and leukemia. Curr. Opin. Pharmacol. 2012;12:444–451. doi: 10.1016/j.coph.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 78.Pirruccello M, Nandez R, Idevall-Hagren O, Alcazar-Roman A, Abriola L, Berwick SA, Lucast L, Morel D, De Camilli P. Identification of inhibitors of inositol 5-phosphatases through multiple screening strategies. ACS Chem. Biol. 2014 doi: 10.1021/cb500161z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balla A, Tuymetova G, Tsiomenko A, Varnai P, Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol. Biol. Cell. 2005;16:1282–1295. doi: 10.1091/mbc.E04-07-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakatsu F, Baskin JM, Chung J, Tanner LB, Shui G, Lee SY, Pirruccello M, Hao M, Ingolia NT, Wenk MR, De Camilli P. PtdIns4P synthesis by PI4KIIIαlpha at the plasma membrane and its impact on plasma membrane identity. J. Cell Biol. 2012;199:1003–1016. doi: 10.1083/jcb.201206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bojjireddy N, Botyanszki J, Hammond G, Creech D, Peterson R, Kemp DC, Snead M, Brown R, Morrison A, Wilson S, Harrison S, Moore C, Balla T. Pharmacological and genetic targeting of the PI4KA enzyme reveals its important role in maintaining plasma membrane phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate levels. J. Biol. Chem. 2014;289:6120–6132. doi: 10.1074/jbc.M113.531426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bianco A, Reghellin V, Donnici L, Fenu S, Alvarez R, Baruffa C, Peri F, Pagani M, Abrignani S, Neddermann P, De Francesco R. Metabolism of phosphatidylinositol 4-kinase IIIalpha-dependent PI4P Is subverted by HCV and is targeted by a 4-anilino quinazoline with antiviral activity. PLoS Pathog. 2012;8:e1002576. doi: 10.1371/journal.ppat.1002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Altan-Bonnet N, Balla T. Phosphatidylinositol 4-kinases: hostages harnessed to build panviral replication platforms. Trends Biochem. Sci. 2012;37:293–302. doi: 10.1016/j.tibs.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toth B, Balla A, Ma H, Knight ZA, Shokat KM, Balla T. Phosphatidylinositol 4-kinase IIIbeta regulates the transport of ceramide between the endoplasmic reticulum and Golgi. J. Biol. Chem. 2006;281:36369–36377. doi: 10.1074/jbc.M604935200. [DOI] [PubMed] [Google Scholar]
- 85.Kawasaki T, Takemura N, Standley DM, Akira S, Kawai T. The second messenger phosphatidylinositol-5-phosphate facilitates antiviral innate immune signaling. Cell Host Microbe. 2013;14:148–158. doi: 10.1016/j.chom.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 86.Sbrissa D, Ikonomov OC, Filios C, Delvecchio K, Shisheva A. Functional dissociation between PIKfyve-synthesized PtdIns5P and PtdIns(3,5)P2 by means of the PIKfyve inhibitor YM201636. Am. J. Physiol. Cell Physiol. 2012;303:C436–C446. doi: 10.1152/ajpcell.00105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cai X, Xu Y, Cheung AK, Tomlinson RC, Alcazar-Roman A, Murphy L, Billich A, Zhang B, Feng Y, Klumpp M, Rondeau JM, Fazal AN, Wilson CJ, Myer V, Joberty G, Bouwmeester T, Labow MA, Finan PM, Porter JA, Ploegh HL, Baird D, De Camilli P, Tallarico JA, Huang Q. PIKfyve a class III PI kinase is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling. Chem. Biol. 2013;20:912–921. doi: 10.1016/j.chembiol.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wymann MP, Schultz C. The chemical biology of phosphoinositide 3-kinases. Chembiochem. 2012;13:2022–2035. doi: 10.1002/cbic.201200089. [DOI] [PubMed] [Google Scholar]
- 89.Laketa V, Zarbakhsh S, Morbier E, Subramanian D, Dinkel C, Brumbaugh J, Zimmermann P, Pepperkok R, Schultz C. Membrane-permeant phosphoinositide derivatives as modulators of growth factor signaling and neurite outgrowth. Chem. Biol. 2009;16:1190–1196. doi: 10.1016/j.chembiol.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Laketa V, Zarbakhsh S, Traynor-Kaplan A, Macnamara A, Subramanian D, Putyrski M, Mueller R, Nadler A, Mentel M, Saez-Rodriguez J, Pepperkok R, Schultz C. PIP(3) induces the recycling of receptor tyrosine kinases. Sci. Signal. 2014;7:ra5. doi: 10.1126/scisignal.2004532. [DOI] [PubMed] [Google Scholar]
- 91.Posor Y, Eichhorn-Gruenig M, Puchkov D, Schoneberg J, Ullrich A, Lampe A, Muller R, Zarbakhsh S, Gulluni F, Hirsch E, Krauss M, Schultz C, Schmoranzer J, Noe F, Haucke V. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature. 2013;499:233–237. doi: 10.1038/nature12360. [DOI] [PubMed] [Google Scholar]
- 92.Reversi A, Loeser E, Subramanian D, Schultz C, De Renzis S. Plasma membrane phosphoinositide balance regulates cell shape during Drosophila embryo morphogenesis. J. Cell Biol. 2014;205:395–408. doi: 10.1083/jcb.201309079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Subramanian D, Laketa V, Muller R, Tischer C, Zarbakhsh S, Pepperkok R, Schultz C. Activation of membrane-permeant caged PtdIns(3)P induces endosomal fusion in cells. Nat. Chem. Biol. 2010;6:324–326. doi: 10.1038/nchembio.348. [DOI] [PubMed] [Google Scholar]
- 94.Mentel M, Laketa V, Subramanian D, Gillandt H, Schultz C. Photoactivatable and cell-membrane-permeable phosphatidylinositol 3,4,5-trisphosphate. Angew. Chem. 2011;50:3811–3814. doi: 10.1002/anie.201007796. [DOI] [PubMed] [Google Scholar]
- 95.Villalba-Galea CA. New insights in the activity of voltage sensitive phosphatases. Cell. Signal. 2012;24:1541–1547. doi: 10.1016/j.cellsig.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 96.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 97.Kurokawa T, Takasuga S, Sakata S, Yamaguchi S, Horie S, Homma KJ, Sasaki T, Okamura Y. 3′ Phosphatase activity toward phosphatidylinositol 3,4-bisphosphate [PI(3,4)P2] by voltage-sensing phosphatase (VSP) Proc. Natl. Acad. Sci. U. S. A. 2012;109:10089–10094. doi: 10.1073/pnas.1203799109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Falkenburger BH, Jensen JB, Hille B. Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J. Gen. Physiol. 2010;135:99–114. doi: 10.1085/jgp.200910345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lacroix J, Halaszovich CR, Schreiber DN, Leitner MG, Bezanilla F, Oliver D, Villalba-Galea CA. Controlling the activity of a phosphatase and tensin homolog (PTEN) by membrane potential. J. Biol. Chem. 2011;286:17945–17953. doi: 10.1074/jbc.M110.201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ho SN, Biggar SR, Spencer DM, Schreiber SL, Crabtree GR. Dimeric ligands define a role for transcriptional activation domains in reinitiation. Nature. 1996;382:822–826. doi: 10.1038/382822a0. [DOI] [PubMed] [Google Scholar]
- 101.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5) P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fili N, Calleja V, Woscholski R, Parker PJ, Larijani B. Compartmental signal modulation: endosomal phosphatidylinositol 3-phosphate controls endosome morphology and selective cargo sorting. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15473–15478. doi: 10.1073/pnas.0607040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Szentpetery Z, Varnai P, Balla T. Acute manipulation of Golgi phosphoinositides to assess their importance in cellular trafficking and signaling. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8225–8230. doi: 10.1073/pnas.1000157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kruse M, Hammond GR, Hille B. Regulation of voltage-gated potassium channels by PI(4,5)P2. J. Gen. Physiol. 2012;140:189–205. doi: 10.1085/jgp.201210806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Umeda N, Ueno T, Pohlmeyer C, Nagano T, Inoue T. A photocleavable rapamycin conjugate for spatiotemporal control of small GTPase activity. J. Am. Chem. Soc. 2011;133:12–14. doi: 10.1021/ja108258d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rivera VM, Clackson T, Natesan S, Pollock R, Amara JF, Keenan T, Magari SR, Phillips T, Courage NL, Cerasoli F, Jr, Holt DA, Gilman M. A humanized system for pharmacologic control of gene expression. Nat. Med. 1996;2:1028–1032. doi: 10.1038/nm0996-1028. [DOI] [PubMed] [Google Scholar]
- 111.Feng S, Laketa V, Stein F, Rutkowska A, MacNamara A, Depner S, Klingmuller U, Saez-Rodriguez J, Schultz C. A rapidly reversible chemical dimerizer system to study lipid signaling in living cells. Angew. Chem. 2014;53:6720–6723. doi: 10.1002/anie.201402294. [DOI] [PubMed] [Google Scholar]
- 112.Idevall-Hagren O, Dickson EJ, Hille B, Toomre DK, De Camilli P. Optogenetic control of phosphoinositide metabolism. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2316–E2323. doi: 10.1073/pnas.1211305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toettcher JE, Gong D, Lim WA, Weiner OD. Light-based feedback for controlling intracellular signaling dynamics. Nat. Methods. 2011;8:837–839. doi: 10.1038/nmeth.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods. 2010;7:973–975. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Toettcher JE, Weiner OD, Lim WA. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell. 2013;155:1422–1434. doi: 10.1016/j.cell.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Strickland D, Lin Y, Wagner E, Hope CM, Zayner J, Antoniou C, Sosnick TR, Weiss EL, Glotzer M. TULIPs: tunable light-controlled interacting protein tags for cell biology. Nat. Methods. 2012;9:379–384. doi: 10.1038/nmeth.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karunarathne WK, Giri L, Kalyanaraman V, Gautam N. Optically triggering spatiotemporally confined GPCR activity in a cell and programming neurite initiation and extension. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E1565–E1574. doi: 10.1073/pnas.1220697110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Karunarathne WK, Giri L, Patel AK, Venkatesh KV, Gautam N. Optical control demonstrates switch-like PIP3 dynamics underlying the initiation of immune cell migration. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E1575–E1583. doi: 10.1073/pnas.1220755110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim N, Kim JM, Lee M, Kim CY, Chang KY, Heo WD. Spatiotemporal control of fibroblast growth factor receptor signals by blue light. Chem. Biol. 2014;21:903–912. doi: 10.1016/j.chembiol.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 122.Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ohno H, Kato S, Naito Y, Kunitomo H, Tomioka M, Iino Y. Role of synaptic phosphatidylinositol 3-kinase in a behavioral learning response in C. elegans. Science. 2014;345:313–317. doi: 10.1126/science.1250709. [DOI] [PubMed] [Google Scholar]
- 124.Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat. Cell Biol. 2004;6:393–404. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- 125.Park WS, Heo WD, Whalen JH, O’Rourke NA, Bryan HM, Meyer T, Teruel MN. Comprehensive identification of PIP3-regulated PH domains from C. elegans to H. sapiens by model prediction and live imaging. Mol. Cell. 2008;30:381–392. doi: 10.1016/j.molcel.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]