Abstract
Breakfast is thought to be beneficial for cognitive and academic performance in school children. However, breakfast is the most frequently skipped meal, especially among adolescents. The aim of the current article was to systematically review the evidence from intervention studies for the effects of breakfast on cognitive performance in children and adolescents. The effects of breakfast were evaluated by cognitive domain and breakfast manipulation. A total of 45 studies reported in 43 articles were included in the review. Most studies considered the acute effect of a single breakfast (n = 34). The acute studies looked at breakfast compared with no breakfast (n = 24) and/or comparisons of breakfast type (n = 15). The effects of chronic school breakfast program interventions were evaluated in 11 studies. The findings suggest that breakfast consumption relative to fasting has a short-term (same morning) positive domain-specific effect on cognition. Tasks requiring attention, executive function, and memory were facilitated more reliably by breakfast consumption relative to fasting, with effects more apparent in undernourished children. Firm conclusions cannot be made about the acute effects of breakfast composition and the effects of chronic breakfast interventions because there are too few studies and these largely report inconsistent findings. This review also highlights methodologic limitations of the existing research. These include a lack of research on adolescents, few naturalistic breakfast manipulations or testing environments, small samples, and insensitive cognitive tests.
Keywords: breakfast, cognitive performance, systematic review, children, adolescents
Introduction
Breakfast is generally accepted to be the most important meal of the day and is purported to confer a number of benefits for diet quality, health, and cognitive and academic performance. Children and adolescents who habitually consume breakfast are more likely to have better micro- and macronutrient intake (1), less likely to be overweight or obese (2, 3), and more likely to have higher physical activity levels (4). Despite the wealth of benefits, several observational studies have reported that between 20% and 30% of children and adolescents skip breakfast (1, 5, 6).
There is also evidence that breakfast consumption is beneficial for cognitive performance in school children (7). Children and adolescents may be particularly sensitive to the nutritional effects of breakfast on brain activity and associated cognitive outcomes. Children have a higher brain glucose metabolism than do adults (8). Moreover, the longer overnight fasting period because of higher sleep demands during childhood and adolescence can deplete glycogen stores overnight (9). To maintain this higher metabolic rate, a continuous supply of energy derived from glucose is needed. Hence breakfast consumption may be vital to providing adequate energy for the morning.
Although a number of published reviews have examined the effect of breakfast on school children’s cognitive performance (7, 10–12), only one is systematic (7). The application of systematic review methodology limits bias and improves the reliability and accuracy of conclusions. The aim of the current review was to provide an updated systematic review of the evidence for the effects of breakfast on objective cognitive performance outcomes from intervention studies in both children and adolescents. The current systematic review extends the published systematic review (7) by highlighting the cognitive domains that are potentially more sensitive to the effects of breakfast consumption. The effects of breakfast were evaluated by cognitive domain and type of breakfast manipulation. The acute effects of breakfast compared with no breakfast, breakfast composition, and the chronic effects of school breakfast programs (SBPs) were considered. The cognitive tasks were grouped into 5 broad categories: memory, attention, executive function, psychomotor function, and language (13).
Methods
Search strategy and search terms
The databases searched were Ovid MEDLINE(R), PsycINFO, and Web of Science for articles published between 1950 and July 2014. The search terms are shown in Table 1.
TABLE 1.
Search statements
| Search terms | ||
| Breakfast manipulation | Cognitive outcomes | Sample |
| “Breakfast” OR “breakfast program” OR “morning meal” OR “first meal” | AND “cogniti*” OR “memory” OR “attention” OR “visual-spatial” OR “visuo-spatial” OR “recall” OR “recognition” OR “problem solving” OR “reaction time” OR “vigilance” OR “executive function” OR “reasoning” OR “psychomotor” | AND “child*” OR “adolescent*” |
The reference lists of existing articles were examined individually to supplement the electronic search.
Inclusion and exclusion criteria
This review was limited to articles published in English in peer-reviewed journals. Papers were included or excluded in this review by using the following criteria.
Participants.
Studies of children or adolescents (4–18-y-old) of either sex were included. All studies that used adult or elderly samples were excluded. Studies were also excluded if they contained patient samples (e.g., those with type 1 or 2 diabetes).
Manipulations.
Studies that included any type of breakfast manipulation were included (e.g., breakfast compared with no breakfast, different breakfast types, and SBPs). Studies of the effects of manipulations at other mealtimes were excluded. Breakfast was defined according to the definition applied within the studies reviewed. Generally, studies defined breakfast as the first food or meal consumed in the day. However, most studies did not include a controlled overnight fasting period. Studies were not excluded on the basis of the content of the meal. For example, studies that included breakfast manipulations with the use of drinks and/or snacks were included and studies that did not report breakfast composition were included.
Outcome measures.
Studies that included any outcome of objectively measured cognitive performance were included.
Design.
Intervention studies examining acute effects (temporary effects occurring shortly after breakfast consumption) and chronic effects (effects occurring after the repeated consumption of breakfast) of breakfast manipulations were included. Observational studies examining associations between habitual breakfast consumption and cognitive performance were excluded.
Study selection process
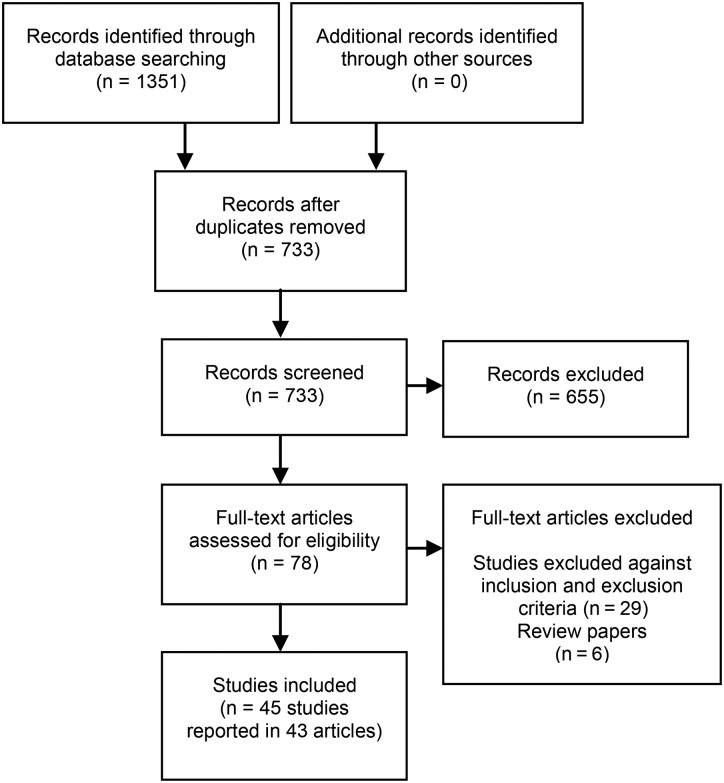
The process for selecting studies for inclusion in this review is detailed in Figure 1. A total of 45 studies reported in 43 articles were included (2 articles reported >1 study)
FIGURE 1.
Flow diagram of the study selection process.
Data extraction and tabulation of studies
The following data were extracted from each included study by 2 reviewers: 1) author and year; 2) study design; 3) characteristics of participants; 4) details of the breakfast intervention; 5) details of the cognitive measures; and 6) findings. A summary of the findings is shown in Tables 2–4. A summary of the characteristics of each study is shown in Tables 5 and 6. The findings are discussed by cognitive domain and type of breakfast manipulation (acute or chronic) and comparison (breakfast compared with no breakfast or breakfast composition).
TABLE 2.
Summary of findings for the acute effects of breakfast compared with no breakfast on cognition in children and adolescents (n = 24 studies)1
| Acute effect of breakfast vs. no breakfast |
||||
| Cognitive domain | Total studies | Advantage of breakfast | No effect | Advantage of no breakfast |
| Attention | 21 | 13 | 14 | 3 |
| Memory | 15 | 8 | 9 | 3 |
| Executive function | 13 | 7 | 8 | 1 |
| Psychomotor function | 4 | 2 | 2 | 1 |
| Language | 4 | 2 | 1 | 1 |
| Total | 24 | 32 | 34 | 9 |
Studies assessed >1 cognitive domain and/or administered >1 measure within the same domain.
TABLE 4.
Summary of findings for the chronic effects of SBPs on cognition in children and adolescents (n = 11 studies)1
| Chronic effect of SBP vs. no SBP |
||||
| Cognitive domain | Total studies | Advantage of SBP | No effect | Advantage of no SBP |
| Attention | 8 | 4 | 7 | 0 |
| Memory | 5 | 1 | 4 | 0 |
| Executive function | 4 | 2 | 2 | 0 |
| Psychomotor function | 3 | 2 | 1 | 0 |
| Language | 0 | 0 | 0 | 0 |
| Total | 11 | 9 | 14 | 0 |
Studies assessed >1 cognitive domain and/or administered >1 measure within the same domain. SBP, school breakfast program.
TABLE 5.
Tabulation of studies investigating the acute effect of breakfast on cognition in children and adolescents1
| Authors | Design | Sample | BF intervention | Cognitive measures | Reported results |
| Amiri et al. (2014) (14) | Acute study. Randomized crossover design. One week washout. |
n = 51; aged 9–11 y. Male: 47% Female: 53% Well-nourished.Iran. |
Three conditions: Fixed isocaloric BF of differing macronutrient content and no BF. 1. High CHO BF 2. High PRO BF 3. No BF |
CT: baseline, 30 mins and 120 mins post-BF. CDR battery: SRT, CRT, digit vigilance, numeric working memory task (Sternberg-like), delayed picture recognition, spatial working memory task. Computerized tasks. |
CRT: Poorer performance after no BF vs. high CHO and high PRO in boys. SRT, CRT, power of attention (SRT, CRT, and digit vigilance factor score): General decline in performance in girls after high-CHO BF. No other effects from BF on CTs. |
| Benton et al. (2007) (15) | Acute school-based study. Randomized crossover design. BF administered during a 4-wk school BF club. |
One primary school. n = 19; mean age: 6 y 10 mo (range: 5 y 11 mo–7 y 8 mo). Male: 47% Female: 53% Low SES school. United Kingdom. |
Three conditions: Ad libitum BF of differing GL designed to be isocaloric, but intake varied. 1. HGL: 25g Cornflakes, 115 mL semiskimmed milk, 2 teaspoons sugar, 1 waffle, 1 tablespoon syrup. Mean intake: GL: 18, 196 kcal, 4.7 g PRO, 1.7 g fat, 33.9 g CHO. 2. MGL: 60 g scrambled egg, 1 slice bread, 10 g jam, 8 g low-fat spread, 125 g yogurt. Mean intake: GL: 12, 168 kcal, 8.9 g PRO, 5.2 g fat, 21.7 g CHO. 3. LGL: 30 g ham, 40 g cheese, 1 slice linseed bread, 8 g low-fat spread. Mean intake: GL: 3, 157 kcal, 10.8 g PRO, 10.2 g fat, 5.7 g CHO |
CT: 140–210 min post-BF. Immediate and delayed object name recall from British Ability Scale, immediate and delayed object location recall, Paradigm of Shakow (respond to visual stimulus after auditory warning). Noncomputerized tasks. |
ANOVA: No main effect from BF condition on all CTs. Correlations: Significant negative correlation between immediate verbal memory and BF GL. Significant negative correlation between BF GL and CHO intake and attention (difficult final trials only). Significant positive relation between fat intake and attention (difficult final trials only). Regression: Lower-GL BF predicted better immediate verbal memory. PRO, fat, CHO did not predict verbal memory. Lower-GL BF predicted better attention (difficult final trials only). |
| Brindal et al. (2012) (16) | Acute laboratory-based study. Randomized crossover design. Three consecutive days. |
n = 39; mean age ± SD: 11.6 ± 0.7 y (range: 10–12). Male: 67% Female: 33% Well-nourished. Australia. |
Three conditions: Fixed isocaloric BF (311 kcal) of differing GL. 1. HGL: 70 g white bread, 10 g margarine, 5 g vegemite/low-sugar jam, 200 mL juice drink (GL: 33, 7 g PRO, 9 g fat, 50 g CHO). 2. MGL: 100 g low-fat yogurt, 20 g full-fat cheese, 35 g white bread, 5 g vegemite/low-sugar jam, 100 mL juice drink (GL: 24, 14 g PRO, 9 g fat, 45 g CHO). 3. LGL: 100 mL full-fat milk, 100 g low-fat yogurt, 20 g cheese, 35 g white bread, 5 g vegemite/low-sugar jam (GL: 18, 18 g PRO, 10 g fat, 38 g CHO). BG monitored. |
CT: baseline, 60, 120, and 180 min post-BF. SRT, CRT, odd-man-out reaction time, attention switching task, letter cancellation, immediate free word recall based on RAVLT word lists, digit span backward from WISC, visual inspection time task. Computerized and noncomputerized tasks. |
No significant effects from BF GL on all CTs. |
| Brindal et al. (2013) (17) | Acute laboratory-based study. Randomized crossover design. Three consecutive days. |
n = 40; mean age ± SD: 11.6 ± 0.1 y (range: 10–12 y). Male: 48% Female: 52% Well-nourished. Australia. |
Three conditions: Fixed isocaloric drink (263 kcal) of differing GL. 1. VHGL: Glucose drink. GL: 65, 0 g PRO, 0 g fat, 65 g CHO. 2. HGL: Glucose drink with 200 mL whole milk. GL: 35, 7 g PRO, 8 g fat, 42 g CHO. 3. LGL: Glucose drink with 400 mL whole milk. GL: 5, 13 g PRO, 15 g fat, 19 g CHO. BG monitored. |
CT: baseline, 60, 120, and 180 min post-BF. SRT, CRT, odd-man-out reaction time, attention switching task, letter cancellation, immediate free word recall based on RAVLT word lists, digit span backward from WISC, visual inspection time task. Computerized and noncomputerized tasks. |
No significant main effects from drink GL on all CTs. Word recall: Significant sex × condition interaction. Post hoc test indicated girls recalled significantly more words after LGL or HGL drink compared with VHGL glucose drink. Opposite pattern in boys, but not statistically significant. |
| Busch et al. (2002) (18) | Acute laboratory-based study. Crossover design. Counter-balanced. One week washout. |
n = 21 male participants; aged 9–12 y. Well-nourished. United States. |
Two conditions: Fixed BF vs. no BF. 1. BF: 25 g confectionary snack (95 kcal, 0 g PRO, 1.1 g fat, 22 g simple CHO). 2. No BF: Aspartame-sweetened drink matched for sweetness (0 kcal). |
CT: 15 min post-BF. CPT (visual), map task (immediate recall), Rey Complex Figure task (copy accuracy), story recall (immediate recall), digit span forward and backward. Computerized and noncomputerized tasks. |
CPT: Significantly higher hit rate, lower miss rate, and lower false alarms after BF vs. no BF. No other significant effects from BF on CTs. |
| Chandler et al. (1995) (19) | Acute school-based study. Randomized crossover design. Two week washout. |
Four schools. n = 197; aged 8–10 y. Male: 51% Female: 49% Stratified by nutritional status: Underweight: n = 97, mean age ± SD: 9.7 ± 0.9 yNormal: n = 100, mean age ± SD: 9.1 ± 0.8 yLow SES. Jamaica. |
Two conditions: Fixed BF vs. no BF. BF also consumed at home before school. 1. School BF: 68 g bread, 28 g cheese, 225 mL chocolate milk (520 kcal, 21.3 g PRO). 2. Low energy control: 60 g orange (18 kcal, 0.3 g PRO). |
CT: Between 0900 and 1200. Letter cancellation, digit span forward, verbal fluency (categorical fluency). Noncomputerized tasks. |
BF × nutrition group interaction: Underweight children generated significantly more words on verbal fluency task after BF vs. no BF, but no change in normal-weight children. No other significant effects from BF on CTs. |
| Conners and Blouin (1982–1983) (20) | Acute laboratory-based study. Crossover design. |
n = 10; aged 9–11 y. Well-nourished. United States. |
Two conditions: Fixed BF vs. no BF 1. BF: RTEC, milk, sugar, egg, juice, toast. 2. No BF. |
CT: 0950, 1100, and 1210. CPT (visual), mental calculation task. EEG recoding. Computerized and noncomputerized tasks. |
CPT: Significantly fewer errors after BF vs. no BF at all time points across morning. Mental calculation: Significantly better performance at 1100 after BF vs. no BF. No other significant effects from BF on CTs. Significant reduction in amplitude of evoked potentials after BF vs. no BF. |
| Cooper et al. (2011) (21) | Acute school-based study. Randomized crossover design. One week washout. |
Five secondary schools. n = 96; mean age ± SD: 13.2 ± 1.2 y (range: 12–15 y). Male: 50% Female: 50% 90% habitual BF consumers. Well-nourished. United Kingdom. |
Two conditions: Ad libitum BF vs. no BF. 1. BF: Choice of RTECs, muesli, semiskimmed milk, bread, fat spreads, jam, yogurt, fruit, and fruit juices. Mean intake: Male: 589 kcal, 14.0 g PRO, 10.7 g fat, 107.6 g CHO. Female: 406 kcal, 9.3 g PRO, 8.2 g fat, 72.8 g CHO. 2. No BF. BG monitored. |
CT: 20 and 140 min post-BF. Tasks differed in difficulty. SRT (2 difficulty levels), Stroop task (2 difficulty levels), Sternberg Paradigm (3 difficulty levels). Computerized tasks. |
SRT: BF × time × difficulty interaction: Significantly better accuracy after BF vs. no BF at 20 min post-BF on more difficult trials. No effect from BF on response times. Stroop: BF × time interaction: Accuracy better maintained across morning after BF vs. no BF on both versions. No effect from BF on response times. Sternberg: BF × time × difficulty interaction: Response times faster across morning after BF vs. no BF on more difficult trials. Response times faster across morning after no BF vs. BF on easier trial. No effect of BF on accuracy. Significantly higher BG after BF vs. no BF across morning. |
| Cooper et al. (2012) (22) | Acute school-based study. Randomized crossover design. One week washout. |
Two secondary schools. n = 41; mean age ± SD: 12.8 ± 0.4 y (range: 12–14 y). Male: 44% Female: 56% Well-nourished. United Kingdom. |
Three conditions: Fixed isocaloric BF (420 kcal) differing in GI and no BF. 1. HGI: 55 g cornflakes, 42 g white bread, 6 g margarine, 216 g 1% fat milk (GI: 72, 14.3 g PRO, 7.2 g fat, 75 g CHO). 2. LGI: 217 g 1% fat milk, 75 g muesli, 150 g apple (GI: 48, 15.5 g PRO, 6.4 g fat, 75 g CHO). 3. No BF. BG and insulin monitored. |
CT: 30 and 120 min post-BF. Tasks differed in difficulty. Stroop task (2 difficulty levels), Flanker task (2 difficulty levels), Sternberg Paradigm (3 difficulty levels). Computerized tasks. |
Stroop task: BF × time × difficulty interaction: Response times improved across morning after LGI vs. no BF on difficult version. BF × time interaction: Greater decline in accuracy across morning after HGI vs. LGI BF. Sternberg: BF × time × interaction: Response times improved more across morning after LGI vs. HGI BF on all versions. BF × time × difficulty interaction: Accuracy better maintained across morning after LGI vs. HGI BF on difficult trial. Flanker task: BF × time × interaction: Response times improved more across the morning after LGI vs. no BF on both versions. BF × time × difficulty interaction: Accuracy better maintained across morning after LGI vs. HGI BF and no BF on difficult trials. |
| Cromer et al. (1990) (23) | Acute laboratory-based study. Randomized independentgroups design. |
n = 34; mean age ± SD: 14.2 ± 0.4 y. Mid-high SES. Well-nourished. United States. |
Two conditions: Fixed BF vs. no BF after overnight stay. 1. BF: Government school BF. 60 g doughnut, 236 g chocolate milk, 118 g orange juice (424 kcal, 11.5 g PRO, 14.1 g fat, 63.9 g CHO). 2. Low-energy control: 236 g sugar-free drink, 1/2 cup sugar-free jelly (12 kcal, 1.6 g PRO, 0 g fat, 1.6 g CHO). BG monitored |
CT: +60, +240 min post-BF Immediate free word recall from RAVLT, digit vigilance, MFFT. Computerized and noncomputerized tasks. |
No significant effect of condition on all CTs. Ceiling effects observed on MFFT. No difference in BG between conditions and no correlation between BG and cognitive performance. Significantly more habitual BF eaters (≥5 d/wk) in control group (81%) vs. BF group (45%) |
| Cueto et al. (1998) (24) | Acute laboratory-based study. Randomized crossover design. One week washout. |
n = 54 male participants. Stratified by nutritional status:Nutritionally at risk: n = 23; mean age ± SD: 10.3 ± 0.7 yNot at risk: n = 31; mean age ± SD: 10.4 ± 0.7 y. Low SES. Peru. |
Two conditions: Fixed BF vs. no BF after standardized evening meal and overnight stay. 1. Government school BF: 80 g cake, 50 g milk-like drink (510 kcal, 14.4 g PRO, 12.1 g fat, 81.9 g CHO). Fortified with iron, vitamins A + C. 2. Low energy control: Sugar- and caffeine-free carbonated drink. BG monitored. |
CT: +180 min post-BF. Digit cancellation, Raven’s Colored Progressive Matrices, Peabody Picture Vocabulary test, CRT, Sternberg Paradigm, stimulus discrimination. Computerized and noncomputerized tasks. |
Nutritionally at risk: Significantly poorer performance on Sternberg paradigm and stimulus discrimination after no BF vs. BF. Nutritionally not at risk: Better performance after no BF vs. BF on Peabody Picture Vocabulary test and stimulus discrimination task. No other significant effects from BF on CTs. BG was not significantly associated with test performance in either nutritional group under both conditions. |
| Defeyter & Russo (2013) (25) | Acute school-based study. Crossover design. Counterbalanced. One week washout. |
One secondary school. n = 40; mean age ± SD: 14.2 ± 0.5 y (range: 13–15 y). Male: 48% Female: 52% BF skippers.Low SES. Well-nourished. United Kingdom. |
Two conditions. Fixed BF vs. no BF. 1. 35 g LGI RTEC: Kellogg’s All-Bran, 125 mL skimmed milk (162 kcal, 9.4 g PRO, 1.2 g fat, 22.7 g CHO). 2. No BF |
CT: Baseline, +135 min post-BF. High and low cognitive load versions of tasks. Order counterbalanced. Delayed free word recall, CRT, RVIP, Stroop task, serial subtractions by 3s and 7s. Computerized tasks. |
Word recall: Significantly better recall after BF vs. no BF on high cognitive load version of task only. Serial 3s and 7s: Significantly better working memory after BF vs. no BF. Cognitive load of task did not interact with effect. No other significant effects from BF on CTs. |
| Dickie and Bender (1982) Expt. 2 (26) | Acute school-based study. Randomized independentgroups design. |
Four boarding schools. Investigation 1: n = 55; mean age: 17 y Investigation 2: n = 53; mean age 16.2 y. Mid-high SES. Well-nourished. United Kingdom. |
Two conditions: Ad libitum BF vs. no BF. 1. Usual boarding school BF: ∼500 kcal. 2. No BF. |
CT: +195 min post BF Investigation 1: Letter cancellation. Investigation 2: Sentence-picture verification task (reasoning task). Noncomputerized tasks. |
No significant effects from BF on all CTs. |
| Ingwersen et al. (2007) (27) | Acute school-based study. Crossover design. Counterbalanced. Two consecutive days. |
One primary school. n = 64; mean age: 9.3 y (range: 6–11 y). Male: 40% Female: 60% Well-nourished. Mixed SES. United Kingdom. |
Two conditions: Fixed BF of differing GI. Not isocaloric. 1. HGI: 35 g Kellogg’s Coco Pops (GI: 77, 133 kcal, 1.6 g PRO, 0.9 g fat, 29.8 g CHO) and 125 mL semiskimmed milk. 2. LGI: 35 g Kellogg’s All-Bran (GI: 42, 98 kcal, 4.9 g PRO, 1.6 g fat, 16.1 g CHO) and 125 mL semiskimmed milk |
CT: Baseline, 10, 70, and 130 min post-BF. CDR battery: Immediate and delayed free word recall, delayed word recognition, SRT, CRT, digit vigilance, numeric working memory (Sternberg-like), delayed picture recognition, spatial working memory. Computerized tasks. |
Significantly better secondary memory (delayed word and picture recognition, immediate and delayed word recall factor score) after LGI vs. HGI at 10 and 130 min but not 70 min post-BF. Significantly better accuracy of attention (SRT, CRT, digit vigilance factor score) after LGI vs. HGI at 130 mins post-BF. No effect of BF on speed of attention, speed of memory, or working memory factor scores. |
| Kral et al. (2012) (28) | Acute laboratory-based study. Randomized crossover design. One week washout. |
n = 21; mean age ± SD: 9.2 ± 0.8 y (range: 8–10 y). Male: 29% Female: 71% Habitual BF consumers. Well-nourished. United States. |
Two conditions: Fixed BF vs. no BF. 1. BF: 32 g RTEC (choice of 3), 192 g 1% fat milk, 60 g banana, 187 g orange juice (∼350 kcal, 9.9–12.4 g PRO, 3.3–5.1 g fat, 68.0–69.1 g CHO). 2. No BF. |
CT: Baseline, 45, 90, and 135 min post-BF. Cogstate battery: PAL, one card learning task (immediate recall), one back task (n-back), chase task, Groton Maze Learning Task, SRT, and CRT. Computerized tasks. |
No significant effects from BF on all CTs. |
| López et al. (1993) (29) | Acute school-based study. Independent group design. |
12 primary schools. n = 279; mean age ± SD: 10.3 ± 0.5 y (range: 8–10 y). Male: 48% Female: 52% Stratified by nutritional status: Normal: n = 106; Underweight: n = 73; Stunted: n = 100. Low SES. Chile. |
Two conditions: Fixed BF vs. no BF. 1. BF: 2 cakes, 200 mL flavored milk (394 kcal; 6g PRO). 2. No BF. |
CT: 60 min post-BF. Domino task, attention task (respond to target geometric figures within continuous stream), digit span. Computerized tasks. |
No significant effects from BF on all CTs. |
| Maffeis et al. (2012) (30) | Acute laboratory-based study. Randomized crossover design. One week washout. |
n = 10; median age: 9.6 y (range: 9–10 y). Male: 40% Female: 60% Well-nourished (obese). Italy. |
Two conditions: Fixed BF vs. no BF. 1. BF: 200 mL full-fat milk, 32 g bread, 25 g marmalade (295 kcal, 9.6 g PRO, 8.8 g fat, 44.9 g CHO). 2. No BF (water). Blood samples: BG, insulin, glucagon, ghrelin, peptide YY, GLP-1 monitored. Indirect calorimetry: REE, meal-induced thermogenesis, and macronutrient oxidation. |
CT: Baseline, 180 min post-BF. Conners’ CPT, immediate free word recall with selective reminding within TOMAL word selective reminding subtest, visual sequential memory subtest within TOMAL (memory for sequences of geometric shapes; immediate recall). Computerized and noncomputerized tasks. |
CPT: Fasting induced a significant decline in performance; no change in performance after BF. Selective reminding: Fasting induced a significant increase in word recall; no change in performance after BF. No other significant effects from BF on CTs. Decline in CPT performance was significantly associated with reduced CHO oxidation. |
| Mahoney et al. (2005) Expt. 1 (31) | Acute school-based study. Crossover design. Counterbalanced. One day per week for 3 consecutive weeks. |
One private primary school. n = 30; aged 9–11 y. Male: 50% Female: 50% 52% habitual BF consumers. Mid-high SES. Well-nourished. United States. |
Three conditions: Fixed isocaloric BF of differing macronutrient content and no BF. 1. 43 g oatmeal, 1/2 cup skimmed milk (200 kcal, 4 g PRO, 2 g fat, 32 g CHO). 2. 36 g RTEC, 1/2 cup skimmed milk (200 kcal, 1 g PRO, 1.5 g fat, 30 g CHO). 3. No BF. |
CT: 60 min post-BF. Map task (immediate and delayed recall), Rey Complex Figure task (immediate and delayed recall, copy accuracy), digit span forward and backward, CPT (auditory and visual), story recall (immediate and delayed recall). Computerized and noncomputerized tasks. |
Map task: Significantly better immediate recall after oatmeal BF vs. no BF. Digit span backward: Girls performed significantly better after oatmeal vs. RTEC and no BF. Rey complex copy: Significantly better copy accuracy after both oatmeal and RTEC vs. no BF. CPT auditory: Fewer false alarms after oatmeal and RTEC vs. no BF early in task. No other significant effects from BF on CTs. |
| Mahoney et al. (2005) Expt. 2 (31) | As Mahoney et al. (2005) Expt. 1. | One private primary school. n = 30; aged 6–8 y. Male: 50% Female: 50% 64% habitual BF consumers. Mid-high SES. Well-nourished. United States |
As Mahoney et al. (2005) Expt. 1. | As Mahoney et al. (2005) Expt. 1 with modifications for use in younger participants. | Map task: Significantly better immediate recall after oatmeal BF vs. no BF. Digit span backward: Girls performed significantly better after oatmeal vs. RTEC. Rey complex copy: Boys had significantly better copy accuracy after RTEC vs. no BF. Significantly better copy accuracy for girls after no BF vs. RTEC. CPT auditory: More hits after oatmeal vs. RTEC with intermediary performance after no BF. Fewer misses after oatmeal or no BF vs. RTEC. No other significant effects from BF on CTs. |
| Micha et al. (2011) (32) | Acute school-based study. 2 × 2 factorial design. Randomized crossover and independent groups design. Independent groups: HGL vs. LGL Crossover: HGI vs. LGI. Two week washout. |
Five secondary schools. n = 74; mean age ± SD: 12.6 ± 0.1 y (range: 11–14 y). Male: 50% Female: 50%Mixed SES. Well-nourished. United Kingdom. |
Two independent groups (differing GL) with crossover conditions (differing GI) within each group. Fixed BFs. HGL (GL: 41–55): 1. LGI: 66 g muesli, 200 mL milk, 245 mL juice, 7 g table sugar (GI: 48, 470 kcal, 14 g PRO, 7.1 g fat, 86.6 g CHO). 2. HGI: 55 g cornflakes, 300 mL milk, 200 mL juice, 7 g table sugar. (GI: 61, 470 kcal, 14 g PRO, 5.3 g fat, 90.4 g CHO). LGL (GL: 21–28): 1. LGI: 40 g muesli, 250 mL milk, 5 g table sugar (GI: 48, 281 kcal, 12.5 g PRO, 6.4 g fat, 43.2 g CHO). 2. HGI: 30 g cornflakes, 300 mL milk, 5 g table sugar (GI: 61, 276 kcal, 12 g PRO, 5.1 g fat, 45.2 g CHO). BG, salivary cortisol monitored. |
CT: 103 min post-BF. Immediate and delayed free word recall, Stroop task, Matrices task (reasoning ability), verbal fluency task (letter fluency), digit cancellation, serial subtractions by 7s. Noncomputerized tasks. |
Verbal fluency task: Significantly higher number of words generated after LGI BF vs. HGI BF. Stroop task: Significantly faster completion after HGI–HGL BF. Digit cancellation: Significantly higher number correct after HGI BF vs. LGI BF. Serial 7s: Significantly higher number correct after HGI BF vs. LGI BF. No other significant effects from BF GI/GL on CTs. Higher BG before test session after HGL and GI vs. LGL and GI BF. Higher cortisol before and after test session after HGI vs. LGI BF. |
| Michaud et al. (1991) (33) | Acute school-based study. Randomized (by school) crossover design. Two week washout. |
n = 319; mean age ± SD: 16.1 ± 1.3 y (range: 13–20 y). Male: 47% Female: 53% Well-nourished. France. |
Two conditions: 1. Habitual BF. 2. Higher energy BF than habitual BF. Stratified by extra energy consumed: • 0–99 kcal • 100–199 kcal • 200–299 kcal • 300–399 kcal • ≥ 400 kcal |
CT: 1100. Scale test (immediate recall for location of boxes), word cancellation. Noncomputerized tasks. |
Scale test: Significantly better recall after additional energy BF vs. habitual BF. Word cancellation: Significantly worse performance after additional energy BF vs. habitual BF. |
| Morrell and Atkinson (1977) (34) | Acute school-based study. Randomized independent groups design. |
n = 52; aged 4–11 y. Well-nourished. United States. |
Two conditions: Fixed BF. 1. Usual school BF: Fruit juice, RTEC or bread, milk, chocolate or syrup, or sweet roll. Meat, fish, poultry, cheese or egg: ∼11 g PRO. 2. High PRO, low CHO school BF: Unsweetened juice, milk, frankfurter, hamburger, burritos: ∼24g PRO. |
CT: Late morning. Digit span forward and backward from WISC. Noncomputerized tasks. |
No significant effects from BF on performance. |
| Muthayya et al. (2007) (35) | Acute laboratory-based study. Randomized crossover design. One week washout. |
n = 69; aged 7–9 y. Stratified by SES: Low SES: n − 34; mean age ± SD: 7.6 ± 0.6 y. 32% wasted, 21% stunted. Male: 44%, Female: 56% High SES: n = 35; mean age ± SD: 7.6 ± 0.6 y. Well-nourished. Male: 63%, Female: 37% India. |
Three conditions. Ad libitum BF of differing energy content with/without midmorning snack. BF: chapatti and potato curry. Midmorning snack: Mango-flavored bar. 1. Small BF (187 kcal) + midmorning snack (153 kcal) + standard lunch (500 kcal). 2. Standard BF (340 kcal) + midmorning snack (153 kcal) + small lunch (347 kcal). 3. Standard BF (340 kcal) + standard lunch (500 kcal). |
CT: Baseline, 30 and 150 min post-BF. Immediate and delayed picture recognition, finger tapping, RVIP. Computerized tasks. |
Low SES: Raw scores: Significantly better immediate picture recognition accuracy 150 min after condition 2 vs. 3. No effect from condition to delayed picture recognition raw scores. Change scores: Decline in accuracy on immediate picture recognition at session 3 relative to baseline was significantly smaller after condition 1 and 2 vs. condition 3. Decline in accuracy on delayed picture recognition at session 3 relative to baseline was significantly smaller after condition 1 and 2 vs. condition 3. High SES: Raw scores: No effect from condition to immediate and delayed picture recognition. Change scores: Decline in accuracy on immediate picture recognition at session 3 relative to baseline was significantly smaller after condition 2 vs. condition 3. Increase in false alarms on delayed picture recognition at session 3 relative to baseline was significantly smaller after condition 2 vs. condition 3. No other significant effects from BF on CTs. |
| Pivik and Dykman (2007) (36) | Acute laboratory-based study. Randomized independent groups design. |
n = 60; aged 8–11 y. Male: 50% Female: 50% Habitual BF consumers.Well-nourished.United States. |
Two conditions: Fixed BF vs. no BF after standardized evening meal and overnight stay. 1. BF: Based on US SBP. 3/4 cup RTEC, 227 mL 2% fat milk, 1 slice white bread, 1/2 cup applesauce (340 kcal, 14 g PRO, 6 g fat, 57 g CHO). 2. No BF. BG monitored. |
CT: Baseline, 40 min post-BF. Go/no-go task. Computerized tasks. EEG recording during task. |
Significant increase in reaction time relative to baseline for no-BF group only; no change in BF group. No effect on task accuracy, but presence of ceiling effect. Increased α wave synchronization in no-BF group. |
| Pivik et al. (2012) (37) | Acute laboratory-based study. Randomized independent groups design. |
n = 81; mean age ± SD: 9.78 ± 0.8 y (range 8–11 y). Male: 46% Female: 54% Habitual BF consumers. Well-nourished. United States. |
Two conditions: Fixed BF vs. no BF after standardized evening meal and overnight stay. 1. BF: Based on US SBP. 3/4 cup RTEC, 227 mL 2% fat milk, 1 slice white bread, 1/2 cup applesauce (340 kcal, 14 g PRO, 6 g fat, 57 g CHO). 2. No BF. BG monitored. |
CT: Baseline, 40 min post-BF. Mental calculation task. Computerized tasks. EEG recording during task. |
Significant increase in accuracy after BF relative to baseline; no change in no-BF group. Significant increase in response time in no-BF group relative to baseline; no change in BF group. EEG: Increased high θ and high and low α band activity in no-BF group vs. BF group. Increased δ and lower θ activity in left frontal recordings in no-BF vs. BF group, indicating increased region-specific activity for working memory. |
| Pollitt et al. (1998) Expt. 1 (38) | Acute laboratory-based study. Randomized crossover design. One week washout. |
n = 32; aged 9–11 y. Male: 28% Female: 72% Well-nourished. United States. |
Two conditions: Fixed BF vs. no BF after standardized evening meal and overnight stay. 1. BF: 535 kcal, 15 g PRO, 20 g fat, 75 g CHO. 2. No BF. BG monitored. |
CT: 180 min post-BF. HCIT (memory for sequences of objects and animals; immediate recall), CPT, MFFT, Peabody Picture Vocabulary test (used as covariate and outcome). Computerized and noncomputerized tasks. |
MFFT: Low-IQ school children made significantly more errors on easy trials after no BF vs. BF. Decrease in BG associated with more errors. HCIT: Recall of last object significantly better after no BF vs. BF. Incidental score better after no BF vs. BF (analysis on first day of testing only). No other significant effects from BF on CTs. |
| Pollitt et al. (1981) (39) | Acute laboratory-based study Randomized crossover design. One week washout. |
n = 34; mean age 10 y 4 mo (range: 9–11). Male: 35% Female: 65% Well-nourished. United States. |
Two conditions: Fixed BF vs. no BF after standardized evening meal and overnight stay. 1. BF: Waffles, syrup, margarine, orange juice, milk (535 kcal, 15 g PRO, 20 g fat, 75 g CHO). 2. No BF. BG monitored. |
CT: 180 min post-BF. HCIT (memory for sequences of objects and animals; immediate recall), CPT (visual), MFFT. Computerized and noncomputerized tasks. |
MFFT: Significantly more errors on easy trials after no BF vs. BF for school children with lower IQ only. Decrease in BG associated with more errors. HCIT: Significantly better recall of last item after no BF vs. BF. No other significant effects from BF on CTs. |
| Pollitt et al. (1982–1983) (40) | Acute laboratory-based study. Randomized crossover design. One week washout. |
n = 39; mean age 10 y 4 mo (range: 9–11 y). Male: 51% Female: 49% Well-nourished. United States. |
Two conditions: Fixed BF vs. no BF after standardized evening meal and overnight stay. 1. BF: 448 kcal, 12 g PRO, 16 g fat, 65 g CHO. No details on type of food provided. 2. No BF. |
CT: 180 min post-BF. HCIT (memory for sequences of objects and animals; immediate recall), digit span, MFFT. Noncomputerized tasks. |
MFFT: Significantly more errors after no BF vs. BF on difficult levels only. No interaction with IQ. HCIT: Significantly higher incidental scores after no BF vs. BF (analysis on first day of testing only). No other significant effects from BF on CTs. |
| Simeon and Grantham-McGregor (1989) (41) | Acute laboratory-based study. Randomized crossover design. One week washout. |
n = 90; aged 9–10.5 y. Stratified by nutritional status: Stunted: n = 30; Previously undernourished: n = 30; Control/well-nourished: n = 30. Effect of wasting also considered. Low SES. Jamaica. |
Two conditions: Fixed BF vs. no BF after standardized evening meal and overnight stay. 1. BF: based on Jamaican government SBP. 105 g Nutribun, 242 g milk, 25 g cheese (590 kcal, 29 g PRO, 12 g fat, 91 g CHO). 2. Low-energy control: 185 mL Aspartame-sweetened tea. |
CT: 180 min post-BF. Digit span forward and backward, mental calculation task from WISC, verbal fluency (categorical fluency), cued story recall (immediate recall), coding test (digit-symbol substitution) from WISC, HCIT (memory for sequences of objects and animals; immediate recall), MFFT. Noncomputerized tasks. |
Stunted, previously undernourished, and wasted: BF × nutrition group interaction indicated worse performance on verbal fluency, coding, digit span backward and forward and MFFT (easy trials) after no BF vs. BF. Well-nourished: BF × nutrition group interaction indicated better performance on calculation task and MFFT after no BF vs. BF. No other significant effects from BF on CTs. |
| Smith and Foster (2008) (42) | Acute laboratory-based study. Randomized independent groups design. |
n = 38; mean age ± SD: 15.6 ± 0.9 y (range: 14–17 y). Male: 50% Female: 50% Mostly habitual BF consumers: average 0.8 d/wk skipped BF. Well-nourished. Australia. |
Two conditions: Fixed BF differing in GL. 1. LGI: 30 g Kellogg’s All-Bran RTEC, 125 mL semiskimmed milk (GI: 30, 218 kcal, 12.7 g PRO, 4.8 g fat, 26.3 g CHO). 2. HGI: 30 g cornflakes RTEC, 125 mL semiskimmed milk (GI: 77, 232 kcal, GI: 77, 10.4 g PRO, 4 g fat, 37 g CHO). BG monitored. |
CT: 20, 60, and 100 min post-BF.Immediate, short, and long delay free and cued word recall from CVLT. Concomitant motor task to increase task demands. Noncomputerized tasks. |
No significant effects from BF GI on raw recall scores. Relative to the number of words recalled at the short delay, significantly fewer words were forgotten after the long delay after HGI RTEC vs. LGI RTEC. No significant effects from BF GI on BG. |
| Vaisman et al. (1996) (43) | Acute school-based study. Randomized independent groups design. |
Five primary schools. n = 569;aged 11–13 y. Male: 51% Female: 49% Test 1 (baseline): n = 491. Test 2 (post-intervention): n = 503. Mixed SES. Israel. |
Test 1 (baseline): Two conditions: Self-reported BF on morning of test vs. no BF. 1. BF at home. 2. No BF. Typical breakfast: cookies, chocolate milk, and a small portion of RTEC. Test 2 (postintervention): Three conditions: Fixed school BF intervention for 14 d vs. BF at home or no BF. 1. School BF: 30 g sugared cornflakes, 200 mL 3% fat milk (∼263 kcal, 7 g PRO, 38 g CHO, 8 g fat). 2. BF at home. 3. No BF. Chronic intervention, but analysis assessed acute effects of BF. |
CT: 30 min postschool BF and 120 min post-BF at home. Immediate and delayed free word recall and recognition from RAVLT, story recall within Wechsler Memory Scale Logical Memory subtest, Benton Visual Retention Test (immediate recall). Noncomputerized tasks. |
Test 1: RAVLT: Significantly better immediate recall after self-reported BF at home vs. no BF. No other significant effects from BF on CTs. Test 2: RAVLT: Significantly better mean learning, best learning, retroactive inhibition, and recognition after school BF vs. no BF and BF at home. Significantly better delayed recall and temporal order after school BF vs. BF at home. Story recall: Significantly better recall after school BF vs. no BF and BF at home. Benton Visual Retention: Significantly better performance after school BF vs. no BF and BF at home. |
| Wesnes et al. (2003) (44) | Acute laboratory-based study. Randomized crossover design. Four consecutive days. |
n = 29; aged 9–16 y. Male: 48%, mean age: 12.1 y. Female: 52%, mean age: 12.3 y. Well-nourished. United Kingdom. |
Four conditions: Fixed BF of differing macronutrient content and no BF. Not isocaloric. Ad libitum water. 1. 45 g Nestlé Shreddies, 125 mL semiskimmed milk (38.3 g CHO, 25.2 g complex CHO). 2. 30 g Nestlé Cheerios, 125 mL semiskimmed milk (28.7 g CHO, 16 g complex CHO). 3. 330 mL orange-flavored drink (38.3 g glucose). 4. No BF. |
CT: Baseline, 30, 90, 150, and 210 min post-BF. CDR battery: Immediate and delayed free word recall, delayed word recognition, SRT, CRT, digit vigilance, numeric working memory (Sternberg-like), delayed picture recognition, spatial working memory. Computerized tasks. |
Significant main effects from BF condition to power of attention (SRT, CRT, digit vigilance factor score) and quality of episodic memory (delayed word and picture recognition, immediate and delayed word recall factor score). No post hoc tests, but observed decline in cognitive performance during morning in no-BF and glucose-drink condition that was reduced by 2 cereal BF conditions. No effect from BF on continuity of attention, speed of memory, and working memory factor scores. |
| Widenhorn-Müller et al. (2008) (45) | Acute school-based study. Randomized crossover design. One week washout. |
One boarding school. n = 104; mean age ± SD: 17.2 ± 1.6 y (range: 13–20 y). Male: 52% Female: 48% 88% habitual BF consumers. Mid-high SES. Well-nourished. Germany. |
Two conditions: Fixed BF vs. no BF. Water and unsweetened peppermint tea consumed ad libitum in both conditions. 1. BF: 60 g whole-grain bread, 28 g butter, 20 g chocolate spread, 30 g jam (476 kcal). 2. No BF. |
CT: 45 min post-BF.d2 Test of Attention, trail route, logos task (picture recognition), Turkish vocabulary, cued recall of factual text, object recall, telephone numbers (all immediate recall). Noncomputerized tasks. |
Significant effect from BF on visual–spatial memory in male subjects, but observed order effects. No other significant effects from BF on CTs. |
| Wyon et al. (1997) (46) | Acute school-based study. Randomized independent groups design. |
Five primary schools. n = 195;n = 165 completed; aged 10 y. Male: 44% Female: 56% Well-nourished. Denmark and Sweden. |
Two conditions: At-home ad libitum BF of differing energy content. 1. High-energy BF: Male participants: 100 g bread, 10 g margarine, 28 g cheese, 20 g ham, 300 mL 3% milk, 20 g cornflakes, 100 g apple, 200 mL juice (mean intake: 536 kcal). Female participants: 50 g bread, 10 g margarine, 20 g ham, 300 mL 1.5% milk, 20 g cornflakes, 100 g apple, 200 mL juice (mean intake: 434 kcal). 2. Low-energy BF: Male participants: 50 g bread, 10 g margarine, 24 g jam, 500 mL sugar free concentrated fruit drink diluted with water (mean intake: 170 kcal). Female participants: 30 g bread, 10 g margarine, 24 g jam, 500 mL sugar free concentrated fruit drink diluted with water (mean intake: 121 kcal). |
CT: Late morning. Mental calculation and multiplication task, digit cancellation, grammatical reasoning, verbal fluency (categorical fluency). Noncomputerized tasks. |
Significantly higher scores on grammatical reasoning task after high-energy BF vs. low-energy BF. No other significant effects from BF on CTs. |
BF, breakfast; BG, blood glucose; CDR, cognitive drug research; CHO, carbohydrate; CPT, continuous performance test; CRT, choice reaction time; CT, cognitive test; CVLT, California Verbal Learning Test; EEG, electroencephalography; GI, glycemic index; GL, glycemic load; GLP-1, glucagon-like peptide 1; HCIT, Hagen Central Incidental Task; HGI, high glycemic index; HGL, high glycemic load; IQ, intelligence quotient; LGI, low glycemic index; LGL, low glycemic load; MFFT, Matching Familiar Figures Test; MGL, medium glycemic load; PAL, paired associates learning; PRO, protein; RAVLT, Rey Auditory Verbal Learning Test; REE, resting energy expenditure; RTEC, ready-to-eat cereal; RVIP, rapid visual information processing; SBP, school breakfast program; SES, socioeconomic status; SRT, simple reaction time; TOMAL, test of memory and learning; VHGL, very high glycemic load; WISC, Wechsler Intelligence Scale for Children.
TABLE 6.
Tabulation of studies investigating the effect of chronic interventions on cognition in children and adolescents1
| Authors | Design | Sample | BF intervention | Cognitive measures | Reported results |
| Cueto and Chinen (2008) (47) | SBP evaluation. Independent groups design. Compared matched schools with SBP (11 schools) vs. no SBP (9 schools). Multiple and full-grade schools. 3 y intervention. |
20 primary schools; n = 590. SBP: n = 300, mean age ± SD: 11.87 ± 1.77 y. Male: 51.7% Female: 48.3% Control: n = 290, mean age ± SD: 11.87 ± 1.90 y. Male: 49.7% Female: 50.3% Comparable nutrition status: 66–69% of school children 9–2 SD height-for-age NCHS. Low SES. Peru. |
Two conditions: 1. Free midmorning SBP: BF during school break time at 1000–1100, milk-like beverage, and 6 cookies (600 kcal, 19.5 g PRO, 20 g fat, 60% RDA for various micronutrients, 100% RDA for iron). 2. Control: No BF or BF at home.Compliance: 82% consumed all of BF. Consumed BF midmorning after BF at home. |
CT: 3 y. Administered after BF at ∼1100. Coding test (digit-symbol substitution) from WISC, picture recognition (immediate recall). Noncomputerized tasks. |
Significantly better picture recognition in multiple-grade intervention schools compared with multiple-grade control schools at postintervention. No other significant effects from BF on CTs. |
| Jacoby et al. (1996) (48) | SBP evaluation. Cluster RCT. Independent groups design. 5 intervention schools, 5 control schools; 1 mo intervention. |
10 primary schools; n = 352. Intervention: n = 201, mean age ± SD: 136.2 ± 18 mo. Male: 46% Female: 54% Control: n = 151, mean age ± SD: 138.9 ± 20 mo. Male: 53% Female: 47% Normal, underweight, and stunted school children. Low SES. Peru. |
Two conditions; SBP. 1. SBP: Milk-like beverage and 6 cookies (600 kcal, 19.5 g PRO, 60% RDA for various micronutrients and 100% RDA iron. 2. Control: No SBP, wait list control. |
CT: Baseline, 1 mo. Digit cancellation, coding test (digit-symbol substitution) from WISC, digit span from WISC. Noncomputerized tasks. |
No significant difference between intervention vs. control schools on all CTs. |
| Lieberman et al. (1976) (49) | SBP evaluation. Independent groups design. Compared matched schools with SBP vs. no SBP. 8 mo intervention. |
2 primary schools; n = 617, aged 8–11 y. SBP: n = 294. Control: n = 323. Well-nourished. Low SES. United States. |
Two conditions: 1. SBP: “Traditional” hot BF designed to provide ∼1/4 of the RDA for 9–10-y-old children. Based on foods from USDA SBP, in addition to eggs, meat, or meat alternatives. 2. Control: No SBP.60% attendance rate at SBP. |
CT: Baseline, 8 mo. Raven’s Colored Progressive Matrices, Rey Complex Figure task (copy accuracy), listening task. |
No significant differences on all CTs in intervention vs. control schools. |
| Moore et al. (2014) (50) | As Murphy et al. (51). Secondary analysis to assess impact of SBP on SES inequalities. | As Murphy et al. (51). Included additional SES measures that used data linkage. SES measures: School-level: 1. % of whole school entitled to FSM. 2. % of participants in school entitled to FSMIndividual level: 3. Yes/No, FSM entitlement. |
As Murphy et al. (51). | As Murphy et al. (51). | School-level analysis:FSM entitlement did not significantly interact with the effects of the intervention on word recall. Individual level analysis:FSM entitlement did not significantly interact with the effects of the intervention on word recall. Main effect of FSM entitlement on word recall: Word recall was significantly poorer in school children in receipt of FSM. |
| Murphy et al. (2011) (51) | SBP evaluation. Clustered RCT. Independent groups design. 56 control schools, 55 intervention schools. 1 y intervention. | 111 primary schools. Subsample of one year 5 and one year 6 class in each school for cognitive assessment. n = 4123 at baseline, n = 4112 at follow-up; aged 9–11 y. Control: n = 2063. Intervention: n = 2049. Well-nourished. Mixed SES. United Kingdom. |
Two conditions: 1. SBP: Welsh Primary School Free BF Initiative: Low-sugar RTEC, milk, bread, fruit. Considered nutritionally balanced. 2. Control: No SBP, wait list control.Compliance: 41% attended SBP 1 d/wk and 30% attended 5 d/wk. 10 schools randomly assigned to intervention did not set up SBP. |
CT: Baseline, 4 mo, 1 y. Administered between 0900 and 1100 in groups of ∼40 participants. Immediate free word recall. |
ITT: No significant differences in word recall in intervention vs. control schools. No difference in prevalence of BF skipping in intervention vs. control schools. PP: No significant differences in word recall in schools that had set up SBP vs. control schools. |
| Nkhoma et al. (2013) (52) | SBP evaluation. Independent groups design. Compared matched school with SBP vs. no SBP. 1 school year intervention. |
2 primary schools; n = 226 at baseline, n = 190 at follow-up. Mean age ± SD: 6.6 ± 0.5 y (range: 6–8 y). Male: 50% Female: 50% Underweight: 25% Stunted: 42% NCHS reference. Low SES. Malawi. |
Two conditions: 1. SBP: 100 g micronutrient-fortified porridge (350 kcal). Reduced ration by 25% because of government funding cut (263 kcal, 11–103% of RNI of various micronutrients). 2. Control: No BF or BF at home. |
CT: Baseline, 1 y. CANTAB battery:PAL (immediate recall), RVIP, Intra-extradimensional set shift (rule acquisition and reversal). Computerized tasks. |
Significantly fewer errors on set shift task at follow-up in SBP vs. no SBP. No other significant effects from BF on CTs. Significant increase in midarm circumference between baseline and follow-up in SBP; no change in no-SBP. |
| Rahmani et al. (2011) (53) | SBP evaluation. Independent groups design. Compared matched schools with SBP vs. no SBP. 3 mo intervention. | 4 single-sex primary schools; n = 469. Male: 49%, mean age ± SD: 7.9 ± 0.8 y. Female: 51%, mean age ± SD: 7.5 ± 0.9 y. Iran. |
Two conditions: 1. SBP: 250 mL 2.5% fat milk at 0930. 2. Control: No milk. |
CT: Baseline, 3 mo. Raven’s Colored Progressive Matrices, WISC. Noncomputerized tasks. |
Boys in intervention group performed significantly better on Raven’s post intervention compared with control group. No effect in girls. Multiple t tests conducted on outcomes at baseline and postintervention (within and between groups). |
| Richter et al. (1997) (54) | SBP evaluation. Independent groups design. Compared matched school with SBP vs. no SBP. 6 wk intervention. | 2 primary schools; n = 108. Intervention vs. control schools poorly matched. Control: n = 55 well-nourished children, mean age ± SD: 8.3 ± 0.8 y, from inner city school. Mid SES. Intervention: n = 53 undernourished school children, mean age ± SD: 10.5 ± 1.9 y from rural school. Low SES. South Africa. |
Two conditions: 1. SBP: 30 g cornflakes, 100 mL semiskimmed milk, banana (∼267 kcal, 7.2 g PRO, 2.5 g fat, 54 g CHO). 2. Control: No SBP. |
CT: Baseline, 6 wk. Letter cancellation, coding test (digit-symbol substitution) from WISC, digit span from WISC. Noncomputerized tasks. |
Digit span and letter cancellation: Mean change scores significantly higher in intervention vs. control group. No other significant effects from BF on CTs. |
| Shemilt et al. (2004) (55) | SBP evaluation. Clustered RCT. Independent groups design. 24 intervention schools, 19 control schools. 1 y intervention. |
43 primary and secondary schools. Subsample of n = 200/school. n = 5837 at baseline, n = 3894 at follow-up. Control: n = 2372, mean age ± SD: 10.13 ± 3.93 y. Male: 52% Female: 48% Intervention: n = 3465, mean age ± SD: 9.59 ± 2.96 y. Male: 49% Female: 51% Well-nourished.Mixed SES. United Kingdom. |
Two conditions: 1. Funding for free SBP. 2. Control: No funding for SBP. Contamination between treatment arms: 72.2% of pupils in intervention and 77.0% of pupils in control had SBP at their school. Evoked PP analysis:School children classified as 1. Nonattendees: Never attended SBP. 2. Attendees: Attended SBP at least once. |
CT: Baseline, 3 mo and 12 mo. Reitan Trail Making Test Part A (primary school children) and Part B (secondary school children). Noncomputerized tasks. |
ITT: Time taken to complete Trail Making Test Part A was significantly shorter in the intervention vs. control at 3 mo follow-up. No other significant effects from BF on CTs. PP: No significant differences in trail making between attendees vs. nonattendees. |
| Worobey and Worobey (1999)Expt. 1 (56) | SBP evaluation. Pre-and postintervention design. 6 wk intervention. |
1 preschool; n = 12. Aged 3 y 10 mo to 5 y 2 mo. Mid SES. Well-nourished. United States. |
Two conditions: 1. Preintervention (baseline): BF at home. Intake record by parents. Mean intake: 275 kcal 2. Intervention: SBP: 1 serving milk, 1 serving fruit/vegetable/fruit juice, 2 servings bread and meat. Mean intake: 262 kcal. |
CT: Baseline, 6 wk. Same or different task, pattern match, mazes task from WPPSI, embedded figures task (nonverbal reasoning), verbal memory scale from MSCA (free word recall; immediate recall), numeric memory scale from MSCA (digit span forward and backward). Noncomputerized tasks. |
Significantly improved performance on mazes, pattern match, and same or different task after SBP BF compared with baseline (BF at home). No other significant effects from BF on CTs. |
| Worobey andWorobey(1999)Expt. 2 (56) | SBP evaluation. Independent groups design. Compared participants attending SBP vs. no SBP. 6 wk intervention. |
1 preschool; n = 16.SBP: n = 9, aged 3 y 11 mo to 4 y 6 mo. Control (BF at home): n = 7, aged 3 y 10 mo to 4 y 5 mo. Mid SES. Well-nourished. United States. |
As in Worobey and Worobey(1999) Expt. 1, with addition of control group. Two conditions: 1. SBP: Mean intake: 158 kcal. 2. Control: BF at home. Intake record by parents. Mean intake: 212 kcal. |
CT: Baseline, 6 wk. Same or different task, cookie hunt task (pattern match), MFFT, animal pegs (place pegs in correct animal locations) from WPPSI. Noncomputerized tasks. |
Animal pegs: Both SBP and control group improved significantly from baseline to follow-up. Follow-up scores significantly faster in SBP vs. control. MFFT: Both SBP and control group improved significantly from baseline to follow-up. Cookie task: Significant decline in performance from baseline to follow-up in control group; no change in SBP group. Same or different task: SBP improved significantly from baseline to follow-up; no change in control group. Follow-up scores significantly higher in SBP vs. control. No other effects from BF on CTs. |
BF, breakfast; CANTAB, Cambridge Neuropsychological Test Automated Battery; CHO, carbohydrate; CT, cognitive test; FSM, free school meal; ITT, intention to treat; MFFT, Matching Familiar Figures Test; MSCA, McCarthy Scales of Children’s Abilities; NCHS, National Center for Health Statistics; PAL, paired associates learning; PP, per protocol; PRO, protein; RCT, randomized controlled trial; RNI, reference nutrient intake; RTEC, ready-to-eat cereal; RVIP, rapid visual information processing; SBP, school breakfast program; SES, socioeconomic status; WISC, Wechsler Intelligence Scale for Children; WPPSI, Wechsler Preschool Primary Scale of Intelligence.
Assessment of strength of evidence
A grading of the strength of evidence for each conclusion was made with the use of the grade definitions in the Academy of Nutrition and Dietetics Evidence Analysis Manual (57).
Results
Summary of studies and their characteristics
Thirty-four studies considered the acute effect of a single breakfast meal in which performance was typically assessed within 4 h postingestion. These studies were further categorized into those using breakfast compared with no breakfast (n = 24) and/or comparisons of breakfast type (n = 15). Five studies examining the effect of breakfast compared with no breakfast also included comparisons of breakfast type (n = 5). The effect of chronic breakfast interventions on cognition was evaluated in 11 studies. Chronic intervention studies were all evaluations of breakfast provision at school as SBPs and were all comparisons of SBP and no SBP.
In the 15 acute intervention studies that compared different breakfast types or size, 7 were comparisons of the glycemic index (GI) or glycemic load (GL) of breakfast meals. An additional 4 studies did not explicitly compare the GI or GL of different breakfast meals, but the macronutrient composition differed across conditions such that the effects were described in terms of differences in glycemic response. The remaining studies were comparisons of high carbohydrate and high protein (2 studies) or comparisons of breakfasts differing in energy (2 studies).
The breakfast manipulations were varied, but a commonality was the use of foods or meals that were carbohydrate-rich. Most studies included ready-to-eat cereals or breads in combination with other foods, including milk, sweet and fat spreads, fruit, fruit juice, yogurt, and cheese. The macronutrient composition of the test meals varied widely between the studies. The energy loads of the breakfast manipulations were also wide-ranging, from 95 to 600 kcal (mean: 225 kcal). In acute intervention studies, the breakfast manipulations were typically fixed rather than ad libitum. In chronic intervention studies, meals were always ad libitum.
Studies were conducted in participants aged 3–20 y, but most studies included children aged 6–11 y (28 studies) with adolescent samples less frequent (10 studies). Two studies included young preschool-aged children (3–6 y). Seven studies included undernourished children. Most studies were carried out in well-nourished children of both sexes.
Most acute studies used crossover designs (74%; 25 of 34 studies), of which 20 were randomized and 5 were nonrandomized, but order of treatment was counterbalanced. Eight studies used parallel-group designs, of which 7 were randomized. Control groups were usually fasting, but some studies attempted to include placebo controls such as very-low-energy conditions. These are not true placebos, but they may account for the extra attention given to children during breakfast provision. Of the 11 chronic studies, 5 were nonrandomized matched school comparison studies, 1 was a nonrandomized matched participant comparison study, 4 were randomized controlled trials (RCTs), and the remaining study was a before-and-after study. Control conditions usually included breakfast at home or no breakfast, depending on participants’ usual habitual breakfast intake.
A wide variety of cognitive tasks were used. Attention and memory were the most frequently assessed cognitive domains. Typically, the acute studies used a battery of cognitive tasks to measure multiple domains. Studies also often administered more than one measure within the same domain. Hence, a single study could show positive, negative, and equivocal findings. The multiple outcomes from these studies are reflected by multiple entries in Tables 2–4. Within the acute studies, the temporal distribution of the cognitive tasks across the morning was variable and ranged from >10 min to ≥210 min postbreakfast. Some studies (38%; 13 of 34 studies) tracked postintervention performance across the morning usually shortly after breakfast (e.g., >60 min), in the midmorning (e.g., ≥120–180 min), and in the late morning (e.g., ≥210 min). However, many acute studies (62%; 21 of 34 studies) included only one postintervention testing period. Furthermore, 25 studies did not include a baseline (pretreatment) test session. In chronic studies, assessment of cognitive function was commonly assessed at one follow-up period after an intervention duration ranging from 1 mo to 3 school years.
Attention
Thirty-eight studies included measures of attention.
Acute intervention studies: Effects of breakfast compared with no breakfast.
Twenty-one acute studies examining attention included breakfast and no breakfast conditions (Tables 2 and 5). Thirteen studies demonstrated a positive effect from breakfast, of which 8 were in well-nourished children and adolescents (18, 20–22, 30, 31, 40, 44). However, of the studies reporting positive effects, 5 of 13 studies (38%) demonstrated effects only in a specific subgroup of the sample under study (14, 24, 38, 39, 41). For example, positive effects of breakfast on attention were confined to male participants (14), participants with an intelligence quotient below the median of the sample (38, 39), and undernourished children (24, 41). Furthermore, 3 studies reported negative effects from breakfast in a specific subgroup of the sample under study (14, 24, 41). For example, the negative effects of breakfast consumption on attention were confined to well-nourished children (24, 41) and female participants (14).
Fourteen studies reported equivocal findings on attention outcomes, of which 4 of 14 studies (29%) were in well-nourished adolescents (23, 25, 26, 45), 7 of 14 studies (50%) were in younger well-nourished children (18, 28, 31, 38–40) and 3 of 14 studies (21%) were in undernourished children (19, 24, 29). However, one study observed a ceiling level of performance on the attention task, which may account for the null effects (23). Moreover, one study assigned participants to a condition based on habitual breakfast intake, which is an obvious bias (29).
Acute intervention studies: Effects of different breakfast types.
Comparisons of the effects of different breakfast types on attention were made in 14 studies (Tables 3 and 5). Nine studies were comparisons of the GI or GL of breakfast meals or foods, of which 6 of 9 studies (67%) demonstrated an advantage for lower-GI or -GL breakfast meals or foods (15, 22, 27, 31, 44). However, lower-GI conditions were not always classified as low GI (e.g., GI value ≤ 55). For example, one study reported that consumption of 2 types of ready-to-eat cereal (GI ∼74) (58) reduced the decline in attention across the morning relative to a glucose drink (GI 100) (44). In addition, conditions were not isocaloric in 3 studies (15, 27, 44). Furthermore, 4 studies demonstrated no effect from breakfast GI or GL on attention (16, 17, 31), and 1 study observed that both high-GI–low-GL and high-GI–high-GL breakfast meals were associated with better attention (32).
TABLE 3.
Summary of findings for the acute effects of breakfast composition on cognition in children and adolescents (n = 15 studies)1
| Acute effect of breakfast type vs. breakfast type |
||||||||||
| GI/GL |
Energy |
High carbohydrate vs. protein |
||||||||
| Cognitive domain | Total studies | Advantage of higher GI/GL | No effect | Advantage of lower GI/GL | Advantage of higher energy | No effect | Advantage of lower energy | Advantage of high carbohydrate | No effect | Advantage of high protein |
| Attention | 14 | 1 | 4 | 6 | 0 | 2 | 1 | 0 | 1 | 1 |
| Memory | 12 | 1 | 5 | 4 | 2 | 0 | 0 | 0 | 1 | 0 |
| Executive function | 5 | 2 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Psychomotor function | 3 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Language | 2 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Total | 15 | 4 | 14 | 12 | 2 | 4 | 1 | 0 | 3 | 1 |
Studies assessed >1 cognitive domain and/or administered >1 measure within the same domain. GI, glycemic index; GL, glycemic load.
The remaining 5 studies compared high protein and high carbohydrate breakfasts or breakfasts that differed in size and/or provision of a midmorning snack. One study reported that consumption of a high carbohydrate breakfast impaired attention relative to a high protein breakfast (14). However, the effects were specific to female participants, and one study found no effect from a high-carbohydrate compared with high-protein breakfast on attention (34). There was no effect from breakfast size or midmorning snack consumption on attention in 2 studies (35, 46). However, one study reported that attention was worse after consumption of a higher-energy breakfast relative to habitual breakfast consumption (33).
Chronic intervention studies: Effects of SBPs.
Eight chronic SBP studies measured attention (Tables 4 and 6). Four studies showed an advantage for SBPs on attention, with effects demonstrable after an intervention period of 1 mo to 3 y (54–56). Of the studies reporting positive effects, 1 of 4 studies (25%) was an RCT, but the trial suffered substantial contamination between treatment arms such that 77% of the children in the control arm had an SBP operating at their school (55). Seven chronic studies demonstrated equivocal findings for attention in well- and undernourished children (47–49, 52, 54, 56).
Memory
Twenty-eight studies took measures of memory.
Acute intervention studies: Effects of breakfast compared with no breakfast.
Comparisons of breakfast and no breakfast were made in 15 acute studies (Tables 2 and 5). An advantage for breakfast on memory was demonstrated in 8 studies in well-nourished children and adolescents (25, 31, 38, 40, 43–45). However, positive effects were only apparent under conditions of greater cognitive load in 1 of 8 studies (13%) (25), and positive effects were specific to male participants in 1 of 8 studies (13%) (45).
Nine studies reported no effect from breakfast compared with no breakfast on memory in well- and undernourished children and adolescents (14, 18, 23, 28, 30, 31, 41, 45). Moreover, 3 studies reported that memory was superior under fasting conditions (30, 38, 39).
Acute intervention studies: Effects of different breakfast types.
Twelve studies investigated the effect of breakfast composition on memory (Tables 3 and 5). Nine studies were comparisons of the GI or GL of breakfast meals or foods, which demonstrated varied results. Four studies reported an advantage of lower-GI or -GL breakfasts for memory (15, 17, 27, 44), of which 1 of 4 (25%) studies demonstrated a lower-GI breakfast facilitation effect in girls only (17), 2 of 4 studies (50%) demonstrated a positive effect of a high-GI breakfast compared with a very high–GI breakfast (17, 44), and 3 of 4 studies (75%) did not include isocaloric conditions (15, 27, 44). Five studies demonstrated no effect from breakfasts differing in GI or GL on memory (15, 16, 31, 32). One study demonstrated an advantage of a high-GI relative to a low-GI breakfast for memory (42).
The remaining 3 studies compared high-protein and high-carbohydrate breakfasts or breakfasts which differed in size and/or a midmorning snack. One study reported no effect from 2 isocaloric breakfasts that were either high carbohydrate or high protein and matched for fat (14). One study observed that memory was better after consumption of a higher-energy breakfast relative to habitual breakfast (33). One study showed that the decline in memory across the morning was reduced by consumption of a midmorning snack (35).
Chronic intervention studies: Effects of SBPs.
Five chronic intervention studies measured memory (Tables 4 and 6). Only one study showed a positive effect from a chronic breakfast intervention on memory, an effect that was specific to schools with more poverty, undernourished children, and lower achievement (47). Four studies found no effect from SBPs on memory in well- and undernourished children (50–52, 56), of which 2 of the 4 studies (50%) reported data from the same RCT (50, 51).
Executive function
Nineteen studies included tests of executive function.
Acute intervention studies: Effects of breakfast compared with no breakfast.
Thirteen acute studies examined executive function after breakfast and no breakfast (see Tables 2 and 5). A positive effect from breakfast on executive function was demonstrated in 7 studies in children and adolescents (20–22, 24, 25, 36, 37), of which 3 of the 7 studies (43%) demonstrated effects under conditions of varying cognitive load (21, 22, 25) and 1 of the 7 studies (14%) demonstrated positive effects in undernourished children only (24). Eight studies showed equivalence for this domain (14, 22, 24–26, 28, 29, 44). Furthermore, 1 study reported that executive function was better under fasting conditions; however, effects were specific to well-nourished children only (41).
Acute intervention studies: Effects of different breakfast types.
Five studies investigated the effect of different types of breakfasts on executive function (Tables 3 and 5). Most studies [4 of 5 studies (80%)] were comparisons of breakfasts differing in GI or GL, which demonstrated mixed results on executive function. Two studies showed an advantage from higher-GI or -GL breakfasts (23, 33), 1 study showed an advantage of lower-GI or -GL breakfasts (23), and 3 studies showed no effect of breakfast GI or GL (27, 32, 44). The remaining study found no effect from a high-carbohydrate compared with a high-protein breakfast on executive function (14).
Chronic intervention studies: Effects of SBPs.
Four chronic intervention studies measured executive function (Tables 4 and 6). Two studies observed a positive effect of SBPs on executive function (52, 53). However, positive effects were only apparent in specific trials of the task (52) or in male participants (53). Two studies found no effect from SBPs on executive function (49, 56).
Psychomotor function
Measures of motor control and coordination were used in 8 studies.
Acute intervention studies: Effects of breakfast compared with no breakfast.
Four acute studies measured psychomotor function after breakfast and no breakfast conditions (Tables 2 and 5). Two studies showed an advantage of breakfast for psychomotor function (31). However, 2 studies showed equivalence (18, 28). Moreover, 1 study found that psychomotor function was better under fasting conditions, an effect that was specific to female participants (31).
Acute intervention studies: Effects of different breakfast types.
Three acute studies found no effect from different breakfast types on psychomotor function (31, 35) (Tables 3 and 5).
Chronic intervention studies: Effects of SBPs.
There were 3 chronic intervention studies (Tables 4 and 6). Two chronic studies showed positive effects on psychomotor outcomes after a 6 wk SBP (56). However, one study found no effect from an 8 mo SBP compared with no SBP on psychomotor function (49).
Language
Measures of language ability were used in 6 acute studies.
Acute intervention studies: Effects of breakfast compared with no breakfast.
Four studies examined the acute effect of breakfast compared with no breakfast on language ability (Tables 2 and 5). An advantage for breakfast on language was demonstrated in 2 studies, both of which showed that effects were specific to undernourished children only (19, 41). One study reported that performance on a vocabulary test was better under fasting conditions in well-nourished children, an effect that was not observed in undernourished children (24). One study demonstrated no effect from breakfast on a vocabulary test in well-nourished children (38).
Acute intervention studies: Effects of different breakfast types.
Two studies investigated the effect of breakfast composition on language (Tables 3 and 5). The effects of both breakfast GI and GL on language were examined by 1 study (32). The 4 breakfast conditions include low GI–high GL, high GI–high GL, low GI–low GL, and high GI–low GL. Verbal fluency was significantly improved after consumption of the low GI compared with the high-GI breakfast, which was consistent across high- and low-GL conditions. One study showed that breakfast energy intake did not affect verbal fluency (46).
Discussion
Principal findings
The present review indicates that breakfast consumption has a transient beneficial effect on cognitive function measured within 4 h postingestion compared with breakfast omission. There was also some evidence that the advantageous effects on cognition of breakfast compared with fasting may be more apparent in undernourished children. It is more difficult to make conclusions about the acute effects of breakfast composition and the chronic effects of SBPs, because there are fewer studies, and these largely report inconsistent findings. Particularly, SBPs seem to have limited effects on cognitive outcomes, but this may be partly attributed to the difficulties in executing these typically large, pragmatic trials. Furthermore, SBP studies tended to use one cognitive measure, whereas acute studies tended to use a battery of tasks and therefore increase the likelihood of finding effects.
Effect of breakfast on specific cognitive domains.
The findings indicate that breakfast-induced cognitive effects are domain-specific. Tasks that required attention, executive function, and memory were facilitated more reliably by breakfast consumption relative to fasting. In acute studies that compared breakfast type, a low-GI breakfast (see information in the section on breakfast composition and cognitive performance) was most consistently associated with positive effects on attention. There were also instances in which effects were specific to certain variables within tasks, although the weight of the evidence was insufficient to draw firm conclusions.
Although the findings suggest that there are domain-specific effects on cognition from breakfast consumption compared with fasting, it should be acknowledged that cognitive functions are not discrete; they overlap. Furthermore, the categorization of certain cognitive tasks to a specific cognitive domain is not consistent.
The timing of the effects of breakfast on cognition across the morning.
Within acute studies, positive effects were noted at many time points across the morning from immediately postingestion (≥10 min) to late morning (≥210 min). However, effects of breakfast consumption relative to fasting appeared most commonly in the mid-late morning (∼180 min postbreakfast). This may be when performance decrements in fasted conditions become apparent, allowing for greater discrimination between conditions. In the studies that tracked performance across the morning with multiple testing sessions, performance often declined across the morning from baseline. However, breakfast consumption functioned to reduce this decline in performance rather than enhance performance above baseline levels.
Breakfast composition and cognitive performance.
Firm conclusions cannot be made regarding the acute effects of breakfast composition. In acute intervention studies comparing breakfast to breakfast omission, effects were demonstrated across a range of breakfast manipulations and energy loads, suggesting that breakfast composition may be unrelated to cognition. There are fewer studies comparing breakfast type than there are those that compare breakfast with no breakfast, and these demonstrate inconsistent findings. Within the limited data comparing breakfast type, the evidence generally suggested that lower-GI breakfasts may facilitate cognition relative to higher-GI breakfasts. This suggests that breakfast foods or meals that elicit a glycemic response characterized by less oscillating glucose concentrations and a sustained blood glucose concentration above fasting concentrations may facilitate cognitive function. This also suggests that the postprandial blood glucose profile may mediate the effects of breakfast on cognitive performance. However, concomitant blood glucose measures were not always taken in studies that reported such effects (27, 44). Furthermore, in studies that used continuous blood glucose monitoring, the evidence indicated that large differences in postprandial glycemic responses elicited by high- and low-GL conditions were apparent in the absence of any cognitive performance effects (16). Moreover, some evidence indicated that cognitive effects were apparent when postprandial blood glucose concentrations had returned to baseline. These temporal relations suggest that other factors associated with ingestion of these low-GI breakfast meals, rather than glucose response per se, may mediate the effects on cognitive performance. Alternatively, cognitive performance could be related to blood glucose concentrations, but not in a tightly, temporally coupled manner.
Methodologic considerations and strength of evidence
A grading of the strength of evidence is shown in Table 7.
TABLE 7.
The strength of the body of evidence supporting the conclusions on the acute effects of breakfast vs. no breakfast, the acute effects of different breakfast types, and the chronic effects of SBPs vs. no SBPs on cognitive test performance in children and adolescents, with the use of the grade definitions for the quality, consistency, quantity, clinical impact, and generalizability of evidence in the Academy of Nutrition and Dietetics Evidence Analysis Manual. Grade definitions: I–Good, II–Moderate, III–Limited, IV–Expert Opinion, and V–Grade Not Assignable (14)1
| Strength of evidence elements | Acute effect of breakfast vs. no breakfast | Acute effect of breakfast type | Chronic effect of SBP vs. no SBP |
| Quality:Scientific rigor/validity; considers design and execution | Grade II Fair: Studies of strong design for the question with minor methodologic concerns. |
Grade III Limited: Studies of weak design for answering the question. |
Grade III Limited: Studies of weak design for answering the question. |
| Consistency:Consistency of findings across studies | Grade II Fair: Inconsistency among results of studies with strong design. |
Grade III Limited: Unexplained inconsistency among results from different studies. |
Grade III Limited: Unexplained inconsistency among results from different studies. |
| Quantity:Number of studies; number ofsubjects in studies | Grade I Good: One to several good quality studies. Large number of subjects studied. Some studies with negative results have sufficiently large sample size for adequate statistical power. |
Grade III Limited: Limited number of studies. Low number of subjects studied, and/or inadequate sample size within studies. |
Grade III Limited: Limited number of studies. Low number of subjects studied, and/or inadequate sample size within studies. |
| Clinical impact:Importance of studied outcomes; magnitude of effect | Grade II Fair: Some doubt about the statistical or clinical significance of the effect. |
Grade III Limited: Size of effect is small or lacks statistical and/or clinical significance. |
Grade III Limited: Size of effect is small or lacks statistical and/or clinical significance. |
| Generalizability:Generalizability to population of interest | Grade III Limited: Serious doubts about generalizability because of narrow or different study population, intervention. |
Grade III Limited: Serious doubts about generalizability because of narrow or different study population, intervention. |
Grade I Good: Generalizability limited to scope of experience. |
SBP, school breakfast program.
Quality.
The quality of the acute studies comparing breakfast with no breakfast or composition was graded as fair and limited, respectively (Grade II and III; Table 7). The quality of chronic intervention studies was graded as limited (Grade III; Table 7). Overall, acute intervention studies comparing breakfast and no breakfast had minor methodologic concerns. For example, compliance to the breakfast manipulation in terms of the amount consumed was rarely reported. Compliance to the fasting requirements before and after breakfast or no breakfast also was not always reported. Furthermore, evening food intake typically was not reported or controlled for. However, 8 acute laboratory studies did include an overnight stay and hence a monitored overnight fasting period. A further limitation was that the cognitive tests used often were not selected on the basis of their capacity to differentiate subtle differences in cognitive performance after nutritional manipulations. The evidence from acute breakfast type comparisons was more limited because breakfast conditions often differed in other characteristics that were not intended to be manipulated.
The quality of evidence from chronic SBP studies was graded as limited. In SBP studies, food consumed before school was not recorded in both conditions and fasting requirements were not prescribed. Hence, participants under the non-SBP conditions could have consumed breakfast and also possibly caffeine as part of breakfast at home. Furthermore, compliance with the intervention in terms of whether or not the SBP breakfast was actually consumed was often not stated. Rather, studies usually described compliance in terms of attendance rates. Hence, these comparisons are more strictly a test of the SBP regimen rather than breakfast per se. Furthermore, these studies reported problems with contamination between treatment arms or poor compliance (51, 55).
Consistency.
The consistency of evidence from acute intervention studies comparing breakfast and no breakfast was graded as fair (Grade II; Table 7). However, the findings of domain-specific effects of breakfast should be interpreted cautiously, because significant positive findings for a particular cognitive domain in some studies were coupled with null effects in other studies. Moreover, when positive effects occurred, there were instances in which effects were apparent only under specific conditions, such as more difficult trials of the task, or in a specific subgroup of the sample under study (e.g., often undernourished children). The failure to detect an effect in some studies but not others may be a result of true lack of effect. However, it may be because of task insensitivity (59). Another factor that may account for the inconsistent findings is the evidence for enhancement effects on specific variables within tasks. For example, some studies suggested that enhancement effects of breakfast on reaction-time tasks are specific to response times rather than accuracy, with other studies showing the reverse pattern. Therefore, a limited analysis of outcomes (e.g., only accuracy) may result in null findings. Finally, the large methodologic variation between studies also may have contributed to the inconsistent findings. The variable findings for particular cognitive domains should also be interpreted in conjunction with an appreciation of the imbalance of research effort across cognitive domains.
The possibility of publication bias should also be acknowledged in relation to the consistency of findings. This review included only published studies. Consequently, a publication bias cannot be excluded. In addition, many of the included studies were funded by a breakfast cereal manufacturer, including this review. Industry funding has been reported to bias conclusions in favor of the sponsor’s products (60). This may have increased the prevalence of the published positive effects of breakfast.
The consistency of evidence from acute studies comparing breakfast type and chronic SBP interventions was limited (Grade III; Table 7). Hence, there is insufficient evidence to support a consistent effect from SBPs or breakfast type on cognitive performance.
Quantity.
Forty-five studies is a reasonable body of evidence. However, most studies are acute, and comparisons with no breakfast, providing a good quantity of studies for this conclusion (Grade I; Table 7). However the number of acute breakfast composition studies and chronic studies is relatively low, providing a limited quantity of studies for conclusions (Grade III; Table 7).
Clinical impact.
The clinical impact of the findings from all studies was fair to limited (Grade II–III; Table 7). The results did not show large breakfast-induced benefits for cognitive performance. The evidence demonstrated that the effects of breakfast consumption on cognitive performance are likely to be subtle, and demonstrable only under specific conditions or in particular subgroups of the samples. The functions assessed could have some wider impact on learning in the classroom and educational achievement. However, objective cognitive tests may not be relevant to a “real world” classroom situation.
Generalizability.
The generalizability of the findings from acute intervention studies was limited (Grade III; Table 7). Many acute studies lacked large representative samples. In addition, children aged 6–10 y were overrepresented. There was also a lack of acute studies using naturalistic breakfast manipulations and contexts, which prevented generalization to “real-life” situations. In acute intervention studies, the breakfast manipulations were typically fixed rather than ad libitum. Ad libitum breakfast manipulations allow participants to choose a breakfast that is palatable and suitable for them in terms of portion size. This should therefore better reflect the participants’ usual eating habits. This is also important given that deviation from habitual intake can adversely affect cognitive performance (61). Furthermore, many acute studies were laboratory-based. School-based studies have the advantage of aligning with the participants’ normal environment and daily routine, providing ecologically valid evidence. In contrast, chronic SBP studies often included much larger samples, more naturalistic breakfast interventions, and settings providing good generalizability of the findings to school children (Grade I; Table 7)
Mechanisms
Physiologic.
Brain extracellular glucose concentrations fluctuate with blood glucose concentrations such that brain extracellular glucose concentrations are ∼20–30% of blood glucose concentrations (62). This suggests that increasing blood glucose by consuming carbohydrates will induce small increases in extracellular glucose in the brain. However, it is unlikely that this will lead to changes in brain activity and thus cognitive function, because neuronal uptake of glucose is driven by neural activity, not by extracellular brain glucose concentration (62, 63). Astrocytes function as a buffering system, regulating availability of brain extracellular glucose for neurons in response to neuronal activity, and also contain glycogen stores to prevent large fluctuations in glucose availability (62, 63). This may explain why the effects of breakfast consumption on cognitive performance can occur when blood glucose concentrations have returned to baseline. Alternatively, mechanisms involving glucose suggest that increased blood glucose may improve cognition by facilitating neuronal glucose uptake specifically in regions in which brain extracellular glucose concentrations are decreased during times of high neuronal glucose uptake (62, 64, 65). This is supported by research demonstrating that increased neuronal glucose uptake driven by active neurons during demanding cognitive tasks can lead to a local deficit in extracellular glucose that is rate-limiting for glucose transfer to neurons (62, 64, 65).
Secondary metabolic events induced by carbohydrate ingestion, such as changes to concentrations of neurotransmitters and hormones, may mediate changes to cognition (62, 63, 65). Glucose-mediated insulin delivery to the brain may facilitate cognitive performance after ingestion of carbohydrates. Insulin crosses the blood–brain barrier, and insulin receptors are located in the brain (66). Furthermore, administration of insulin intranasally has been shown to facilitate cognitive performance in adults (67). Other mechanisms may involve the excitatory neurotransmitter acetylcholine, given that synthesis of acetylcholine requires glucose. Glucose ingestion may increase acetylcholine synthesis, which could influence cognitive function. Cortisol secretion as a result of the combination of carbohydrate consumption and an arousing situation (cognitive testing) may interact to facilitate effects on cognitive performance (32). Cortisol receptors are abundant in the hippocampus. Ingestion of glucose can interact with a stressful task and provoke a greater cortisol response (68), which in turn has dose-dependent and bidirectional effects on cognitive function (63). This may account for the fact that beneficial cognitive effects are most apparent at times when postprandial blood glucose concentrations have returned to or below baseline values after breakfast consumption, but when differences in cortisol are most likely to occur (i.e., during cognitive testing).
Subjective state.
Breakfast may affect cognitive performance indirectly through changes in feelings or subjective state (e.g., mood or alertness) caused by the consumption of breakfast. There is consistent evidence that breakfast consumption is associated with improved subjective feelings of mood and alertness in school children (21, 25, 28, 44). The positive changes in mood, alertness, and motivation after breakfast may in turn facilitate cognitive performance by increasing children’s ability to concentrate and/or motivation to try hard on cognitive tasks.
Research recommendations
Further studies are needed with well-matched study conditions to establish the effect of breakfast composition on school children’s cognitive performance. Studies are also needed with larger samples with sufficient power to detect a statistically significant effect and in samples with adolescents, in whom there are far fewer studies. Evidence is also needed in more ecologically valid research conditions, such as school-based studies and/or ad libitum breakfast manipulations. This review also highlights the need for more studies that use cognitive tasks sensitive to nutritional manipulations (59).
Conclusion
From the studies reviewed, the data suggest that consuming breakfast has a short-term positive, domain-specific effect on cognitive function measured within 4 h postingestion in children and adolescents. The potential for breakfast to have an impact upon cognitive performance appears to be more pronounced in undernourished children. However, the effects of breakfast composition and the long-term effects of consuming breakfast are unclear because of insufficient studies in this area and problematic experimental designs.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: GI, glycemic index; GL, glycemic load; RCT, randomized controlled trial; SBP, school breakfast program.
References
- 1.Deshmukh-Taskar PR, Nicklas TA, O’Neil CE, Keast DR, Radcliffe JD, Cho S. The relationship of breakfast skipping and type of breakfast consumption with nutrient intake and weight status in children and adolescents: the National Health and Nutrition Examination Survey 1999–2006. J Am Diet Assoc 2010;110:869–78. [DOI] [PubMed] [Google Scholar]
- 2.de la Hunty A, Gibson S, Ashwell M. Does regular breakfast cereal consumption help children and adolescents stay slimmer? A systematic review and meta-analysis. Obes Facts 2013;6:70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szajewska H, Ruszczynski M. Systematic review demonstrating that breakfast consumption influences body weight outcomes in children and adolescents in Europe. Crit Rev Food Sci Nutr 2010;50:113–9. [DOI] [PubMed] [Google Scholar]
- 4.Sandercock GRH, Voss C, Dye L. Associations between habitual school-day breakfast consumption, body mass index, physical activity and cardiorespiratory fitness in English schoolchildren. Eur J Clin Nutr 2010;64:1086–92. [DOI] [PubMed] [Google Scholar]
- 5.Hoyland A, McWilliams KA, Duff RJ, Walton JL. Breakfast consumption in UK schoolchildren and provision of school breakfast clubs. Nutr Bull 2012;37:232–40. [Google Scholar]
- 6.Vereecken C, Dupuy M, Rasmussen M, Kelly C, Nansel TR, Al Sabbah H, Baldassari D, Jordan MD, Maes L, Niclasen BV, et al. Breakfast consumption and its socio-demographic and lifestyle correlates in schoolchildren in 41 countries participating in the HBSC study. Int J Public Health 2009;54:180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoyland A, Dye L, Lawton CL. A systematic review of the effect of breakfast on the cognitive performance of children and adolescents. Nutr Res Rev 2009;22:220–43. [DOI] [PubMed] [Google Scholar]
- 8.Chugani HT. A critical period of brain development: Studies of cerebral glucose utilization with PET. Prev Med 1998;27:184–8. [DOI] [PubMed] [Google Scholar]
- 9.Thorleifsdottir B, Björnsson JK, Benediktsdottir B, Gislason T, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res 2002;53:529–37. [DOI] [PubMed] [Google Scholar]
- 10.Grantham-McGregor S. Can the provision of breakfast benefit school performance? Food Nutr Bull 2005;26 Suppl 2:S144–58. [DOI] [PubMed] [Google Scholar]
- 11.Rampersaud GC, Pereira MA, Girard BL, Adams J, Metzl JD. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Diet Assoc 2005;105:743–60. [DOI] [PubMed] [Google Scholar]
- 12.Pollitt E, Mathews R. Breakfast and cognition: an integrative summary. Am J Clin Nutr 1998; 67(4, Suppl)804S–13S. [DOI] [PubMed] [Google Scholar]
- 13.Lezak M, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. Fifth ed. Oxford, 2012. [Google Scholar]
- 14.Amiri F, Amani R, Khajemogahi N, Rashidkhani B, Saxby B, Wesnes K. Assessing the effects of two breakfasts (high-carbohydrate vs high-protein) on cognitive function, mood and satiety status of 9–11 year-old primary school children with a new technology in Iran. Brain Inj 2014;28:754–5. [Google Scholar]
- 15.Benton D, Maconie A, Williams C. The influence of the glycemic load of breakfast on the behavior of children in school. Physiol Behav 2007;92:717–24. [DOI] [PubMed] [Google Scholar]
- 16.Brindal E, Baird D, Danthiir V, Wilson C, Bowen J, Slater A, Noakes M. Ingesting breakfast meals of different glycemic load does not alter cognition and satiety in children. Eur J Clin Nutr 2012;66:1166–71. [DOI] [PubMed] [Google Scholar]
- 17.Brindal E, Baird D, Slater A, Danthiir V, Wilson C, Bowen J, Noakes M. The effect of beverages varying in glycemic load on postprandial glucose responses, appetite and cognition in 10–12-year-old school children. Br J Nutr 2013;110:529–37. [DOI] [PubMed] [Google Scholar]
- 18.Busch CR, Taylor HA, Kanarek RB, Holcomb PJ. The effects of a confectionery snack on attention in young boys. Physiol Behav 2002;77:333–40. [DOI] [PubMed] [Google Scholar]
- 19.Chandler AM, Walker SP, Connolly K, Grantham-McGregor SM. School breakfast improves verbal fluency in undernourished Jamaican children. J Nutr 1995;125:894–900. [DOI] [PubMed] [Google Scholar]
- 20.Conners CK, Blouin AG. Nutritional effects on behavior of children. J Psychiatr Res 1982–1983;17:193–201. [DOI] [PubMed] [Google Scholar]
- 21.Cooper SB, Bandelow S, Nevill ME. Breakfast consumption and cognitive function in adolescent schoolchildren. Physiol Behav 2011;103:431–9. [DOI] [PubMed] [Google Scholar]
- 22.Cooper SB, Bandelow S, Nute ML, Morris JG, Nevill ME. Breakfast glycemic index and cognitive function in adolescent school children. Br J Nutr 2012;107:1823–32. [DOI] [PubMed] [Google Scholar]
- 23.Cromer BA, Tarnowski KJ, Stein AM, Harton P, Thornton DJ. The school breakfast program and cognition in adolescents. J Dev Behav Pediatr 1990;11:295–300. [PubMed] [Google Scholar]
- 24.Cueto S, Jacoby E, Pollitt E. Breakfast prevents delays of attention and memory functions among nutritionally at-risk boys. J Appl Dev Psychol 1998;19:219–33. [Google Scholar]
- 25.Defeyter MA, Russo R. The effect of breakfast cereal consumption on adolescents’ cognitive performance and mood. Front Hum Neurosci 2013;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickie NH, Bender AE. Breakfast and performance in schoolchildren. Br J Nutr 1982;48:483–96. [DOI] [PubMed] [Google Scholar]
- 27.Ingwersen J, Defeyter MA, Kennedy DO, Wesnes K, Scholey AB. A low glycemic index breakfast cereal preferentially prevents children’s cognitive performance from declining throughout the morning. Appetite 2007;49:240–4. [DOI] [PubMed] [Google Scholar]
- 28.Kral TV, Heo M, Whiteford LM, Faith MS. Effects on cognitive performance of eating compared with omitting breakfast in elementary schoolchildren. J Dev Behav Pediatr 2012;33:9–16. [DOI] [PubMed] [Google Scholar]
- 29.López I, Deandraca I, Perales CG, Heresi E, Castillo M, Colombo M. . Breakfast omission and cognitive performance of normal, wasted, and stunted school children. Eur J Clin Nutr 1993;47:533–42. [PubMed] [Google Scholar]
- 30.Maffeis C, Fornari E, Surano MG, Comencini E, Corradi M, Tommasi M, Fasan I, Cortese S. Breakfast skipping in prepubertal obese children: hormonal, metabolic and cognitive consequences. Eur J Clin Nutr 2012;66:314–21. [DOI] [PubMed] [Google Scholar]
- 31.Mahoney CR, Taylor HA, Kanarek RB, Samuel P. Effect of breakfast composition on cognitive processes in elementary school children. Physiol Behav 2005;85:635–45. [DOI] [PubMed] [Google Scholar]
- 32.Micha R, Rogers PJ, Nelson M. Glycemic index and glycemic load of breakfast predict cognitive function and mood in school children: a randomized controlled trial. Br J Nutr 2011;106:1552–61. [DOI] [PubMed] [Google Scholar]
- 33.Michaud C, Musse N, Nicolas JP, Mejean L. Effects of breakfast size on short term memory, concentration, mood and blood glucose. J Adolesc Health 1991;12:53–7. [DOI] [PubMed] [Google Scholar]
- 34.Morrell G, Atkinson D. Effects of breakfast program on school performance and attendance of elementary school children. Education 1977;98:111–6. [Google Scholar]
- 35.Muthayya S, Thomas T, Srinivasan K, Rao K, Kurpad AV, van Klinken J-W, Owen G, de Bruin EA. Consumption of a mid-morning snack improves memory but not attention in school children. Physiol Behav 2007;90:142–50. [DOI] [PubMed] [Google Scholar]
- 36.Pivik RT, Dykman RA. Event-related variations in alpha band activity during an attentional task in preadolescents: Effects of morning nutrition. Clin Neurophysiol 2007;118:615–32. [DOI] [PubMed] [Google Scholar]
- 37.Pivik RT, Tennal KB, Chapman SD, Gu Y. Eating breakfast enhances the efficiency of neural networks engaged during mental arithmetic in school-aged children. Physiol Behav 2012;106:548–55. [DOI] [PubMed] [Google Scholar]
- 38.Pollitt E, Cueto S, Jacoby E. Fasting and cognition in well- and undernourished schoolchildren: a review of three experimental studies. Am J Clin Nutr 1998;67:779S–84S. [DOI] [PubMed] [Google Scholar]
- 39.Pollitt E, Leibel R, Greenfield D. Brief fasting, stress, and cognition in children. Am J Clin Nutr 1981;34:1526–33. [DOI] [PubMed] [Google Scholar]
- 40.Pollitt E, Lewis NL, Garza C, Shulman RJ. Fasting and cognitive function. J Psychiatr Res 1982–1983;17:169–74. [DOI] [PubMed] [Google Scholar]
- 41.Simeon DT, Grantham-Mcgregor S. Effects of missing breakfast on the cognitive functions of school-children of differing nutritional status. Am J Clin Nutr 1989;49:646–53. [DOI] [PubMed] [Google Scholar]
- 42.Smith MA, Foster JK. The impact of a high versus a low glycemic index breakfast cereal meal on verbal episodic memory in healthy adolescents. Nutr Neurosci 2008;11:219–27. [DOI] [PubMed] [Google Scholar]
- 43.Vaisman N, Voet H, Akivis A, Vakil E. Effect of breakfast timing on the cognitive functions of elementary school students. Arch Pediatr Adolesc Med 1996;150:1089–92. [DOI] [PubMed] [Google Scholar]
- 44.Wesnes KA, Pincock C, Richardson D, Helm G, Hails S. Breakfast reduces declines in attention and memory over the morning in schoolchildren. Appetite 2003;41:329–31. [DOI] [PubMed] [Google Scholar]
- 45.Widenhorn-Müller K, Hille K, Klenk J, Weiland U. Influence of having breakfast on cognitive performance and mood in 13- to 20-year-old high school students: Results of a crossover trial. Pediatrics 2008;122:279–84. [DOI] [PubMed] [Google Scholar]
- 46.Wyon DP, Abrahamsson L, Jartelius M, Fletcher RJ. An experimental study of the effects of energy intake at breakfast on the test performance of 10-year-old children in school. Int J Food Sci Nutr 1997;48:5–12. [DOI] [PubMed] [Google Scholar]
- 47.Cueto S, Chinen M. Educational impact of a school breakfast program in rural Peru. Int J Educ Dev 2008;28:132–48. [Google Scholar]
- 48.Jacoby E, Cueto S, Pollitt E. Benefits of a school breakfast program among Andean children in Huaraz, Peru. Food Nutr Bull 1996;17:54–64. [Google Scholar]
- 49.Lieberman HM, Hunt IF, Coulson AH, Clark VA, Swendseid ME, Ho L. Evaluation of a ghetto school breakfast program. J Am Diet Assoc 1976;68:132–8. [PubMed] [Google Scholar]
- 50.Moore GF, Murphy S, Chaplin K, Lyons RA, Atkinson M, Moore L. Impacts of the Primary School Free Breakfast Initiative on socio-economic inequalities in breakfast consumption among 9–11-year-old schoolchildren in Wales. Public Health Nutr 2014;17:1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy S, Moore GF, Tapper K, Lynch R, Clarke R, Raisanen L, Desousa C, Moore L. Free healthy breakfasts in primary schools: a cluster randomized controlled trial of a policy intervention in Wales, UK. Public Health Nutr 2011;14:219–26. [DOI] [PubMed] [Google Scholar]
- 52.Nkhoma OWW, Duffy ME, Cory-Slechta DA, Davidson PW, McSorley EM, Strain JJ, O’Brien GM. Early-stage primary school children attending a school in the Malawian School Feeding Program (SFP) have better reversal learning and lean muscle mass growth than those attending a non-SFP school. J Nutr 2013;143:1324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahmani K, Djazayery A, Habibi MI, Heidari H, Dorosti-Motlagh AR, Pourshahriari M, Azadbakht L. Effects of daily milk supplementation on improving the physical and mental function as well as school performance among children: results from a school feeding program. J Res Med Sci 2011;16:469–76. [PMC free article] [PubMed] [Google Scholar]
- 54.Richter LM, Rose C, Griesel RD. Cognitive and behavioral effects of a school breakfast. S Afr Med J 1997;87 Suppl:93–100. [PubMed] [Google Scholar]
- 55.Shemilt I, Harvey I, Shepstone L, Swift L, Reading R, Mugford M, Belderson P, Norris N, Thoburn J, Robinson J. A national evaluation of school breakfast clubs: Evidence from a cluster randomized controlled trial and an observational analysis. Child Care Health Dev 2004;30:413–27. [DOI] [PubMed] [Google Scholar]
- 56.Worobey J, Worobey HS. The impact of a two-year school breakfast program for preschool-aged children on their nutrient intake and pre-academic performance. Child Study J 1999;29:113–31. [Google Scholar]
- 57.Academy of Nutrition and Dietetics. Evidence analysis manual: Steps in the Academy evidence analysis process. Chicago: Academy of Nutrition and Dietetics, 2012. [Google Scholar]
- 58.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5–56. [DOI] [PubMed] [Google Scholar]
- 59.de Jager CA, Dye L, de Bruin EA, Butler L, Fletcher J, Lamport DJ, Latulippe ME, Spencer JPE, Wesnes K. Criteria for validation and selection of cognitive tests for investigating the effects of foods and nutrients. Nutr Rev 2014;72:162–79. [DOI] [PubMed] [Google Scholar]
- 60.Lesser LI, Ebbeling CB, Goozner M, Wypij D, Ludwig DS. Relationship between funding source and conclusion among nutrition-related scientific articles. PLoS Med 2007;4:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lloyd HM, Green MW, Rogers PJ. Mood and cognitive performance effects of isocaloric lunches differing in fat and carbohydrate content. Physiol Behav 1994;56:51–7. [DOI] [PubMed] [Google Scholar]
- 62.Messier C. Glucose improvement of memory: A review. Eur J Pharmacol 2004;490:33–57. [DOI] [PubMed] [Google Scholar]
- 63.Gibson EL. Carbohydrates and mental function: Feeding or impeding the brain? Nutr Bull 2007;32:71–83. [Google Scholar]
- 64.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. P Natl Acad of Sci 2000;97:2881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith MA, Riby LM, Eekelen JA, Foster JK. Glucose enhancement of human memory: A comprehensive research review of the glucose memory facilitation effect. Neurosci Biobehav Rev 2011;35:770–83. [DOI] [PubMed] [Google Scholar]
- 66.Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 2005;16:59–65. [DOI] [PubMed] [Google Scholar]
- 67.Shemesh E, Rudich A, Harman-Boehm I, Cukierman-Yaffe T. Effect of intranasal insulin on cognitive function: A systematic review. J Clin Endocrinol Metab 2012;97:366–76. [DOI] [PubMed] [Google Scholar]
- 68.Kirschbaum C, Gonzalez Bono E, Rohleder N, Gessner C, Pirke KM, Salvador A, Hellhammer DH. Effects of fasting and glucose load on free cortisol responses to stress and nicotine. J Clin Endocrinol Metab 1997;82:1101–5. [DOI] [PubMed] [Google Scholar]