Abstract
Background
The J tip uses air instead of a needle to push lidocaine into the skin. No studies have investigated its use for venipuncture in young children.
Objective
Determine if J tip decreased venipuncture pain in young children compared to vapocoolant spray.
Methods
Children ages 1 to 6 years were randomized into 3 groups: Intervention – J tip, Control –vapocoolant spray, and Sham –vapocoolant spray and “pop” of an empty J tip. The procedure was videotaped and scored using the FLACC tool at three times; Baseline - before approach, Device - J tip deployment and Venipuncture - venipuncture. The FLACC tool was scored 0(none) to 10(severe). Comparisons of pain scores over time were made using the Generalized Estimating Equation. Venipuncture success and adverse effects were assessed and compared using Χ2.
Results
205 children enrolled; Intervention=96, Control=53, Sham=56. There were no between group differences in baseline characteristics. There was no mean change in pain scores from Device to Venipuncture in the Intervention group (0.25, 95% CI (−0.31, 0.82) but there was an increase in pain in the Control (2.82, 95% CI (1.91, 3.74) and Sham (1.68, 95% CI (0.83, 2.52) groups. This change was greater for the Control and Sham compared to the Intervention group. There was no difference in venipuncture success between groups. No severe adverse events occurred. Minor adverse events were the same between groups.
Conclusion
Use of the J tip for children ages 1 to 6 years reduced venipuncture pain compared to vapocoolant spray or sham treatment.
Introduction
Background
Needle-sticks are a common source of pain for pediatric patients. Untreated pain has been correlated with strong negative responses and greater pain with subsequent needle-sticks.1,2 Additionally, painful medical experiences during childhood are associated with increased adult pain sensitivity and failure to seek health care.1 Efforts to improve pain treatment for children are needed to reduce these unwanted effects.
Venipuncture can cause moderate to severe pain, especially in young children. A study of children aged 3 to 17 years found that 36% of children age 3 to 6 and 13% of children aged 7 to 17 experienced moderate to severe levels of pain during venipuncture. As the youngest children report higher pain, they may benefit the most from interventions to improve their pain experience.3 Recommendations by the American Academy of Pediatrics (AAP) advise minimizing pain during pediatric procedures including venipuncture.4 Evidence-based interventions are needed to reduce the pain experienced by the youngest children during venipuncture.
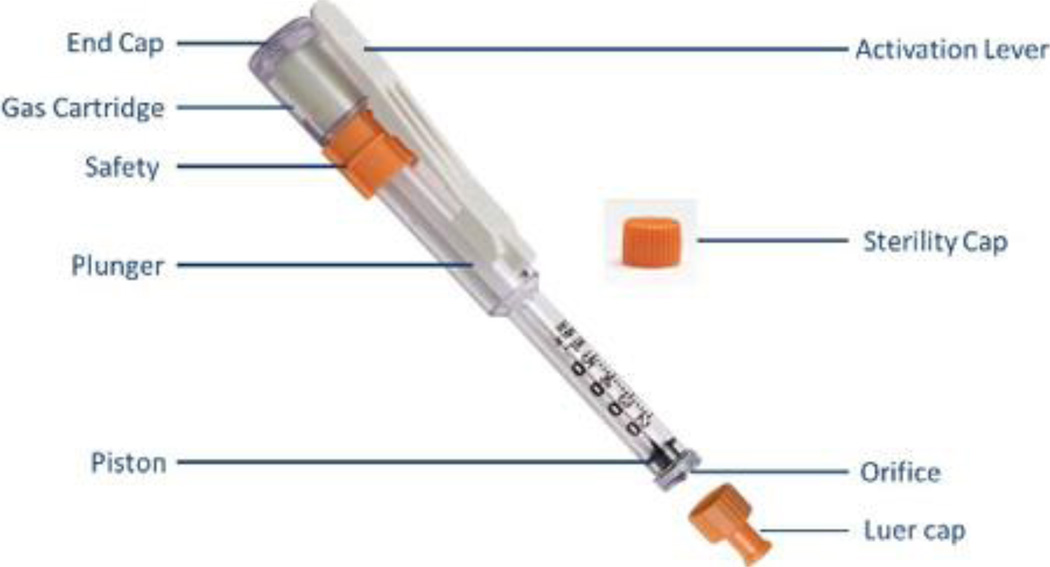
The Needle Free Jet Injection system with buffered lidocaine (J tip) uses air instead of a needle to push lidocaine in to the skin (Figure 1). This provides local anesthetic at the site of administration in less than a minute, making it ideal for pre-venipuncture anesthetic. Prior randomized clinical trials in children ages 8 to 15 years and 7 to 19 years found that the J tip was more effective than lidocaine/prilocaine cream for the treatment of pain during venipuncture for intravenous line (IV) placement.5,6 Use of the J tip device, itself, was also not painful or associated with adverse events for children.6 A randomized clinical trial in children ages 5 to 18 years undergoing IV insertion or venipuncture for blood draw found the device treated pain more effectively than no treatment, but found no difference in reported pain if the device injected lidocaine or normal saline.7 These studies were conducted in the emergency department or pre-operative settings.
Figure 1. Photo of J tip Device.
This image was used with permission from National Medical Products, Inc
Importance
There is no published research evaluating the efficacy and safety of the J tip in children younger than 5 years old and only limited data in children 5 to 6 years old.
Goals of This Investigation
The objective of this study was to determine if the J tip decreased venipuncture pain in children ages 1 to 6 years. We hypothesized the J tip would decrease venipuncture pain in young children compared to vapocoolant spray.
Methods
Study Design and Setting
This was a randomized single-dose clinical trial comparing the efficacy of the J tip device to vapocoolant spray for the reduction of venipuncture pain in young children. The children’s pain experience was videotaped and later scored by physicians blinded to the group assignment. The study received IRB approval and was registered with clinicaltrials.gov (NCT01890642). Verbal and written consent were obtained from all parents. This study was conducted from July 1, 2013 – August 8, 2013 in the outpatient laboratory at a tertiary care children’s hospital. A convenience sample of eligible patients was approached for enrollment when the research team was available Monday through Friday between the hours of 9 am and 5 pm.
Selection of Participants
Children ages 1 to 6 years with orders for a blood draw were eligible for the study. Patients were excluded if they were allergic to lidocaine or vapocoolant spray, had a blood or connective tissue disorder that predisposed them to bruising, experienced a previous blood draw the same day, were not having blood draw via venipuncture (children with a central line for blood draws or with finger-stick blood draws), were not accompanied by a parent or legal guardian, the parents or legal guardians were non-English speaking, or children were unable to cry or move their extremities.
After eligibility criteria were confirmed, data on personal characteristics and previous venipuncture experience were gathered from parent report. A random number table was used to assign children at a 2:1:1 ratio to: Intervention, Control and Sham groups. Uneven group numbers were used to give patients a 50% chance of receiving the intervention and a 50% chance of being in the control or sham group. Study group assignment was not revealed to the research team prior to completion of the consent process.
Interventions
The Intervention group received 0.2 ml of 1% buffered lidocaine administered with a J tip followed by a spray of normal saline prior to venipuncture. Children in the Control group received only vapocoolant spray at the site just prior to venipuncture. Vapocoolant is usual care at this institution. The Sham group had an empty J tip deployed near the venipuncture site to create the loud “pop” effect and also received vapocoolant spray at the site just prior to venipuncture. The Sham group included an empty J tip to evaluate if the sound of the J tip was distressing to young children and to assess for placebo effect. The researcher (MML), a pediatric emergency medicine physician, administered each J tip dose, both empty and with lidocaine, as well as the vapocoolant and normal saline sprays. The Control group was approached by the researcher (MML) to “look at their arm” at the time when a J tip was administered in the Sham and Intervention groups to standardize timing.
Methods and Measurements
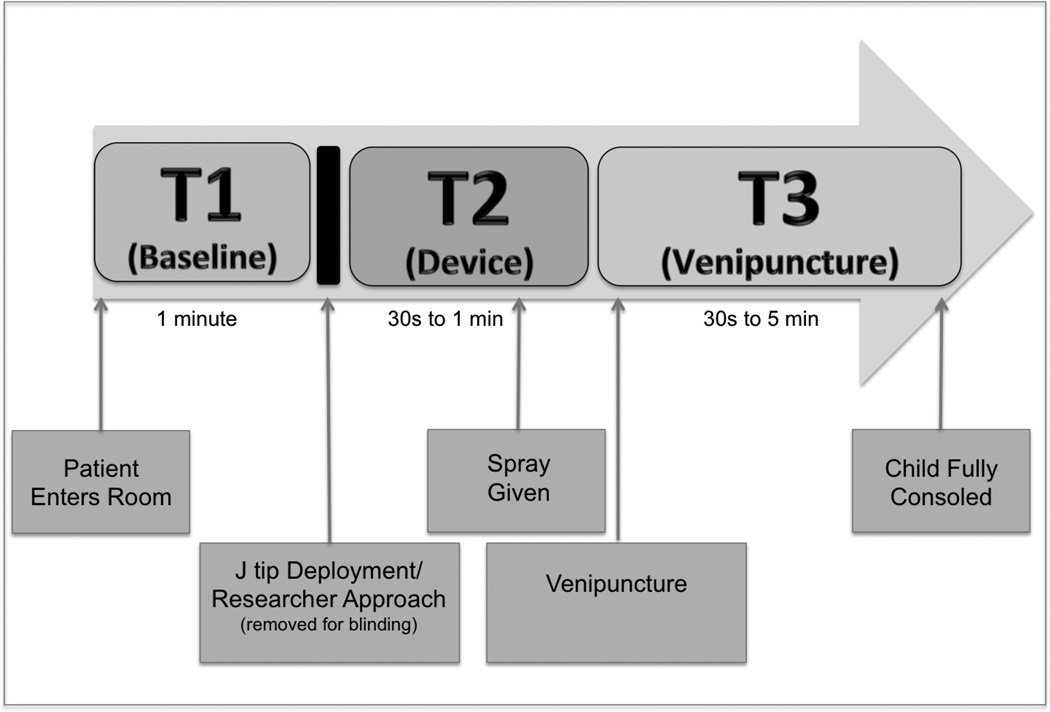
The procedure was videotaped from the time the patient arrived in the room until they consoled after the procedure. Each video was edited by a member of the research team (MML) into 3 separate time frames: Baseline (T1) preceding any intervention while the phlebotomist palpated the vein but prior to any researcher approach [1 minute in duration], Device (T2) following J tip deployment or researcher approach of the child and included vapocoolant spray or normal saline spray at the venipuncture site [30 seconds to 1 minute in duration], and Venipuncture (T3) at the time of needle insertion until child was consoled [30 seconds to 5 minutes in duration]. The administration of the J tip and researcher approach of the child was removed from the videos to preserve blinding (Figure 2).
Figure 2. Study flow.
This depicts the procedure that was videotaped from the time the patient enters the room (left), until the patient was fully consoled (right). The video was then edited to show 3 time frames depicted above: Baseline (T1) – from the time the patient enters the room until right before J tip deployment or researcher report. Device (T2): Immediately after the J tip deployment or Researcher approach until immediately prior to venipuncture, including administration of spray and Venipuncture (T3): At venipuncture until the child was fully consoled. The actual J tip deployment or Researcher approach was removed for blinding.
Children requiring multiple attempts for successful venipuncture only had the first attempt recorded. Children were assessed by a physician (MML) immediately after the procedure for adverse events. Parents were contacted by phone the following day to inquire about adverse events since leaving the lab.
Two pediatric emergency medicine physicians (ALD, MNL) blinded to group assignment reviewed the videos and assigned Face, Legs, Activity, Cry and Consolability (FLACC) pain scores to each time frame. These physicians underwent training and were tested on proper use of the FLACC scale prior to review of the study videos. The videotapes were viewed in a random order to avoid reviewers recognizing or remembering patients. Inter-rater reliability, defined as a difference in scores of 2 or less, was assessed. Videos with a difference in FLACC score between reviewers of more than 2 were re-reviewed by a pediatric anesthesiologist with pain expertise (SW) to assign an additional FLACC score.
Outcomes
Pain Experience
The primary outcome of interest was the child’s pain experience measured by the FLACC pain score assigned by two reviewers blinded to the study group assignment. The FLACC pain scale uses 5 elements to measure pain: Face, Legs, Activity, Cry and Consolability.8 Each element is scored from 0 to 2 and these scores are added to give a total score of 0 to 10. The FLACC pain scale is a validated observation pain measure that is widely used and has been recommended for the evaluation of procedural pain for children ages 1 year and older.9,10
A FLACC score was measured once for each time frame by the two reviewers and averaged, resulting in 3 FLACC scores for each patient. Change in pain score between time frames (T1–T2, T2–T3 and T1–T3) was calculated for each patient. The primary outcome was the change in pain from Device (T2) to Venipuncture (T3). The FLACC score at Venipuncture (T3) was categorized for analysis: 0=no pain, 1–3=mild pain, 4–6=moderate pain and 7–10=severe pain. For classification, FLACC scores were rounded to the nearest whole number.
Inter-rater reliability between the initial two reviewers was 90%. When scores from the third reviewer were averaged in to pain scores of the 10% of patients with discrepancies between reviewers, results did not change. Therefore, all scores used in analysis were the average of the two initial reviewers.
Venipuncture Success
Successful blood draw was defined as obtaining the volume of blood needed to perform tests ordered for the patient. First attempt success was compared among the 3 groups.
Adverse Events
Immediately after venipuncture, the researcher (MML) observed the child and examined the venipuncture site for any adverse events. Severe adverse events were defined as anaphylaxis, difficulty breathing or systemic reaction. Minor adverse events included redness, rash, bruising or itching at the venipuncture site. Families provided a phone number and preferred time of day for follow up contact. Families were called the following day and parents reported if any of these adverse events occurred in the time since they returned home. A total of 3 phone calls per patient were attempted the following day and voice messages were left if possible. After 3 unsuccessful calls, the patient was considered lost to follow up for adverse events.
Analysis
Sample Size Calculation
The primary analysis was powered to demonstrate a three point difference in FLACC scores related to venipuncture: defined as the difference between the FLACC scores at Venipuncture (T3) and Device (T2). A difference of three points is the difference between mild and moderate as well as moderate and severe pain on the scale and therefore was considered clinically significant. To maximize the likelihood of a patient receiving an intervention that may help with pain, uneven group sizes were used so the total number of subjects in the Control and Sham groups was equal to the number in the Intervention group. To maintain an alpha=0.05 and a power of 0.80 for the above difference required 96 patients in the Intervention group, 48 patients in the Control group and 48 patients in the Sham group for a total of 192 patients in a 2:1:1 randomization. To account for a 15% dropout rate an additional 28 patients were included totaling 220 patients planned for enrollment.
This sample size could detect a difference of +/− 7% between the intervention group and the control or sham groups for analysis of adverse events and venipuncture success.
Data Analysis
Descriptive statistics were used to examine patient characteristics. Discrete group characteristics were compared using the X2/Fisher’s exact test and continuous and ordinal variables were compared using the Kruskal-Wallis test with the Mann-Whitney Test for pairwise comparisons. Comparisons of median pain scores at the same time point were made using the Wilxocon rank sum non-parametric tests. Confidence intervals for the medians were estimated using the bootstrap. Comparisons of pain scores over the three time points were made using the Generalized Estimating Equation (GEE) with unstructured covariance matrix. Adverse events, venipuncture success and proportion of patients with no to mild pain were compared using the Χ2/Fisher’s exact test. Analyses were performed using SAS 9.2 software and R 3.0.3 software.
Results
Characteristics of Study subjects
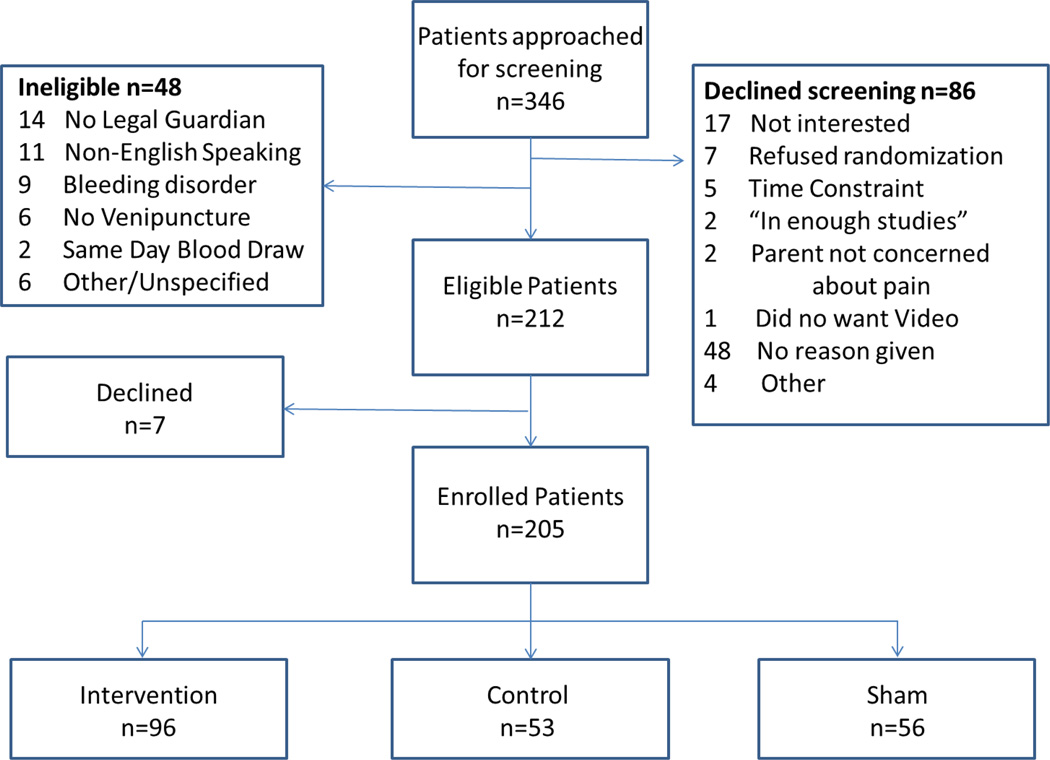
During the study enrollment period, 346 patients ages 1 to 6 years presented to the outpatient laboratory for venipuncture. At approach, 86 declined participation prior to screening, 48 were not eligible (Figure 3) and 7 eligible patients declined after screening, resulting in 205 patients enrolled; Intervention=96, Control=53, Sham=56.
Figure 3. Enrollment Flow Diagram.
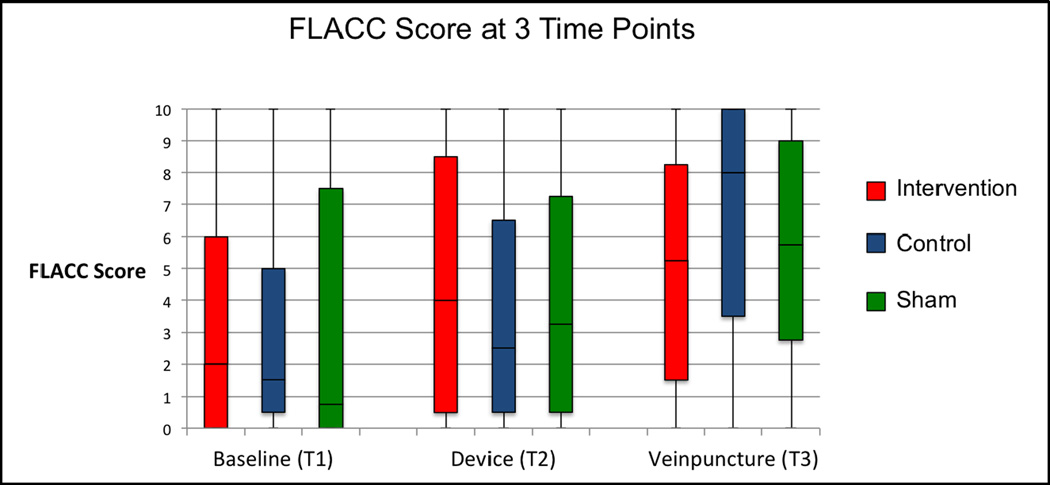
Baseline characteristics were similar between the three groups (Table 1). The overall mean age was 3.2 years; 88.8% of children had experienced a previous venipuncture, but this occurrence was not different between groups. The Baseline (T1) median pain score was not different between groups. (Figure 4)
Table 1.
Patient characteristics by group
| Intervention | Control | Sham | |
|---|---|---|---|
| Male (% (95% CI)) | 56.3 (45.7, 66.4) | 69.8 (55.7, 81.7) | 55.4 (41.5, 68.7) |
| Age in years (mean (SD)) | 3.3 (1.9) | 2.9 (1.7) | 3.4 (1.6) |
| Race (% (95% CI)) | |||
| White | 46.9 (36.6, 57.3) | 58.5 (44.1, 71.9) | 60.7 (46.8, 73.5) |
| Black | 21.9 (14.1, 31.5) | 17 (8.1, 29.8) | 14.3 (6.4, 26.2) |
| Hispanic | 12.5 (6.6, 20.8) | 15.1 (6.7, 27.6) | 14.3 (6.4, 26.2) |
| Other | 18.8 (11.5, 28.0) | 9.4 (3.1, 20.7) | 10.7 (4.0, 21.9) |
| Previous Venipuncture (% (95% CI)) | 87.5 (79.2, 93.4) | 94.4 (84.3, 98.8) | 85.7 (73.8, 93.6) |
| Baseline Pain Score (T1) (median (95% CI)) | 2.00 (0.50, 3.50) | 1.00 (0.50, 3.00) | 0.75 (0, 4.70) |
Figure 4. FLACC pain scores at each time: (T1) Baseline (T2) Device and (T3) Venipuncture. (solid horizontal line represents median, box represents interquartile range and whiskers represent range).
Main Results
Pain Experience
Pain scores increased significantly from Baseline to Device (T1 to T2) in the Intervention group (mean change=1.35; 95% CI 0.80 to 1.91) and the Control group (mean change=0.89; 95% CI 0.28 to 1.50), but not the Sham group (mean change=0.65; 95% CI −0.07 to 1.37). The change in pain scores from Baseline to Device (T1 to T2) in the Intervention group was similar to both the Control group (mean difference = 0.48; 95% CI −0.02 to 0.98 ) and the Sham group (mean difference= 0.75; 95% CI −0.11 to 1.18). The median pain scores at T2 were not different between groups. (Figure 4)
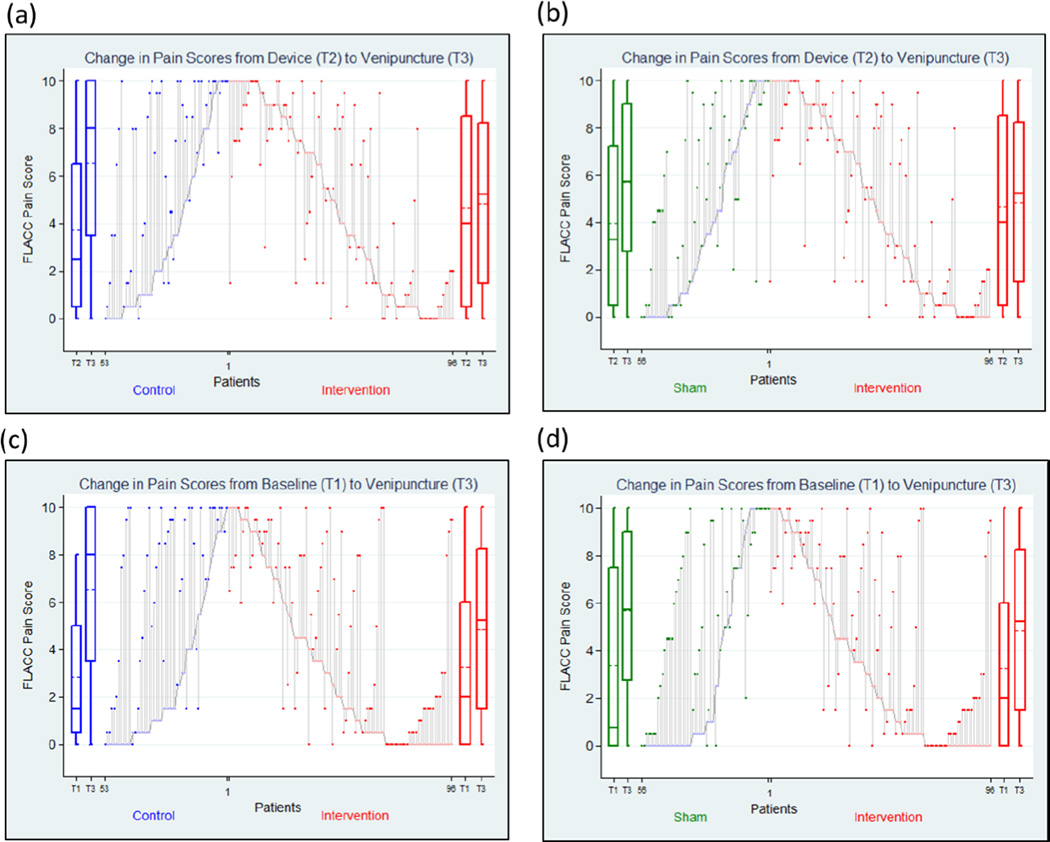
With regards to the primary outcome, pain scores did not significantly increase from Device to Venipuncture (T2 to T3) in the Intervention group (mean change=0.26; 95% CI −0.31 to 0.82), but significantly increased in both the Control group (mean change=2.82; 95% CI 1.91 to 3.74), and the Sham group (1.68; 95% CI 0.83 to 2.52). The change in pain scores from Device to Venipucture (T2 to T3) in the Intervention group was significantly less than both the Control group (mean difference = −2.59; 95% CI −1.13 to −2.82) and the Sham group (mean difference= −1.48; 95% CI −1.96 to −1.630). (Figure 5a and 5b)
Figure 5. Changes in FLACC pain scores for individual patients.
Parallel lines represent changes in FLACC pain score and changes in FLACC pain score from Device (T2) to Venipuncture (T3) compared between Intervention and (a) Control and (b) Sham and from Baseline (T1) to Venipuncture (T3) compared between Intervention and (c) Control and (d) Sham. The boxplots on the sides represent FLACC pain scores. The solid horizontal lines represent median, the dashed line represents mean, boxes represent interquartile range and whiskers represent range.
Overall, pain scores increased significantly from Baseline (T1) to Venipucture (T3) in all three groups; Intervention: (mean change=1.61; 95%CI 0.98 to 2.23), Control: (mean change=3.71; 95%CI 2.71 to 4.70), and Sham: (mean change=2.33; 95%CI 1.37 to 3.29). The Intervention group had a significantly lower mean change in pain scores from Baseline to Venipuncture (T1 to T3) than the Control group (mean difference=−2.11; 95% CI −1.84 to −2.33) but had a similar mean change to the Sham group (mean difference= −.73; 95% CI −1.85 to 0.38). (Figure 5c and 5d) The median pain score at Venipuncture (T3) was lower in the Intervention group 5.25 (95% CI 2.5 to 7.5) than the control group 8.00 (95% CI 6.0 to 9.5) but similar to the sham group 5.75 (95% CI 4.5 to 8.0). (Figure 4)
At Venipuncture (T3) 45% (95% CI 34% to 55%) of children in the Intervention group had no or mild pain compared to 23% (95% CI 12% to 36%) in Controls and 30% (95% CI 18% to 44%) in the Sham group. Overall, 9% (95% CI 6% to 14%) of patients had no pain at Venipuncture (T3) and the proportion with no pain was similar between groups; 13% (95% CI 6% to 21%) in the Intervention group, 4% (95% CI 0 % to 13 %) in the Control group and 7% (95% CI 2% to 17%) in the Sham group.
Venipuncture success
Overall first attempt venipuncture success was 90.7% (95% CI (85.9%, 94.3%)) and was not different between groups; the Intervention group-89.6% (95% CI (81.7%, 94.9%)), the Control group-90.6% (95% CI (79.3%, 96.9%)) and the Sham group-92.9% (95% CI (82.7%, 98%)).
Adverse Events
No severe adverse events were reported. Immediately after venipuncture, 1 child in the Intervention group had excess bleeding that was controlled with pressure and 1 child in the Sham group had a hematoma. Next day phone follow up was successfully performed in 85.7% of patients. Bruising (13.7%) and redness (1.1%) were the most common reactions reported next day overall. No difference in reported adverse events was found between groups (Table 2).
Table 2.
Adverse events by group
| Intervention (n=96) |
Control (n=53) |
Sham (n=56) |
|
|---|---|---|---|
| In lab % (95% CI) | 1.0%, (0, 5.7) | 0%, (0, 6.7) | 1.8%, (0,9.6) |
| Hematoma | 0% (0, 3.8) | 0% (0, 6.7) | 1.8%, (0, 9.6) |
| Excess Bleeding | 1.0% (0, 5.7) | 0% (0, 6.7) | 0% (0, 6.4) |
| At Home% (95% CI) | 15.7%, (8.6, 25.3) | 13.0% (4.9, 26.2) | 10.9% (3.6, 23.6) |
| Bruise | 14.4% (7.7, 23.9) | 8.6% (2.4, 20.8) | 6.5% (1.4, 17.9) |
| Redness | 0% (0, 4.3) | 0% (0, 7.8) | 4.3% (0.5, 14.8) |
| Other | 1.2% (0, 6.5) | 4.3% (0.5, 14.8) | 0% (0, 7.7) |
Limitations
The gold standard for pain assessment is self-report, however young children are not able to provide self-report. Instead, observational pain scales are widely used to assess pain in young children. The FLACC score is a valid measure of pain, but may also capture anxiety.11 This is a potential problem with all observational pain scales since pain and anxiety behaviors can be difficult to distinguish. To better differentiate pain from anxiety, future studies may consider utilizing an observational anxiety scale to evaluate the role of anxiety on the venipuncture pain experience.
Additionally, patient characteristics that can affect venipuncture experience may not have been captured in this study design. These may include patient and parent anxiety, analgesic use with any prior venipuncture experience, time since last venipuncture or personality match between the patient, parent and phlebotomist. Though randomization should randomly allocate these variables across groups, further studies may explore the relationship between these patient characteristics and overall experience.
Due to ethical concerns about withholding usual care, a control group with no pain treatment could not be used, so vapocoolant spray was used as a control. Studies of vapocoolant spray in older children have shown both positive and negative results regarding efficacy and very few studies examine efficacy in young children.12,13,14 A recent systematic review concluded that vapocoolant spray was ineffective in reducing venipuncture pain in children.13 It is unclear if patients in the Control group experienced anesthesia from the vapocoolant spray. Additionally, the use of normal saline spray in the intervention group was done to enhance blinding of the family, phlebotomists and video reviewers. Though there is no evidence that a room temperature saline spray would affect pain experienced by children undergoing venipuncture, it is possible that it altered the children’s experience in some way.
The trained physician reviewers in this study were blinded to group assignment. However, some conversations between the patient and families on the videos may have revealed the patient group. Additionally, though patients and their families were not told their group assignment, families in the Control group may have been aware that they were not receiving the Intervention and those familiar with the device may have been able ascertain their assignment to the Sham or Intervention groups. This may have affected the family or child’s perception of the pain treatment.
When assessing for adverse events, this study evaluated for known adverse events from the J tip such as local skin reaction or allergic reaction. A recently published case series reported on 4 children with elevated serum lidocaine levels after J tip lidocaine administration.15 Though the clinical significance of this is unknown, this study did not evaluated for toxic lidocaine levels in children and this may have been unrecognized. However, based on direct observation of the children immediately after the administration of the J tip, no children showed clinical signs of lidocaine toxicity including dizziness, disorientation, seizures, chest pain or difficulty breathing.
Finally, this study was done in a tertiary care children’s hospital where many of the participants in the study had extensive health care exposure. Nearly 90% of children enrolled had experienced a previous venipuncture. Research has shown children with untreated procedural pain during their first exposure report increased pain at subsequent procedures despite adequate pain treatment, which may influence the findings of this study.2 Given this information, the device may have had less of an impact on our population than would be expected in a population naïve to venipuncture.
Discussion
Children administered a J tip prior to venipuncture had no significant increase in pain at needle insertion and 45% had no or mild pain at the time of venipuncture. Venipuncture success was not affected by the intervention. Adverse effects were minor including bleeding and bruising and were not increased compared to the Control and Sham groups. This is the first study to provide evidence that the J tip improved the pain experience for children ages 1–6 years undergoing venipuncture.
These results are consistent with studies involving older children.5,6,7 Those studies that enrolled older children used self-reported pain scores (the visual analog scale and color analog scale) during IV cannulation and venipuncture. Each study showed a lower pain score at time of needle insertion for children administered a J tip compared to lidocaine/prilocaine cream and for children administered a J tip compared to no pain treatment.5,6,7 The findings of this study add to the evidence that the J tip reduces needle stick pain for children ages 1 to 6 years.
The J tip administration itself is not painful, as the increase in FLACC pain score after administration of the J tip was not different from the increased score noted after researcher approach. Though the absolute score at Device was highest in the Intervention group, it was not significantly different than the Sham or Control groups. This was also consistent with previous studies in older children where the majority (84–91%) of patients reported no to mild pain at J tip deployment.5,6 This also refutes the misperception that the analgesia provided by the J tip is over shadowed by distress at its deployment. Though the device is not painful, some may perceive the “pop” noise as distressing and further studies might examine anxiety experienced by patients receiving the device.
Interestingly, the FLACC scores at Venipuncture for the Sham group were significantly lower than the Control group. Similar findings were noted in a study of children administered a J tip with lidocaine compared to children administered a J tip with normal saline.7 The authors proposed the gate theory of pain to explain this diminished pain response. The gate theory of pain proposes that afferent nerve impulses from the peripheral nervous system are modulated in the spinal cord, therefore the first stimulation by the saline may result in decreased pain sensitivity to the second stimulus; the venipuncture.7,16 In this study, the Sham group did not have a tactile stimulus resulting in direct nerve stimulation. However, the children did experience the loud “pop” noise that may have been a distraction. Distraction techniques have been used to decrease distress in young children undergoing procedures.17 Even passive distraction techniques, such as a caregiver talking about a toy or movie, where children are not actively engaged, have been shown to reduce distress at venipuncture.18 The distraction due to the noise may have decreased distress at venipuncture in both the sham and intervention groups. Alternatively, the lower FLACC score in the sham group may be explained by the placebo effect. The placebo effect can be present in children, though little literature exists on the phenomenon in very young children and the effect may be dependent on the explicit descriptions of the placebo’s intended effect.19,20 This study did not include a specific description to the children of perceived benefit of the Sham treatment, making the magnitude of the placebo effect unclear.
This study was set in the outpatient phlebotomy lab, an environment where a large number of venipuncture procedures are performed daily but where the J tip has not previously been studied. Though the AAP advises the use of local anesthetics for painful procedures, actual hospital protocols vary by institution.4 This variation mirrors inconsistent practices seen in emergency departments regarding the treatment of procedural pain in children.21,22 Though the children seen in the outpatient lab were generally well at the time of their venipuncture, the experience of a needle stick for outpatient venipuncture is similar to that of children undergoing venipuncture in the emergency department. Similarly, the act of using a needle to obtain blood is less invasive but similar to the placement of an IV catheter. For these reasons, the results of this study are likely generalizable to children undergoing venipuncture or IV placement in other settings including the emergency department, outpatient clinic or pre-operative settings.
Though cost analysis was not planned, the issue of cost is always present in clinical practice. Reported cost of the J tip device varies, ranging from $0.98 per unit to $4.10.5, 6, 7, 23 (Sana Patel, National Medical Products, Inc, email communication, 10/6/2014) Additionally, pharmacy labor costs for filling the device vary by institution. The cost of a single application of vapocoolant spray is approximately $0.50.24 The cost difference between J tip and vapocoolant spray may deter the use of the J tip in clinical practice. A previous survey of parents in an emergency department found that 77% of parents would be willing to pay at least $15 out of pocket for a “painless” IV start and 37% would be willing to pay $100.25 Though the J tip rendered the IV placement “painless” for only 13.5% of patients, the financial cost should be weighed against the value of patient comfort, parental desire for decreased pain and avoidance of long term consequences of untreated needle-stick pain. A previous cost analysis in adults found the cost of generating a patient with no more than moderate pain to be $3.40.26 Additionally, a cost analysis in children found the J tip to be the most cost effective option for ED physicians aiming to reduce pain for IV insertion.27
In summary, the J tip reduces venipuncture pain in young children with no change in venipuncture success and no increase in adverse events compared to vapocoolant spray or sham treatment. This study supports the use of the J tip as an effective intervention to reduce the pain experienced during venipuncture in children ages 1 to 6 years.
Acknowledgments
Grant: Support received by grant 8UL1TR000055 from the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose
Author Contributions: MML, ALD, SJW and DCB conceived the study and designed the trial and supervised the conduct of the trial and data collection. MML undertook recruitment of patients and managed data, including quality control. MNL, ALD and SJW assisted with subject analysis. RGH provided statistical advice on study design and MD and RGH analyzed the data. MML drafted the manuscript and all the authors contributed substantially to its revision. MML takes responsibility for the paper as a whole.
References
- 1.Cohen LL. Behavioral Approaches to Anxiety and Pain Management for Pediatric Venous Access. Pediatrics. 2008;112:S134–S139. doi: 10.1542/peds.2008-1055f. [DOI] [PubMed] [Google Scholar]
- 2.Weisman SJ, Bernstein B, Schechter NL. Consequences of Inadequate Analgesia During Painful Procedures in Children. Arch Pediatr Adolesc Med. 1998;152:147–149. doi: 10.1001/archpedi.152.2.147. [DOI] [PubMed] [Google Scholar]
- 3.Fradet C, McGrath PJ, Kay J, Adams S, Luke B. A prospective survey of reactions to blood tests by children and adolescents. Pain. 1990;40:53–60. doi: 10.1016/0304-3959(90)91050-S. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics, Committee on Psychosocial Aspects of Child and Family Health; American Pain Society Task Force on Pain in Infants, Children and Adolescents. The assessment and management of acute pain in infants, children and adolescents. Pediatrics. 2001:793–797. [Google Scholar]
- 5.Spanos S, Booth R, Koenig H, Sikes K, Gracely E, Kim IK. Jet Injection of 1% Buffered Lidocaine versus Topical ELA-Max for Anesthesia Before Peripheral Intravenous Catheterization in Children. Pediatr Emerg Care. 2008;24:511–515. doi: 10.1097/PEC.0b013e31816a8d5b. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez N, Bradford H, Seidel KD, Sousa M, Lynn AM. A comparison of Needle-Free Injection System for Local Anesthesia Versus EMLA for Intravenous Catheter Insertion in the Pediatric Patient. Anesth Analg. 2006;102:411–414. doi: 10.1213/01.ane.0000194293.10549.62. [DOI] [PubMed] [Google Scholar]
- 7.Auerbach M, Tunik M, Mojica M. A randomized, double-blind controlled study of jet lidocaine compared to jet placebo for pain relief in children undergoing needle insertion in the emergency department. Acad Emerg Med. 2009;16:388–393. doi: 10.1111/j.1553-2712.2009.00401.x. [DOI] [PubMed] [Google Scholar]
- 8.Merkel S, Voepel-Lewis T, Malviya S. Pain Assessment in Infants and Young children: The FLACC Scale. Am J Nurs. 2002;102(10):55–57. doi: 10.1097/00000446-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 9.McGrath PJ, Walco Ga, Turk DC, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9:771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Crellin D, Sullivan TP, Babl FE, O’Sullivan R, Hutchinson A. Analysis of the validation of existing behavioral pain and distress scales for use in the procedural setting. Paediatr Anaesth. 2007;17:720–733. doi: 10.1111/j.1460-9592.2007.02218.x. [DOI] [PubMed] [Google Scholar]
- 11.Babl FE, Crellin D, Cheng J, Sullivan TP, O’Sullivan R, Hutchinson A. The use of faces, legs, activity, cry and consolability scale to assess procedural pain and distress in young children. Pediatr Emerg Care. 2012;28:1281–1296. doi: 10.1097/PEC.0b013e3182767d66. [DOI] [PubMed] [Google Scholar]
- 12.Farion KJ, Splinter KL, Newhook K, Gaboury I, Splinter WM. The effect of vapocoolant spray on pain due to intravenous cannulation in children: a randomized controlled trial. CMAJ. 2008;179(1):31–36. doi: 10.1503/cmaj.070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan ME, Smart S, Shah V, Taddio A. A Systematic Review of Vapocoolants for Reducing Pain from Venipuncture and Venous Cannulcation in Children and Adults. J Emerg Med. 2014 doi: 10.1016/j.jemermed.2014.06.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Reis EC, Holubkov R. Vapocoolant Spray Is Equally Effective as EMLA Cream in Reducing Immunization Pain in School-aged Children. Pediatrics. 1997;100(6):e5. doi: 10.1542/peds.100.6.e5. [DOI] [PubMed] [Google Scholar]
- 15.Gular P, et al. Elevated lidocaine serum concentration after subcutaneous lidocaine administration using a needle-free device in pediatric patients. Pediatr Emerg Care. 2014 doi: 10.1097/PEC.0000000000000178. in press. [DOI] [PubMed] [Google Scholar]
- 16.Melzack R, Wall PD. Pain Mechanisms: A New Theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 17.Blount RL, Piira T, Cohen LL. Management of Pediatric Pain and Distress Due to Medical Procedures. In: Roberts M, editor. Handbook of Pediatric Psychol. 3rd. New York, USA: Guilford; pp. 216–233. [Google Scholar]
- 18.MacLauren JE, Cohen LL. A Comparison of Distraction Strategies for Venipuncture Distress in Children. J Pediatr Psychology. 2005;30:387–396. doi: 10.1093/jpepsy/jsi062. [DOI] [PubMed] [Google Scholar]
- 19.Goodenough B, Kampel L, Champion GD, Laubreaux L, Nicholas MK, Ziegler JB, McInerney M. An investigation of the placebo effect and age-related factors in the report of needle pain from venipuncture in children. Pain. 1997;72(3):383–391. doi: 10.1016/s0304-3959(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 20.Krummenacher P, Kossowsky J, Schwarz C, Brugger P, Kelley JM, Meyer A, Gaab J. Expectancy-Induced Placebo Analgesia in Children and the Role of Magical Thinking. J Pain. 2014 doi: 10.1016/j.jpain.2014.09.005. In Press. [DOI] [PubMed] [Google Scholar]
- 21.Ferrante P, Cuttini M, Zangardi T, Tomasello C, Messi G, Pirozzi N, Losacco V, Piga S, Benini F PIPER Study Group. Pain management policies and practices in pediatric emergency care: a nationwide survey of Italian hospitals. BMC Pediatr. 2013;13:139. doi: 10.1186/1471-2431-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLean S, Obispo J, Young KD. The gap between pediatric emergency department procedural pain management treatments available and actual practice. Pediatr Emerg Care. 2007;23(2):87–93. doi: 10.1097/PEC.0b013e31803. [DOI] [PubMed] [Google Scholar]
- 23.Frequently Asked Questions. University of Illinois Medical Center at Chicago; [Accessed August 28. 2014]. “A Model Program – Implementation of Needle-free Jet Injection Technology in an Academic Health Setting”. http://www.uic.edu/orgs/hpmo/needle_free/Text/faq.html Published 2004. [Google Scholar]
- 24.Pain Ease – Pediatrics – Gebauer Company. [Accessed August 28, 2014]; http://www.gebauer.com/products/gebauer-s-pain-ease/pediatrics/ Published 2014. [Google Scholar]
- 25.Walsh BM, Bartfield JM. Survey of Parental Willingness to Pay and Willingness to Stay for “Painless” Intravenous Catheter Placement. Pediatr Emerg Care. 2006;22:699–703. doi: 10.1097/01.pec.0000238743.96606.69. [DOI] [PubMed] [Google Scholar]
- 26.Lysakowski C, Dumon L, Tramer MR, Tassonyi E. A needle-free jet-injection system with lidocaine for peripheral intravenous cannula insertion: a randomized controlled trial with cost-effectiveness analysis. Anesth Analg. 2003 Jan;96(1):215–219. doi: 10.1097/00000539-200301000-00044. [DOI] [PubMed] [Google Scholar]
- 27.Parshad J, Steinbery S, Waters TW. Cost-effective Analysis of Anesthetic Agents During Peripheral Intravenous Cannulation in the Pediatric Emergency Department. Arch Pediatr Adolesc Med. 2008;162(10):952–961. doi: 10.1001/archpedi.162.10.952. [DOI] [PubMed] [Google Scholar]