Abstract
Study Objectives:
Coma and chronic sleepiness are common after traumatic brain injury (TBI). Here, we explored whether injury to arousal-promoting brainstem neurons occurs in patients with fatal TBI.
Methods:
Postmortem examination of 8 TBI patients and 10 controls.
Results:
Compared to controls, TBI patients had 17% fewer serotonergic neurons in the dorsal raphe nucleus (effect size: 1.25), but the number of serotonergic neurons did not differ in the median raphe nucleus. TBI patients also had 29% fewer noradrenergic neurons in the locus coeruleus (effect size: 0.96). The number of cholinergic neurons in the pedunculopontine and laterodorsal tegmental nuclei (PPT/LDT) was similar in TBI patients and controls.
Conclusions:
TBI injures arousal-promoting neurons of the mesopontine tegmentum, but this injury is less severe than previously observed in hypothalamic arousal-promoting neurons. Most likely, posttraumatic arousal disturbances are not primarily caused by damage to these brainstem neurons, but arise from an aggregate of injuries, including damage to hypothalamic arousal nuclei and disruption of other arousal-related circuitries.
Citation:
Valko PO, Gavrilov YV, Yamamoto M, Noain D, Reddy H, Haybaeck J, Weis S, Baumann CR, Scammell TE. Damage to arousal-promoting brainstem neurons with traumatic brain injury. SLEEP 2016;39(6):1249–1252.
Keywords: traumatic brain injury, arousal, coma, locus coeruleus, raphe nucleus
Significance.
Direct damage to arousal-promoting neurons is one of several potential causes of chronic sleep-wake disturbances after traumatic brain injury (TBI). We measured the degree of cell loss in arousal structures of the brainstem and hypothalamus in subjects with severe TBI. We found mild neuronal loss in the dorsal raphe nucleus and locus coeruleus, and overall, the monoaminergic and cholinergic arousal nuclei in the rostral brainstem appeared less injured than the arousal structures in the dorsal hypothalamus. The location of these nuclei and local tissue characteristics may better protect these neurons from contusions, shearing, and other trauma-induced forces than those in the hypothalamus.
INTRODUCTION
Impaired arousal is common after traumatic brain injury (TBI). With severe TBI, coma often persists for days or weeks, and massively injured patients may remain in a permanent coma or persistent vegetative state. Even among TBI patients with otherwise excellent neurological recovery, up to 60% report chronic daytime sleepiness or hypersomnia (i.e., increased sleep need per 24 hours).1 Over the last decade, researchers have developed a better clinical understanding of posttraumatic sleep-wake disturbances, but the underlying neuropathology remains little understood, and treatment options are limited.2,3
The hypothalamus contains key arousal-promoting neurons, including those that make orexin (hypocretin) in the laterodorsal hypothalamus and those that make histamine in the tuberomammillary nucleus (TMN).4 In a series of 12 patients with severe TBI and 16 matched controls, we found a 41% loss of histaminergic TMN neurons and a 21% loss of hypocretin neurons.5 Damage to these hypothalamic neurons may contribute to the chronic sleepiness, coma, and other disorders of arousal frequently seen after TBI.
Since the 1930s, researchers have also known that the rostral brainstem contains crucial arousal-promoting neurons. In 1971, Crompton reported that brainstem lesions occurred in a significant proportion of patients with fatal TBI, and affected most often the tegmental area of the midbrain.6 Similarly, in patients with brainstem stroke, Parvizi and Damasio found that acute coma was associated with magnetic resonance imaging (MRI) lesions in the pontine tegmentum extending from the upper pons into the midbrain.7 In fact, this region contains the rostral raphe complex, locus coeruleus, laterodorsal teg-mental nucleus, and other arousal-promoting nuclei. Still, it remains unknown whether TBI injures these specific arousal-promoting neurons.
Here, we examined whether severe TBI injures monoaminergic and cholinergic arousal-promoting nuclei of the rostral brainstem and whether TBI differentially affects hypothalamic and mesopontine arousal regions.
METHODS
Human Subjects
We examined 8 patients with fatal TBI and adequately preserved brainstems throughout the entire midbrain and pons. All TBI patients remained in a coma after their head injury and died after a period of 20 ± 10 days (range: 11–36 days). We also examined the brainstems of 10 age- and sex-matched controls. We have previously reported hypothalamic cell counts of all controls and all but one of these TBI patients.2 The local ethics committees of all involved institutes approved the study protocol.
Brain Tissue Processing and Immunohistochemistry
We used the same techniques for fixation, cutting of brainstem blocks and cryoprotection as previously described.8 We assessed 5 arousal-promoting populations in the rostral brainstem: noradrenergic neurons in the locus coeruleus (LC), serotonergic neurons in the dorsal (DRN) and median (MRN) raphe nuclei, and cholinergic neurons in the pedunculopontine (PPT) and laterodorsal (LDT) tegmental nuclei. We used the following primary antibodies: rabbit anti-tyrosine hydroxylase antibody (1:800; Millipore; Product# AB 152; Lot# 1951919), sheep anti-tryptophan hydroxylase antibody (1:800; Millipore; Product# AB 1541; Lot# 1972784), and goat anti-choline acetyltransferase (ChAT) antibody (1:200; Millipore; Product# AB 144P; Lot# GN 1978747). Otherwise, the immunohistochemical methods were the same as previously reported.8 After the initial reaction with hydrogen peroxide, we used antigen retrieval to improve immunostaining for ChAT by incubating sections in tris-buffered saline (TBS; pH 11.0) at 95°C for 20 min. In addition to immunostaining, a trained neuropathologist (H.R.) examined the pattern and severity of neuronal damage using standard microscopy analysis of hematoxylin and eosin-stained sections.
Stereological Cell Counts
The counting grid and counting frame were 150 × 150 μm and 50 × 50 μm for LC, and 400 × 400 μm and 100 × 100 μm for serotonergic and cholinergic nuclei. We counted brainstem neurons in a blinded manner using the same stereo-logical procedures as previously described.9 The Gundersen coefficients of error were < 8% for all cell types, indicating good accuracy of our cell counts.
Statistics
Group data are reported as means and standard deviations. We compared cell counts between groups using Student's t test. Statistical significance was accepted at P < 0.05. To estimate effect size, we used Cohen's d to compare standardized mean differences between groups.
RESULTS
General Neuropathological Findings
With H&E staining, only 2 of the 8 TBI patients (25%) showed pathological findings in the brainstem, including acute perivascular “agonal-type” hemorrhages and microglial nodules in the pons, around the 4th ventricle and in the solitary tract. These 2 TBI patients had fewer noradrenergic LC and serotonergic DRN neurons than the other 6 TBI patients.
Injury to Mesopontine Arousal Nuclei
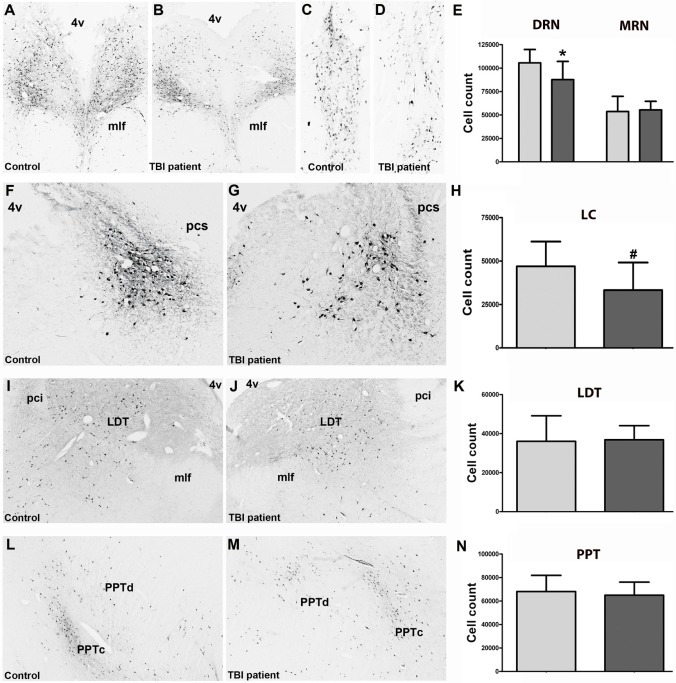
TBI patients had 17% fewer DRN neurons than controls (87,733 ± 19,428 vs. 105,584 ± 14,272, P = 0.04; effect size: d = 1.25), but a similar number of MRN neurons (55,375 ± 9,216 vs. 53,706 ± 16,225, P = 0.80) (Figure 1A–1E). TBI patients also had 29% fewer LC neurons than controls (33,259 ± 15,865 vs. 46,928 ± 14,276, (P = 0.07; effect size: d = 0.96) (Figure 1F–1H). The numbers of cholinergic neurons did not differ in the LDT (36,027 ± 13,103 vs. 36,825 ± 7,265, P = 0.87) and PPT (65,024 ± 11,108 vs. 68,048 ± 13,825, P = 0.61) (Figure 1I–1N).
Figure 1.
Photomicrographs and cell counts. Photomicrographs illustrate the soma, proximal dendrites, and axons of serotonergic neurons in the dorsal (A,B) and median raphe nucleus (C,D) of a TBI patient and a control subject. Compared to controls, TBI patients had 17% fewer serotonergic DRN neurons (effect size: d = 1.25), but similar numbers of serotonergic MRN neurons (E). Photomicrographs of noradrenergic neurons in the locus coeruleus (LC) of a TBI patient and a control subject (F,G). Compared to controls, TBI patients had 29% fewer noradrenergic LC neurons (effect size: d = 0.96) (H). Photomicrographs of cholinergic neurons in the laterodorsal tegmental nucleus (LDT) (I,J) and in the pedunculopontine tegmental nucleus (L,M). Controls and TBI patients had similar numbers of LDT (K) and PPT neurons (N). *P < 0.05. # P = 0.07. 4v, 4th ventricle; mlf, medial longitudinal fasciculus; pci, inferior cerebellar peduncle; pcs, superior cerebellar peduncle; PPTc, pars compacta of the PPT; PPTd, pars dissipata of the PPT.
Comparison between Hypothalamic and Brainstem Injury
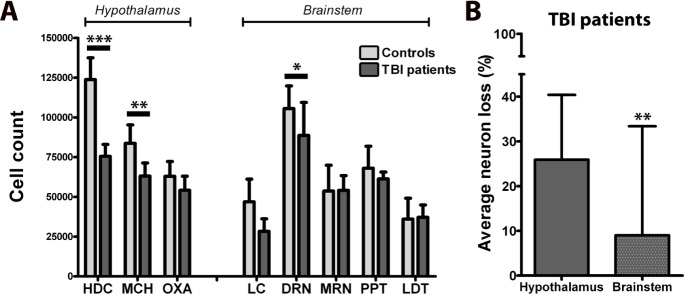
To contrast brainstem and hypothalamic injury, we compared the present findings with the numbers of hypothalamic neurons producing orexin A (OxA), melanin-concentrating hormone (MCH), and histidine-decarboxylase (HDC) in these same patients.5 Neuron loss was more severe in the hypothalamus (−25.9 ± 14.5%, as averaged across HDC, MCH, OxA) than in the brainstem (−9.0 ± 24.4%, as averaged across LC, DRN, MRN, PPT, LDT) (P = 0.001) (Figure 2). There was no correlation between cell numbers in the hypothalamic and brainstem nuclei.
Figure 2.
Overview of damage to specific sleep-wake regulating nuclei in the hypothalamus and brainstem of 7 TBI patients and 10 controls (A). The average neuron loss is more pronounced in sleep-wake regulating nuclei of the hypothalamus than of the brainstem (B). The hypothalamic cell counts were published previously.8 *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
The cause of impaired arousal after TBI has been unclear, and we found that severe TBI is associated with a 17% loss of serotonergic DRN neurons and a 29% loss of noradrenergic LC neurons. However, other arousal-promoting neurons of the midbrain and pons appear less injured, as indicated by similar cell counts in the MRN, PPT and LDT of TBI patients and controls. These results build upon prior work to suggest that coma and sleepiness after TBI are likely the result of multiple factors, including direct damage to some arousal-promoting neurons, as well as cortical and thalamic injury, diffuse axonal injury, and alterations in cerebral energy metabolism.9
Our results suggest that head trauma differentially injures sleep-wake regulating neurons in the hypothalamus and the brainstem. Possibly, due to its location and tissue properties, the brainstem may be less vulnerable to contusions, shearing, and other trauma-induced forces than the hypothalamus. In this line, Ommaya and Gennarelly demonstrated that the brainstem is more protected from axonal injury than other brain areas.10 Moreover, in a comprehensive neuropathology study of 35 TBI patients with vegetative state who survived for 5 weeks to more than 8 years (24 patients survived at least 3 months), brainstem damage was found in only 14% of patients, suggesting that brainstem damage may be less common in TBI than previously thought.11 Most likely, the brainstem is relatively protected from TBI as it may flex less during TBI and is sturdier due to its abundant white matter. Additional factors that may protect the brainstem include lower metabolic requirements and a blood supply less likely to be damaged by direct traumatic forces.9
Within the brainstem, neuronal loss was most apparent in the serotonergic neurons of the DRN and the noradrenergic neurons of the LC. Like most of the brainstem arousal structures, the DRN and LC are located in the mesopontine tegmentum and lay close to the floor of the 4th ventricle. While the cause of the more pronounced neuronal loss in the DRN and LC remains elusive, the observed pattern of injury is in keeping with other studies which have shown the greatest brainstem damage with TBI is often near the dorsolateral surface of the mesopontine junction,12,13 perhaps because this region is vulnerable to direct impact from the tentorium cerebelli.
The mesopontine serotonergic neurons innervate the cerebral cortex, amygdala, basal forebrain, thalamus, hypothalamus, and other nuclei of the ascending arousal system.4 They are most active during wakefulness, whereas their firing rate decreases by about 50% during slow wave sleep to become almost completely silent during REM sleep.4 TBI survivors with posttraumatic arousal disturbances often manifest anxiety or depression as comorbidities, and deficient serotonergic signaling is a well-known contributor to mood disorders. Thus, drugs that enhance serotonergic signaling should be considered a promising treatment strategy for patients with posttraumatic arousal disturbances, especially when associated with depression or anxiety.
Like the neurons of the raphe nuclei, the LC neurons discharge maximally during wakefulness. LC neurons receive abundant excitatory afferents from the orexin neurons, and recent optogenetic studies indicate that orexin neurons can promote wakefulness through LC activation.14 LC neurons are particularly important for arousal in a novel environment and in response to salient or potentially threatening stimuli that require reorientation and adaptive reaction.15
Postmortem studies of TBI patients reported significant loss of cholinergic neurotransmission in the inferior temporal gyrus, cingulate gyrus and superior parietal cortex. Cognitive slowing, impaired attention, and reduced working memory are common after TBI,16 and a deficiency in cholinergic signaling probably contributes to these posttraumatic neurobehavioral deficits.17 Thus, our finding of a normal number of cholinergic neurons in the LDT and PPT suggests that damage to cortically projecting cholinergic neurons in the basal forebrain is a more likely cause of posttraumatic cholinergic deficiency.
This study has limitations. All patients suffered from severe TBI, with death occurring within one month after TBI. This could produce some inclusion bias, and our findings should therefore not be regarded as representative for all TBI patients. In addition, we may have overlooked some brainstem damage by focusing on neuronal loss instead of axonal injury. Finally, none of our TBI subjects survived more than 1 month, so we cannot correlate their neuropathological findings with subjective complaints or sleep physiology. Secondary changes, including circuit level neural degeneration among connected arousal regions, may for the same reason not be apparent yet in our patients.
In conclusion, severe TBI mildly damages neurons of the dorsal raphe nucleus and the locus coeruleus, whereas the MRN, LDT, and PPT are more protected. Arousal nuclei of the rostral brainstem may be less injured than those in the hypothalamus, and the compound effects of multi-level injury to arousal nuclei likely contributes to the frequent arousal disturbances in TBI survivors.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by the Swiss National Science Foundation (grant No. 32003B-125504) and the Clinical Research Priority Program “Sleep and Health” of the University of Zurich. Dr. Scammell has received research support from Eisai and has consulted for Merck, Symphony, Prexa, Jazz, Cereve, Purdue Pharma, Ferrer, Heptares, and Synageva. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. G. Rosen and Dr. L. Slomianka for use of their Stereo Investigator software.
REFERENCES
- 1.Imbach LL, Valko PO, Li T, et al. Increased sleep need and daytime sleepiness 6 months after traumatic brain injury: a prospective controlled clinical trial. Brain. 2015;138:726–35. doi: 10.1093/brain/awu391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouellet MC, Beaulieu-Bonneau S, Morin CM. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. 2015;14:746–57. doi: 10.1016/S1474-4422(15)00068-X. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser PR, Valko PO, Werth E, et al. Modafinil ameliorates excessive daytime sleepiness after traumatic brain injury. Neurology. 2010;75:1780–5. doi: 10.1212/WNL.0b013e3181fd62a2. [DOI] [PubMed] [Google Scholar]
- 4.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valko PO, Gavrilov YV, Yamamoto M, et al. Damage to histaminergic tuberomammillary neurons and other hypothalamic neurons with traumatic brain injury. Ann Neurol. 2015;77:177–82. doi: 10.1002/ana.24298. [DOI] [PubMed] [Google Scholar]
- 6.Crompton MR. Brainstem lesions due to closed head injury. Lancet. 1971;1:669–73. doi: 10.1016/s0140-6736(71)92680-8. [DOI] [PubMed] [Google Scholar]
- 7.Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524–36. doi: 10.1093/brain/awg166. [DOI] [PubMed] [Google Scholar]
- 8.Valko PO, Gavrilov YV, Yamamoto M, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol. 2013;74:794–804. doi: 10.1002/ana.24019. [DOI] [PubMed] [Google Scholar]
- 9.Prins M, Greco T, Alexander D, Giza CC. The pathophysiology of traumatic brain injury at a glance. Dis Model Mech. 2013;6:1307–15. doi: 10.1242/dmm.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ommaya AK, Gennarelli TA. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain. 1974;97:633–54. doi: 10.1093/brain/97.1.633. [DOI] [PubMed] [Google Scholar]
- 11.Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123:1327–38. doi: 10.1093/brain/123.7.1327. [DOI] [PubMed] [Google Scholar]
- 12.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to non-missile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–63. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 13.Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564–74. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- 14.Carter ME, Brill J, Bonnavion P, et al. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci U S A. 2012;109:E2635–44. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–41. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Murdoch I, Perry EK, Court JA, Graham DI, Dewar D. Cortical cholinergic dysfunction after human head injury. J Neurotrauma. 1998;15:295–305. doi: 10.1089/neu.1998.15.295. [DOI] [PubMed] [Google Scholar]
- 17.Arciniegas DB. The cholinergic hypothesis of cognitive impairment caused by traumatic brain injury. Curr Psychiatry Rep. 2003;5:391–9. doi: 10.1007/s11920-003-0074-5. [DOI] [PubMed] [Google Scholar]