Abstract
Study Objectives:
Individual differences in sleep timing have been widely recognized and are of particular relevance in adolescents and young adults who often show mild to severely delayed sleep. The biological mechanisms underlying the between-subject variance remain to be determined. Recent human genetics studies showed an association between sleep timing and melanopsin gene variation, but support for functional effects on downstream pathways and behavior was not demonstrated before. We therefore investigated the association between the autonomic (i.e., pupil diameter) and behavioral (i.e., sleep timing) readouts of two different downstream brain areas, both affected by the same melanopsin-dependent retinal phototransduction: the olivary pretectal nucleus (OPN) and the suprachiasmatic nucleus (SCN).
Methods:
Our study population included 71 healthy individuals within an age range with known vulnerability to a delayed sleep phase (16.8–35.7 y, 37 males, 34 females). Pupillometry was performed to estimate functionality of the intrinsic melanopsin-signaling circuitry based on the OPN-mediated post-illumination pupil response (PIPR) to blue light. Sleep timing was quantified by estimating the SCN-mediated mid-sleep timing in three different ways in parallel: using a chronotype questionnaire, a sleep diary, and actigraphy.
Results:
All three measures consistently showed that those individuals with a later mid-sleep timing had a more pronounced PIPR (0.03 < P < 0.05), indicating a stronger blue-light responsiveness of the intrinsic melanopsin-based phototransduction circuitry.
Conclusions:
Trait-like individual differences in the melanopsin phototransduction circuitry contribute to individual differences in sleep timing. Blue light-sensitive young individuals are more prone to delayed sleep.
Citation:
van der Meijden WP, Van Someren JL; te Lindert BH, Bruijel J, van Oosterhout F, Coppens JE, Kalsbeek A, Cajochen C, Bourgin P, Van Someren EJ. Individual differences in sleep timing relate to melanopsin-based phototransduction in healthy adolescents and young adults. SLEEP 2016;39(6):1305–1310.
Keywords: circadian, light, melanopsin, sleep timing, post-illumination pupil response
Significance.
Biological mechanisms underlying individual differences in sleep timing are insufficiently understood. We show an association of individual differences in sleep timing with specific functionality of melanopsin-containing intrinsically photosensitive retinal ganglion cells (ipRGCs). Focusing on adolescents and young adults, an age range with known vulnerability to a delayed sleep phase, we measured the post-illumination pupil response after blue light. This measure of intrinsic ipRGC functionality was consistently associated with three independent measures of sleep timing (i.e., a chronotype questionnaire, a sleep diary, and actigraphy). Individuals with a later sleep timing had a stronger intrinsic ipRGC responsiveness to blue light. Clinical interventions for delayed sleep phase syndrome may benefit from considering hypersensitivity to blue light.
INTRODUCTION
The suprachiasmatic nucleus (SCN) in the hypothalamus is the central pacemaker for endocrine, physiological, and behavioral rhythms.1 The rhythms orchestrated by this endogenous clock are characterized by a periodicity of approximately 24 h and are therefore dubbed “circadian rhythms” (circa = about, dies = day).2 In order to be in phase with the environment (i.e., a day on earth), however, these circadian rhythms need to be synchronized to an exact 24-h cycle. The most important environmental information used for this entrainment is the light-dark cycle.3 Key players in this so called circadian photoentrainment are the intrinsically photosensitive retinal ganglion cells (ipRGCs)4,5: a small subset of retinal ganglion cells expressing the photopigment melanopsin (peak sensitivity 460–480 nm), which renders them intrinsically photosensitive.6,7 The ipRGCs integrate their intrinsic photoresponse with the extrinsic input from the classic rod and cone phototransduction pathways.8 As a result of direct connections between ipRGCs and the brain, the integrated information on environmental light is directly transduced from the retina to downstream areas, with the SCN as one of the main targets.9 From an evolutionary perspective, ambient light solely consisted of sunlight, but since the introduction of electric light, the 24-h exposure profile is increasingly becoming a mixture of solar and artificial light.10 Findings from previous studies suggest that this “light pollution” affects photoentrainment of the biological clock and may shift circadian rhythms.11,12
The direction of photoinduced circadian shifts is dependent on the diurnal profile of solar and artificial light exposure according to a so-called phase response curve.13 Morning bright light advances circadian phase,14 whereas bright light exposure in the evening causes a phase delay.15 Classic studies on circadian rhythms identified circadian phase changes from shifts in the core body temperature minimum or the dim light onset of the sleep-promoting hormone melatonin.16,17 Photo-induced shifts were also found in timing of the sleep-wake cycle,18 which is the most explicitly expressed behavioral circadian rhythm.19,20 Interestingly, the phase shifting effects of light on sleep-wake timing show a considerable interindividual variation.21 Whereas entrainment in “early birds” is more likely to result in a sleep-wake schedule that is advanced relative to the environmental light-dark cycle, the entrainment of “night owls” is more likely to result in a sleep-wake schedule that is relatively delayed. These individual differences in internal-external phase relationships are thought to result, in part, from polymorphisms in core clock genes.22–24 Recent findings moreover suggest that the individual differences may involve as well polymorphisms in genes of the phototransduction circuitry.25,26 Human genetics studies found an association between sleep timing and melanopsin gene variation. Previous work also showed chronotype-dependent differential responses to light.27,28 It remains to be evaluated, however, whether such individual differences actually involve a specific functionality of the melanopsin phototransduction circuitry. We here address this question, by assessing the association of individual differences in a pupillary response that is independent from the inputs from rods and cones and specific to intrinsic melanopsin-dependent phototransduction effects on the downstream olivary pretectal nucleus (OPN), with those in three different markers of sleep timing involving the SCN.29 We focus on healthy adolescents and young adults, a population in which a mild to severely delayed sleep timing is highly prevalent.30,31 Functionality of the intrinsic melanopsin-driven phototransduction circuitry is assessed from the post-illumination pupil response (PIPR) after bright blue light (i.e., the sustained pupil constriction), which indicates the strength of the photoresponsiveness of the intrinsic melanopsin-signaling phototransduction circuitry.32 Individual differences in PIPR are correlated with sleep timing assessed in three ways: using the Munich Chronotype Questionnaire (MCTQ),33 the Consensus Sleep Diary,34 and actigraphy.35
METHODS
Participants
We included participants with an age between 16 and 36 y. Other inclusion criteria were self-acclaimed health and no use of medication, as indicated by the sleepregistry.nl36 implementation of the health-related questions of the Duke Structured Interview for Sleep Disorders.37 The sleepregistry.nl implementation includes questions on current and past health issues according to the 10 categories of the International Classification of Diseases. Shift workers were excluded. Nagel anomaloscope tests were used to exclude participants suffering from color vision deficiency.38 Participants received oral and written information on the study and signed informed consent before participation. The study was approved by the Medical Ethical Committee of the VU University Medical Center Amsterdam.
Our study population included 37 males and 34 females with a mean age ± standard deviation [range] of 22.7 ± 5.0 [16.8 – 35.7] y, and was composed of participants included in three different studies. As part of these studies, all 71 participants were subjected to a PIPR assessment, and completed the MCTQ to obtain a measure for habitual sleep timing. Recent sleep timing was measured in 56 of the 71 participants using a sleep diary and actigraphy: in 29 participants immediately following the PIPR assessment and in 27 participants one month prior to the PIPR assessment.
Post-illumination Pupil Response
Most characteristics of the pupillary light reflex are determined by the combined activation of rods, cones, and melanopsin.39 Conversely, the PIPR is a feature of the pupillary light reflex that is almost entirely driven by melanopsin activation, with only limited contribution of rod and cone activity, and can therefore be used to quantify the functionality of the intrinsic melanopsin-signaling system.32 Details of the pupillometry paradigm for PIPR assessment are described elsewhere.40 In brief, using a custom-made infrared pupillometry setup, the pupil diameter of the left eye was measured while the dilated (tropicamide 0.5%) pupil of the right eye underwent a light exposure protocol. This protocol contained five consecutive 5-min blocks: baseline dark; monochromatic red light (peak wavelength (full width half maximum): 630 (20) nm, luminance: 375 cd/m2) to maximize the effect of subsequent blue light41; dark; monochromatic blue light (peak wavelength (full width half maximum): 470 (20) nm, luminance: 375 cd/m2); and postblue dark. We calculated the PIPR by subtracting pupil diameter during post-blue dark from pupil diameter during baseline dark.42,43 All PIPR assessments were performed between 8:30 and 17:00 to avoid the evening and nighttime modulation of the melanopsin-based phototransduction circuitry.44 We previously showed that this outcome parameter is sensitive to individual differences and has very high within-subject test-retest reliability.40
Habitual Sleep Timing: Munich Chronotype Questionnaire
From the MCTQ we obtained habitual lights-out time, sleep onset latency, and final wake-up time separately for work days and free days. Sleep onset time was calculated by subtracting sleep onset latency from lights-out time.33 Sleep duration was calculated as the duration between sleep onset time and final wake-up time and mid-sleep as the midpoint between them.
Recent Sleep Timing: Consensus Sleep Diary
As a second measure of sleep timing, 56 participants kept a sleep diary for 1 w. From the sleep diaries we obtained lights-out time, sleep onset latency, and final wake-up time. For each of the 7 days, sleep onset time, mid-sleep time, and sleep duration were calculated as previously described.
Recent Sleep Timing: Actigraphy
To obtain an objective estimate of sleep timing, 56 participants wore an actigraph during the week they kept a sleep diary. Participants were equipped with either a traditional actigraph (Philips Actiwatch Spectrum, Philips Respironics, Murrysville, PA, USA) or a microelectromechanical accelerometer (Move II, Movisens GmbH, Karlsruhe, Germany). We previously showed almost perfect agreement between these two types of acti-graphs in discriminating between sleep and wake.45 Sleep onset time and final wake-up time were automatically estimated using a detection algorithm46 implemented in Matlab (Version 2014A, The Mathworks Inc., Natick, MA, USA). The algorithm searches for these two parameters between the lights-out time and final wake-up time as obtained from the sleep diary (https://github.com/btlindert/actant-1). Mid-sleep and sleep duration were calculated as previously described.
Statistical Analysis
Mixed-effect regression models were used to estimate how individual differences in the PIPR predicted individual differences in sleep timing. Mid-sleep was used as a the main marker for sleep timing.47 Ancillary analyses with sleep onset time and final wake-up time as outcome measures were run to determine whether mid-sleep shifts arose from changes in sleep onset time, wake-up time, or both. Finally, it was evaluated whether individual differences in the PIPR predicted individual differences in sleep duration.
The data from all three measurement techniques represented two-level hierarchies. For each participant, the MCTQ provided two sleep timing measures, one for work days and one for free days. The sleep diaries and actigraphy provided seven sleep timing measures: five for work days and two for free days. Type of day (work day versus free day) was included as within-subject time-varying covariate, dummy coded as 0 for work days and 1 for free days. Age and sex were initially included as possible confounding covariates.31 Sex was dummy coded as 0 for females and 1 for males. Covariates were selected for inclusion in the final model using backward elimination and comparing models with likelihood-ratio tests. The PIPR and age variable were centered around the grand mean to optimize interpretation of the model's intercept and slope parameters.48 Q-Q plots were made for all models to evaluate the assumption of a normal distribution of the residuals. Mixed-effect regression models were conducted using the “lme4” package for R (Version 3.1.1, R Foundation for Statistical Computing, Vienna, Austria). The significance of the effects of the independent variables on the sleep timing variables was evaluated using t-tests with the denominator degrees of freedom based on Satterthwaite approximation.
RESULTS
Underscoring the value of multiple outcome measures, sleep timing derived from the MCTQ shared no more than 48.3% of its variance with the variance in mid-sleep timing estimated from sleep diaries and 51.2% of its variance with the variance in mid-sleep timing estimated from actigraphy. The mid-sleep timing estimates from sleep diaries and actigraphy shared 94.5% of their interindividual variance. Regression analyses for all three measures consistently indicated that people with more pronounced PIPR showed a later mid-sleep timing (MCTQ: P = 0.03; sleep diary: P = 0.046; actigraphy: P = 0.04) (Figure 1). The association between the magnitude of the PIPR and mid-sleep timing arose from later sleep onset (MCTQ: P = 0.01; sleep diary: P = 0.02; actigraphy: P = 0.04) rather than a delay in wake-up time (MCTQ: P = 0.15; sleep diary: P = 0.30; actigraphy: P = 0.17) (Table 1). MCTQ data indicated that individuals with a more pronounced PIPR had a shorter sleep duration (P = 0.04), but this association was not confirmed using sleep duration estimates from sleep diaries (P = 0.12) or actigraphy recordings (P = 0.25).
Figure 1.
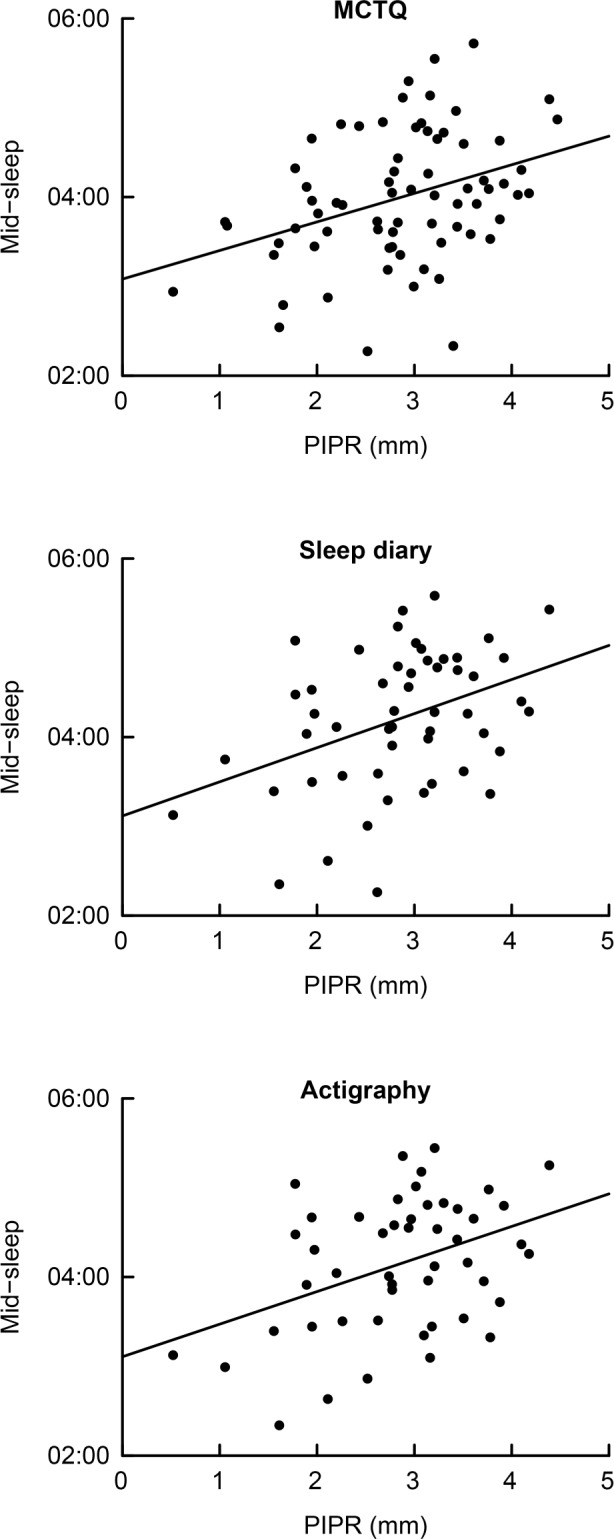
The relationship between post-illumination pupil response (PIPR) and mid-sleep timing. Mid-sleep was obtained using the Munich Chronotype Questionnaire (MCTQ; top panel), the Consensus Sleep Diary (sleep diary; center panel), and actigraphy (bottom panel). Each dot represents the weekly average of mid-sleep per participant. For the MCTQ the average mid-sleep for 1 w was calculated using the following formula: [ (5 * mid-sleep during work days + 2 * mid-sleep during free days) / 7 ]. The lines indicate the association between PIPR and mid-sleep timing as obtained from the MCTQ (r = 0.36), sleep diaries (r = 0.39), and actigraphy (r = 0.40).
Table 1.
Model estimates of the effects of post-illumination pupil response, age, and type of day on sleep timing.
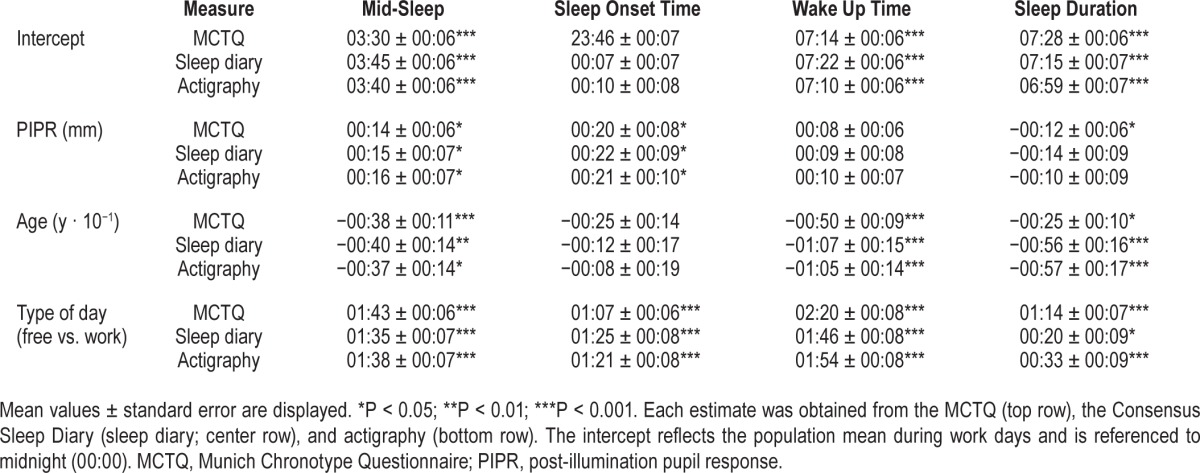
The association of individual differences in PIPR magnitude and sleep timing was not secondary to confounding by common effects of age or sex. Sex did not affect either PIPR magnitude (P = 0.82) or any of the three mid-sleep variables (0.19 < P < 0.51). Whereas PIPR magnitude decreased with increasing age (P = 0.03) and mid-sleep timing on all three measures became earlier with increasing age (6 · 10−4 < P < 0.01), these changes did not confound the association between PIPR and sleep timing: the inclusion of age as a covariate in the linear regression models did not eliminate the association between PIPR and sleep timing. Type of day explained a considerable part of the within-subject variance in mid-sleep timing (MCTQ: 78.2%; sleep diary: 41.0%; actigraphy: 43.1%). Inclusion of type of day as a covariate therefore improved estimation of individual sleep timing and allowed for a more reliable evaluation of the relationship between individual differences in sleep timing and interindividual variation in PIPR (see Figure S1 in the supplemental material for the relationship between PIPR and mid-sleep during free days). Predictably, the effect of type of day on mid-sleep timing was significant for all three measures: mid-sleep during free days was later than during work days (all: P < 1 · 10−15).
DISCUSSION
The aim of the current study was to assess the relationship between interindividual variation in functionality of the intrinsic melanopsin-dependent circuitry and individual differences in the timing of sleep in a healthy young population with known vulnerability to a delayed sleep phase. We found a significant association showing that individuals with a stronger blue-light responsiveness of the intrinsic melanopsin-based phototransduction circuitry slept at a later phase of the 24-h light-dark cycle.
Previous studies showed that individual differences in the habitual timing of sleep are related to the magnitude of the effect of light exposure on acute melatonin levels. One study reported that two individuals with early habitual bedtimes lacked the melatonin-suppressing effect of light.28 In contrast, another study showed enhanced light-induced melatonin suppression in individuals with very late habitual bedtimes due to delayed sleep phase disorder.27 Interestingly, others showed that individuals with an increased photoinduced delay of melatonin secretion had a stronger PIPR.49 These endocrine findings are in line with our results on behavioral circadian measures, indicating that an increased PIPR strength is associated with a larger photoinduced delay of sleep timing. For future studies it would therefore be interesting to integrate endocrine and behavioral circadian measures and relate them to the PIPR to more specifically investigate to what extent individual phase differences are related to individual differences at the very earliest stage of circadian photoentrainment (i.e. the melanopsin-expressing ipRGCs in the retina). If a robust association is demonstrated, it is tempting to imagine the feasibility of estimating an individual's circadian phase from the PIPR in combination with one's 24-h light exposure profile. This would be a major advantage, because such measures may be less costly than dim light melatonin onset assessment (i.e., one of the most reliable markers of the phase of the circadian pacemaker in the SCN).40,50
A limitation of our study was that our study design does not allow for a causal interpretation of the association between individual differences in sleep timing and interindividual variation in functionality of the intrinsic melanopsin-based phototransduction circuitry. However, we consider it more likely that an individual's melanopsin-based phototransduction circuitry affects habitual sleep, rather than that an individual night affects the PIPR, because we have previously shown that the PIPR magnitude is highly replicable across multiple assessments within subjects,40 whereas sleep timing is quite variable over subsequent nights.51 An adequate estimate of habitual sleep timing requires averaging 1 w of data to overcome most of the day-to-day variance,51 as employed in the current study. A limitation of the study was that information on the participant's use of alcohol, health food supplements, or recreational drugs was incomplete. The intake of such substances may alter the pupil response52 and sleep timing53 and thus have increased the unexplained variance in our data. We did obtain the number of alcohol consumptions during the week in which the participants filled out the sleep diary and wore an actigraph. We found no association between alcohol intake and mid-sleep during work days (P = 0.35), indicating that the possible confounding effect of substance use on our results would be only small, if present. Another limitation of our study was that the PIPR assessment only allows for a quantification of the functionality of the intrinsic melanopsin-based photo-transduction circuitry and not for estimating functionality of the extrinsic rod and cone pathways. For future studies it may therefore be interesting to include functional measures on both the intrinsic as well as the extrinsic circuitry in order to get a more complete picture of the individual differences in ipRGC functionality. Another possible limitation of our study was that we did not measure light history. Differences in habitual environmental light exposure may contribute to interindividual variation in sleep timing.13 However, whereas a different way of assessing the PIPR is sensitive to one's light history,41 the measure we here applied is more robust and does not seem to be confounded by it.40
We have previously shown that the PIPR, assessed in the way presented here, may be a very reliable biomarker with considerable interindividual differences, yet marginal sensitivity to environmental and behavioral changes. We therefore consider it likely that these individual differences in functionality of the intrinsic melanopsin-dependent circuitry result from the natural variation in the melanopsin gene. Indeed, melanopsin-driven characteristics of pupillary light reflex have previously been associated with melanopsin polymorphisms.54 Interestingly, other studies reported that melanopsin gene polymorphisms were also associated with the timing of sleep.25,26 Future studies should ideally combine assessment of melanopsin polymorphisms, PIPR, dim light melatonin onset, and sleep timing in order to elucidate the mechanisms underlying their associations and to assess the value of the PIPR assessment to understand individual differences in circadian regulation.
DISCLOSURE STATEMENT
This was not an industry supported study. This project has been funded with support from the NeuroTime Erasmus+: Erasmus Mundus programme of the European Commission. This publication/communication reflects the views only of the author, and the Commission cannot be held responsible for any use that may be made of the information contained therein. This work was supported by Project NeuroSIPE 10738, of the Dutch Technology Foundation STW, which is part of the Netherlands Organization for Scientific Research (NWO) and partly funded by the Ministry of Economic Affairs, Agriculture and Innovation. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Brit Giesbertz, Kim Dekker, Jeske Hendrinks, Liz Vink and Jesminne Castricum (Netherlands Institute for Neuroscience), and Denise Bijlenga and Sandra Kooij (PsyQ The Hague) for their contributions to the realization of this study.
ABBREVIATIONS
- DLMO
Dim Light Melatonin Onset
- ipRGC
intrinsically photosensitive retinal ganglion cell
- MCTQ
Munich ChronoType Questionnaire
- OPN
olivary pretectal nucleus
- PIPR
post-illumination pupil response
- SCN
suprachiasmatic nucleus
REFERENCES
- 1.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind's clock. Oxford University Press. 1991 [Google Scholar]
- 2.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–8. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- 4.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 5.Panda S, Sato TK, Castrucci AM, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–6. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 6.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–54. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 9.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navara KJ, Nelson RJ. The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res. 2007;43:215–24. doi: 10.1111/j.1600-079X.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- 11.Stevens RG, Blask DE, Brainard GC, et al. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 2007;115:1357–62. doi: 10.1289/ehp.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright KP, Jr, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–8. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy JF, Kronauer RE, Czeisler CA. Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. J Physiol. 1996;495(Pt 1):289–97. doi: 10.1113/jphysiol.1996.sp021593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235:352–4. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- 15.Czeisler CA, Allan JS, Strogatz SH, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–71. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 16.Czeisler CA, Kronauer RE, Allan JS, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–33. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 17.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honma K, Honma S, Wada T. Phase-dependent shift of free-running human circadian rhythms in response to a single bright light pulse. Experientia. 1987;43:1205–7. doi: 10.1007/BF01945525. [DOI] [PubMed] [Google Scholar]
- 19.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–8. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 20.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–9. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 21.Santhi N, Thorne HC, van der Veen DR, et al. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2012;53:47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 22.Ebisawa T, Uchiyama M, Kajimura N, et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2:342–6. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–3. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 24.Jones CR, Campbell SS, Zone SE, et al. Familial advanced sleep-phase syndrome: a short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–5. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 25.Lee SI, Hida A, Kitamura S, Mishima K, Higuchi S. Association between the melanopsin gene polymorphism OPN4*Ile394Thr and sleep/wake timing in Japanese university students. J Physiol Anthropol. 2014;33:9. doi: 10.1186/1880-6805-33-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roecklein KA, Wong PM, Franzen PL, et al. Melanopsin gene variations interact with season to predict sleep onset and chronotype. Chronobiol Int. 2012;29:1036–47. doi: 10.3109/07420528.2012.706766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001;18:263–71. doi: 10.1081/cbi-100103190. [DOI] [PubMed] [Google Scholar]
- 28.Higuchi S, Motohashi Y, Maeda T, Ishibashi K. Relationship between individual difference in melatonin suppression by light and habitual bedtime. J Physiol Anthropol Appl Human Sci. 2005;24:419–23. doi: 10.2114/jpa.24.419. [DOI] [PubMed] [Google Scholar]
- 29.Baver SB, Pickard GE, Sollars PJ. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–70. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 30.Millman RP. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115:1774–86. doi: 10.1542/peds.2005-0772. [DOI] [PubMed] [Google Scholar]
- 31.Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–38. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–54. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 34.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Benjamins JS, Migliorati F, Dekker K, et al. The sleep registry. An international online survey and cognitive test assessment tool and database for multivariate sleep and insomnia phenotyping. Sleep Med. 2013;14:e293–4. doi: 10.1016/j.sleep.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Edinger J, Kirby A, Lineberger M, Loiselle M, Wohlgemuth W, Means M. Durham, NC: Veterans Affairs and Duke University Medical Centers; 2004. The Duke Structured Interview Schedule for DSM-IV-TR and ICSD-2 Sleep Disorder Diagnoses. [Google Scholar]
- 38.Schmidt I. Some problems related to testing color vision with the Nagel anomaloscope. J Opt Soc Am. 1955;45:514–22. doi: 10.1364/josa.45.000514. [DOI] [PubMed] [Google Scholar]
- 39.Lall GS, Revell VL, Momiji H, et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–28. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Meijden WP, Te Lindert BH, Bijlenga D, et al. Post-illumination pupil response after blue light: reliability of optimized melanopsin-based phototransduction assessment. Exp Eye Res. 2015;139:73–80. doi: 10.1016/j.exer.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Mure LS, Cornut PL, Rieux C, et al. Melanopsin bistability: a fly's eye technology in the human retina. PLoS One. 2009;4:e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kankipati L, Girkin CA, Gamlin PD. The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci. 2011;52:2287–92. doi: 10.1167/iovs.10-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roecklein K, Wong P, Ernecoff N, et al. The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res. 2013;210:150–8. doi: 10.1016/j.psychres.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zele AJ, Feigl B, Smith SS, Markwell EL. The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS One. 2011;6:e17860. doi: 10.1371/journal.pone.0017860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.te Lindert BH, Van Someren EJ. Sleep estimates using microelectromechanical systems (MEMS) Sleep. 2013;36:781–9. doi: 10.5665/sleep.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 47.Terman JS, Terman M, Lo ES, Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 48.Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12:121–38. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- 49.Munch M, Leon L, Collomb S, Kawasaki A. Comparison of acute non-visual bright light responses in patients with optic nerve disease, glaucoma and healthy controls. Sci Rep. 2015;5:15185. doi: 10.1038/srep15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Technol. 2013;45:421–34. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Someren E. Improving actigraphic sleep estimates in insomnia and dementia: how many nights? J Sleep Res. 2007;16:269–75. doi: 10.1111/j.1365-2869.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 52.Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: dose and time profiles of marijuana, amphetamine, alcohol, and diazepam. J Anal Toxicol. 1993;17:264–72. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- 53.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 54.Lee SI, Hida A, Tsujimura S, Morita T, Mishima K, Higuchi S. Association between melanopsin gene polymorphism (I394T) and pupillary light reflex is dependent on light wavelength. J Physiol Anthropol. 2013;32:16. doi: 10.1186/1880-6805-32-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


