Abstract
Study Objectives:
Pediatric type 1 narcolepsy is often challenging to diagnose and remains largely undiagnosed. Excessive daytime sleepiness, disrupted nocturnal sleep, and a peculiar phenotype of cataplexy are the prominent features. The knowledge available about the regulation of circadian rhythms in affected children is scarce. This study compared circadian rest-activity rhythm and actigraphic estimated sleep measures of children with type 1 narcolepsy versus healthy controls.
Methods:
Twenty-two drug-naïve type 1 narcolepsy children and 21 age- and sex- matched controls were monitored for seven days during the school week by actigraphy. Circadian activity rhythms were analyzed through functional linear modeling; nocturnal and diurnal sleep measures were estimated from activity using a validated algorithm.
Results:
Children with type 1 narcolepsy presented an altered rest-activity rhythm characterized by enhanced motor activity throughout the night and blunted activity in the first afternoon. No difference was found between children with type 1 narcolepsy and controls in the timing of the circadian phase. Actigraphic sleep measures showed good discriminant capabilities in assessing type 1 narcolepsy nycthemeral disruption.
Conclusions:
Actigraphy reliably renders the nycthemeral disruption typical of narcolepsy type 1 in drug-naïve children with recent disease onset, indicating the sensibility of actigraphic assessment in the diagnostic work-up of childhood narcolepsy type 1.
Citation:
Filardi M, Pizza F, Bruni O, Natale V, Plazzi G. Circadian rest-activity rhythm in pediatric type 1 narcolepsy. SLEEP 2016;39(6):1241–1247.
Keywords: actigraphy, circadian rhythms, motor activity, narcolepsy, pediatrics
Significance.
Circadian rest-activity rhythm is altered in pediatric type 1 narcolepsy, showing nighttime increased activity, and a decrease of activity in the early afternoon hours. Recording motor activity by actigraphy promises to be a reliable objective marker in the complex diagnostic pathway of type 1 narcolepsy in children. This simple and cheap screening could help to improve diagnosis, and may prove useful to assess disease severity and ecologically monitor treatments response.
INTRODUCTION
Type 1 narcolepsy (NT1) is a lifelong central nervous system disorder characterized by chronic hypersomnolence with multiple sleep attacks during daytime, cataplexy (sudden and transient loss of muscular tone usually evoked by emotions), dissociated rapid eye movement (REM) sleep manifestations such as sleep paralysis (temporary inability to move voluntary muscles) and/or hallucinations at the wake-sleep transition, and nocturnal sleep disruption.1,2 The disease is linked to the loss of hypothalamic hypocretin-producing neurons, which leads to cerebrospinal fluid (CSF) hypocretin-1 (hcrt-1) deficiency.1,3
NT1 is regarded as rare with estimated prevalence between 25 and 50 per 100,000 in the general population4; however, a large proportion of the expected cases are undiagnosed or remain misdiagnosed in both adults and children.5,6 Until recently, NT1 has been poorly recognized in children, although most patients report the onset of symptoms in childhood and adolescence.5 Indeed, the epidemiological data on pediatric NT1 are scarce7; nevertheless, the improving disease awareness and the recent peak of incidence after H1N1 influenza pandemic and vaccine has led to a relevant increase in NT1 diagnoses in children and adolescents.8,9
Diagnosis of childhood NT1 often remains challenging, especially close to symptom onset, given the frequent paradoxical presentation of hypersomnolence as hyperactivity and the peculiar cataplexy phenotype with persistent and spontaneous hypotonic features and falls, intermingled with active movements and further enhanced by emotions.10–12 This variable clinical presentation, and the still-scarce awareness about this peculiar feature, has frequently led to misdiagnosis with behavioral, psychiatric, or neurological disorders, further delaying proper diagnosis and treatment.6,13
Recently, several studies have highlighted that actigraphy, an objective method for quantitative assessment of motor activity and indirect assessment of sleep based on wearable technology, offers unique potentialities in the clinical work-up of adult NT1 cases.14,15 Actigraphic monitoring discriminated NT1 from other central hypersomnias in patients and healthy controls,14 and quantified treatment response to wake-promoting drugs and sodium oxybate.16,17
In addition to sleep assessment,18 prolonged actigraphic monitoring also offers a way to evaluate the robustness of the endogenous rhythms driven and synchronized by the master circadian pacemaker through direct evaluation of rest-activity rhythm.19 To date only a limited number of studies has investigated circadian rhythms in NT1, describing increased daytime secretion of melatonin,20,21 whereas circadian rhythms of core body temperature and cortisol were essentially preserved.22 However, these studies examined exclusively adult patients with a long disease history.
The main purpose of the current study is to investigate the rest-activity rhythm in pediatric NT1 patients versus healthy children by means of actigraphy. Actigraphy is a noninvasive method that provides circadian measures collected in the subject's natural environment, thus representing a particularly valuable approach to assess rest\activity behavior among pediatric populations.23,24 Furthermore, prolonged actigraphic monitoring allows the extraction of densely recorded time series of motor activity suitable to be analyzed with advanced techniques for data modeling.25 The secondary aim of the study is to compare actigraphic estimated nocturnal and diurnal sleep measures of NT1 children with age- and sex- matched controls to explore whether actigraphic assessment shows good discriminant capability in pediatric NT1 cases, together with the analyses of correlates between clinical (body mass index [BMI] and hcrt-1), neurophysiological, and actigraphic-derived sleep measures.
METHODS
Subjects
The study included 22 patients, drug-naïve children and adolescents (10 males, mean age 12.09 ± 2.37 y, range 7–15 y), with a final diagnosis of NT1 evaluated at the Outpatient Clinic for Narcolepsy of the Department of Biomedical and Neuro-motor Sciences, University of Bologna from January 2012 to September 2013.
Patients underwent the following diagnostic procedures: clinical assessment, at-home actigraphic monitoring, cerebral magnetic resonance imaging (to rule out secondary cases), and then hospitalization with 48-h continuous polysomnographic (PSG) recording, multiple sleep latency test (MSLT), cataplexy video documentation, human leukocyte antigen (HLA) typing to confirm DQB1*06:02 haplotype and, whenever possible, CSF hcrt-1 assay.26
Clinical evaluation was systematically conducted by the same sleep specialist (GP); it included the assessment of subjective sleepiness with the Epworth Sleepiness Scale adapted for children and adolescents (aESS),27 and of circadian preferences by means of the Italian version of the reduced Morningness-Eveningness questionnaire for Children and Adolescents (rMEQ-CA).28
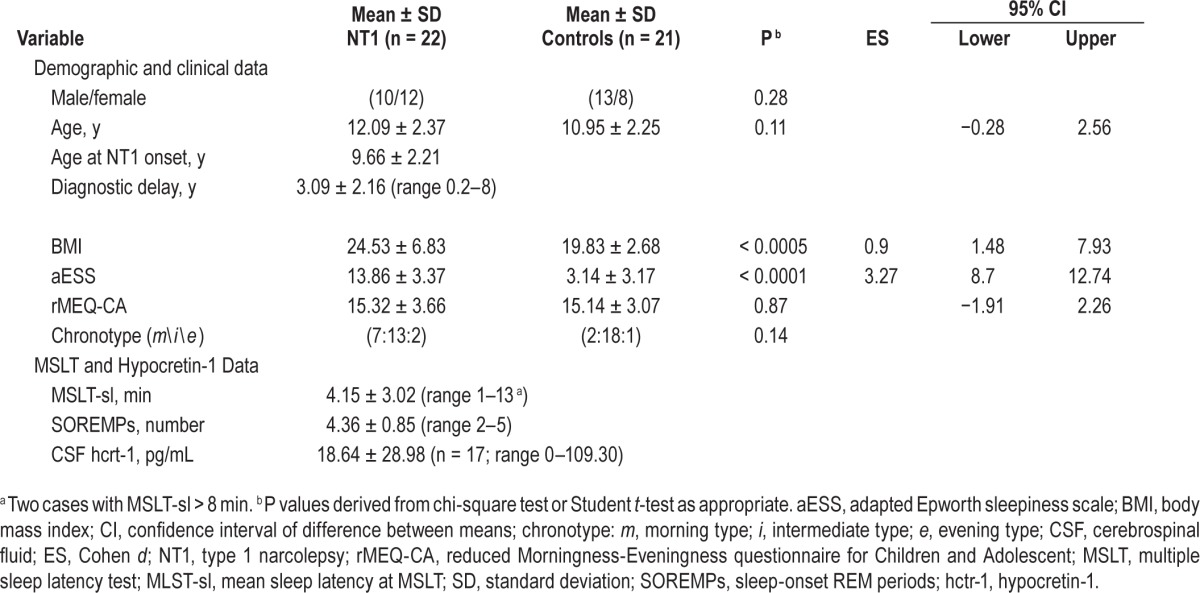
All patients fulfilled the current International Classification of Sleep Disorders, Third Edition clinical criteria for NT1 presenting severe cataplexy (n = 22/22) and daytime sleepiness (aESS score: 13.86 ± 3.37). Twenty of 22 cases had mean MSLT sleep latency (MSLT-sl) < 8 min with multiple sleep-onset REM periods (SOREMPs). Seventeen patients (including the two cases with MSLT-sl > 8 min) underwent lumbar puncture and all had low (≤ 110 pg/mL) or undetectable CSF hcrt-1 levels; all patients carried the HLA DQB1*06:02 allele.
Twenty-one age- and sex-matched healthy children (13 males, mean age 10.95 ± 2.25 y, range 7–16 y), recruited at a school in Rome, were selected from the anonymous database of the Pediatric Sleep University Center, Sapienza University, Rome. This series of children belong to the same group of controls used in a previously published study.29
The study was approved by the internal review board and written informed consent was signed by parents of children.
Procedure
Rest-activity rhythm was monitored during the school week, outside of holidays and vacation. Participants were required to wear the actigraph on the nondominant wrist for 1 w (before hospitalization for NT1 patients), providing five complete nycthemeral cycles, which are necessary to obtain a reliable description of sleep and rest-activity rhythm in children.30
The Micro Motionlogger Watch actigraph (Ambulatory Monitoring, Inc, Ardsley, NY), consisting of a triaxial accelerometer with case temperature and ambient light sensors, was used in the current study. Actigraphs quantify motor activity exceeding 0.01g at a sampling frequency of 32 Hz, the values for each sample are used to compute the average activity counts within the chosen time window (epoch). Devices were initialized for zero-crossing mode to collect data in 1-min epochs in accordance with the practice parameters for the use of actigraphy.23
Participants were asked to maintain their usual sleep/wake schedule during the recording period. Children wore the device continuously throughout the 24 h, except when bathing/ showering, and were instructed to push the event-marker button on the device to mark time in and out of bed.
In addition, a sleep diary was used to obtain subjective information from the children, for children younger than 11 y (i.e. five NT1 and 9 control children) we asked parents/caregivers to fill in daily the sleep diary and help them with the event-marker procedure, if necessary.
The information contained in the sleep diary included the bedtime, the wake time, and the rise time; additionally, we asked to record events that might bias the actigraphic recording such as periods of device removal.
Actigraphic recording was visually edited by an experienced scorer who used the information provided by event-marker points and sleep dairy to identify the major nocturnal sleep period. Periods of device removal detailed in the diary were further verified from the case temperature channel and excluded from analysis.
Circadian Rest-Activity Rhythm and Actigraphic Sleep Assessment
For circadian motor activity analysis we extracted raw activity data per minute (time series) using Action 4 software version 1.16 (Ambulatory Monitoring, Inc) and processed them with R statistical software to apply Functional Linear Modeling (FLM) according to the model put forth by Wang and coauthors.31 FLM belongs to a broader family of statistical techniques (Functional Data Analysis) that represent observations arising from time-series in the form of functions.25,32 This approach allows the analysis of the circadian features of motor activity through direct analysis of raw activity data. FLM replaces the motor activity counts with a function that models the data, reduces variability, and compares sets of functions to explore whether and when they statistically differ between groups.
We considered only data from 20:00 Sunday to 20:00 Friday, in order to avoid possible variations related to weekend days. Activity gaps during the daytime due to device removal were filled up with average activity values from the same time period of the remaining days; days containing gaps longer than 1 h were excluded from analysis.
The five continuous nycthemeral cycles of actigraphic data were averaged into a single 24-h motor activity pattern and converted into a function adopting a Fourier expansion model with n = 9 basis permutation fitted at a 24-h periodicity.
Actigraphic estimated sleep measures were computed with the Action W-2 software version 2.7.1 (Ambulatory Monitoring, Inc); this software identified each epoch as sleep or wake using the mathematical model validated by Sadeh et al.33
Actigraphic recordings were divided into nighttime and daytime periods according to individual bedtime and wake-up time; mean daytime and nighttime parameters were computed across the school week for each subject.
We considered the following actigraphic measures for sleep timing: bed time (BT – clock time, in hours and minutes, when subject goes to bed and turns off the light), get up time (GUT – clock time, in hours and minutes, when subject gets out of bed in the morning), time in bed (TIB – time, in minutes, from BT to GUT), and midpoint of sleep (MS – clock time, in hours and minutes, that split in half the TIB). For the nighttime period the following measures were considered: estimated sleep onset latency (eSOL – interval, in minutes, between BT and sleep onset, the latter determined as the first epoch of a block of 20 consecutive min after BT with no more than one epoch scored as wake); estimated total sleep time (eTST – sum, in minutes, of all sleep epochs between sleep onset and GUT); estimated wake after sleep onset (eWASO – sum of minutes scored as wake between sleep onset and GUT); estimated sleep efficiency (eSE% – the ratio of TST to TIB multiplied by 100); number of estimated awakenings (eAwk – number of wake episodes between sleep onset and GUT); estimated awakenings lasting more than 5 consecutive min (eAwk > 5); and sleep motor activity (SMA – sum of all activity counts in 1-min epochs during TIB divided by TIB duration in minutes). From the daytime period we considered the following measures: daytime motor activity (DMA – sum of all activity counts in 1-min epochs for the time period between GUT and BT divided by its duration in minutes); daytime estimated total sleep time (eDTST – sum of minutes scored as sleep between GUT and BT); estimated nap frequency (eNap – number of sleep episodes between GUT and BT, where nap is defined as an interval of at least 10 min up to 3 h scored as sleep, preceded and followed by a period of at least 30 continuous min scored as wake); and mean duration of longest estimated nap (eNapD – mean duration, in minutes, of the longest daytime sleep episode).
Statistical Analysis
All continuous and categorical data were explored with descriptive (mean ± standard deviation) and frequency statistics for each group. Differences between groups in demographical data, BMI, and scale scores were analyzed with chi-square and independent samples t-test.
Circadian activity patterns analysis was undertaken with R and the Actigraphy library in R; FLM was used to test differences in the time-course of motor activity between groups.
For each actigraphic measure independent sample t-tests were performed to compare NT1 and control children, followed by effect size (Cohen d) computation.34
Finally, the relationship between clinical data (BMI and Hcrt-1 levels), questionnaire scores (aESS and rMEQCA), neurophysiological measures (MSLT-sl and number of SOREMPs), and actigraphic-derived sleep measures were explored, separately for each group, with Pearson correlation coefficient analyses.
Statistical analyses were conducted using SPSS 19.0 (SPSS, Inc. Chicago, IL). Results with P < 0.05 were considered statistically significant.
RESULTS
Table 1 shows demographic, clinical, and neurophysiological characteristics of the sample and questionnaire scores. Detailed nocturnal PSG features of the NT1 sample are reported in the Table S1 in the supplemental material. NT1 patients displayed higher aESS scores and BMI than controls without differing in the distribution of circadian typologies or rMEQCA scores.
Table 1.
Demographics, scale scores, neurophysiological data, and hypocretin-1 levels of children with type 1 narcolepsy and healthy controls.
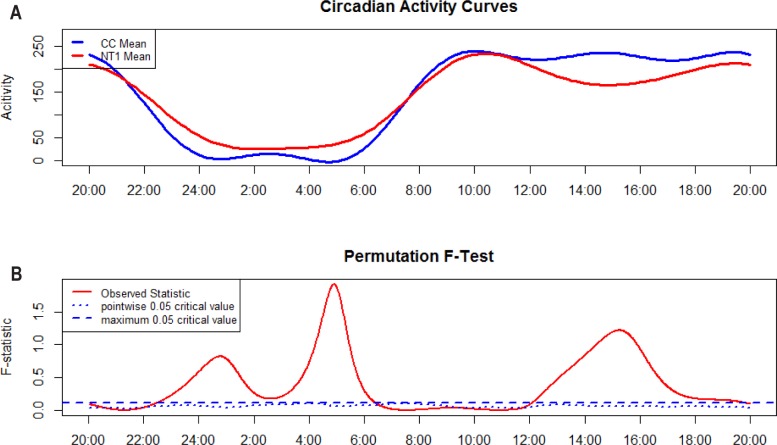
Circadian mean motor activity profiles of each group resulting from Fourier expansion are shown in Figure 1 (upper panel) together with F-statistics results (lower panel). Where the observed statistic (i.e., the red solid line) is above the blue dashed line (i.e., global critical test of significance with α = 0.05) the groups have statistically different activity counts at that specific time point (1 min time window). Both groups showed a preserved overall structure, with lower activity during night hours and higher activity during daytime, and comparable TIB. NT1 patients had significantly higher motor activity throughout nighttime (from 23:00 until 06:00), similar motor activity during morning between 07:00 and 12:00, and a significantly marked decrease of motor activity in the afternoon starting from 12:00 until approximately 18:00, with no further differences from the latter time to 23:00 compared to controls.
Figure 1.
Functional linear modeling for NT1 and controls. Plot (A) shows estimated activity patterns for the two groups. Plot (B) shows F-test result, the red curve represents the observed statistic, and the blue dashed and dotted lines correspond to a global and point-wise test of significance at α = 0.05. NT1, type 1 narcolepsy; CC, controls.
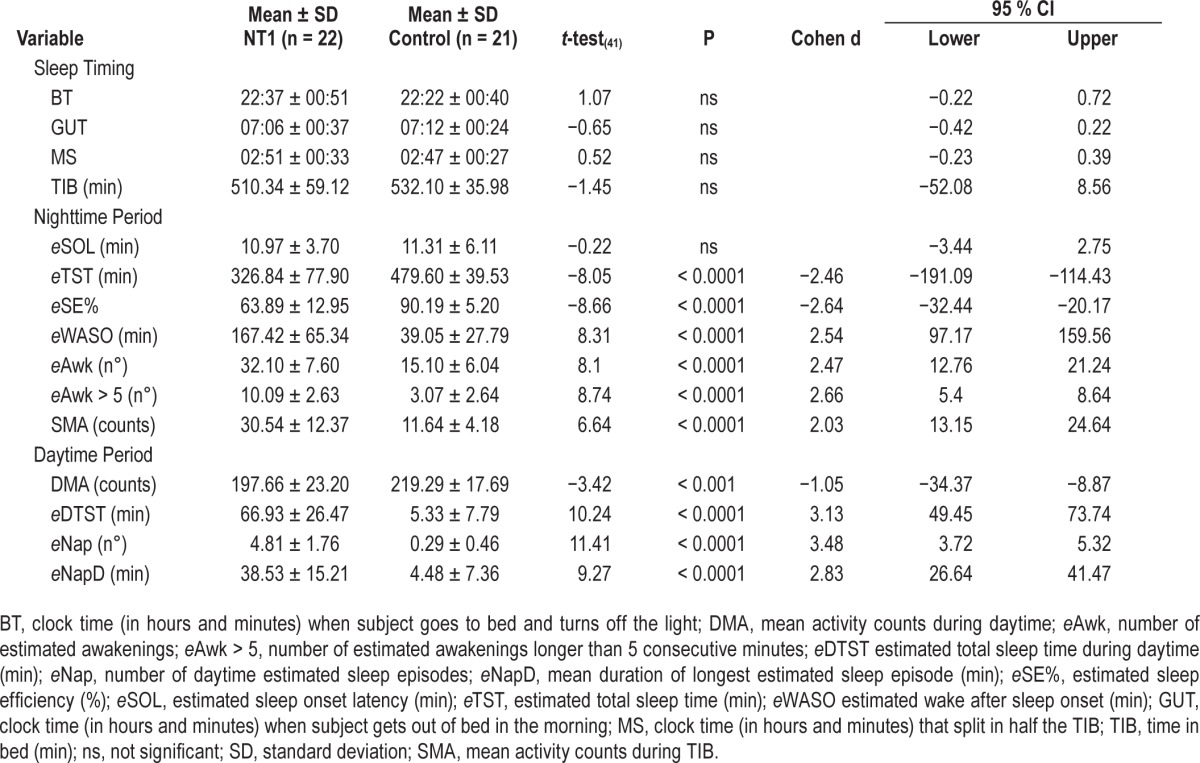
Actigraphic sleep measures are reported in Table 2, together with significance values at t-test, Cohen d, and 95% confidence intervals (CI). NT1 patients and controls went to bed (BT) and woke up (GUT) at similar time, thus displaying comparable sleep phase. All actigraphic nighttime measures except TIB, eSOL, and sleep timing differed between the two groups: NT1 patients slept less during nighttime (eTST), displayed lower eSE% with increased frequency of sleep interruptions (eAwk) and prolonged awakenings (eAwk > 5), higher amount of eWASO, and enhanced SMA than controls. During daytime NT1 children had more eDTST with more frequent eNap occurrence, longer nap duration (eNapD), and lower motor activity during wakefulness (DMA) than controls.
Table 2.
Actigraphic nighttime, daytime, and sleep timing measures (means and standard deviation) of children with type 1 narcolepsy and healthy controls.
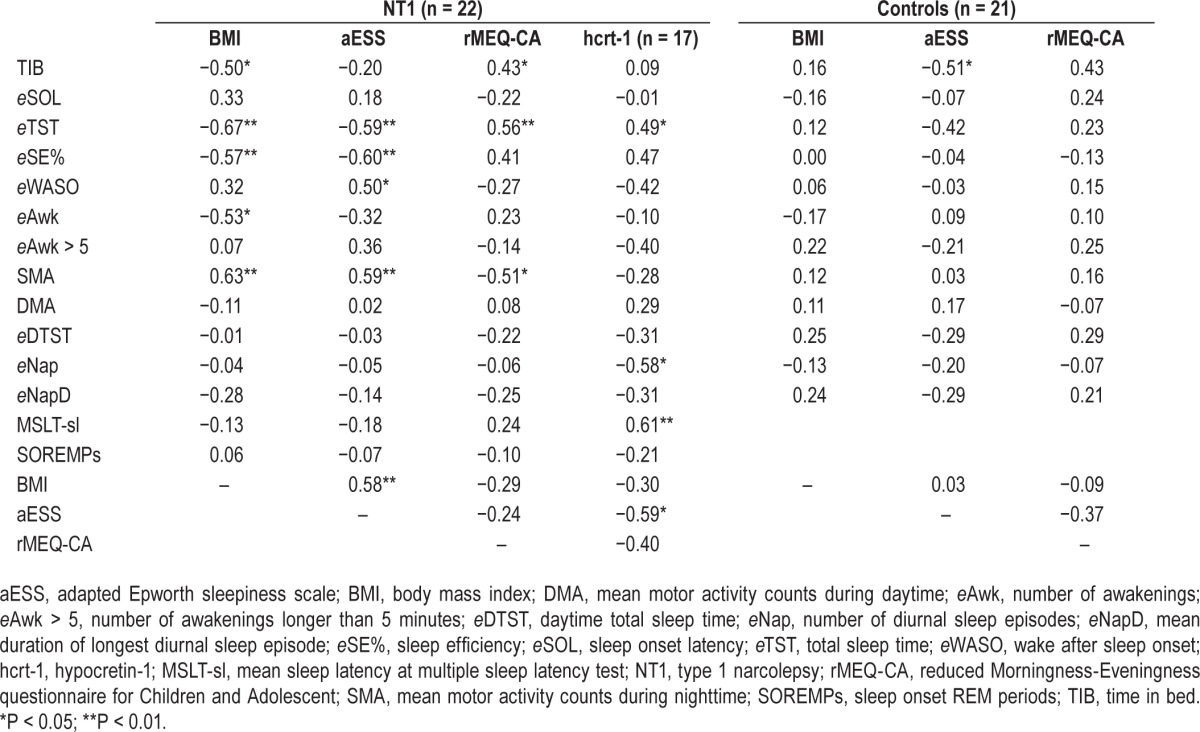
Pearson correlation analyses are reported in Table 3 for NT1 patients and controls. In the NT1 group we found that: (1) BMI was positively correlated with aESS and SMA, and negatively with TIB, eTST, eSE%, eAwk, and DMA; (2) aESS was directly related to SMA and eWASO, and inversely related to eTST, and eSE%; (3) rMEQ-CA was positively correlated with TIB and eTST, and negatively correlated with SMA; and (4) CSF hcrt-1 level was positively correlated with eTST and MSLT-sl, and negatively with eNap and aESS. In the control group, only a negative correlation between aESS and TIB reached statistical significance.
Table 3.
Pearson correlations between clinical, neurophysiological data, scale scores, and actigraphic-derived measures.
DISCUSSION
Our study was the first specifically aimed at analyzing rest-activity rhythm in a sizeable group of drug-naïve NT1 children, monitored in real-life setting during the school week. We found that, despite a comparable sleep phase, NT1 children showed an altered circadian rest-activity rhythm compared to age- and sex- matched healthy children.
Circadian analyses revealed that the most striking differences between our cohort of NT1 versus control children were time-locked to nighttime, when NT1 children presented higher motor activity levels maintained throughout the nocturnal period, and to the early afternoon, when NT1 children displayed lower motor activity. Conversely, NT1 and control children showed similar activity levels during morning and evening hours. To our knowledge, the current report is the first investigation on circadian rhythms in pediatric NT1 patients; nonetheless, the circadian rhythm abnormalities highlighted herewith are remarkably similar to those reported on adult NT1 patients.
In line with our findings, a recent study compared circadian pattern of melatonin secretion (through assay of plasma melatonin concentration) of adult NT1 patients and controls and showed that, although average hormone concentrations across the 24-h did not differ between groups, the circadian pattern of melatonin release was altered in NT1, with patients presenting a higher proportion of melatonin secreted during daytime and a major peak of secretion in the early afternoon between 14:00 and 16:00.21
Further evidences for altered circadian rhythmicity in NT1 is supported by cognitive studies: Schneider and coauthors compared daytime variations of performances in cognitive task assessing alertness and selective attention in four groups of adults with sleep disorders (NT1, psychophysiological insomnia, and treated or untreated obstructive sleep apnea syndrome) and controls, highlighting a peculiar pattern of daytime fluctuations in NT1 patients, along with the lowest mean performance in all cognitive functions.35 Performance of NT1 patients was higher in the early morning (08:00); thereafter it quickly deteriorates until reaching a nadir in the early afternoon (14:00) before rebounding again to levels similar to those of morning session at approximately 18:00. On the contrary, healthy controls and patients with other sleep disorders showed an initial increase in performance and did not presented such major fluctuations during daytime.
The aforementioned findings, along with our observations, suggest that the loss of hypocretinergic neurons may lead to an imbalance between the sleep-wake regulating homeostatic and circadian processes,36,37 weakening the circadian waking drive and its ability to oppose the homeostatic sleep pressure.38 As a result, the ultradian and semicircadian fluctuation of sleep propensity may became predominant with untimely intrusion of sleep, regardless of circadian phase.39,40
Analyzing actigraphic-derived nocturnal and diurnal sleep measures we highlighted the good discriminant capability of actigraphic monitoring in depicting the marked impairment of both nocturnal sleep and daytime wakefulness in a young cohort of drug-naïve NT1 patients. Indeed, our cohort of drug-naïve NT1 children presented numerous sleep episodes and lower motor activity counts during daytime, associated with major nocturnal sleep fragmentation and enhanced motor activity during nighttime, as previously reported in adult NT1 drug-naïve cases.14 This peculiar nycthemeral disruption, already detectable by means of actigraphy in NT1 children close to disease onset, should be regarded as an intrinsic disease hallmark.
Although actigraphy recommendations in the diagnostic work-up of NT1 are confined to rule out sleep deprivation and circadian rhythm disorders prior to MSLT,1 we showed the good discriminant capabilities of actigraphic assessment, both in adult and pediatric NT1 cases.14 Actigraphy also offers the possibility to monitor patients in their own environment, allowing us to document long-lasting diurnal sleep episodes that are often reported in childhood NT1.41 The standard diagnostic approach, based on the nocturnal polysomnography followed by the MSLT, allows a proper neurophysiological diagnosis with high sensitivity and specificity, but does not give insight into this very common aspect of NT1 hypersomnolence.1
Finally, we reported that increased BMI, high subjective sleepiness and low CSF hcrt-1 levels were associated with the severity of nycthemeral disruption in childhood NT1, pointing to the possibility to further objectively stratify disease severity.
Some limitations of the current study need to be acknowledged. First, although this cohort represents the largest acti-graphic study on pediatric NT1, the sample is still relatively small and prevented us from categorizing children according to pubertal maturation. Second, we did not evaluate other markers of the circadian clock (e.g., melatonin or cortisol) in addition to the rest-activity rhythm to test whether the blunted motor activity pattern during daytime was coupled with altered endocrine secretions.
Although actigraphy remains a screening method that cannot substitute the gold standard diagnostic protocol for NT1 (namely nocturnal polysomnography followed by MSLT and CSF hcrt-1 measurement), this monitoring may offer a complementary measure to support the diagnosis of pediatric NT1, especially enhancing the diagnostic probability of questionable cases, to track disease course over time, and to tailor supportive strategies. First, we showed that actigraphy can document different daytime and nighttime impairments in a more ecological and cost-effective way compared to laboratory procedures, and could represent an objective tool to assess disease burden in real-life settings. Second, given that behavioral treatment (i.e., regularly scheduled naps) is a major management strategy for NT1,42 actigraphy can be used to objectively assess, and possibly adjust, the napping schedule. Third, different studies have shown the ability of actigraphy in assessing wake-promoting drugs and sodium oxybate effects, highlighting that actigraphy could represent a less expensive and ecological approach to assess treatment outcome and prospectively track disease course.16,17 Fourth, the observation of a discrete circadian profile of blunted motor activity in NT1 children provided additional insight into the nature of diurnal variation and suggested that the quantitative assessment of motor activity is a promising behavioral biomarker of NT1 in young patients. Further studies are needed to test the reliability of actigraphy for wide-scale epidemiological studies as a screening tool to steer toward proper diagnosis of NT1 and hopefully reduce diagnostic delay and disease burden.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Plazzi has participated on the advisory boards for UCB Pharma and Jazz Pharmaceuticals. The other authors have indicated no financial conflicts of interest. The work was performed at the Department of Biomedical and Neuromotor Sciences, University of Bologna, Italy; Department of Psychology, University of Bologna, Bologna, Italy; and Department of Developmental and Social Psychology, Sapienza University, Rome, Italy.
ACKNOWLEDGMENTS
The authors are indebted to all the children and families participating in this study, most notably the patients of the Italian Association of Narcolepsy (AIN onlus). Without their contributions, this study would not have been possible. We also thank Cecilia Baroncini for editing the English text.
REFERENCES
- 1.American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2014. International Classification of Sleep Disorders, 3rd ed. [Google Scholar]
- 2.Pizza F, Vandi S, Iloti M, et al. Nocturnal sleep dynamics identify narcolepsy type 1. Sleep. 2015;38:1277–84. doi: 10.5665/sleep.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 4.Longstreth WT, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30:13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15:502–7. doi: 10.1016/j.sleep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Kryger MH, Walid R. Manfreda J. Diagnoses received by narcolepsy patients in the year prior to diagnosis by a sleep specialist. Sleep. 2002;25:36–41. doi: 10.1093/sleep/25.1.36. [DOI] [PubMed] [Google Scholar]
- 7.Rocca LR, Pizza F, Ricci E, Plazzi G. Narcolepsy during childhood: an update. Neuropediatrics. 2015;46:181–98. doi: 10.1055/s-0035-1550152. [DOI] [PubMed] [Google Scholar]
- 8.Partinen M, Saarenpaa-Heikkila O, Ilveskoski I, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han F, Lin L, Warby SC, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70:410–7. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 10.Nevsimalova S. The diagnosis and treatment of pediatric narcolepsy. Curr Neurol Neurosci Rep. 2014;14:469. doi: 10.1007/s11910-014-0469-1. [DOI] [PubMed] [Google Scholar]
- 11.Plazzi G, Pizza F, Palaia V, et al. Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain. 2011;134:3480–92. doi: 10.1093/brain/awr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain. 2013;136:3787–95. doi: 10.1093/brain/awt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kauta SR, Marcus CL. Cases of pediatric narcolepsy after misdiagnoses. Pediatr Neurol. 2012;47:362–5. doi: 10.1016/j.pediatrneurol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Filardi M, Pizza F, Martoni M, Vandi S, Plazzi F, Natale V. Actigraphic assessment of sleep/wake behavior in central disorders of hypersomnolence. Sleep Med. 2015;16:126–30. doi: 10.1016/j.sleep.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Alakuijala A, Sarkanen T, Partinen M. Polysomnographic and actigraphic characteristics of patients with H1N1-vaccine-related and sporadic narcolepsy. Sleep Med. 2015;16:39–44. doi: 10.1016/j.sleep.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Bruck D, Kennedy GA, Cooper A, Apel S. Diurnal actigraphy and stimulant efficacy in narcolepsy. Hum Psychopharmacol. 2005;20:105–13. doi: 10.1002/hup.666. [DOI] [PubMed] [Google Scholar]
- 17.Poryazova R, Tartarotti S, Khatami R, et al. Sodium oxybate in narcolepsy with cataplexy: Zurich sleep center experience. Eur Neurol. 2011;65:175–82. doi: 10.1159/000324549. [DOI] [PubMed] [Google Scholar]
- 18.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 20.Blazejova K, Illnerova H, Hajek I, Nevsimalova S. Circadian rhythm in salivary melatonin in narcoleptic patients. Neurosci Lett. 2008;437:162–4. doi: 10.1016/j.neulet.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 21.Donjacour CEHM, Kalsbeek A, Overeem S, et al. Altered circadian rhythm of melatonin concentrations in hypocretin-deficient men. Chronobiol Int. 2012;29:356–62. doi: 10.3109/07420528.2012.655869. [DOI] [PubMed] [Google Scholar]
- 22.Mayer G, Hellmann F, Leonhard E, Meier-Ewert K. Circadian temperature and activity rhythms in unmedicated narcoleptic patients. Pharmacol Biochem Behav. 1997;58:395–402. doi: 10.1016/s0091-3057(97)00241-4. [DOI] [PubMed] [Google Scholar]
- 23.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 24.Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16:463–75. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey JO, Silverman BW. New York, NY: Springer-Verlag; 2005. Functional Data Analysis, 2nd ed. [Google Scholar]
- 26.Pizza F, Moghadam KK, Vandi S, et al. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res. 2013;22:32–40. doi: 10.1111/j.1365-2869.2012.01032.x. [DOI] [PubMed] [Google Scholar]
- 27.Murali H, Kotagal S. Off-label treatment of severe childhood narcolepsy-cataplexy with sodium oxybate. Sleep. 2006;29:1025–9. doi: 10.1093/sleep/29.8.1025. [DOI] [PubMed] [Google Scholar]
- 28.Tonetti L, Adan A, Di Milia L, Randler C, Natale V. Measures of circadian preference in childhood and adolescence: a review. Eur Psychiatry. 2015;30:576–82. doi: 10.1016/j.eurpsy.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Bruni O, Russo PM, Violani C, Guidetti V. Sleep and migraine: an actigraphic study. Cephalalgia. 2004;24:134–9. doi: 10.1111/j.1468-2982.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 30.Acebo C, Sadeh A, Seifer R, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Xian H, Licis A, et al. Measuring the impact of apnea and obesity on circadian activity patterns using functional linear modeling of actigraphy data. J Circad Rhythms. 2011;9:11. doi: 10.1186/1740-3391-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ullah S, Finch CF. Applications of functional data analysis: a systematic review. BMC Med Res Methodol. 2013;13:43. doi: 10.1186/1471-2288-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadeh A, Lavie P, Scher A, Tirosh E, Epstein R. Actigraphic home-monitoring sleep-disturbed and control infants and young children: a new method for pediatric assessment of sleep-wake patterns. Pediatrics. 1991;87:494–9. [PubMed] [Google Scholar]
- 34.Cohen J. New York, NY: Routledge Academic; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 35.Schneider C, Fulda S, Schulz H. Daytime variation in performance and tiredness/sleepiness ratings in patients with insomnia, narcolepsy, sleep apnea and normal controls. J Sleep Res. 2004;13:373–83. doi: 10.1111/j.1365-2869.2004.00427.x. [DOI] [PubMed] [Google Scholar]
- 36.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–68. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 37.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones GJ. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17:1298–303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broughton R, Mullington J. Circasemidian sleep propensity and the phase-amplitude maintenance model of human sleep/wake regulation. J Sleep Res. 1992;1:93–8. doi: 10.1111/j.1365-2869.1992.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 40.Nobili L, Ferrillo F, Besset A, Rosadini G, Schiavi G, Billiard M. Ultradian aspects of sleep in narcolepsy. Neurophysiol Clin. 1996;26:51–9. doi: 10.1016/0987-7053(96)81534-6. [DOI] [PubMed] [Google Scholar]
- 41.Nevsimalova S. Narcolepsy in childhood. Sleep Med Rev. 2009;13:169–80. doi: 10.1016/j.smrv.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep. 2001;24:385–91. doi: 10.1093/sleep/24.4.385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.