Abstract
Study Objectives:
To evaluate changes in muscle and cerebral oxygenation during intermittent hypoxia (IH).
Methods:
Fifteen healthy subjects were exposed to 45-min IH (2-min cycles). Arterial blood oxygen saturation (SpO2), prefrontal cortex and brachial biceps muscle oxygenation (assessed by near-infrared spectroscopy), heart rate, and ventilation were continuously recorded.
Results:
During 2-min IH cycles, changes in SpO2 (9.2% ± 3.3%) were associated with significant changes in cortex oxygenation (3.2% ± 1.8%), minute ventilation, and heart rate, but no change in muscle oxygenation (0.2% ± 1.0%).
Conclusions:
Fluctuations of blood oxygen levels comparable to severe obstructive sleep apnea translate into distinct pattern of oxygenation changes in the muscle and cortex.
Citation:
Rupp T, Peyrard A, Tamisier R, Pepin JL, Verges S. Cerebral and muscle oxygenation during intermittent hypoxia exposure in healthy humans. SLEEP 2016;39(6):1197–1199.
Keywords: intermittent hypoxia, sleep apnea, tissue oxygenation, brain, muscle
Significance.
While the consequences of sleep apnea in terms of oxygenation are generally described at the arterial blood level (i.e. intermittent hypoxemia), data from animal models suggest that changes in oxygenation may differ between tissues. In a model of intermittent hypoxia in healthy humans, the present study demonstrates that intermittent arterial hypoxemia translates into significant oscillations in cerebral oxygenation but no significant change in muscle oxygenation. These data emphasize differences between organs regarding changes in oxygenation during intermittent hypoxemia and indicate that some tissues such as the brain may be specifically vulnerable to intermittent hypoxemia. This should be taken into account when considering the pathophysiological consequences of sleep apnea.
INTRODUCTION
Obstructive sleep apnea (OSA) is associated with two major harmful consequences—cardiometabolic comorbidities and neurocognitive impairments. Intermittent hypoxia (IH) exposure leads to tissue remodeling owing to local oxidative stress and inflammation.1,2 Clinical and experimental literature have generally assumed that repetitive blood oxygen desaturation-reoxygenation sequences, which is the OSA hallmark, translate into similar patterns of hypoxia-reoxygenation in different organs and tissues, then triggering oxidative stress, inflammation and consequently local insulin resistance and cerebral impairments.1,3 However, while IH in OSA patients is routinely characterized by pulse oximetry, how changes in arterial blood oxygen saturation (SpO2) convert into changes in tissue oxygenation remains relatively unknown. This might strongly influence our understanding of the organ-specific responses to IH leading to cardiometabolic dysfunctions in OSA.4
Tissue oxygenation during IH has been studied by invasive measurements in animal models showing significant oxygen swings in the brain and skeletal muscle, whereas the amplitude of the swings is attenuated in visceral fat.5 Reinke et al. established that IH generates large hypoxia-reoxygenation swings in the liver and smaller amplitude swings in skeletal muscle, whereas white adipose tissue exhibits a sustained hypoxic pattern.6 The differences in tissue oxygenation during IH in human have never been investigated.
Experimental IH exposure in healthy human provides the opportunity to address OSA pathophysiological mechanisms without confounding factors such as age, obesity, and cardio-metabolic comorbidities. IH exposure during sleep in healthy subjects has been shown, for instance, to increase 24-hour blood pressure and sympathetic activity.7 The same model of IH exposure is used here to describe the effect of IH on muscle and cerebral oxygenation in awake healthy subjects.
METHODS
Fifteen healthy male subjects (age: 26 ± 5 years, weight: 73 ± 12 kg, height: 176 ± 11 cm) were studied. All subjects were non-smokers and had no history of cardiometabolic disease. The study was approved by the local ethics committee and performed according to the Declaration of Helsinki; subjects gave their written consent.
Subjects were exposed for 45 min to IH (inspiratory oxygen fraction, FiO2 = 12% with 20 s of oxygen administration at 2 L/ min every 2 min, as previously described7). The hypoxic gas mixture was delivered by an IsoCap-Altitrainer 200 (SMTEC, Nyon, Switzerland) via a facemask while oxygen was administered via a nasal cannula. Earlobe SpO2, prefrontal cortex and brachial biceps muscle near-infrared spectroscopy (NIRS) signals, heart rate, and ventilation (Medisoft, Dinant, Belgium) were continuously recorded.
Oxy[O2Hb]-, deoxy[HHb]-, total-hemoglobin[HbTot] concentration changes and tissue saturation index (TSI) were estimated using a multichannel NIRS system (Oxymon MkIII, Artinis Medical Systems, the Netherlands) as previously described.8
Data from all 2-min IH cycles were averaged and analyzed over twelve 10-s averaged periods. One- (time) or two-way (time × tissue) repeated-measures ANOVA were used to detect changes over the 2-min IH cycle, with Fischer LSD tests for post hoc analysis. A two-tailed alpha level of 0.05 was used as the cutoff for significance. All data are presented as mean values ± standard deviation.
RESULTS
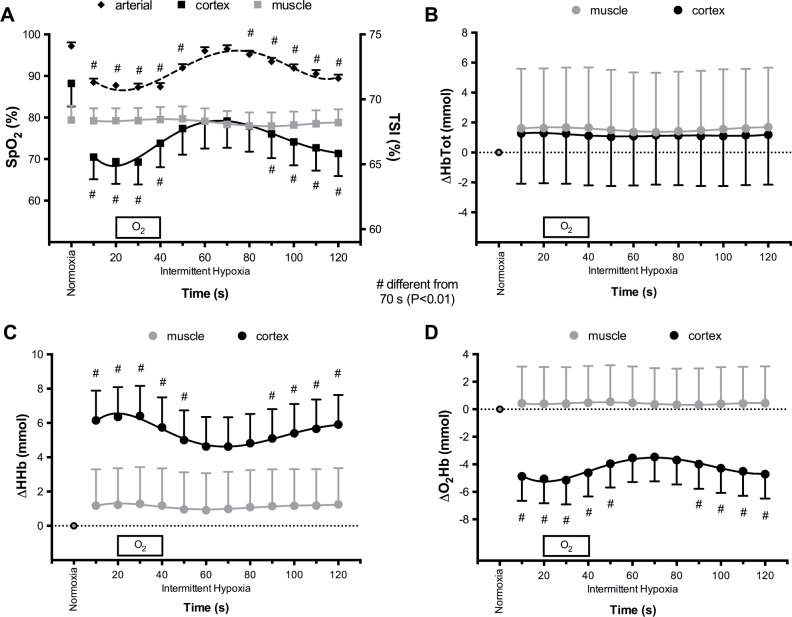
Changes in SpO2, prefrontal cortex and muscle NIRS variables during IH are shown in Figure 1A–1D. During 2-min IH, SpO2 showed significant oscillations of 9.2% ± 3.3% on average. Mean values of prefrontal cortex NIRS variables during the 2-min IH cycles were all significantly different compared to normoxic baseline except HbTot. Conversely, mean values of muscle NIRS variables during IH were not significantly modified compared to normoxic baseline. Prefrontal cortex O2Hb, HHb, and TSI exhibited significant oscillation over the 2-min IH cycles, while no significant swing in muscle NIRS variables was observed.
Figure 1.
Changes in arterial blood, prefrontal cortex, and brachial biceps muscle oxygenation during 2-min intermittent hypoxia cycles. (A) Arterial (SpO2), cortex and muscle oxygen (TSI) saturation. (B) Changes in total hemoglobin concentration (HbTot). (C) Changes in deoxyhemoglobin concentration (HHb). (D) Changes in oxyhemoglobin concentration (O2Hb). Only arterial oxygen saturation, prefrontal cortex oxygen saturation, HHb and O2Hb were significantly different during intermittent hypoxia compared to normoxia. #Time point during intermittent hypoxia significantly different from 70 s (P < 0.01).
During 2-min IH cycles, significant changes in minute ventilation (minimum 8.6 ± 1.7 L/min, maximum 12.8 ± 2.4 L/min; P < 0.05), end-tidal CO2 partial pressure (minimum 32.6 ± 3.9 mm Hg, maximum 37.1 ± 4.7 mm Hg; P < 0.05) and heart rate (minimum 62 ± 6 bpm, maximum 70 ± 9 bpm; P < 0.05) were observed.
DISCUSSION
To the best of our knowledge, this is the first report of simultaneous muscle and cerebral oxygenation during IH exposure in healthy human. The present data demonstrate that fluctuations of blood oxygen levels comparable to severe OSA translate into distinct pattern of oxygenation changes in the muscle and cortex. While prefrontal cortex exhibited large oxygenation swings, muscle oxygenation did not change. Moreover, while average cortex oxygenation was reduced during IH compared to normoxia, muscle oxygenation during IH did not differ from normoxia.
These striking differences between tissues may arise from several potential mechanisms. First, cerebral basal metabolism is higher compared to the muscle at rest, and changes in arterial oxygen supply may have greater impact in tissue with larger metabolic activity. Second, additional capillary recruitment is possible at the muscle level but this mechanism is not available to improve cerebral perfusion and oxygen delivery when arterial oxygen content is reduced or metabolic requirements are increased. This mechanism might allow the muscle to compensate for reduced arterial oxygen delivery more efficiently than the brain. Third, cerebral perfusion is highly sensitive to changes in arterial CO2, and one could suggest that IH-induced hypocapnia may contribute to the reduction in cerebral blood flow and consequently cerebral oxygenation. The unchanged total hemoglobin concentration during IH (Figure 1B) does not suggest, however, that local prefrontal cortex blood volume was modified. Almendros et al. measured brain tissue oxygenation by using a microelectrode in rats exposed to obstructive apneas or IH.5 Oscillations in brain oxygenation occurred in both conditions but at higher oxygenation levels during obstructive apneas, probably because of hypercapnia-induced vasodilation associated with obstructive apneas. Interestingly, reduced cerebral glutathione and increased lipid peroxidation were associated with obstructive apneas only. Hence, while the present study evaluates cerebral oxygenation during IH for the first time in human, future studies should address the combined cerebral effects of IH and hypercapnia in human.
The pattern of prefrontal cortex and muscle oxygenation during IH in the present study suggest that hypoxia-reoxygenation stress may be larger at the cortex level compared to the muscle. As a consequence, greater oxidative stress might occur explaining, at least in part, the cerebral impairments commonly reported in OSA1 and the neuroinflammation and microglial activation reported in rodent models.2,9 Neurocognitive impairments represent the main complaint of OSA patients but the link with classical markers of OSA severity is weak. Direct measurement of brain hypoxia might be relevant to explain different susceptibilities to IH regarding neurocognitive dysfunction. Our data showing no change in oxygenation levels in the muscle suggest that this organ might be relatively protected against deleterious IH consequences such as insulin resistance. Previous results in animal IH models reported significant oscillations in muscle tissue oxygenation.5,6 This apparent discrepancy may due to (i) different types of muscle oxygenation measurement, i.e., noninvasive NIRS measures oxy/deoxy-hemoglobin concentration changes occurring in the tissue microcirculation while previous studies in animal IH models used invasive microelectrodes measuring oxygen tension directly into the tissue5,6; and (ii) more severe intermittent hypoxemia in previous animal models compared to the present study in humans.
While the present report describes tissue oxygenation during acute hypoxic exposure, it is conceivable that chronic IH during sleep would result in different tissue oxygenation profile. The present results might also be modulated by comorbidities commonly associated with OSA and ageing.6,10 Adipose tissue and circulating free fatty acids are, for instance, likely to influence IH tissue responses in obese OSA patients.6
DISCLOSURE STATEMENT
This work was supported by the “Fond de dotation AGIR pour les maladies chroniques.” The authors have indicated no financial conflicts of interest. This work was performed at Univ. Grenoble Alpes, HP2 Laboratory.
ACKNOWLEDGMENTS
Author contributions: Drs. Rupp, Tamisier, Pepin, and Verges conceived and designed the study; Dr. Rupp, Peyrard and Verges performed the experiment and analyzed the data; Dr. Rupp, Peyrard, Tamisier, Pepin, and Verges interpreted the data and wrote the manuscript; Dr. Rupp, Peyrard, Tamisier, Pepin, and Verges approved the final version of the manuscript.
REFERENCES
- 1.Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia - Revisited - The bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45. doi: 10.1016/j.smrv.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Sapin E, Peyron C, Roche F, et al. Chronic intermittent hypoxia induces chronic low-grade neuroinflammation in the dorsal hippocampus of mice. Sleep. 2015;38:1537–46. doi: 10.5665/sleep.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briancon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr. 2015;7:25. doi: 10.1186/s13098-015-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepin JL, Tamisier R, Levy P. Obstructive sleep apnoea and metabolic syndrome: put CPAP efficacy in a more realistic perspective. Thorax. 2012;67:1025–7. doi: 10.1136/thoraxjnl-2012-202807. [DOI] [PubMed] [Google Scholar]
- 5.Almendros I, Farre R, Planas AM, et al. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep. 2011;34:1127–33. doi: 10.5665/SLEEP.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol. 2011;111:881–90. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamisier R, Pepin JL, Remy J, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37:119–28. doi: 10.1183/09031936.00204209. [DOI] [PubMed] [Google Scholar]
- 8.Rupp T, Leti T, Jubeau M, et al. Tissue deoxygenation kinetics induced by prolonged hypoxic exposure in healthy humans at rest. J Biomed Optics. 2013;18:095002. doi: 10.1117/1.JBO.18.9.095002. [DOI] [PubMed] [Google Scholar]
- 9.Zhang SX, Wang Y, Gozal D. Pathological consequences of intermittent hypoxia in the central nervous system. Compr Physiol. 2012;2:1767–77. doi: 10.1002/cphy.c100060. [DOI] [PubMed] [Google Scholar]
- 10.Dalmases M, Torres M, Marquez-Kisinousky L, et al. Brain tissue hypoxia and oxidative stress induced by obstructive apneas is different in young and aged rats. Sleep. 2014;37:1249–56. doi: 10.5665/sleep.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]