Abstract
Study Objectives:
New and effective strategies are needed to manage the autonomic and cardiovascular sequelae of obstructive sleep apnea (OSA). We assessed the effect of daily inspiratory muscle strength training (IMT) on sleep and cardiovascular function in adults unable to use continuous positive airway pressure (CPAP) therapy.
Methods:
This is a placebo-controlled, single-blind study conducted in twenty four adults with mild, moderate, and severe OSA. Subjects were randomly assigned to placebo or inspiratory muscle strength training. Subjects in each group performed 5 min of training each day for 6 w. All subjects underwent overnight polysomnography at intake and again at study close.
Results:
We evaluated the effects of placebo training or IMT on sleep, blood pressure, and plasma catecholamines. Relative to placebo-trained subjects with OSA, subjects with OSA who performed IMT manifested reductions in systolic and diastolic blood pressures (−12.3 ± 1.6 SBP and −5.0 ± 1.3 DBP mmHg;
P < 0.01); plasma norepinephrine levels (536.3 ± 56.6 versus 380.6 ± 41.2 pg/mL; P = 0.01); and registered fewer nighttime arousals and reported improved sleep (Pittsburgh Sleep Quality Index scores: 9.1 ± 0.9 versus 5.1 ± 0.7; P = 0.001). These favorable outcomes were achieved without affecting apneahypopnea index.
Conclusions:
The results are consistent with our previously published findings in normotensive adults but further indicate that IMT can modulate blood pressure and plasma catecholamines in subjects with ongoing nighttime apnea and hypoxemia. Accordingly, we suggest IMT offers a low cost, nonpharmacologic means of improving sleep and blood pressure in patients who are intolerant of CPAP.
Citation:
Vranish JR, Bailey EF. Inspiratory muscle training improves sleep and mitigates cardiovascular dysfunction in obstructive sleep apnea. SLEEP 2016;39(6):1179–1185.
Keywords: hypertension, obstructive sleep apnea, respiratory training
Significance.
5 minutes of inspiratory muscle training a day improves sleep and reduces blood pressure and plasma catecholamines in adults with obstructive sleep apnea.
INTRODUCTION
As the prevalence of hypertension grows worldwide, novel approaches are needed to combat what is fast becoming a public health epidemic.1,2 Of the nonpharmacologic treatments available, a long-term respiratory strength training protocol has yielded surprising results including improved blood pressure (BP) and autonomic balance in hypertensive patients3 and improved baroreflex sensitivity in a rodent model of heart failure.4 Despite some enticing findings and its common use in clinical practice, the benefits and applications for respiratory muscle training remain a subject of debate.5
Recently, our group tested five distinct respiratory training protocols to determine which of them may modulate BP. The results of this placebo-controlled and blinded study conducted in normotensive adults (n = 50) showed the daily generation of large (positive or negative) intrathoracic pressures was the most effective means of lowering systolic [−8.96 mmHg] and diastolic [−5.25 mmHg] BP. Importantly, the lowering of BP was achieved in the absence of any change in heart rate,6 highlighting an important but previously unappreciated distinction between the effects obtained with traditional aerobic training7,8 and respiratory muscle strength training.
In light of these results, we were interested in assessing the suitability of respiratory muscle strength training within the context of a clinical population. Given the significant risk for worsening hypertension9 and cardiovascular morbidity and mortality in patients with obstructive sleep apnea (OSA),10,11 we recruited men and women with mild, moderate, and severe OSA to test the hypothesis that IMT lowers BP in adults who are unable to adhere to nightly continuous positive airway pressure (CPAP) use.12,13
METHODS
This prospective, randomized, single-blind, control physiological experiment was conducted on 24 adults with mild, moderate, and severe OSA, who did not use or had discontinued using CPAP, dental devices, or other OSA-related therapies for at least 6 mo prior to the study intake. Exclusion criteria were: CPAP use, presence of diabetes, chronic heart failure, unstable angina, myocardial infarction, smoking, respiratory or neuromuscular disease. Note that none of the participants were taking antihypertensive medication(s) or medication(s) for cardiovascular disease. All procedures were approved by the Human Subjects Protection Program at The University of Arizona and subjects gave their written informed consent prior to participation.
Overnight Polysomnography
All subjects underwent standard montage overnight polysomongraphy (PSG), including electroencephalogram, electro-oculogram, electrocardiogram signals with pulse oximetry, airflow, and thoracic and abdominal inductive plethysmography. In accordance with American Sleep Association guidelines, an apnea was defined as a cessation in airflow (≥ 90% baseline) for ≥ 10 sec and a hypopnea was defined as a reduction in airflow (≥ 30% baseline) for ≥ 10 sec which resulted in a ≥ 4% oxygen desaturation and/or electroencephalographic arousal from sleep. Sleep apnea severity was rated in accordance with the individual's apnea-hypopnea index (AHI), defined as the total number of apneas and hypopneas per hour of sleep and oxygen desaturation per hour of sleep, where an AHI of 5 to 15 is designated mild, 16 to 30 moderate, and > 30 events per hour designated as severe OSA. Additionally, we asked subjects questions regarding their overall sleep quality using the Pittsburgh Sleep Quality Index (PSQI).14
Respiratory Assessment
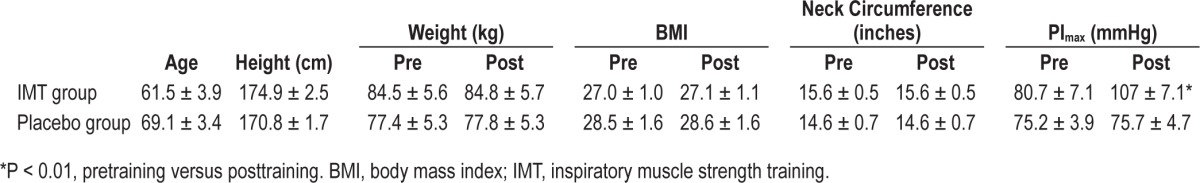
Lung function was assessed by standard spirometry and included forced expiratory volume in 1.0 sec (FEV1.0), forced vital capacity (FVC), forced inspiratory volume in 1.0 sec (FIV1.0), forced inspiratory vital capacity (FIVC), FEV1.0/FVC, FIV1.0/ FIVC, peak expiratory flow, and peak inspiratory flow in accordance with the guidelines of The American Thoracic Society. Maximal inspiratory pressure (PImax) was assessed by having subjects perform a maximal inspiratory effort against a constant resistance. PImax was measured via a pressure transducer (Omega-dyne Inc., Sunbury, OH) and determined from the average of the three largest pressure values generated (Table 1).
Table 1.
Average (± SEM) values for age, anthropomorphic data, and maximal inspiratory pressures (PImax) generated by subjects assigned to either inspiratory muscle strength training (n = 12) or to placebo training (n = 12) groups, at intake and at study close.
Training Groups
Subjects were randomly assigned to inspiratory muscle strength training (IMT) or placebo training groups. For both groups, training comprised 30 breaths each day for 6 w using an inspiratory threshold training device (K3 series, POWER-breathe, Warwickshire, UK). The training device records each training session and data from each session were uploaded at the end of each week. The IMT group trained against a resistance set to 75% of PImax. Note that because IMT increases respiratory muscle strength, PImax typically will increase over the course of training and thus, it is necessary to set resistance to a new, higher training level at the end of each week. Subjects in the placebo group trained daily against a resistance set to 15% PImax.
Autonomic-Cardiovascular Assessment
BP was determined via sphygmomanometer and stethoscope at the brachial artery, in accordance with American Heart Association guidelines.15
Plasma samples were obtained to assay circulating dopa-mine, epinephrine, and norepinephrine content in a subset of participants (n = 7 per group, 14 total). Importantly, pretraining and posttraining blood draws were performed at the same time of day for each subject and, in accordance with previously published methods, subjects fasted overnight and were instructed to refrain from caffeine and over-the-counter pain or allergy medications for the 12 h leading up to the blood draw.16 Venous blood samples were collected between 07:00–10:00 from the antecubital region following 30 min of supine rest in a quiet, temperature-controlled room. Samples were placed on ice in lithium-heparin coated tubes (BD Vacutainer, Franklin Lakes, NJ), immediately centrifuged (4°C, 1,500 RPM, 15 min) and the plasma frozen at −80°C. Plasma samples were analyzed via quantitative high-performance liquid chromatography (Associated Regional and University Pathologists - ARUP Laboratories, Salt Lake City, UT).
Statistical Analyses
A general linear model analysis of variance was used to assess between-group differences in sleep, respiratory, cardiovascular, and immune parameters for the independent variables: sex, pretraining versus posttraining, and training group. Significance was set at P < 0.05. Within-group comparisons were performed using paired t-tests, with significance adjusted according to the Bonferroni correction.
RESULTS
Twenty-six subjects were consecutively recruited and randomized to IMT or placebo training groups. Two subjects were disqualified after commencing the study due to noncompliance with training, resulting in a retention rate of 92.3%. At intake, there were no between-group differences in sex, age, weight, height, systolic and diastolic BP, heart rate, plasma epinephrine, norepinephrine, AHI, apnea duration, oxygen desaturation, sleep architecture, sleep efficiency, sleep onset latency, arousals, limb movements, PSQI scores, or PImax (P > 0.1), nor was there any interaction between sex and any of the independent variables (P > 0.2) (Table 1).
Respiratory Measures
Spirometry values at intake were not different between IMT or placebo groups and nor were there any changes in spirometric measures pretraining versus posttraining for subjects in either group (P > 0.05). Average PImax in the IMT group increased by ∼33%, rising from 80.7 ± 7.1 to 107.3 ± 7.1 mmHg (P < 0.001), consistent with an increase in muscle strength. There was no change in the average PImax (pretraining 75.33 ± 12.3 mmHg versus posttraining 75.58 ± 14.98 mmHg) for the placebo group (P = 0.87) (Table 1).
Sleep and Sleep Quality
Despite significant gains in inspiratory muscle strength, subjects who performed IMT showed no improvement in nighttime AHI. Thus, no changes were found for measures of apnea frequency, apnea duration, severity of oxygen desaturations, total sleep time, sleep architecture, sleep efficiency, or latency to sleep onset (P > 0.1) pretraining versus posttraining for either IMT or placebo trained subjects. The percentage of obstructions that resulted in arterial oxygen saturation < 90% was not different between the two groups at intake and nor was any difference noted in desaturations pretraining versus post-training (81.8% and 87.6%, pretraining versus posttraining for the IMT group and 94.4% and 90.0%, pretraining versus post-training for the placebo group) (P > 0.3). Despite no change in AHI, IMT subjects registered fewer awakenings after sleep onset (P = 0.001), fewer arousals per hour of sleep (P < 0.001), and fewer periodic limb movements (P < 0.001) pretraining versus posttraining (Table 2). IMT subjects also reported better sleep quality preintervention versus postintervention (PSQI scores: 9.1 ± 0.9 versus 5.1 ± 0.7; P = 0.001) (Figure 1) whereas PSQI scores were unchanged in the placebo-training group. (Please refer to Table S1 in the supplemental material for individual subject observations).
Table 2.
Mean (± standard error of the mean) for parameters measured via overnight polysomnography, pretraining versus posttraining for each group.
Figure 1.
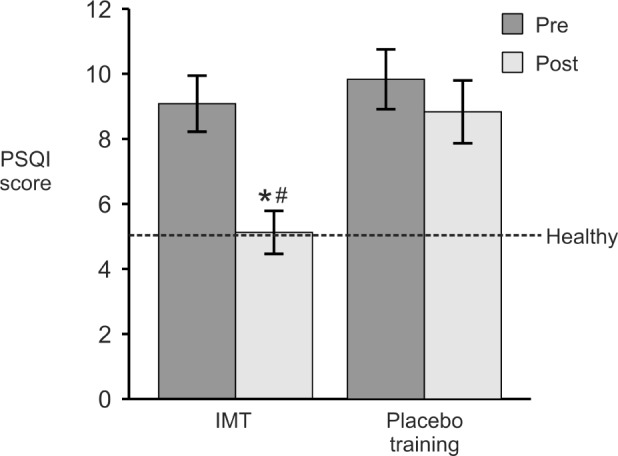
Mean (± standard error of the mean) Pittsburgh Sleep Quality Index (PSQI) scores pretraining versus posttraining (n = 12 per group). PSQI scores range from 0–21, with scores ≤ 5 considered indicative of healthy sleep. IMT, inspiratory muscle strength training. *P < 0.01, pretraining versus posttraining; # P < 0.01, relative to placebo group.
Blood Pressure
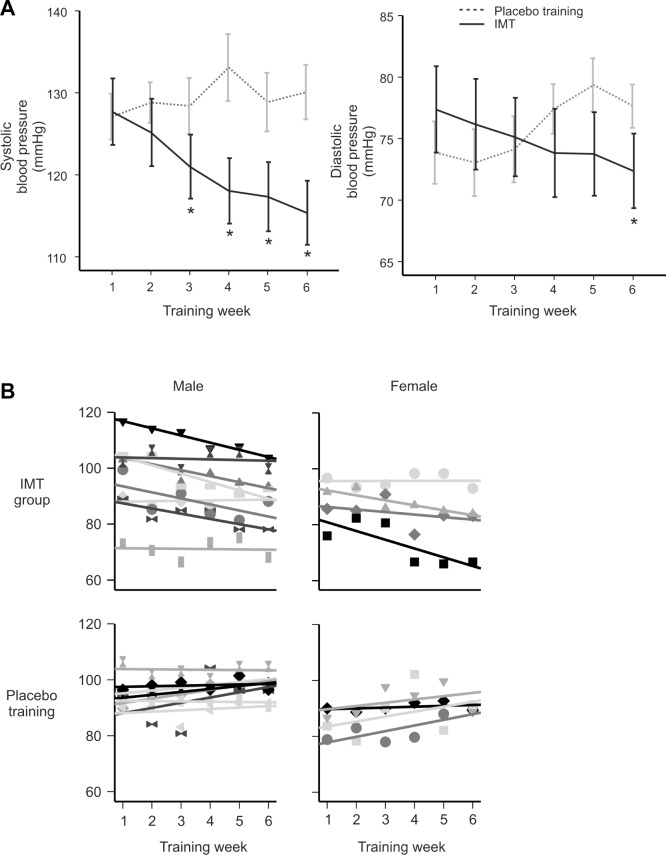
By study close, the IMT group had lower average systolic and diastolic BP (−12.3 ± 1.6 and −5.0 ± 1.3 mmHg, respectively) relative to pressures at intake (P < 0.01) (Table 3). The average weekly BP for both groups is presented in Figure 2, along with a breakdown of the results for male (n = 8) and female (n = 4) participants in each training group. As shown, systolic BP showed the most precipitous decline and attained significance by week 3. The decline in diastolic BP was more gradual and did not attain significance until the final week (P < 0.01). BP was unchanged in the placebo group (P > 0.05). There was no effect of training on resting heart rate for either group (IMT group: pretraining 73.5 ± 5.7 versus posttraining 76.8 ± 2.8; P = 0.35) and (placebo group: pretraining 72.8 ± 3.8 versus posttraining 73.9 ± 3.0; P = 0.62) (data not shown).
Table 3.
Mean (± standard error of the mean) blood pressure (n = 12 per group) and plasma catecholamines (n = 7 per group), pretraining versus posttraining.
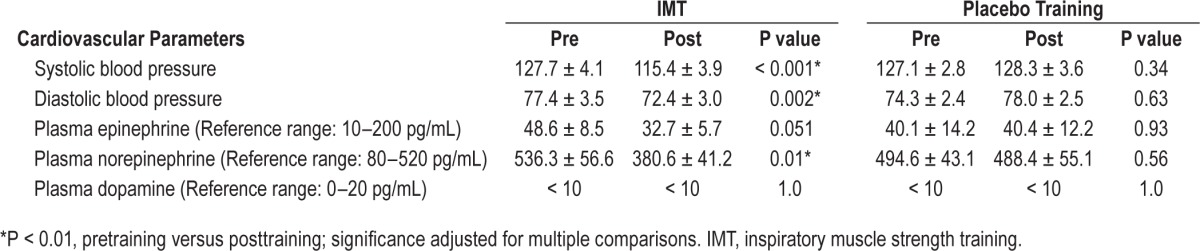
Figure 2.
(A) Average (± standard error of the mean) systolic and diastolic blood pressure (n = 12 per group). Note a significant decline in systolic blood pressure for the training group that begins in week 3. (*P < 0.01, relative to week 1). (B) Mean arterial blood pressure at the end of each training week for male and female subjects in IMT and placebo training groups. IMT, inspiratory muscle strength training
Plasma Catecholamines
There was no effect of either training protocol on plasma dopamine levels (P = 1.0). Plasma norepinephrine and epinephrine levels declined in the IMT group pretraining versus posttraining. Norepinephrine dropped by ∼30% (P = 0.01) and plasma epinephrine also declined but just failed to attain statistical significance (P = 0.051) (Table 3). There was no change in plasma epinephrine (P = 0.93) or norepinephrine (P = 0.56) for participants performing placebo training.
DISCUSSION
This is the first study to assess IMT in the context of OSA. Our results highlight a rapid course of improvement in BP, plasma catecholamine levels and sleep indices achieved with just 5 min of training each day. Importantly, for our understanding of the management of the disorder, these improvements occurred against a background of unchanged AHI demonstrating potential to mitigate cardiovascular risk in adults who are either noncompliant or intolerant of CPAP.
Inspiratory Muscle Training Protocol
The protocol implemented here falls within the range of published guidelines for IMT protocols in regard to the inspiratory effort level (e.g., 50% to 80% PImax) and training duration (e.g., 5 to 7 days/w, for 4 to 12 w).3,17–20 The resistance setting for the placebo group was set to 15% of PImax to approximate “paced” or slow breathing protocols21 and in our hands, placebo training had no effect on BP or plasma catecholamines in agreement with recent publications.22,23 In regard to the IMT group, one previous study also documented improved BP in hypertensive subjects after 8 w of training. In that case, training was for 20 min each day with inspiratory pressures set to ∼30%PImax.3 Here, we show a more rapid time course of improvement is attainable in subjects that typically have a limited capacity for more traditional forms of exercise24 with just 5 min of training each day and inspiratory pressures set to ∼75%PImax.
Because the intrathoracic pressures generated during IMT resemble pressures generated in sleep-disordered breathing, it is reasonable to ask how a stimulus might both mitigate and exacerbate sleep disruption and cardiovascular dysfunction. Certainly, IMT differs from sleep apnea in that the stimulus is (briefly) administered when the subject is awake and well oxygenated. By comparison, the pressure swings that are the hallmark of airway obstruction are sustained and give rise to hypoxemia and occur during sleep when BP and sympathetic activities normally attain their nadir. In this regard, the effects of IMT may be considered more analogous to traditional forms of aerobic exercise, wherein repeated acute increases in BP experienced during training result in its longer term reduction.16,19
Effects on Autonomic-Cardiovascular Function
At intake, mean arterial BP for both groups was comparable to BP documented previously in patients with mild and moderate OSA (AHI: 15–30, BP: 127.5/80)25 encompassing normotensive (n = 15), prehypertensive (n = 8), and hypertensive (n = 1) individuals. Accordingly, as a group, the subjects were considered pre-hypertensive for systolic BP and normotensive for diastolic BP. Plasma epinephrine (30–40 pg/mL) and norepinephrine (400–500 pg/mL) also were within range of reports in OSA.26 Whereas BP and plasma catecholamines were unchanged in the placebo group, systolic and diastolic BP in the IMT group (∼12 mmHg systolic and ∼5 mmHg diastolic) declined to levels that matched or exceeded those reported with medication alone (i.e., 2–6 mmHg over 5 y),27 or with CPAP.28 Most importantly, the approximate 30% reduction in plasma NE attained with IMT is equivalent to that attained with nighttime CPAP or following 4 mo of aerobic exercise training performed 60 min/day, three times a week, at 70% to 80% maximal heart rate.16 Interestingly, the decline in norepinephrine was independent of subject AHI (P = 0.997). We note here that plasma epinephrine levels also declined to an extent that likely is of physiological if not statistical significance (P = 0.051). Certainly these reductions may have contributed to long-term BP changes secondary to changes in volume regulation and vascular reactivity.
At this point we can only speculate as to the mechanism by which IMT improves BP. As we have documented previously, IMT lowers BP but does not affect resting heart rate3,6 and in this regard the effects of training are inconsistent with increased vagal modulation of heart rate and with results obtained with traditional aerobic training.16 Remarkably, despite there being no change in average AHI for either group, subjects in the IMT group registered fewer arousals, fewer periodic limb movements, and overall better sleep quality posttraining. Fewer nighttime arousals are consistent with improved sleep quality and because periodic limb movements are accompanied by surges in BP and heart rate29 and heightened sympathetic activation,30 their reduction may lower plasma catecholamines and vascular resistance.31 In view of the effect on plasma catacholamines and the robust link between norepinephrine, BP,32 and sympathetic nerve activity,33 it seems reasonable to consider that IMT modifies sympathetic output.34 To what extent that modification results from better sleep and/or training-related improvements in baroreflex sensitivity35 and/or arterial compliance remain to be determined.
Study Limitations
As with any investigation performed in human subjects and in a clinical population more specifically, the current experiment is not without limitations. We acknowledge that as a first study of its type conducted in subjects with OSA, we drew from the population broadly. Thus, whereas the total number of participants in each group represents the breadth of severity of OSA in the general population,36 the numbers of participants in each group is limited and our ability to discern differences in the effect of training as a function of disease severity, sex, and/or subject age is hampered as a result. BP measures were performed at the end of each week and as such, we are unable to report on the potential effect of training on day-to-day variability. Certainly, access to ambulatory BP monitoring would provide much-needed insight into the diurnal and nocturnal fluctuations in arterial BP over the course of the intervention.37,38 These limitations aside, the results provide strong rationale for future studies that reasonably might target a larger cohort of men and women with mild, moderate, and severe OSA that may/may not depend on nighttime CPAP.
CONCLUSION
OSA is a nighttime disorder characterized by repeated airway collapse that results in hypoxemia and frequent arousal from sleep. OSA coexists with hypertension in ∼50% of patients9 and with an increased risk for cardiovascular morbidity and mortality.11 Here we show that a simple and cost-effective respiratory training protocol holds promise as an adjunct treatment for hypertension secondary to OSA in patients who are unwilling or unable to tolerate CPAP. Subsequent studies reasonably should assess the effects of training on nocturnal BP, cardiac output, baroreflex sensitivity, and muscle sympathetic nervous system activity in an effort to pinpoint the mechanism(s) contributing to the improvement in BP. Important secondary (clinical) objectives reasonably should focus on determining the optimal training frequency (i.e., every day versus every other day or once a week) and the intensity of training (i.e., 75% versus 60% versus 50% PImax) for each segment (men versus women; mild versus moderate versus severe AHI) of this population.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by start-up monies (Department of Physiology, University of Arizona) awarded to Dr. Bailey and by The Finley and Florence Brown Pre-Doctoral Fellowship (Sarver Heart Center, The University of Arizona, College of Medicine) awarded to Dr. Vranish. The work does not entail off-label or investigational drug use. There are no conflicts of interest to declare. The authors have indicated no financial conflicts of interest. This work was performed in the Department of Physiology at The University of Arizona College of Medicine, Tucson, AZ.
ACKNOWLEDGMENTS
The authors thank Dr. Mark Borgstrom for assistance with statistical analyses and Claire DeLucia for assistance with experiments. We thank Dr. Sairam Parthasarathy and Carmine Martinez for assistance in completing blood draws and the Department of Pathology for assistance with plasma catcholamine analyses. Finally, we thank Dr. Richard (Dick) Bootzin (deceased) and all the members of the Sleep Research Laboratory in the Department of Psychology for completing the overnight sleep study and PSG evaluations and for providing critical feedback and support in the very early stages of this project. We acknowledge the support of the Sarver Heart Center (University of Arizona) and express our sincere thanks to Florence and Finley “Brownie” Brown for the Pre-Doctoral Scholarship awarded to Dr. Vranish. Both authors participated in conception and design of the experiments, collection, analysis, and interpretation of data, and draft and revision of the article.
REFERENCES
- 1.Brook RD, Appel LJ, Rubenfire M, et al. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the american heart association. Hypertension. 2013;61:1360–83. doi: 10.1161/HYP.0b013e318293645f. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2014;23:3–16. doi: 10.3109/08037051.2014.868629. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira JB, Plentz RD, Stein C, Casali KR, Arena R, Lago PD. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: a randomized controlled trial. International journal of cardiology. 2013;166:61–7. doi: 10.1016/j.ijcard.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 4.Jaenisch RB, Hentschke VS, Quagliotto E, et al. Respiratory muscle training improves hemodynamics, autonomic function, baroreceptor sensitivity, and respiratory mechanics in rats with heart failure. J Appl Physiol. 2011;111:1664–70. doi: 10.1152/japplphysiol.01245.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosselink R, Decramer M. Inspiratory muscle training: where are we? Eur Respir J. 1994;7:2103–5. doi: 10.1183/09031936.94.07122103. [DOI] [PubMed] [Google Scholar]
- 6.Vranish JR, Bailey EF. Daily respiratory training with large intrathoracic pressures, but not large lung volumes, lowers blood pressure in normotensive adults. Respir Physiol Neurobiol. 2015;216:63–9. doi: 10.1016/j.resp.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Arakawa K. Antihypertensive mechanism of exercise. Journal of hypertension. 1993;11:223–9. doi: 10.1097/00004872-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Halliwill JR. Mechanisms and clinical implications of post-exercise hypotension in humans. Exercise and sport sciences reviews. 2001;29:65–70. doi: 10.1097/00003677-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension: an update. Hypertension. 2014;63:203–9. doi: 10.1161/HYPERTENSIONAHA.113.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 11.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muxfeldt ES, Margallo V, Costa LM, et al. Effects of continuous positive airway pressure treatment on clinic and ambulatory blood pressures in patients with obstructive sleep apnea and resistant hypertension: a randomized controlled trial. Hypertension. 2015;65:736–42. doi: 10.1161/HYPERTENSIONAHA.114.04852. [DOI] [PubMed] [Google Scholar]
- 13.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama T, Mizuno T, Kojima M, et al. Criterion validity of the Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale for the diagnosis of sleep disorders. Sleep Med. 2014;15:422–9. doi: 10.1016/j.sleep.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) European heart journal. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 16.Duncan JJ, Farr JE, Upton SJ, Hagan RD, Oglesby ME, Blair SN. The effects of aerobic exercise on plasma catecholamines and blood pressure in patients with mild essential hypertension. JAMA. 1985;254:2609–13. [PubMed] [Google Scholar]
- 17.Held HE, Pendergast DR. The effects of respiratory muscle training on respiratory mechanics and energy cost. Respir Physiol Neurobiol. 2014;200:7–17. doi: 10.1016/j.resp.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Hostettler S, Illi SK, Mohler E, Aliverti A, Spengler CM. Chest wall volume changes during inspiratory loaded breathing. Respir Physiol Neurobiol. 2011;175:130–9. doi: 10.1016/j.resp.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 19.McConnell AK, Griffiths LA. Acute cardiorespiratory responses to inspiratory pressure threshold loading. Medicine and science in sports and exercise. 2010;42:1696–703. doi: 10.1249/MSS.0b013e3181d435cf. [DOI] [PubMed] [Google Scholar]
- 20.Ray AD, Pendergast DR, Lundgren CE. Respiratory muscle training reduces the work of breathing at depth. European journal of applied physiology. 2010;108:811–20. doi: 10.1007/s00421-009-1275-3. [DOI] [PubMed] [Google Scholar]
- 21.Landman GW, Drion I, van Hateren KJ, et al. Device-guided breathing as treatment for hypertension in type 2 diabetes mellitus: a randomized, double-blind, sham-controlled trial. JAMA internal medicine. 2013;173:1346–50. doi: 10.1001/jamainternmed.2013.6883. [DOI] [PubMed] [Google Scholar]
- 22.Landman GW, van Hateren KJ, van Dijk PR, et al. Efficacy of device-guided breathing for hypertension in blinded, randomized, active-controlled trials: a meta-analysis of individual patient data. JAMA internal medicine. 2014;174:1815–21. doi: 10.1001/jamainternmed.2014.4336. [DOI] [PubMed] [Google Scholar]
- 23.van Hateren KJ, Landman GW, Logtenberg SJ, Bilo HJ, Kleefstra N. Device-guided breathing exercises for the treatment of hypertension: an overview. World J Cardiol. 2014;6:277–82. doi: 10.4330/wjc.v6.i5.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beitler JR, Awad KM, Bakker JP, et al. Obstructive sleep apnea is associated with impaired exercise capacity: a cross-sectional study. J Clin Sleep Med. 2014;10:1199–204. doi: 10.5664/jcsm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strollo PJ, Jr., Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. The New England journal of medicine. 2014;370:139–49. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 26.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–8. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 27.Collins R, Peto R, MacMahon S, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–15. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 29.Pennestri MH, Montplaisir J, Fradette L, Lavigne G, Colombo R, Lanfranchi PA. Blood pressure changes associated with periodic leg movements during sleep in healthy subjects. Sleep Med. 2013;14:555–61. doi: 10.1016/j.sleep.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Bertisch SM, Muresan C, Schoerning L, Winkelman JW, Taylor JA. Impact of restless legs syndrome on cardiovascular autonomic control. Sleep. 2016;39:565–71. doi: 10.5665/sleep.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa Y, Kanbayashi T, Saito Y, et al. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep. 2003;26:986–9. doi: 10.1093/sleep/26.8.986. [DOI] [PubMed] [Google Scholar]
- 32.Shimada K, Kitazumi T, Sadakane N, Ogura H, Ozawa T. Age-related changes of baroreflex function, plasma norepinephrine, and blood pressure. Hypertension. 1985;7:113–7. doi: 10.1161/01.hyp.7.1.113. [DOI] [PubMed] [Google Scholar]
- 33.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- 34.Pinto P, Barbara C, Montserrat JM, et al. Effects of CPAP on nitrate and norepinephrine levels in severe and mild-moderate sleep apnea. BMC Pulm Med. 2013;13:13. doi: 10.1186/1471-2466-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Convertino VA, Ratliff DA, Ryan KL, et al. Effects of inspiratory impedance on the carotid-cardiac baroreflex response in humans. Clin Auton Res. 2004;14:240–8. doi: 10.1007/s10286-004-0180-4. [DOI] [PubMed] [Google Scholar]
- 36.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bixler EO, Kales A, Vela-Bueno A, Niklaus DE, Shubert DD, Soldatos CR. Nocturnal sleep and blood pressure in essential hypertension. Int J Neurosci. 1988;40:1–11. doi: 10.3109/00207458808985721. [DOI] [PubMed] [Google Scholar]
- 38.Grote L, Heitmann J, Kohler U, Penzel T, Peter JH, Wichert P. Assessment of the nocturnal blood pressure relative to sleep stages in patients with obstructive sleep apnea. Zeitschrift fur Kardiologie. 1996;85(Suppl 3):112–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.