Abstract
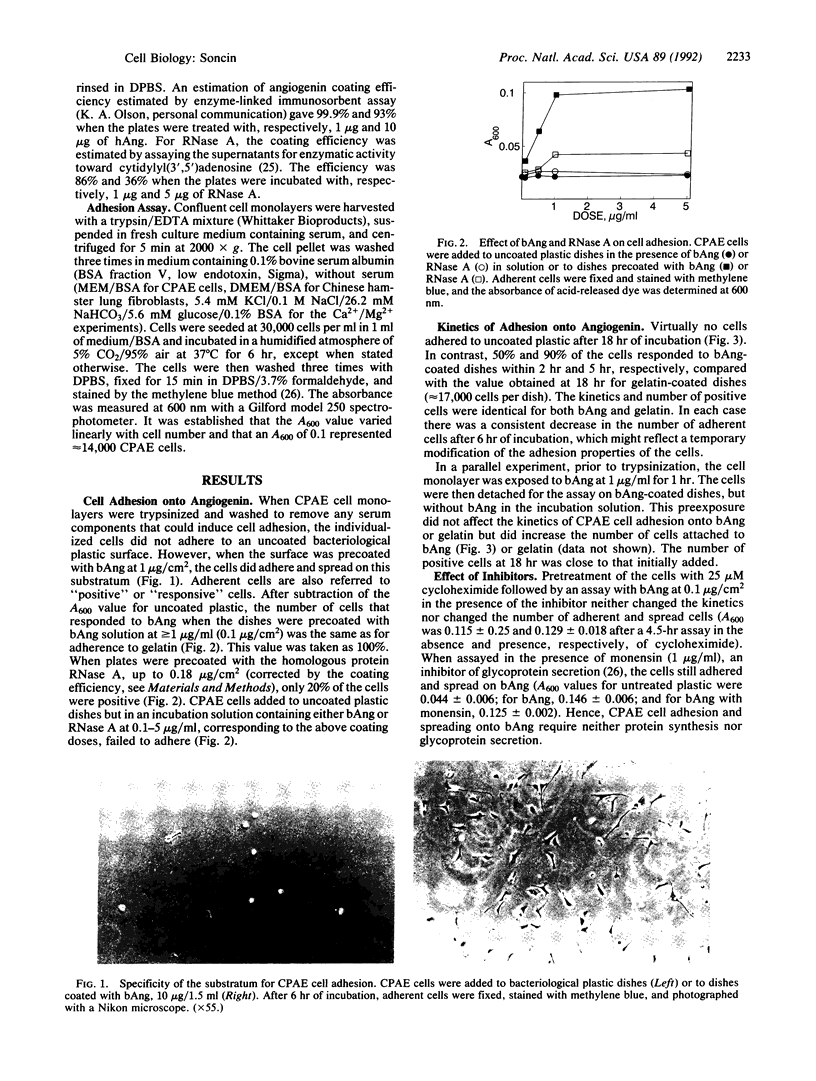
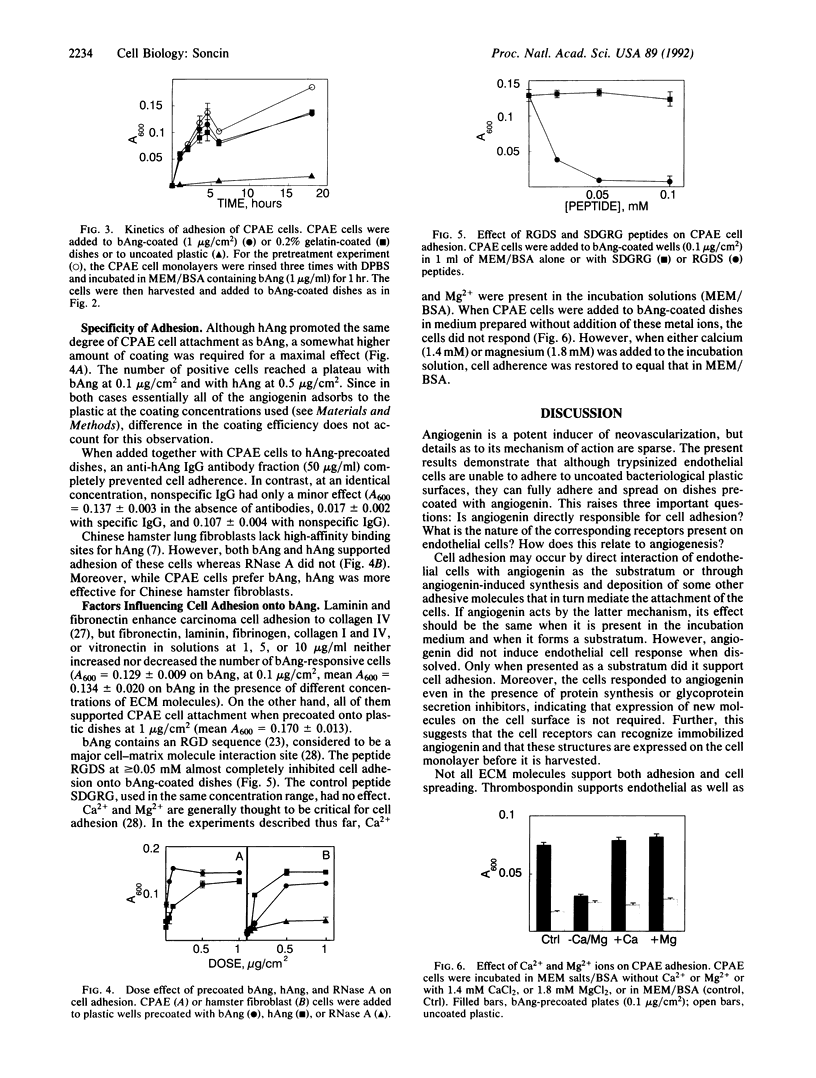
When coated on bacteriological plastic at doses greater than or equal to 0.1 microgram/cm2, human and bovine angiogenin support calf pulmonary artery endothelial and Chinese hamster fibroblast cell adhesion and spreading, but do not affect cell adhesion when in solution. The kinetics of endothelial cell attachment to angiogenin are indistinguishable from those in the presence of gelatin. Calcium and/or magnesium ions are critical for cell adhesion or spreading onto angiogenin but protein synthesis and glycoprotein secretion are not necessary. Adhesion to angiogenin is not altered by the addition to the incubation solution of fibronectin, fibrinogen, laminin, collagen I and IV, or vitronectin. The peptide Arg-Gly-Asp-Ser inhibits endothelial cell response to angiogenin whereas the reverse peptide Ser-Asp-Gly-Arg-Gly has no effect. These findings show that angiogenin can serve as an effective substratum for cell adhesion by inducing an interaction similar to but independent from that of other extracellular matrix molecules. Induction of cell adhesion and subsequent migration may be critical steps in the process of angiogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres J. L., Stanley K., Cheifetz S., Massagué J. Membrane-anchored and soluble forms of betaglycan, a polymorphic proteoglycan that binds transforming growth factor-beta. J Cell Biol. 1989 Dec;109(6 Pt 1):3137–3145. doi: 10.1083/jcb.109.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badet J., Soncin F., Guitton J. D., Lamare O., Cartwright T., Barritault D. Specific binding of angiogenin to calf pulmonary artery endothelial cells. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8427–8431. doi: 10.1073/pnas.86.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A., Schubert D., Ling N., Guillemin R. Receptor- and heparin-binding domains of basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2324–2328. doi: 10.1073/pnas.85.7.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R., Sabbah V., Lampugnani M. G., Marchisio P. C., Fenton J. W., 2nd, Vlodavsky I., Dejana E. An Arg-Gly-Asp sequence within thrombin promotes endothelial cell adhesion. J Cell Biol. 1991 Jan;112(2):335–344. doi: 10.1083/jcb.112.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson C. T., Knowles W. J., Bell L., Albelda S. M., Castronovo V., Liotta L. A., Madri J. A. Spatiotemporal segregation of endothelial cell integrin and nonintegrin extracellular matrix-binding proteins during adhesion events. J Cell Biol. 1990 Mar;110(3):789–801. doi: 10.1083/jcb.110.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R., Vallee B. L. Angiogenin activates endothelial cell phospholipase C. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5961–5965. doi: 10.1073/pnas.85.16.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R., Vallee B. L. Angiogenin stimulates endothelial cell prostacyclin secretion by activation of phospholipase A2. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1573–1577. doi: 10.1073/pnas.86.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood C. H., Zetter B. R. Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta. 1990 Jun 1;1032(1):89–118. doi: 10.1016/0304-419x(90)90014-r. [DOI] [PubMed] [Google Scholar]
- Bond M. D., Strydom D. J. Amino acid sequence of bovine angiogenin. Biochemistry. 1989 Jul 11;28(14):6110–6113. doi: 10.1021/bi00440a057. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Nannizzi L., Smith J. W., Cheresh D. A. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J Cell Biol. 1990 Dec;111(6 Pt 1):2795–2800. doi: 10.1083/jcb.111.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Good D. J., Polverini P. J., Rastinejad F., Le Beau M. M., Lemons R. S., Frazier W. A., Bouck N. P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Foidart J. M., Savion N. The production and localization of laminin in cultured vascular and corneal endothelial cells. J Cell Physiol. 1981 May;107(2):171–183. doi: 10.1002/jcp.1041070203. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Vlodavsky I., Alvarado J., Johnson L. K. The identification and localization of fibronectin in cultured corneal endothelial cells: cell surface polarity and physiological implications. Exp Eye Res. 1979 Nov;29(5):485–509. doi: 10.1016/0014-4835(79)90151-9. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J., Braun D., Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Hallahan T. W., Shapiro R., Vallee B. L. Dual site model for the organogenic activity of angiogenin. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2222–2226. doi: 10.1073/pnas.88.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath W. F., Jr, Moore F., Bicknell R., Vallee B. L. Modulation of mitogenic stimuli by angiogenin correlates with in vitro phosphatidylinositol bisphosphate synthesis. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2718–2722. doi: 10.1073/pnas.86.8.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G. F., Chang S. I., Riordan J. F., Vallee B. L. An angiogenin-binding protein from endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2227–2231. doi: 10.1073/pnas.88.6.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Ingber D. E. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci U S A. 1990 May;87(9):3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989 Jul;109(1):317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Mosher D. F. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978 Jun 1;147(6):1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney M. C., Benya P. D., Nimni M. E., Smith R. E. Stability of the collagen phenotype and decreased collagen production in serial subcultures of rabbit corneal endothelial cells. Exp Eye Res. 1981 Aug;33(2):131–140. doi: 10.1016/s0014-4835(81)80061-9. [DOI] [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol. 1988 Jul;132(1):18–27. [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Vassalli J. D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D. High and low affinity binding sites for basic fibroblast growth factor on cultured cells: absence of a role for low affinity binding in the stimulation of plasminogen activator production by bovine capillary endothelial cells. J Cell Physiol. 1987 Apr;131(1):123–130. doi: 10.1002/jcp.1041310118. [DOI] [PubMed] [Google Scholar]
- Presta M., Maier J. A., Rusnati M., Ragnotti G. Basic fibroblast growth factor is released from endothelial extracellular matrix in a biologically active form. J Cell Physiol. 1989 Jul;140(1):68–74. doi: 10.1002/jcp.1041400109. [DOI] [PubMed] [Google Scholar]
- Rogelj S., Klagsbrun M., Atzmon R., Kurokawa M., Haimovitz A., Fuks Z., Vlodavsky I. Basic fibroblast growth factor is an extracellular matrix component required for supporting the proliferation of vascular endothelial cells and the differentiation of PC12 cells. J Cell Biol. 1989 Aug;109(2):823–831. doi: 10.1083/jcb.109.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Fox E. A., Riordan J. F. Role of lysines in human angiogenin: chemical modification and site-directed mutagenesis. Biochemistry. 1989 Feb 21;28(4):1726–1732. doi: 10.1021/bi00430a045. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Harper J. W., Fox E. A., Jansen H. W., Hein F., Uhlmann E. Expression of Met-(-1) angiogenin in Escherichia coli: conversion to the authentic less than Glu-1 protein. Anal Biochem. 1988 Dec;175(2):450–461. doi: 10.1016/0003-2697(88)90569-6. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Riordan J. F., Vallee B. L. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986 Jun 17;25(12):3527–3532. doi: 10.1021/bi00360a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Strydom D. J., Olson K. A., Vallee B. L. Isolation of angiogenin from normal human plasma. Biochemistry. 1987 Aug 11;26(16):5141–5146. doi: 10.1021/bi00390a037. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Site-directed mutagenesis of histidine-13 and histidine-114 of human angiogenin. Alanine derivatives inhibit angiogenin-induced angiogenesis. Biochemistry. 1989 Sep 5;28(18):7401–7408. doi: 10.1021/bi00444a038. [DOI] [PubMed] [Google Scholar]
- Strydom D. J., Fett J. W., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985 Sep 24;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Taraboletti G., Roberts D., Liotta L. A., Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990 Aug;111(2):765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Johnson L. K., Greenburg G., Gospodarowicz D. Vascular endothelial cells maintained in the absence of fibroblast growth factor undergo structural and functional alterations that are incompatible with their in vivo differentiated properties. J Cell Biol. 1979 Nov;83(2 Pt 1):468–486. doi: 10.1083/jcb.83.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITZEL H., BARNARD E. A. Mechanism and binding sites in the ribonuclease reaction. II. Kinetic studies on the first step of the reaction. Biochem Biophys Res Commun. 1962 May 4;7:295–299. doi: 10.1016/0006-291x(62)90194-8. [DOI] [PubMed] [Google Scholar]




