Abstract
Study Objectives:
To examine the REM sleep response to stress and fearful memories as a potential marker of stress resilience and vulnerability and to assess the role of the basolateral amygdala (BLA) in mediating the effects of fear memory on sleep.
Methods:
Outbred Wistar rats were surgically implanted with electrodes for recording EEG and EMG and with bilateral guide cannulae directed at the BLA. Data loggers were placed intraperitoneally to record core body temperature. After recovery from surgery, the rats received shock training (ST: 20 footshocks, 0.8 mA, 0.5-s duration, 60-s interstimulus interval) and afterwards received microinjections of the GABAA agonist muscimol (MUS; 1.0 μM) to inactivate BLA or microinjections of vehicle (VEH) alone. Subsequently, the rats were separated into 4 groups (VEH-vulnerable (VEH-Vul; n = 14), VEH-resilient (VEH-Res; n = 13), MUS-vulnerable (MUS-Vul; n = 8), and MUS-resilient (MUS-Res; n = 11) based on whether or not REM was decreased, compared to baseline, during the first 4 h following ST. We then compared sleep, freezing, and the stress response (stress-induced hyperthermia, SIH) across groups to determine the effects of ST and fearful context re-exposure alone (CTX).
Results:
REM was significantly reduced on the ST day in both VEH-Vul and MUS-Vul rats; however, post-ST MUS blocked the reduction in REM on the CTX day in the MUS-Vul group. The VEH-Res and MUS-Res rats showed similar levels of REM on both ST and CTX days. The effects of post-ST inactivation of BLA on freezing and SIH were minimal.
Conclusions:
Outbred Wistar rats can show significant individual differences in the effects of stress on REM that are mediated by BLA. These differences in REM can be independent of behavioral fear and the peripheral stress response, and may be an important biomarker of stress resilience and vulnerability.
Citation:
Wellman LL, Fitzpatrick ME, Hallum OY, Sutton AM, Williams BL, Sanford LD. Individual differences in animal stress models: considering resilience, vulnerability, and the amygdala in mediating the effects of stress and conditioned fear on sleep. SLEEP 2016;39(6):1293–1303.
Keywords: basolateral amygdala, fear conditioning, muscimol, REM sleep, resilience, vulnerability
Significance.
Stress-induced disturbances in sleep have been linked to the development of psychopathology, but have been difficult to examine because of the wide range of effects that stress can have on sleep as well as differences in responsivity to stress. Here we report individual differences in sleep responses to an identical stressor and to memories formed during stress, and that the amygdala mediates how stress-related memories impact future sleep. These findings demonstrate that complex sleep responses to stress and stressful memories as well as their neurobiological bases can be studied in animal models. This provides the potential for assessing the role of sleep in mediating stress resilience and vulnerability which can provide better models for disorders such as posttraumatic stress disorder.
INTRODUCTION
In conditioned fear, an association is formed between an explicit neutral stimulus (generally a light or auditory stimulus) or situational context and an aversive stimulus (usually footshock).1,2 Afterwards, the explicit stimulus or context can evoke fear and produce behavioral and physiologic outcomes similar to those produced by the aversive event. Evoking fearful memories can also produce changes in subsequent sleep that are similar to those that occur after the initial fearful stressor. These qualities have resulted in fear conditioning becoming one of our most important experimental models for understanding the mechanisms underlying posttraumatic stress disorder (PTSD) as well as of other anxiety disorders.2–8
The relative effects of conditioned fear may depend on the genetic predisposition of individual animals or strains.9,10 These differences in stress resilience and vulnerability are likely very important for models related to PTSD, as only 20% to 30% of individuals who experience traumatic events develop PTSD, whereas others do not appear to suffer significant long-lasting effects.11,12 Attempts to develop animal models that better represent individual differences in clinical populations have included selecting low and high responders to stressors in genetically heterogeneous outbred strain rats.11,12 Another approach is to compare inbred strains, which are genetically identical within strain but which vary genetically and pheno-typically across strain, to identify animals that vary in level of responsiveness to conditioned fear and other stressors. Our work in mice demonstrated that strains that exhibited greater anxiety-like behaviors in response to challenges in wakefulness exhibited correspondingly greater and longer duration alterations in sleep after shock training, and after fearful cues and contexts.13–15 In general, more “anxious” mouse strains also showed greater decreases in sleep in situations with un-learned responses including after exposure to an open field,16 after cage change and after novel objects were placed in the home cage.17 These findings led us to suggest that mouse strains that have greater emotional responses when faced with various types of environmental challenges also have greater reductions in subsequent sleep.13,15–17
It should also be noted that the relationship between fear conditioning and sleep is complex, and that neither fear conditioning nor the stress response are predictive of subsequent alterations in sleep. For example, extensive training using inescapable shock (IS) as the aversive stimulus can result in significant reductions in rapid eye movement sleep (REM), and training with escapable shock (ES) can result in significant increases in REM,18,19 whereas indices of fear (freezing) and stress (stress-induced hyperthermia [SIH]) are similar for both conditions.18 These studies demonstrate that the interpretive context in which stress is experienced, or the perception of stress, can be a significant factor in determining post-stress sleep. In general, however, the relative roles of individual resilience and vulnerability in mediating the effects of conditioned fear on sleep are poorly understood.
Understanding the neural processes by which fear and stress can produce directionally different alterations in sleep is likely key to understanding sleep disturbances in disorders such as PTSD, which are viewed as arising from abnormal functioning in the brain's fear system.20 The amygdala, medial prefrontal cortex, and hippocampus have established roles in fear conditioning and fear extinction and are central to current concepts of PTSD.21–23 Of these regions, the amygdala, especially its basolateral nucleus (BLA), has an established role in regulating fear- and stress-induced alterations in sleep, especially REM sleep, as well as in the acquisition and consolidation of fear conditioning.24–33 BLA also appears to play a critical role determining how fear memories impact sleep. For example, administration of antalarmin, a corticotropin releasing factor antagonist, into BLA of rats prior to shock training (ST) blocked both ST-induced reductions in REM sleep and the formation of memories that alter sleep without blocking fear memory as indicated by contextual freezing.25 By comparison, global inactivation of BLA with microinjections of the GABAA agonist, muscimol (MUS), prior to ST, blocked the post-training reduction in REM sleep seen in vehicle treated rats and attenuated contextual freezing and subsequent reductions in REM.10 Together, these data indicate that BLA plays a significant role in regulating the initial effects of stress and fear on sleep, and in mediating the subsequent effects of fearful memories.
Several studies have reported stable and robust inter-individual differences in anxiety-like behavior in Wistar rats that have been exploited to examine anxiety-related behavior and neurobiology,34–38 as well as to generate high and low anxiety strains.39 High and low anxiety-like behavior on the elevated plus maze was also positively related to fear conditioned behavior and vocalizations.37 As an outbred strain, we have also noted inter-individual variability in ST-induced and conditioned alterations in sleep and behavior. Over the last few years, we have noted that a large number of Wistar rats exhibit increased REM in the post-stress and post-context period instead of the significant decreases our lab13,15,40,41 and others42,43 have typically reported for uncontrollable ST. Given the important linkages between stress-induced alterations in sleep and the development of psychopathology,44–46 we systematically examined these differences in the REM response to ST to establish their consistency and to explore their potential neurobiological basis. We separated Wistar rats into high-REM and low-REM responders (as potential models of stress-resistant and stress-vulnerable individuals), based on REM amounts in the first 4 hours after ST, and examined stress-induced and conditioned changes in sleep across subgroups. In a subset of animals, we inactivated BLA with microinjections of MUS immediately after ST to determine whether it had a role in mediating individual differences in ST-induced and conditioned changes in sleep. Additionally, we recorded core body temperature in order to assess SIH as an index of the stress response and we examined behavioral freezing as an index of fear memory.
METHODS
Subjects
The subjects were 46 seventy-day-old Wistar rats obtained from Harlan Laboratories (Frederick, MD). Upon arrival, the rats were individually housed in polycarbonate cages and given ad lib access to food and water. The rooms were kept on a 12:12 light-dark cycle with lights on from 07:00 to 19:00. Light intensity during the light period was 100–110 lux and less than 1 lux during the dark period. Ambient room temperature was maintained at 24.5 ± 0.5 C.
Surgery
Beginning one week following arrival, the rats were anesthetized with isoflurane (5% induction; 2–3% maintenance) and implanted with skull screw electrodes for recording their electroencephalogram (EEG), and stainless steel wire electrodes sutured to the dorsal neck musculature for recording their electromyogram (EMG). Leads from the recording electrodes were routed to a 9-pin miniature plug that mated to one attached to a recording cable. Bilateral guide cannulae (26 g) for micro-injections into BLA were implanted with their tips aimed 1.0 mm above BLA (A 2.6, ML ± 4.8, DV 8.047). The recording plug and cannulae were affixed to the skull with dental acrylic and stainless steel anchor screws. During the same surgery, temperature recorders (SubCue Standard Dataloggers, Canadian Analytical Technologies Inc. Calgary, Alberta, Canada) were implanted intraperitoneally. Ibuprofen (15 mg/kg) was made available in their water supply for relief of postoperative pain. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School's Animal Care and Use Committee (Protocol # 13-003).
Drugs
MUS (muscimol hydrobromide, 5-aminomethyl-3-hydroxyisoxazole) was obtained from Sigma-Aldrich, St. Louis, MO, USA. It was prepared in pyrogen-free distilled water as a vehicle (VEH; 1.0 μM) and was sonicated for 20 min to ensure that the drug was dissolved completely. A fresh solution was prepared for each experimental day.
Procedures
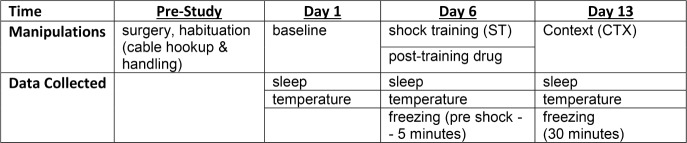
A general outline of the experimental timeline is presented in Figure 1. All experimental manipulations were conducted during the fourth hour of the light period, such that sleep recording would begin at the start of the fifth hour. This resulted in 8 h of light period recording on each experimental day.
Figure 1.
Outline of experimental time frame, manipulations and data collection.
Home cages were changed at least 3 days prior to each treatment day. The same room was used for animal housing and sleep recording. The microinjections and behavioral testing were conducted in a separate room from that used for recording.
Sleep Recording
For recording sleep, each animal, in its home cage, was placed on a rack outfitted for electrophysiological recording and a lightweight, shielded cable was connected to the miniature plug on the rat's head. The cable was attached to a commutator that permitted free movement of the rat within its cage. EEG and EMG signals were processed by a Grass, Model 12 polygraph equipped with model 12A5 amplifiers and routed to an A/D board (Model USB-2533, Measurement Computing) housed in a personal computer. The signals were digitized at 256 Hz and collected in 10 s epochs using the SleepWave (Biosoft Studio) data collection program.
The rats were allowed a post-surgery recovery period of 14 days prior to beginning the experiment. Once recovered, the animals were randomly assigned to 1 of 2 groups: MUS after ST (MUS; n = 19) or VEH after ST (VEH; n = 27) for studies of its effects on ST and fear. All rats were habituated to the recording cable and chamber over 3 consecutive days. Then the rats were habituated to the 5 min handling procedure necessary for microinjections over 2 consecutive days and a baseline following handling (BH) was recorded on day 1 of study.
Microinjections
For microinjections, injection cannulae (33 g) were secured in place within the guide cannulae, and projecting 1.0 mm beyond the tip of the guide cannulae for delivery of drug into the target region. The injection cannulae were connected to one end of a section of polyethylene tubing that had the other end connected to 5.0 μL Hamilton syringes. The injection cannulae and tubing were prefilled with the solution to be injected. Once the cannulae were in place, 0.5 μL of either drug or vehicle was bilaterally infused over 3 min. The cannulae were left in place one min pre- and post-injection to allow for maximal absorption of the solution.
Fear Conditioning
On day 6, animals were subjected to a ST session lasting 30 min. During this procedure, individual rats were placed in shock chambers (Coulbourn Habitest cages equipped with grid floors [Model E10-18RF] that were housed in Coulbourn Isolation Cubicles [Model H10-23]) and were allowed to freely explore for 5 min. Over the next 20 min, they were presented with 20 footshocks (0.8 mA, 0.5 s duration) at 1.0-min intervals. Shock was produced by Coulbourn Precision Regulated Animal Shockers (Model E13-14) and presented via the grid floor of the shock chamber. Five min after the last shock, the rats were injected with either MUS or vehicle, as noted above, then returned to their home cages.
On day 13 (7 days after training), the rats were placed back in the shock chambers and allowed to explore freely for 30 min (no shock presented) before being returned to their home cage. This context re-exposure (CTX) was used to test for fear memory (assessed by behavioral freezing) and for post-exposure alterations in sleep.
The shock chamber was thoroughly cleaned with diluted alcohol following each session. Each session was videotaped using mini video cameras (Weldex, WDH-2500BS, 3.6 mm lens) attached to the center of the ceiling of the shock chamber for subsequent visual scoring of freezing.
Data Analyses
Sleep
Computerized EEG and EMG records were visually scored by trained observers blind to drug condition in 10-s epochs to determine wakefulness, NREM, and REM. Wakefulness was scored based on the presence of low-voltage, fast EEG and high amplitude, tonic EMG levels. NREM was characterized by the presence of spindles interspersed with slow waves, lower muscle tone and no gross body movements. REM was scored continuously during the presence of low voltage, fast EEG, theta rhythm, and muscle atonia. Data were collapsed into two 4-h blocks (B1 and B2) and the total 8-h light period. The following sleep parameters were examined in the data analyses: total NREM (min), total REM (min); total sleep (REM + NREM), and duration and and number of NREM and REM episodes (defined as contiguous 10-s epochs of a given state).
After an initial assessment that revealed 2 distinct sleep responses after ST and ascertaining that baseline sleep was not significantly different among groups, the rats were separated into 4 groups: VEH-vulnerable (VEH-Vul; n = 14), VEH-resilient (VEH-Res; n = 13), MUS-vulnerable (MUS-Vul; n = 8), and MUS-resilient (MUS-Res; n = 11). The groups were formed based on whether, compared to baseline, individual rats showed a decrease in REM or either no decrease or an increase in REM during the first 4 h (B1) following ST. The designations of vulnerable or resilient were based on accumulating data that REM plays an adaptive role in processing emotional memories (see Discussion for expanded rationale).48–50 The sleep data were analyzed with 2-way mixed factors (Group (VEH-Vul; VEH-Res; MUS-Vul; MUS-Res) × Treatment (Bas; ST; CTX) ANOVAs with repeated measures on Treatment. The Holm-Sidak method was used to determine differences among means as appropriate.
Freezing and Core Body Temperature
Videotapes of the ST and CTX sessions were scored for freezing, defined as the absence of body movement except for respiration.51,52 Freezing was scored by a trained observer blind to condition in 5-s intervals during 1.0 min observation periods over the course of the 30 min of the CTX trials. The percentage time spent freezing was calculated (FT%: freezing time/observed time × 100) for each animal for each observation period.
Freezing was scored during the 5-min pre-shock period to obtain baseline levels prior to ST. Freezing data were analyzed for the entire 30-min context exposure and compared to the pre-shock period on the ST day and across groups on the CTX test day. The freezing data were analyzed with a two-way mixed factors (Group (VEH-Vul; VEH-Res; MUS-Vul; MUSRes) by Treatment (Pre-shock; CTX) ANOVAs with repeated measures on Treatment.
The Subcue Dataloggers were programmed to record an animal's temperature every 15 min over the course of the experiment. To determine the effect of fear and shock on SIH and its relationship to sleep, temperature data for the time in the shock chamber and for the first 4 h of the sleep recording period were compared to the 30-min period immediately prior to ST and across treatment conditions (ST and CTX). The temperature data were analyzed with two-way mixed factors (Group (VEHVul; VEH-Res; MUS-Vul; MUS-Res) × Treatment (ST; CTX) ANOVAs with repeated measures on Treatment. Post hoc comparisons were conducted with the Holm-Sidak method.
Histology
To localize the microinjection sites in, brain slices (40 μm) were made through the amygdala and the sections were mounted on slides and stained with cresyl violet. The sections were then examined in conjunction with a stereotaxic atlas47 to confirm cannulae placements. Though there were rostral-caudal variations in the placements among animals, the histology indicated that MUS or VEH would have been infused into BLA and adjacent areas in all the rats, and all animals were used in the data analyses.
RESULTS
Sleep
Rapid Eye Movement Sleep
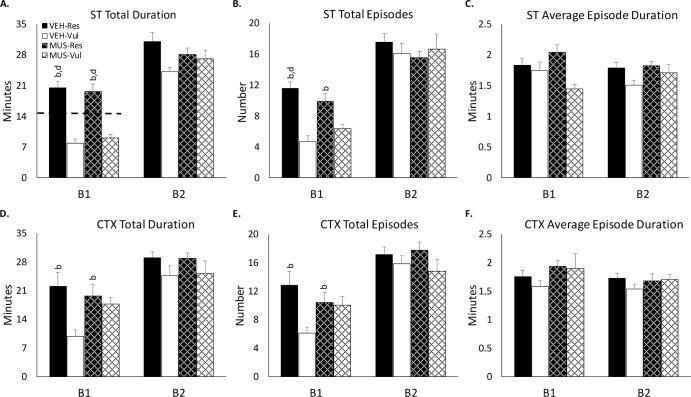
As indicated in the Methods section, the rats were grouped based on differences in REM amounts in B1 after ST and the subsequent analysis compared these groups during baseline, ST and CTX. The groups did not significantly differ in baseline REM amounts (Mean ± SEM; Dw-Res: 17.2 ± 1.8; Dw-Vul: 13.6 ± 1.5; Mus-Res: 15.4 ± 2.1; Mus-Vul: 14.1 ± 2.4, P = 0.49) and, because of selection, during B1 of ST, the VEH-Res and MUS-Res groups showed significantly greater REM than the VEH-Vul and MUS-Vul groups, and the collective Res and Vul groups did not differ (Figure 2A). However, on B1 of the CTX day, REM amount was decreased in the VEH-Vul group compared to that recorded in the VEH-Res (P < 0.001), MUS-Res (P < 0.01) and MUS-Vul (P < 0.05) groups, which did not significantly differ due to MUS inactivation of BLA blocking the reduction in REM expected in the MUS-Vul group (Figure 2D). There were mostly parallel changes in the number of REM episodes (Figure 2B, 2E) whereas there was no significant alteration in REM episode duration in either ST or CTX (Figure 2C, 2F). In B2, REM amount did not differ between ST and CTX, and both ST and CTX were greater compared to baseline, but did not significantly differ among the groups.
Figure 2.
Parameters of rapid eye movement sleep after shock training (ST) and context re-exposure (CTX). ST: (A) total REM; (B) REM episode number; (C) average episode duration. CTX: (D) total REM; (E) REM episode number; (F) average episode duration. Parameters of rapid eye movement sleep after ST and CTX for the two 4-h blocks (B1, B2) during the 8-h light recording periods in rats receiving distilled water vehicle (VEH) or muscimol (MUS) microinjected into the basolateral amygdala immediately after ST. VEH-Vul, distilled water-vulnerable; VEH-Res, distilled water-resilient; MUS-Vul, muscimol-vulnerable; MUS-Res, muscimol-resilient. b P < 0.05 compared to VEH-Vul; d P < 0.05 compared to MUS-Vul. Dashed line in panel A indicates group baseline mean used for separating groups.
Non-Rapid Eye Movement Sleep
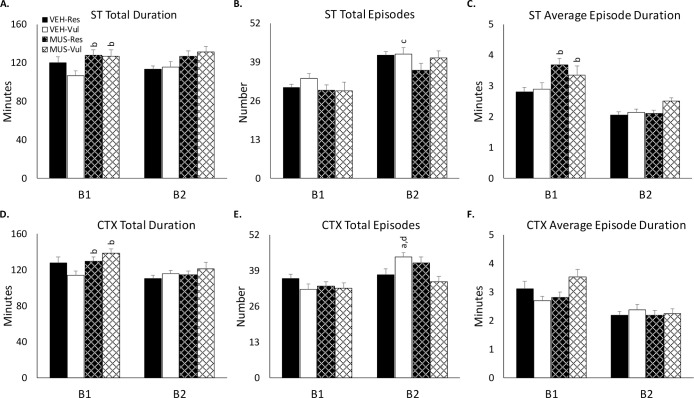
The only significant alterations in NREM amounts were found in comparisons of the VEH-Vul rats to the other groups. NREM amount was reduced in VEH-Vul rats compared to MUS-Res (P < 0.01) and MUS-Vul (P < 0.01) rats during B1 of both ST and CTX (Figure 3A, 3D). There also was a significant difference in NREM episode duration in VEH-Vul rats compared to MUS-Res (P < 0.05) and MUS-Vul (P < 0.05) rats during B1 of both ST (Figure 3C). There were no differences across days, or any significant differences between the VEH-Res, MUS-Res or Mus-Vul groups.
Figure 3.
Parameters of non-rapid eye movement sleep after shock training (ST) and context re-exposure (CTX). ST: (A) total NREM; (B) NREM episode number; (C) average episode duration. CTX: (D) total NREM; (E) NREM episode number; (F) average episode duration. Parameters of non-rapid eye movement sleep after ST and CTX for the two 4-h blocks (B1, B2) during the 8-h light recording periods in rats receiving distilled water vehicle (VEH) or muscimol (MUS) microinjected into the basolateral amygdala immediately after ST. VEH-Vul, distilled water-vulnerable; VEH-Res, distilled water-resilient; MUS-Vul, muscimol-vulnerable; MUS-Res, muscimol-resilient. a P < 0.05 compared to VEH-Res; b P < 0.05 compared to VEH-Vul; c P < 0.05 compared to MUS-Res; d P < 0.05 compared to MUS-Vul.
Freezing
None of the animals showed freezing during the pre-shock period, and all showed freezing during the context re-exposure. However, there were no significant differences among the groups in the amount of freezing behavior exhibited during re-exposure to the context (Figure 4).
Figure 4.
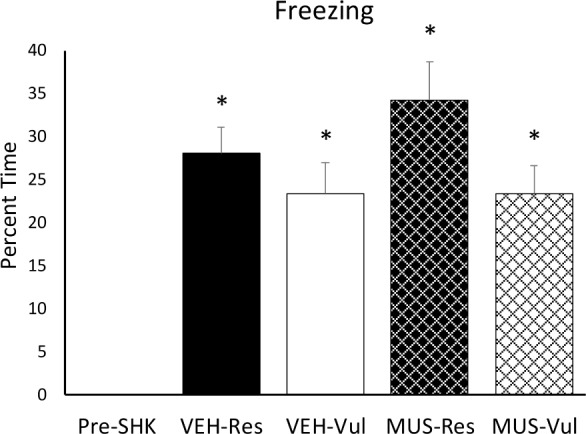
Behavioral freezing. Freezing plotted as percentage for the pre-shock time in the chamber averaged for all rats and for context re-exposure (CTX) in rats receiving distilled water vehicle (VEH) or muscimol (MUS) microinjected into the basolateral amygdala after shock training. VEH-Vul, distilled water-vulnerable; VEH-Res, distilled water-resilient; MUS-Vul, muscimol-vulnerable; MUS-Res, muscimol-resilient; *P < 0.05 compared to pre-shock.
Temperature
We examined temperature 60 minutes prior to ST and CTX as a baseline. Temperature during this time period did not vary across groups or days (VEH-Res: ST: 36.73 ± 0.10; CTX: 36.75 ± 0.08; VEH-Vul: ST: 36.96 ± 0.10; CTX: 36.98 ± 0.08; MUS-Res: ST: 36.73 ± 0.10; CTX: 36.85 ± 0.06; MUS-Vul: ST: 36.66 ± 0.12; CTX: 36.86 ± 0.10; data are mean ± SEM). Subsequently, we examined temperature in two 15-min periods during exposure to the shock chamber on the ST and CTX days and we examined temperature hourly for 4 h on each day after sleep recording had begun. Each was also compared to their respective baselines.
Each of the groups showed similar increases in temperature during both 15-min ST periods, and core body temperature was greater on ST than on baseline and CTX, and greater on CTX than on baseline for all groups (Figure 5). On the CTX day, temperature was greater in the VEH-Vul group compared to VEHRes (P < 0.001), MUS-Res (P < 0.01), and MUS-Vul (P < 0.05) for the first 15 minutes in the shock context. Temperature in the VEH-Res, MUS-Res, and MUS-Vul groups did not differ. There was no difference among groups for the second 15-min period.
Figure 5.
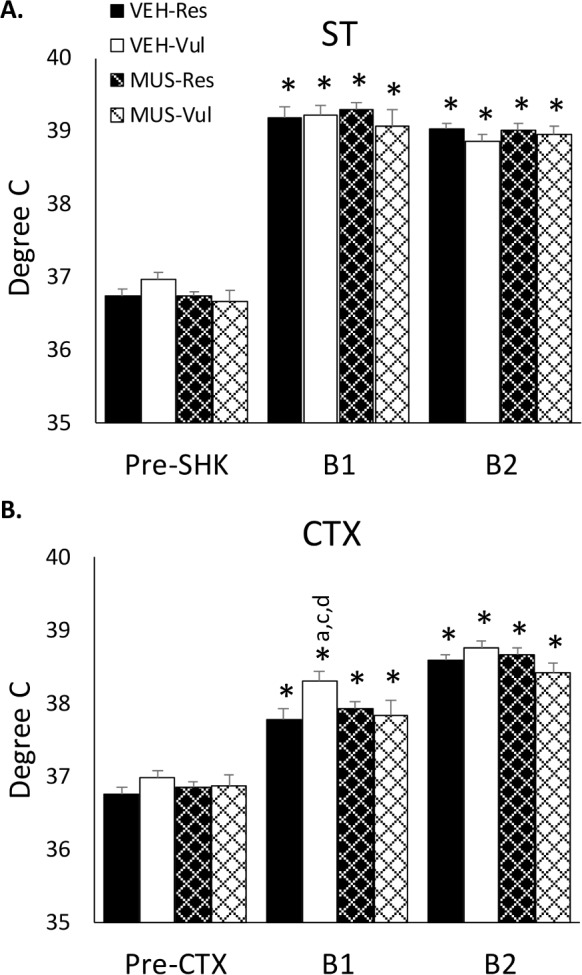
Core body temperature prior to and during the time in the shock chamber. Body temperature plotted in 15 minute intervals for the shock training day (ST) and for context re-exposure (CTX) in rats receiving distilled water vehicle (VEH) or muscimol (MUS) microinjected into the basolateral amygdala after ST. Pre-Shk/Pre-CTX, 60 min prior to ST or CTX; B1, first 15 min of ST or CTX; B2, second 15 min of ST or CTX; VEH-Vul, distilled water-vulnerable; VEH-Res, distilled water-resilient; MUS-Vul, muscimol-vulnerable; MUS-Res, muscimol-resilient. *P < 0.05 compared to Pre-Shk/Pre/CTX; a P < 0.05 compared to VEHRes; c P < 0.05 compared to MUS-Res; d P < 0.05 compared to MUS-Vul.
For each of the hourly periods, temperature on ST and CTX were greater than on baseline and ST was greater than on CTX. There were no significant differences between groups for any hourly period (Figure 6).
Figure 6.
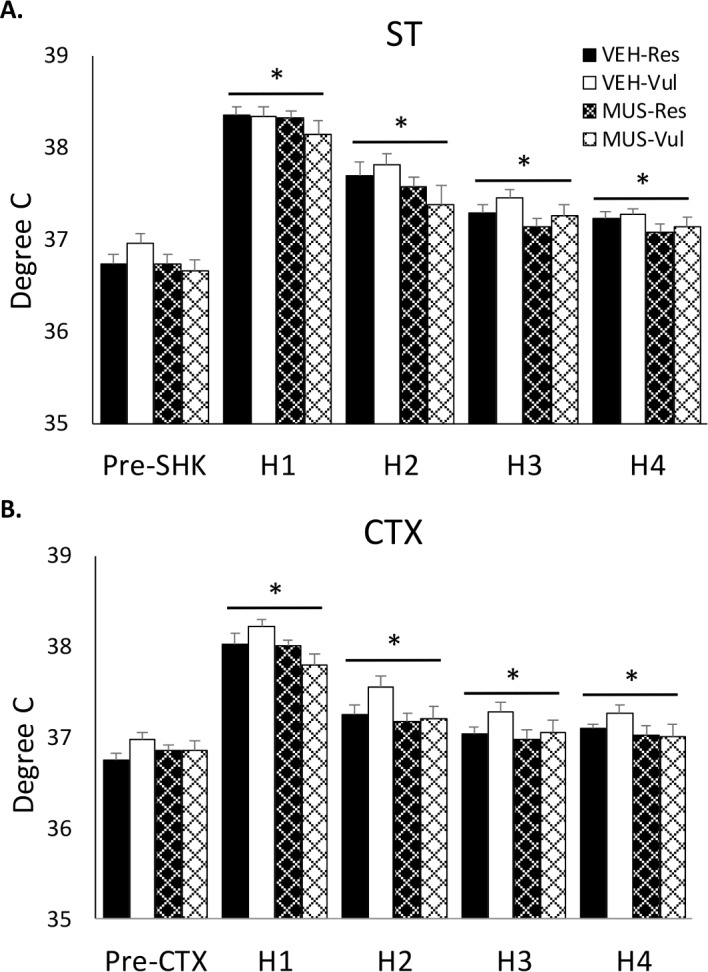
Core body temperature. Body temperature plotted hourly for four hours after shock training and after context re-exposure (CTX) in rats receiving distilled water vehicle (VEH) or muscimol (MUS) microinjected into the basolateral amygdala after ST. Pre-Shk/Pre-CTX, 60 min prior to ST or CTX; H1–4, hourly average for 4 hours after ST or CTX; VEH-Vul, distilled water-vulnerable; VEH-Res, distilled water-resilient; MUS-Vul, muscimol-vulnerable; MUS-Res, muscimol-resilient. *P < 0.05 compared to Pre-Shk/Pre-CTX. There were no differences among groups.
DISCUSSION
This study demonstrated that individual, outbred Wistar strain rats can show marked differences in post-stress sleep even though they experienced an identical stressor, and showed virtually identical stress responses and fear behavior. Differences were found mainly in REM with some rats showing marked reductions in post-stress REM and others showing normal or greater amounts of post-stress REM. Importantly, the rats showed similar changes in REM to both ST and to CTX indicating that the respective increases and decreases in REM were associated with fear memories formed during ST. A second primary finding of this study was that the BLA plays a significant role in mediating alterations in sleep associated with fearful memories and that it can regulate fear-induced alterations in sleep without altering behavioral fear or the stress response. This is indicated by the finding that post-ST inactivation of BLA blocked the reduction in REM expected following CTX in MUS-Vul animals. Thus, these findings extend and refine prior work that has focused primarily on the reduction in REM that occurs after training with inescapable shock,13,15,40–43 but is fully consistent with evidence that stress-induced alterations in sleep can occur independently of fear memory (as assessed by freezing) and the stress response (as assessed by SIH).
Fear Memory, Stress, and Sleep
Greater behavioral freezing in rodents is considered to indicate greater associative learning53 as well as stronger fear reactions.51,52,54–56 Sleep has long been thought to play a role in memory consolidation and several studies have indicated a role for sleep in the consolidation of contextual fear memory associated with brief or mild fearful experiences57–64 (see 53 for recent review). However, there is no evidence that sleep is necessary for the formation of contextual fear memory associated with intensely stressful experiences, such as are modeled by our experiments. For example, similar freezing is found in both groups of a yoked escapable and inescapable shock (ES and IS) paradigm that produces significant differences in REM: IS significantly reduces REM sleep whereas training with ES can produce significant increases in REM sleep.18,19 In the current study, there were no significant differences in freezing of rats that showed significant individual differences in amounts of REM after ST and CTX.
Both the initial stressor and fearful memories can induce similar physiological responses including increased body temperature (SIH65,66), HPA activation, and corticosteroid release, as well as increases in respiration and heart rate.66 In this study, we examined SIH as an index of the stress response. It is rapid and stable across repeated presentations of a stressor.66–68 Psychological SIH persists as long as psychologically stressful situations last,69 and it has a time course that parallels that of HPA activation.70,71 As with prior studies, we found similar SIH responses occurring in rats with either decreases or increases in post-stress REM.72 Thus, similar to freezing, SIH does not predict subsequent changes in sleep72 and did not distinguish between rats that showed post-stress increases or decreases in REM.
There was only one significant group difference in SIH. In the first 15 min of re-exposure to the shock context, temperature in the VEH-Vul rats was elevated over that in the VEH-Res, MUS-Res and MUS-Vul groups, which did not differ. This suggests that the stress response may have been slightly reduced in the two Res groups, and also the possibility that MUS inactivation of BLA may have attenuated the initial stress response in the MUSVul group. However, SIH did not differ across groups in the second 15 min in the shock context, and the apparent elevation in the VEH-Vul rats did not reach significance for any of the hourly intervals. Thus, there were only minimal differences between groups with respect to SIH and further work would be required to determine whether the slight difference in temperature on re-exposure to CTX has functional significance.
The Amygdala as a Regulator of Stress- and Fear-Induced Alterations in Sleep
The relationship between fear memory and sleep is complex. For example, blocking the formation of contextual fear memory that produces behavioral freezing also blocks fear memory that alters sleep.73 Additionally, increased REM and normalization of sleep is associated with extinction of contextual fear arising from extensive fear training.41 However, as discussed above, behavioral fear can be dissociated from subsequent sleep based on stressor controllability18,19 and neurobiologically such as with microinjections of antalarmin into BLA of rats prior to ST which blocked both ST-induced reductions in REM sleep and the formation of memories that alter sleep without affecting freezing.25 The current work extends the evidence for potential dissociation to include individual differences in the sleep response to fear training.
Subsequent freezing in the shock context also did not appear to be altered by post-ST inactivation of BLA in either the MUS-Res or MUS-Vul rats. This indicates that consolidation of memory for behavioral fear was not interrupted by inactivating BLA. Overtraining can overcome the effects of BLA lesions on contextual fear,74–76 indicating that circuitry outside BLA can be involved in contextual fear under some circumstances, though pre-training lesions of BLA have been reported to impair acquisition with up to 25 training trials.74 Thus, it seems likely that the moderately extensive training paradigm (with respect to number of trials) we used engaged other regions involved with memory of contextual fear, and was sufficient to produce consolidation of fear memory even with post-ST inactivation of BLA. However, the current work does point to BLA as a critical region for mediating the effects of fear memory on subsequent sleep, though other brain regions may mediate behavioral fear upon re-exposure to the shock context.
Consistent with prior studies, the primary effect of ST was on REM and the changes in NREM were minimal. The only significant change in NREM we observed were increases relative to the VEH-Vul rats in the MUS-Res and MUS-Vul rats on the ST and CTX days. This suggests that inactivation of BLA may have produced a modest increase in NREM. This suggestion is supported by findings that bilateral electrolytic and chemical lesions of BLA increased NREM and total sleep time without altering REM in rats,77 and that bilateral chemical lesions of the amygdala in chair-restrained Rhesus monkeys produced more consolidated sleep.78 However, the effect was small and the data do not allow unequivocal conclusions to be drawn. Indeed, across studies, changes in NREM have been more variable with some strains showing increases and some showing overall decreases compared to handling controls.79
The current work also suggests that BLA plays a role in mediating individual differences in the effects of stressful memories on sleep. That is, although the VEH-Res and MUS-Res rats showed similar sleep on the ST and CTX days, post-stress inactivation of BLA significantly attenuated the reduction in REM expected in the MUS-Vul group. The fact that REM was high or at least at baseline levels in the MUS-Res and DW-Res animals after both ST and CTX suggests that reduced activity in BLA may normally be associated with increased or normalized sleep after stress. Consistent with this suggestion is our recent finding that optogenetic inhibition of glutamatergic neurons in BLA during the presentation of footshock can block subsequent reductions in REM, again without significantly altering freezing or SIH (unpublished data). Thus, BLA may be an important target for understanding the effects of stress-related pathology on sleep.
Conditioned Fear, Sleep, and Models of Psychopathology
PTSD is characterized by a pathological activation of fear systems8 that is thought to arise from fear learning associated with a traumatic event.7 As such, experimental fear conditioning would seem to be a natural model for examining processes that can lead to PTSD and other stress-related mental disturbances. However, it also is important to note that fear conditioning normally underlies adaptive behavior that is easily extinguished, and that only a percentage of individuals who experience traumatic stress develop PTSD.80,81 Thus, fear conditioning based on brief stressful experiences, as typically examined in the literature, is unlikely to produce pathological processes. Even strong stressors may produce variable outcomes across animals given differences in individual resilience and vulnerability. Our work suggests that standard measures used to measure fear (freezing) and stress responses (SIH) are not predictive of different post-stress neurobehavioral outcomes including post-stress sleep. Together, these lines of evidence indicate that conceptions of fear memory and fear circuitry will need to be significantly refined if they are to provide insight into the role of conditioned fear in psychopathology, which almost always involves aberrant sleep.
REM sleep figures prominently in hypotheses regarding the processing of emotion including proposals that it functions in “decoupling” memory from its emotional charge48 and in the processing of traumatic memories.49,50 However, the alterations in REM associated with PTSD have not been clearly delineated. Indeed, there have been various reports of reduced, normal, enhanced, and fragmented REM sleep following trauma.79 Mellman et al.49,50 have made the point that studies conducted months, years or even decades after the traumatic event may be influenced by factors not related to the development of PTSD and have recently suggested82 that PTSD may be associated with initial trauma-induced reductions in REM followed by increases over time as secondary processes promote REM in ways that may benefit recovery. However, based on positive correlations of REM amounts with fear recall in human subjects, others have suggested that REM sleep deprivation be considered as a means to attenuate fear memory associated with distressing events.57
These contradictory ideas regarding the role of REM sleep in the processing of memory and emotion illustrate that delineating the relationship between PTSD and sleep is critical, both for understanding the etiology of PTSD and the way that sleep may play into treatments of the disorder. Additionally, our data indicate that actual fear memory, as typically defined, is independent of REM. However, REM may well play a role in mediating the emotional processes regulating subsequent appropriate or inappropriate engagement of fear, including fear generalization and resistance to extinction. These conceptual differences point out the need to distinguish adaptive from non-adaptive responses to fearful and stressful events and to establish the role of sleep in processing emotion associated with memories of each. These efforts need to go beyond simply describing sleep across various stress paradigms as the outcomes can be impacted by stressor parameters that alter how stress is perceived as well as by differences in vulnerability and resilience of the subject pool. Useful insight into the complex relationship between stress, stress-related learning and sleep are thus unlikely without considering contextual and genetic factors that can impact the perception of stress.
Experimental Design and Interpretation
Wistar rats are an outbred strain and as such we have noted occasional outliers in responses to conditioned fear training in our paradigm. This is particularly true as we have reduced the number of ST sessions to meet the requirements of additional experimental manipulations. However, over the last few years, the number of animals showing increased REM after ST and fearful contexts has dramatically increased. Other investigators have also recently reported increases in REM in Wistar rats after relatively intense sessions of inescapable shock.83 Whether these difference are due to genetic drift in the supply colonies or simply a sampling variance is not known. However, the present results demonstrate that the Wistar strain contains distinct phenotypes with respect to sleep outcomes of stress and stressful memories.
The rats were separated into groups based on changes in REM after ST, and this limits our conclusions regarding the potential functional difference between high and low levels of post-stress REM and the relevance of REM for adaptive coping. However, a positive role for post-stress increases in REM is supported by indications that REM plays a role in adaptive processing of emotional learning and memories,48–50 its increase after controllable stress,18,19 and the fact that REM is increased after fear extinction relative to animals that continue to show fear41 (it is worth noting that REM-related pontine wave activity may be critical for extinction84). Additionally, post-stress increases in sleep have been reported for a variety of stressors (avoidable footshock,85,86 restraint,87–89 water maze,85 novel object,17,90,91 open field,16,91 cage change16,91 and social stress92,93) not linked to enduring psychopathology thereby suggesting that increased post-stress REM sleep is normal after many mild to moderately challenging situations. It should be noted however that the nature of sleep in the immediate aftermath of traumatic stress in humans is virtually unknown. Nevertheless, disrupted sleep both before and after significant stress is implicated in stress-related pathology,44–46,94 and continued sleep disturbances have been suggested to be a hallmark symptom of PTSD.61
Developing methods to predict prior to training, perhaps via performance on an elevated plus maze34–38 or other behavioral method designed to assess anxiety, which rats will show increases and which will show decreases in REM after ST and fearful contexts could increase the ability to exploit individual differences as an experimental variable. However, as has been noted in other experimental contexts,95 the payoff for considering differences in the responses of individual animals will likely be significantly increased validity for models related to PTSD.
CONCLUSION
The data demonstrate that outbred rats can show significant individual differences in post-stress sleep that are independent of behavioral markers of fear and activation of the peripheral stress system. The differences in sleep and evidence that REM is involved in the processing of emotional memories suggest that post-stress REM may be a useful biomarker of resilience and vulnerability that may enable improved animals models of PTSD and anxiety disorders. These data also suggest that the BLA may play a role in regulating differences in sleep, and that individual differences in amygdalar functioning may be a significant factor in resilience and vulnerability to stress. Further work is needed to clearly delineate the association of increased or decreased REM in adaptive or non-adaptive stress outcomes, and the role that the amygdala may play in mediating them.
DISCLOSURE STATEMENT
This was not an industry supported study. The work was supported by NIH research grant MH64827. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Davis M. The role of the amygdala in conditioned fear. In: Aggleton J, editor. The Amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY: Wiley-Liss, Inc; 1992. pp. 255–305. [Google Scholar]
- 2.Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 3.Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and the fear-potentiated startle effect. Pharmacol Ther. 1990;47:147–65. doi: 10.1016/0163-7258(90)90084-f. [DOI] [PubMed] [Google Scholar]
- 4.Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112:218–38. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- 5.Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Mol Psychiatry. 1996;1:278–97. [PubMed] [Google Scholar]
- 6.Shalev AY, Ragel-Fuchs Y, Pitman RK. Conditioned fear and psychological trauma. Biol Psychiatry. 1992;31:863–5. doi: 10.1016/0006-3223(92)90113-e. [DOI] [PubMed] [Google Scholar]
- 7.Lissek S, van Meurs B. Learning models of PTSD: theoretical accounts and psychobiological evidence. Int J Psychophysiol. 2015;98:594–605. doi: 10.1016/j.ijpsycho.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez P, Martinez KG. The role of stress and fear in the development of mental disorders. Psychiatr Clin North Am. 2014;37:535–46. doi: 10.1016/j.psc.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paylor R, Tracy R, Wehner J, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–7. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- 10.Falls WA, Carlson S, Turner JG, Willott JF. Fear-potentiated startle in two strains of inbred mice. Behav Neurosci. 1997;111:855–61. [PubMed] [Google Scholar]
- 11.Cohen H, Zohar J, Matar M. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2003;53:463–73. doi: 10.1016/s0006-3223(02)01909-1. [DOI] [PubMed] [Google Scholar]
- 12.Cohen H, Zohar J, Matar MA, Zeev K, Loewenthal U, Richter-Levin G. Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 2004;29:1962–70. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- 13.Sanford LD, Tang X, Ross RJ, Morrison AR. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Genet. 2003;33:43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- 14.Sanford LD, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003;147:193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- 15.Sanford LD, Yang L, Tang X. Influence of contextual fear on sleep in mice: a strain comparison. Sleep. 2003;26:527–40. doi: 10.1093/sleep/26.5.527. [DOI] [PubMed] [Google Scholar]
- 16.Tang X, Xiao J, Liu X, Sanford LD. Strain differences in the influence of open field exposure on sleep in mice. Behav Brain Res. 2004;154:137–47. doi: 10.1016/j.bbr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Tang X, Xiao J, Brian PB, Fang J, Sanford LD. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/J mice. Physiol Behav. 2005;85:419–29. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Wellman LL, Ambrozewicz MA, Sanford LD. Effects of stressor predictability and controllability on sleep, temperature, and fear behavior in mice. Sleep. 2011;34:759–71. doi: 10.5665/SLEEP.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanford LD, Yang L, Wellman LL, Liu X, Tang X. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep. 2010;33:A621–30. doi: 10.1093/sleep/33.5.621. Abstract Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shvil E, Rusch HL, Sullivan GM, Neria Y. Neural, psychophysiological, and behavioral markers of fear processing in PTSD: a review of the literature. Curr Psychiatr Rep. 2013;15:358. doi: 10.1007/s11920-013-0358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–50. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 22.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–28. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bremner JD, Vermetten E, Schmahl C, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Yang L, Wellman LL, Tang X, Sanford LD. GABAergic antagonism of the central nucleus of the amygdala attenuates reductions in rapid eye movement sleep after inescapable footshock stress. Sleep. 2009;32:A888–96. doi: 10.1093/sleep/32.7.888. Abstract Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellman LL, Yang L, Ambrozewicz MA, Machida M, Sanford LD. Basolateral amygdala and the regulation of fear-conditioned changes in sleep: role of corticotropin-releasing factor. Sleep. 2013;36:471–80. doi: 10.5665/sleep.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Wellman LL, Yang L, Ambrozewicz MA, Tang X, Sanford LD. Antagonizing corticotropin-releasing factor in the central nucleus of the amygdala attenuates fear-induced reductions in sleep but not freezing. Sleep. 2011;34:A1539–49. doi: 10.5665/sleep.1394. Abstract Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cousens G, Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav Neurosci. 1998;112:1092–103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- 28.Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–62. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J Neurosci. 1998;18:3088–97. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110:718–26. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- 31.Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J Neurosci. 1999;19:9570–8. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108:1005–9. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- 33.Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–91. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- 34.Naslund J, Studer E, Pettersson R, et al. Differences in anxiety-like behavior within a batch of wistar rats are associated with differences in serotonergic transmission, enhanced by acute SRI administration, and abolished by serotonin depletion. Int J Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider P, Ho YJ, Spanagel R, Pawlak CR. A novel elevated plus-maze procedure to avoid the one-trial tolerance problem. Front Behav Neurosci. 2011;5:43. doi: 10.3389/fnbeh.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho YJ, Eichendorff J, Schwarting RK. Individual response profiles of male Wistar rats in animal models for anxiety and depression. Behav Brain Res. 2002;136:1–12. doi: 10.1016/s0166-4328(02)00089-x. [DOI] [PubMed] [Google Scholar]
- 37.Borta A, Wohr M, Schwarting RK. Rat ultrasonic vocalization in aversively motivated situations and the role of individual differences in anxiety-related behavior. Behav Brain Res. 2006;166:271–80. doi: 10.1016/j.bbr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Ho YJ, Hsu LS, Wang CF, et al. Behavioral effects of d-cycloserine in rats: the role of anxiety level. Brain Res. 2005;1043:179–85. doi: 10.1016/j.brainres.2005.02.057. [DOI] [PubMed] [Google Scholar]
- 39.Liebsch G, Montkowski A, Holsboer F, Landgraf R. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res. 1998;94:301–10. doi: 10.1016/s0166-4328(97)00198-8. [DOI] [PubMed] [Google Scholar]
- 40.Wellman LL, Fitzpatrick ME, Machida M, Sanford LD. The basolateral amygdala determines the effects of fear memory on sleep in an animal model of PTSD. Exp Brain Res. 2014;232:1555–65. doi: 10.1007/s00221-014-3850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wellman LL, Yang L, Tang X, Sanford LD. Contextual fear extinction ameliorates sleep disturbances found following fear conditioning in rats. Sleep. 2008;31:1035–42. [PMC free article] [PubMed] [Google Scholar]
- 42.Pawlyk AC, Jha SK, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbances in posttraumatic stress disorder: the role of context after fear conditioning. Biol Psychiatry. 2005;57:268–77. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Jha SK, Brennan FX, Pawlyk AC, Ross RJ, Morrison AR. REM sleep: a sensitive index of fear conditioning in rats. Eur J Neurosci. 2005;21:1077–80. doi: 10.1111/j.1460-9568.2005.03920.x. [DOI] [PubMed] [Google Scholar]
- 44.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345:1825–32. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 45.Lavie P, Hefez A, Halperin G, Enoch D. Long-term effects of traumatic war-related events on sleep. Am J Psychiatry. 1979;136:175–8. doi: 10.1176/ajp.136.2.175. [DOI] [PubMed] [Google Scholar]
- 46.Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–7. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- 47.Kruger L, Saporta S, Swanson L. New York, NY: Cambridge University Press; 1995. Photographic atlas of the rat brain. [Google Scholar]
- 48.Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–48. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 50.Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20:893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- 51.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 52.Doyere V, Gisquet-Verrier P, de Marsanich B, Ammassari-Teule M. Age-related modifications of contextual information processing in rats: role of emotional reactivity, arousal and testing procedure. Behav Brain Res. 2000;114:153–65. doi: 10.1016/s0166-4328(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 53.Havekes R, Meerlo P, Abel T. Animal studies on the role of sleep in memory: from behavioral performance to molecular mechanisms. Curr Topics Behav Neurosci. 2015;25:183–206. doi: 10.1007/7854_2015_369. [DOI] [PubMed] [Google Scholar]
- 54.Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68:129–35. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- 55.Paylor R, Tracy R, Wehner J, Rudy J. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–7. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- 56.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 57.Menz MM, Rihm JS, Salari N, et al. The role of sleep and sleep deprivation in consolidating fear memories. NeuroImage. 2013;75:87–96. doi: 10.1016/j.neuroimage.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Greenwood BN, Thompson RS, Opp MR, Fleshner M. Repeated exposure to conditioned fear stress increases anxiety and delays sleep recovery following exposure to an acute traumatic stressor. Front Psychiatry. 2014;5:146. doi: 10.3389/fpsyt.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rossi VC, Tiba PA, Moreira KD, Ferreira TL, Oliveira MG, Suchecki D. Effects of sleep deprivation on different phases of memory in the rat: dissociation between contextual and tone fear conditioning tasks. Front Behav Neurosci. 2014;8:389. doi: 10.3389/fnbeh.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Memy. 2003;10:168–76. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hagewoud R, Bultsma LJ, Barf RP, Koolhaas JM, Meerlo P. Sleep deprivation impairs contextual fear conditioning and attenuates subsequent behavioural, endocrine and neuronal responses. J Sleep Res. 2011;20:259–66. doi: 10.1111/j.1365-2869.2010.00895.x. [DOI] [PubMed] [Google Scholar]
- 62.Kumar T, Jha SK. Sleep deprivation impairs consolidation of cued fear memory in rats. PloS One. 2012;7:e47042. doi: 10.1371/journal.pone.0047042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silvestri AJ. REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiol Behav. 2005;84:343–9. doi: 10.1016/j.physbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Hellman K, Abel T. Fear conditioning increases NREM sleep. Behav Neurosci. 2007;121:310–23. doi: 10.1037/0735-7044.121.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006;5:139–49. doi: 10.1111/j.1601-183X.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- 66.Vinckers CH, van Oorschot R, Olivier B, Groenink L. Stress-induced hyperthermia in the mouse. In: Gould TD, editor. Mood and Anxiety-related Phenotypes in Mice: Characterization Using Behavioral Tests. New York, NY: Humana Press; 2009. pp. 139–52. [Google Scholar]
- 67.Clement JG, Mills P, Brockway B. Use of telemetry to record body temperature and activity in mice. J Pharmacol Methods. 1989;21:129–40. doi: 10.1016/0160-5402(89)90031-4. [DOI] [PubMed] [Google Scholar]
- 68.Krarup A, Chattopadhyay P, Bhattacharjee AK, Burge JR, Ruble GR. Evaluation of surrogate markers of impending death in the galactosamine-sensitized murine model of bacterial endotoxemia. Lab Anim Sci. 1999;49:545–50. [PubMed] [Google Scholar]
- 69.Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom Med. 2001;63:476–86. doi: 10.1097/00006842-200105000-00018. [DOI] [PubMed] [Google Scholar]
- 70.Groenink L, van der Gugten J, Zethof T, van der Heyden J, Olivier B. Stress-induced hyperthermia in mice: hormonal correlates. Physiol Behav. 1994;56:747–9. doi: 10.1016/0031-9384(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 71.Veening JG, Bouwknecht JA, Joosten HJ, et al. Stress-induced hyperthermia in the mouse: c-fos expression, corticosterone and temperature changes. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:699–707. doi: 10.1016/j.pnpbp.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Yang L, Wellman LL, Ambrozewicz MA, Sanford LD. Effects of stressor predictability and controllability on sleep, temperature and fear behavior in mice. Sleep. 2011;34:759–71. doi: 10.5665/SLEEP.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999;113:283–90. doi: 10.1037//0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- 74.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zimmerman JM, Maren S. The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiol Learn Mem. 2011;95:199–205. doi: 10.1016/j.nlm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–44. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu GQ, Zhong MK, Zhang JX, et al. [Role of basolateral amygdaloid nuclei in sleep and wakeful state regulation] Sheng Li Xue Bao. 1998;50:688–92. [PubMed] [Google Scholar]
- 78.Benca RM, Obermeyer WH, Shelton SE, Droster J, Kalin NH. Effects of amygdala lesions on sleep in rhesus monkeys. Brain Res. 2000;879:130–8. doi: 10.1016/s0006-8993(00)02761-x. [DOI] [PubMed] [Google Scholar]
- 79.Sanford LD, Tang X. Effect of stress on sleep and its relationship to PTSD. In: Shiromani P, Keane T, LeDoux JE, editors. Neurobiology of PTSD. Totowa, NJ: Humana Press; 2009. pp. 231–53. [Google Scholar]
- 80.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55:626–32. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 81.Weiss DS, Marmar CR, Schlenger WE, et al. The prevalence of lifetime and partial post-traumatic stress disorder in Vietnam theater veterans. J Trauma Stress. 1992;5:365–76. [Google Scholar]
- 82.Mellman TA, Kobayashi I, Lavela J, Wilson B, Hall Brown TS. A relationship between REM sleep measures and the duration of posttraumatic stress disorder in a young adult urban minority population. Sleep. 2014;37:1321–6. doi: 10.5665/sleep.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O'Malley MW, Fishman RL, Ciraulo DA, Datta S. Effect of five-consecutive-day exposure to an anxiogenic stressor on sleep-wake activity in rats. Front Neurol. 2013;4:15. doi: 10.3389/fneur.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Datta S, O'Malley MW. Fear extinction memory consolidation requires potentiation of pontine-wave activity during REM sleep. J Neurosci. 2013;33:4561–9. doi: 10.1523/JNEUROSCI.5525-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith C. Sleep states and memory processes. Behav Brain Res. 1995;69:137–45. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 86.Sanford LD, Xiao J, Liu X, Yang L, Tang X. Influence of avoidance training (AT) and AT cues on sleep in C57BL/6J (B6) and BALB/cJ (C) mice. Sleep. 2005;28:A6. (Abstract Suppl) [Google Scholar]
- 87.Gonzalez MM, Debilly G, Valatx JL, Jouvet M. Sleep increase after immobilization stress: role of the noradrenergic locus coeruleus system in the rat. Neurosci Lett. 1995;202:5–8. doi: 10.1016/0304-3940(95)12209-5. [DOI] [PubMed] [Google Scholar]
- 88.Meerlo P, Easton A, Bergmann BM, Turek FW. Restraint increases prolactin and REM sleep in C57BL/6J mice but not in BALB/cJ mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R846–54. doi: 10.1152/ajpregu.2001.281.3.R846. [DOI] [PubMed] [Google Scholar]
- 89.Rampin C, Cespuglio R, Chastrette N, Jouvet M. Immobilisation stress induces a paradoxical sleep rebound in rat. Neurosci Lett. 1991;126:113–8. doi: 10.1016/0304-3940(91)90532-x. [DOI] [PubMed] [Google Scholar]
- 90.Schiffelholz T, Aldenhoff JB. Novel object presentation affects sleep-wake behavior in rats. Neurosci Lett. 2002;328:41–4. doi: 10.1016/s0304-3940(02)00452-4. [DOI] [PubMed] [Google Scholar]
- 91.Tang X, Liu X, Yang L, Sanford LD. Rat strain differences in sleep after acute mild stressors and short-term sleep loss. Behav Brain Res. 2005;160:60–71. doi: 10.1016/j.bbr.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 92.Meerlo P, Pragt BJ, Daan S. Social stress induces high intensity sleep in rats. Neurosci Lett. 1997;225:41–4. doi: 10.1016/s0304-3940(97)00180-8. [DOI] [PubMed] [Google Scholar]
- 93.Meerlo P, Turek FW. Effects of social stimuli on sleep in mice: non-rapid-eye-movement (NREM) sleep is promoted by aggressive interaction but not by sexual interaction. Brain Res. 2001;907:84–92. doi: 10.1016/s0006-8993(01)02603-8. [DOI] [PubMed] [Google Scholar]
- 94.Bryant RA, Felmingham K, Kemp A, et al. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol Med. 2008;38:555–61. doi: 10.1017/S0033291707002231. [DOI] [PubMed] [Google Scholar]
- 95.Holly EN, Miczek KA. Capturing individual differences: challenges in animal models of posttraumatic stress disorder and drug abuse. Biol Psychiatry. 2015;78:816–8. doi: 10.1016/j.biopsych.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]