Abstract
Previous research on animals indicates flavonoid compounds have immunomodulatory properties; however, human research remains inconclusive. The aim of this systematic review was to assess the efficacy of dietary flavonoids on upper respiratory tract infections (URTIs) and immune function in healthy adults. A created search strategy was run against Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE and EMBASE classic, CINAHL, and AMED. The returned studies were initially screened, and 2 reviewers independently assessed the remaining studies for eligibility against prespecified criteria. Fourteen studies, of 387 initially identified, were included in this review, and the primary outcome measure was the effect of flavonoids on URTI incidence, duration, and severity. Of the included studies, flavonoid supplementation ranged from 0.2 to 1.2 g/d. Overall, flavonoid supplementation decreased URTI incidence by 33% (95% CI: 31%, 36%) compared with control, with no apparent adverse effects. Sick-day count was decreased by 40% with flavonoid supplementation, although unclear. Differences in bio-immune markers (e.g., interleukin-6, tumor necrosis factor-α, interferon-γ, neutrophils) were trivial between the intervention and control groups during the intervention and after exercise when a postintervention exercise bout was included. These findings suggest that flavonoids are a viable supplement to decrease URTI incidence in an otherwise healthy population.
Keywords: flavonoids, quercetin, upper respiratory tract infection, common cold, systematic review, meta-analysis, immune function, exercise, polyphenols
Introduction
Upper respiratory tract infections (URTIs)5 include an array of acute illnesses that affect the upper respiratory system, including sinusitis, tonsillitis, pharyngitis, otitis media, laryngitis, and the “common cold” (1). URTIs are a common health problem in society; 23.1% of a population of US adults reported a URTI in a 4-wk period, which resulted in a loss of 5.9 h work/wk per URTI episode (2). In general, adults have 2 or 3 URTIs each year, whereas children can average up to 5 (3). URTI symptoms usually appear and peak 24–72 h after infection but can last up to 7–14 d (3–5). More than 200 viruses are reported to cause URTIs, which can be classified into 7 categories, each with different seasonal and viral rates: orthomyxoviruses, parainfluenza, coronaviruses, paramyxoviruses (respiratory syncytial virus; RSV), herpes virus, adenoviruses, and picornaviruses (6). Bacterial URTIs alone are rare with <10% of total causation; however, coinfection with viruses may occur (7, 8).
The current treatment options for the common cold are typically symptom related, but currently there is no effective cure. The first-line treatment for the common cold is rest, fluids, maintenance of hydration status, and prevention of viral/bacterial spread (3). Antibiotics are ineffective to treat viral infections, but analgesics and antipyretics can be prescribed and purchased over the counter to relieve symptoms such as pain and/or fever (3).
URTIs are also a common health problem in athletes, as reported in several studies (9–12). Engebretsen et al. (9, 10) completed 2 retrospective studies on the 2010 Winter and 2012 Summer Olympics. In both studies, Engebretsen et al. (9, 10) found that 6.7% and 7.1%, respectively, of athletes reported an illness that was mostly respiratory related. This trend is also seen when people are training constantly. Nieman et al. (13) showed that 40% of the study participants had ≥1 URTI episodes in a 2-mo training period before the Los Angeles marathon. In New Zealand, a study reported >50% of a professional Super 15 Rugby team experienced URTI symptoms during a 4-wk training period (14). Consequently, researchers are looking for new and novel ways to decrease URTI incidence, one of which is flavonoids.
Flavonoids are “the most common group of polyphenolic compounds in the human diet and are found ubiquitously in plants” (15). Flavonoids are found in many different species of plants and can be subdivided into flavonols, flavones, flavanols, flavanones, anthocyanidins, proanthocyanidins, and isoflavones, each with their own individual compounds (16, 17). The widespread distribution of flavonoids and their tolerability mean that humans ingest appreciable quantities in their diet.
Flavonoids are proposed to reduce the incidence of URTIs because they have a range of physiologic effects in humans, including antiviral, anti-inflammatory, cytotoxic, antimicrobial, and antioxidant (18). Studies report flavonoids have both an antiproliferative and antireplicative effect on 2 common viral sources of URTIs and reduce inflammation by decreasing NF-κB (19–22). These mechanisms, and others, may have the potential to decrease URTI incidence, which makes flavonoids a current field of interest in human immunity (23–26).
Herbal and “natural” products are a growing industry in today’s society because they reportedly help with numerous diseases and ailments (27). The purpose of this current systematic review is to investigate the efficacy of all food-related and supplementary forms of flavonoids on URTIs and immune function. Because athletes are reported to have an increased URTI risk, the effect of flavonoids on immune function after exercise is also investigated. In addition, analyzing the adverse effects assesses the applicability of a flavonoid supplement to society.
Methods
Search strategy and selection criteria.
A search of all current literature that investigated the effect of flavonoids on URTIs was conducted by 2 reviewers (VSS and AJB) independently with the use of a created search strategy. The search was optimized for each database by using Medical Subject Headings, Boolean operators, and the Cochrane sensitive-maximizing randomized controlled trial (RCT) filter, when appropriate. The search strategy was run against Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE and EMBASE classic, CINAHL, and AMED. The search strategy consisted of the following 3 main concepts: flavonoids, immune biomarkers, and URTIs. The meta-register of controlled trials, clinicaltrials.gov, the WHO International Clinical Trials Registry Platform, the System for Information on Gray Literature in Europe, and Google Scholar were also searched with the terms “Flavonoid and Immune.”
Once all studies were identified, 2 reviewers (VSS and AJB) screened the titles and abstracts for potential inclusion and also deleted duplicates. Subsequently, 78 full-text articles remained, and their reference lists were reviewed for additional studies, but no further studies were identified. The 78 studies were evaluated against the inclusion and exclusion criteria independently by the 2 reviewers.
The inclusion criteria included the following:
Single- or double-blind RCT
Human participants aged between 18 and 65 y
Flavonoid intervention for ≥4 d
URTI incidence diagnosed by a medical professional or predetermined “illness log” as defined by the individual studies
Primary outcomes included incidence of URTIs and their respective duration and severity (days missed from work/school). Secondary outcomes included adverse effects and measures of immune status with the use of the following biomarkers: IL-6, IL-8, IL-10, IL-1 receptor antagonist (IL-1ra), TNF-α, IFN-γ, natural killer (NK) cell concentrations, neutrophil concentrations, and both CD4+ and CD8+ T-lymphocyte counts, and immune function after exercise (intervention compared with control).
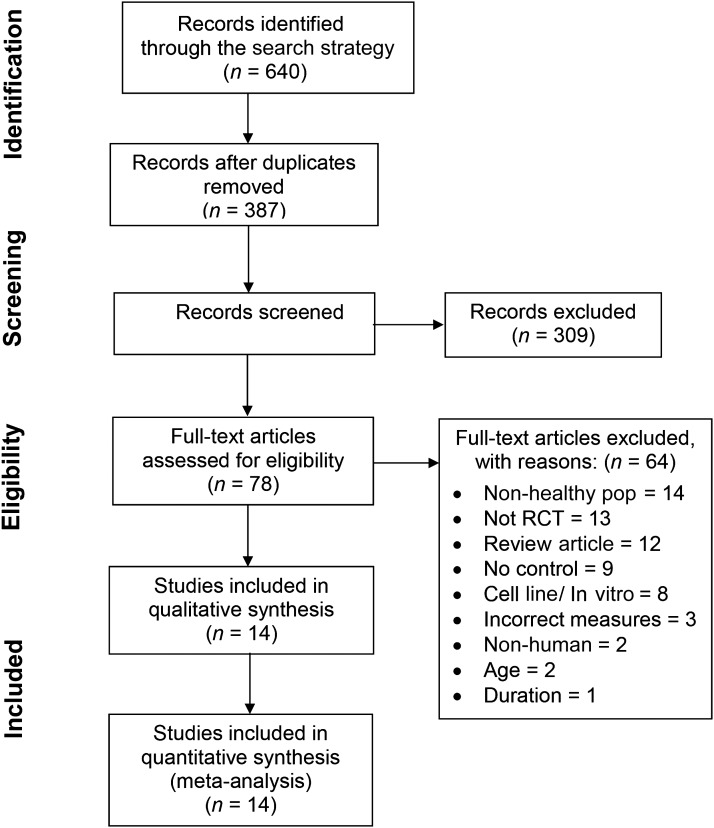
The exclusion criteria were participants who smoked or had a history of, or current, cardiovascular/pulmonary/endocrinologic disease, malignancy, obesity, and/or immune defects or allergies (i.e., allergic rhinitis). Two authors (VSS and AJB) then came to a consensus as to which studies would be included. After this process, 14 studies remained for inclusion in the systematic review (Figure 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. Pop, population; RCT, randomized controlled trial.
Data collection and assessment of bias.
The review of abstracts and full texts retrieved followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (28). Collected data included characteristics of participants and study, intervention type and dose, study description/overview/setting, study recruitment, risk of bias, and outcomes. Unsuccessful attempts were made to contact 2 investigators for additional statistics (29, 30).
With the use of the following 6 categories, 2 reviewers also completed the assessment of the risk of bias for each study independently:
Sequence generation
Allocation concealment
Blinding
Missing outcome data
Risk of reporting bias
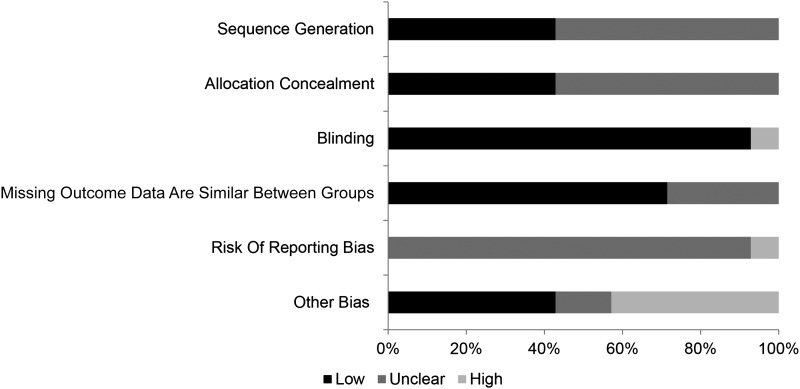
Other sources of bias according to the Cochrane Handbook (31) (Figure 2)
FIGURE 2.
Risk of bias summary of the included studies in the systematic review.
Data synthesis.
For inclusion of a study estimate in the meta-analysis, investigators had to provide an SE, an exact P value, or other information that would allow calculation of the SE. URTI incidence and count of sick days were the only outcome measures reported with sufficient data for meta-analysis.
URTI incidence was adjusted for intervention duration by calculating the hazard (incidence per unit of time). The hazard in each group was assumed to be constant and given by hazard = −ln(1 − incidence/n)/t, where t = duration of study. The HR was calculated by dividing the intervention hazard by the control hazard. The effect of the intervention on the count of sick days was also expressed as a ratio for meta-analysis. The ratio of sick days was calculated by dividing the intervention ratio (count of sick days/n) by the control ratio.
Data analysis.
Meta-analyses were performed with the mixed model (Proc Mixed) in the Statistical Analysis System (version 9.4; SAS Institute) by using the method of holding residual variance to zero (32) or, when convergence failed, by using the method of holding variances of study estimates to their observed values (33).
The assumption was made that the P value provided by the investigators for their outcome could be applied to the ln of the HR and that the sampling distribution of the ratio was normally distributed. These assumptions allowed estimation of the SE of each estimate for the meta-analysis. The meta-analysis of count of sick days was performed with the ln of the ratio. The SE for the log of the ratio of sick days per participant in the intervention and control groups was derived by assuming the count of sick days had a Poisson distribution. Study estimates were weighted by the inverse of the squares of their SEs. Meta-analyzed outcomes were back transformed to HR or count ratios. All analyses included a random effect for true between-study variance, allowing for negative variance in SAS. We back transformed the square root of the variance and its CIs to create a real (free of within-study variation) between-study SD.
The remaining outcomes with insufficient data for meta-analysis are displayed in a log scatter plot (bio-immune markers) or described (count of symptoms, days missed, and adverse effects). For change in bio-immune markers over time, the ratio (day measure/baseline measure) of intervention and control were both plotted against time. For change in bio-immune markers before compared with after exercise, the ratios (postexercise measure/pre-exercise measure) of intervention and control were both plotted in bio-immune marker columns.
Uncertainty of the effect estimates are reported as 95% CIs. In the analysis, magnitude-based inferences were made about the true value of the effect (34). In the interpretation of the HR, the outcome was deemed unclear if there was a possibility of benefit (P > 0.25) but unacceptable risk of harm (P > 0.005). If the result was deemed clear, thresholds were used to interpret the magnitude of the effect. The factor thresholds used for HR were 0.9, 0.7, and 0.5 for small, moderate, and large reductions, respectively, and inverse for increases (34). To interpret the magnitude of the bio-immune marker data, the baseline variability of the bio-immune marker was calculated as a ×/÷ factor SD. The factor SD was calculated by expressing each study SD as a factor [(SD + mean)/mean], log transforming, squaring, calculating the mean weighted by the degrees of freedom for the SD, then back transforming the outcome (Equation 1). To assess the magnitude of change, thresholds of 0.20, 0.60, and 1.20 of the log-transformed factor SD were used, which represent small, moderate, and large change, respectively (34).
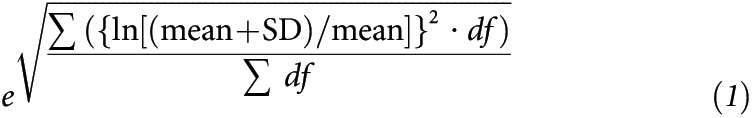 |
Results
Study selection
After individual screening of the 387 articles and deletion of articles that did not fit the research according to title/abstract, then mutual consensus decision by the 2 reviewers of the remaining 78 articles, 14 studies were included for review. The selection process, including the number of studies at each stage and reasons for exclusion, are presented in Figure 1.
Study characteristics
Some of the extracted data from the studies are shown in Table 1. In summary, all of the studies were RCTs, 3 used a crossover design (38, 39, 47) and 11 used a parallel design (30, 35–37, 40–46). The studies were conducted in a range of countries, including 9 in the United States (36, 38–43, 45, 46), 3 in Germany (37, 44, 47), 1 in the United Kingdom (35), and 1 in Brazil (30). A total of 4 studies used individual flavonoid supplements, either anthocyanidins (35) or quercetin (36, 42, 43), whereas the remaining used a combination of flavonoids (30, 37–41, 44–47). In 5 trials, the specific flavonoid and/or amount were not reported (30, 37, 40, 44, 45). Of the remaining 9 trials that mentioned the dose, 5 used an intervention dose > 350 mg/d (35, 36, 41–43), which is above the average daily intake of flavonoids (48–53). The total dose ranged from 0.2 to 1.2 g of flavonoid daily.
TABLE 1.
Summary of included studies on association between dietary flavonoids and URTIs and associated immune markers in healthy adults1
| Study (ref) | Participants | Study design | Intervention | Outcomes measured |
| Bell et al., 2014 (35) | 16 Well-trained male cyclists from the United Kingdom | Double-blind parallel RCT, 4-d supplement loading then exercise trial on days 5, 6, and 7 | 1.1 g anthocyanins/d for 7 d | Bio-immune markers (IL-6, IL-8, and TNF-α) |
| Henson et al., 2008 (36) | 39 Adults from the United States completing the WSER | Double-blind parallel RCT, with 21-d supplementation before the WSER | 1 g quercetin/d for 21 d | URTI (incidence and sick days) and bio-immune markers (NK cells and neutrophils) |
| Huber et al., 2011 (37) | 41 Adults from Germany | Double-blind parallel RCT | Iscucin Populi (0.0125%, 0.25%, 5%), and Viscum Mali e planta tota (1:1000, 1:100, 2%) for 28 d | Bio-immune markers (IL-6, TNF-α, NK cell, neutrophils, CD4+ and CD8+ cells) and adverse effects |
| Knab et al., 2014 (38) | 9 Elite male swimmers at SwimMAC Carolina | Single-blind crossover RCT | 230 mg flavonoids/d by juice for 10 d, 21-d washout | Bio-immune markers (IL-6 and IL-10) |
| Luna et al., 2011 (30) | 14 Adult men running the Sao Paulo marathon | Double-blind parallel RCT, 28-d supplementation before the marathon | 72 g/d of (unquantifiable) intervention for 28 d | Bio-immune markers (IL-6, IL-8, IL-10, and TNF-α) and adverse effects |
| McAnulty et al., 2004 (39) | 9 Moderately trained men of the Appalachian University area | Double-blind crossover RCT | 150 g blueberries/d = 142 mg anthocyanins,2 7-d washout | Bio-immune markers (IL-6, IL-8, IL-10, IL-1ra, and TNF-α) |
| Nantz et al., 2012 (40) | 112 Men and women from the University of Florida campus and Gainesville | Double-blind parallel RCT | 2.56 g aged-garlic extract/d for 90 d | URTI (incidence, sick days, days missed at work, and total symptoms), bio-immune markers (TNF-α and IFN-γ), and adverse effects |
| Nantz et al., 2013 (41) | 45 Men and women from the University of Florida campus and Gainesville | Double-blind parallel RCT | 292.5–346.5 mL proanthocyanidins/d | URTI (incidence, sick days, days missed at work or school, and total symptoms), bio-immune markers (TNF-α and IFN-γ), and adverse effects |
| 30.6–50.85 mL anthocyanins/d | ||||
| 30.6–45 mL flavonols/d for 70 d | ||||
| Nieman et al., 2007a (42) | 40 Trained male cyclists in North Carolina | Double-blind parallel RCT, supplementation 21 d before exercise, during 3-d period of intensified exercise, and 14 d after | 1 g quercetin/d for 38 d | URTI (incidence and sick days) and bio-immune markers (NK cells) |
| Nieman et al., 2007b (43) | 40 Trained male cyclists in North Carolina | Double-blind parallel RCT, supplementation 21 d before exercise, during 3-d period of exercise, and 14 d after | 1 g quercetin/d for 38 d | Bio-immune markers (IL-6, IL-8, IL-10, IL-1ra, and TNF-α) |
| Riede et al., 2013 (44) | 187 Men and woman from Germany | Double-blind parallel RCT | 4.5 g proprietary water-based extract from larch tree (RestAid, contains taxifolin and quercetin)/d for 84 d | URTI (incidence) and adverse effects |
| Rowe et al., 2007 (45) | 108 Men and women from University of Florida or the Florida community | Double-blind parallel RCT | Unknown quantity of l-theanine and epigallocatechin gallate for 84 d | URTI (incidence and sick days) and adverse effects |
| Ryan-Borchers et al., 2006 (46) | 52 Postmenopausal women from the United States | Double-blind parallel RCT | Soymilk + control = 30.9 ± 1.5 mg diadzein/d, 37.4 ± 1.3 mg genistein/d, and 3.6 ± 0.5 mg glycitein/d = 71.6 ± 3.1 mg/d for 112 dCow's milk + isoflavones = 30 mg diadzein/d, 33 mg genistein/d, and 7 mg glycitein/d = 70 mg/d for 112 d | Bio-immune markers (TNF-α, IFN-γ, and NK cells) |
| Schwarz et al., 2002 (47) | 15 Men from the United States | Double-blind crossover RCT | Echinacea purpurea herb harvested without roots and containing 22% (vol:vol) ethanol for 14 d, washout 28 d | Bio-immune markers (TNF-α) and adverse effects |
IL-1ra, IL-1 receptor antagonist; NK, natural killer; RCT, randomized controlled trial; ref, reference; URTI, upper respiratory tract infection; WSER, Western States Endurance Run.
Flavonoid content estimated from the USDA Flavonoid database.
The 14 studies can be subdivided into 3 durations. Bell et al. (35) and McAnulty et al. (39) had an intervention duration of 1 wk; 6 studies had a duration of 1–4 wk (30, 36, 38, 42, 43, 47), and 6 studies had an intervention duration of 10–13 wk (37, 40, 41, 44–46).
The studies varied in their reported outcomes, although they all measured ≥1 of the predetermined primary or secondary outcomes, including 7 studies that reported adverse effects (30, 37, 40, 41, 44, 45, 47). Of the 14 studies included, 6 studies measured URTI incidence (36, 40–42, 44, 45); 4 measured sick days (36, 40, 42, 45); and only Nantz et al. (40, 41) measured days missed and URTI symptom count.
Bias
In summary, 6 studies reported adequate sequence of allocating participants to treatment (37, 40, 41, 44, 46, 47), whereas the remaining 8 did not state the method of allocation and therefore had unclear bias (30, 35, 36, 38, 39, 42, 43, 45) (Figure 2).
Similarly, 6 studies reported a satisfactory method of concealing allocation (35, 39–41, 45, 46), and the remaining 8 studies were categorized as unclear because the method was not reported by the investigators (30, 36–38, 42, 43, 45, 46).
All studies were double-blind RCTs except Knab et al. (38), which was a single-blind RCT. As a consequence, the 13 double-blind RCTs had a low blinding bias, and Knab et al. (38) had high blinding bias.
Most studies had zero withdrawals during the study period (30, 35, 37–41, 44, 46, 47); therefore, no outcome data were missing. Henson et al. (36) and Rowe et al. (45) provided differing allocation and follow-up numbers, but the reviewers were unable to confirm if the withdrawals were from the intervention or control group; consequently, these studies were allocated as unclear. Another 2 studies (42, 43) reported the total number of participants allocated rather than number of participants allocated to intervention and control, so it was not possible to derive dropout rates; subsequently, the studies were categorized as unclear.
Once the studies were included in the systematic review, a search for their associated protocol was conducted to assess the risk of reporting bias in each study. In 13 of the 14 studies, no protocol was found. These 13 studies were subsequently assigned as unclear bias. The protocol for Riede et al. (44) was found; however, one of the secondary outcomes mentioned in this protocol was not presented in the final study, so it was subsequently classified as high bias.
The main source of “other bias” resulted from funding. In 6 studies there was no concern about funding sources (30, 36, 40, 42, 43, 46). Dole Food Company, who had a vested interest, funded the study by Knab et al. (38). Although the investigators produced negative results, the study was still published. McAnulty et al. (39) was sponsored by the North American Blueberry Council; however, it is not a direct wholesaler of blueberries. Because it was not possible to estimate the impact funding had on these 2 studies, they were both allocated unclear. The remaining 6 studies were assigned high bias for various reasons; 4 studies had funding provided from the intervention supplement company (35, 37, 41, 45), Schwarz et al. (47) was funded by the investigators, and in Riede et al. (44), the funding company made the final decision about publication.
URTI incidence
Six RCTs reported URTI incidence, totaling 531 participants (36, 40–42, 44, 45). All participants were given either a flavonoid intervention (n = 266) or control (n = 265). The meta-analysis of URTI incidence displayed as an HR, in which the hazard is ≥1 URTI episode in the study duration, is shown in Table 2. A clear moderate reduction was seen in participants who experienced ≥1 URTI on flavonoid supplementation compared with control. The real between-study variance reported by SAS was negative and expressed as a factor was 0.83 (95% CI: 0.75, 1.11). No meta-regression that controlled for any of our subanalysis categories was completed, because data were insufficient.
TABLE 2.
Pooled study characteristics and meta-analysis (SAS) of studies that investigated URTI incidence, displayed as an HR, whereby the hazard is the occurrence of ≥1 URTI episode in the study duration1
| Intervention |
Control |
||||||
| Study (ref) | Incidence | n | Incidence | n | Duration, d | P-value | HR2 |
| Henson et al., 2008 (36) | 4 | 18 | 5 | 21 | 14 | 0.88 | 0.92 |
| Nantz et al., 2013 (41) | 15 | 22 | 20 | 23 | 70 | 0.62 | 0.56 |
| Nantz et al., 2012 (40) | 26 | 56 | 28 | 56 | 90 | 0.85 | 0.9 |
| Nieman et al., 2007a (42) | 1 | 20 | 9 | 20 | 14 | <0.01 | 0.09 |
| Riede et al., 2013 (44) | 58 | 97 | 67 | 90 | 84 | 0.03 | 0.67 |
| Rowe et al., 2007 (45) | 23 | 53 | 35 | 55 | 84 | 0.04 | 0.56 |
ref, reference; SAS, Statistical Analysis System; URTI, upper respiratory tract infection.
Overall effect: HR = 0.67 (95% CI: 0.64, 0.69).
URTI sick days
Four studies reported sick days as a result of URTIs with intervention (n = 147) or control (n = 152) over a cumulative period of 202 d (36, 40, 42, 45). The meta-analysis of the URTI sick days is shown in (Table 3). Flavonoid supplementation had an unclear reduction in URTI sick days (P = 0.16). The real difference between studies, expressed as a factor, was 1.63 (95% CI: 0.62, 2.32). Data were insufficient to perform a meta-regression.
TABLE 3.
Study characteristics and meta-analysis (SAS) of studies that investigated URTI sick-day count, displayed as a sick-day ratio (intervention sick-day count/control sick-day count), whereby the sick-day count is the number of sick-days divided by the number of participants1
| Intervention |
Control |
||||||
| Study (ref) | Incidence | n | Incidence | n | Duration, d | P-value | Sick-day ratio2 |
| Henson et al., 2008 (36) | 21 | 18 | 29 | 21 | 14 | 0.84 | 0.84 |
| Nantz et al., 2012 (40) | 317 | 56 | 358 | 56 | 90 | 0.13 | 0.89 |
| Nieman et al., 2007a (42) | 8 | 20 | 61 | 20 | 14 | <0.01 | 0.13 |
| Rowe et al., 2007 (45) | 360 | 53 | 559 | 55 | 84 | 0.02 | 0.67 |
ref, reference; SAS, Statistical Analysis System; URTI, upper respiratory tract infection.
Overall effect: sick-day ratio = 0.60 (95% CI: 0.24, 1.47).
URTI severity
Days missed.
Days missed from work/school give an estimate of URTI severity. Nantz et al. (40) reported a statistically significant reduction in work days missed in the flavonoid supplementation cohort compared with the control cohort (P = 0.035). Nantz et al. (41) described a similar reduction in work days missed; however, the result was not significant (P = 0.33).
Symptoms.
The severity of URTIs can also be represented by the number of URTI symptoms of that episode. Only Nantz et al. (40, 41) investigated the number of symptoms due to URTI episodes. Both studies reported statistically significant reductions in URTI symptoms with flavonoid intervention compared with control intervention (P < 0.001 and P = 0.031, respectively).
Effects of intervention on secondary outcomes
Bio-immune markers.
The bio-immune markers were unable to be meta-analyzed and are therefore displayed in log scatter plots.
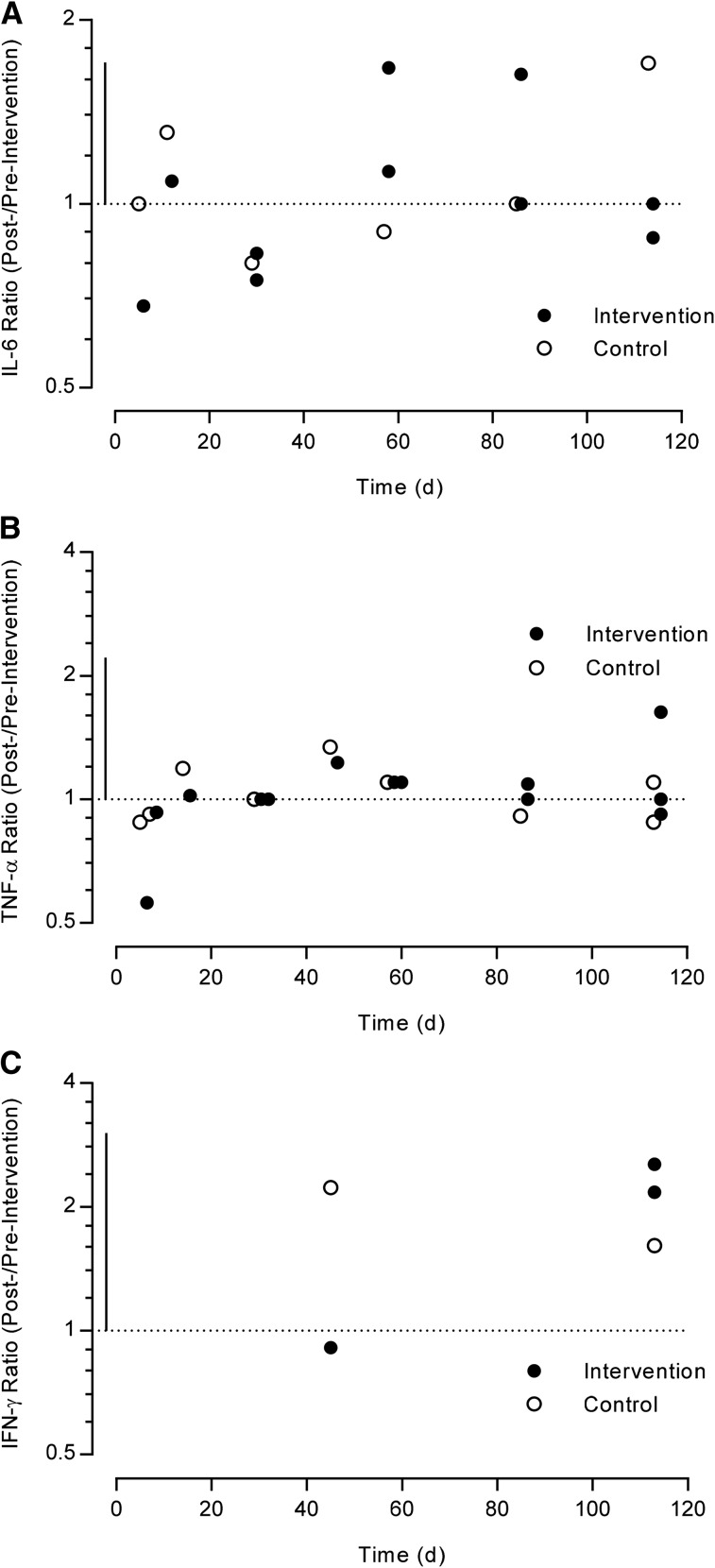
The intervention group reported small-to-moderate changes in IL-6 and IFN-γ and trivial-to-small changes in TNF-α (Figure 3). The magnitudes of the change in the intervention group, however, were similar in size to the control group. Data on IL-8 and IL-10 are not presented because they are only reported by Bell et al. (35) and Knab et al. (38), respectively. No studies reported IL-1ra measures through the trial duration.
FIGURE 3.
Log scale scatter plot of the studies that investigated cytokine concentration during their intervention period compared with baseline, expressed as a ratio (after:before intervention). The horizontal dotted line represents no change compared with baseline, and the vertical solid line represents 1-factor SD. (A) IL-6, (B) TNF-α, and (C) IFN-γ ratios (after:before intervention or control) in healthy adults at different time points.
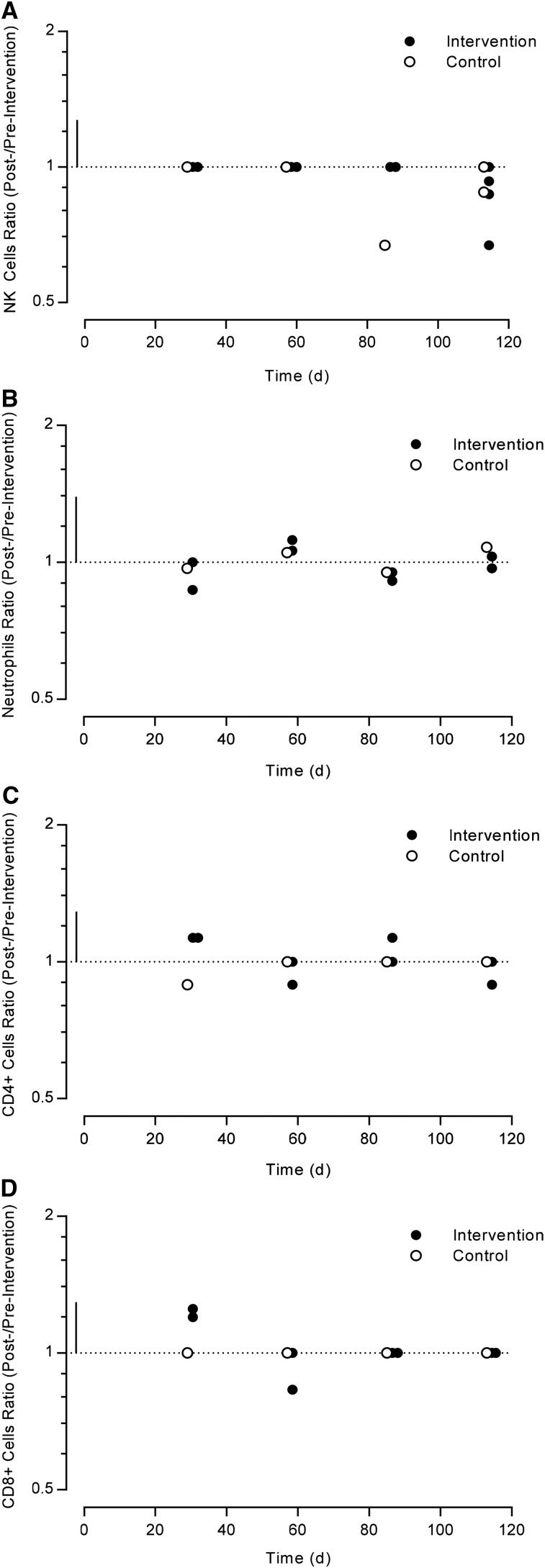
All cell concentrations (NK, neutrophil, CD4+, and CD8+) similarly had a range of small, moderate, and large changes with flavonoid intervention over time (Figure 4). The intervention changes were, again, of similar magnitude to the control changes.
FIGURE 4.
Log scale scatter plot of the studies that investigated cell concentration during their intervention period compared with baseline, expressed as a ratio (after:before intervention or control). The horizontal dotted line represents no change compared with baseline, and the vertical solid line represents 1-factor SD. (A) NK cell, (B) neutrophil, (C) CD4+, and (D) CD8+ ratios in healthy adults at different time points. NK, natural killer.
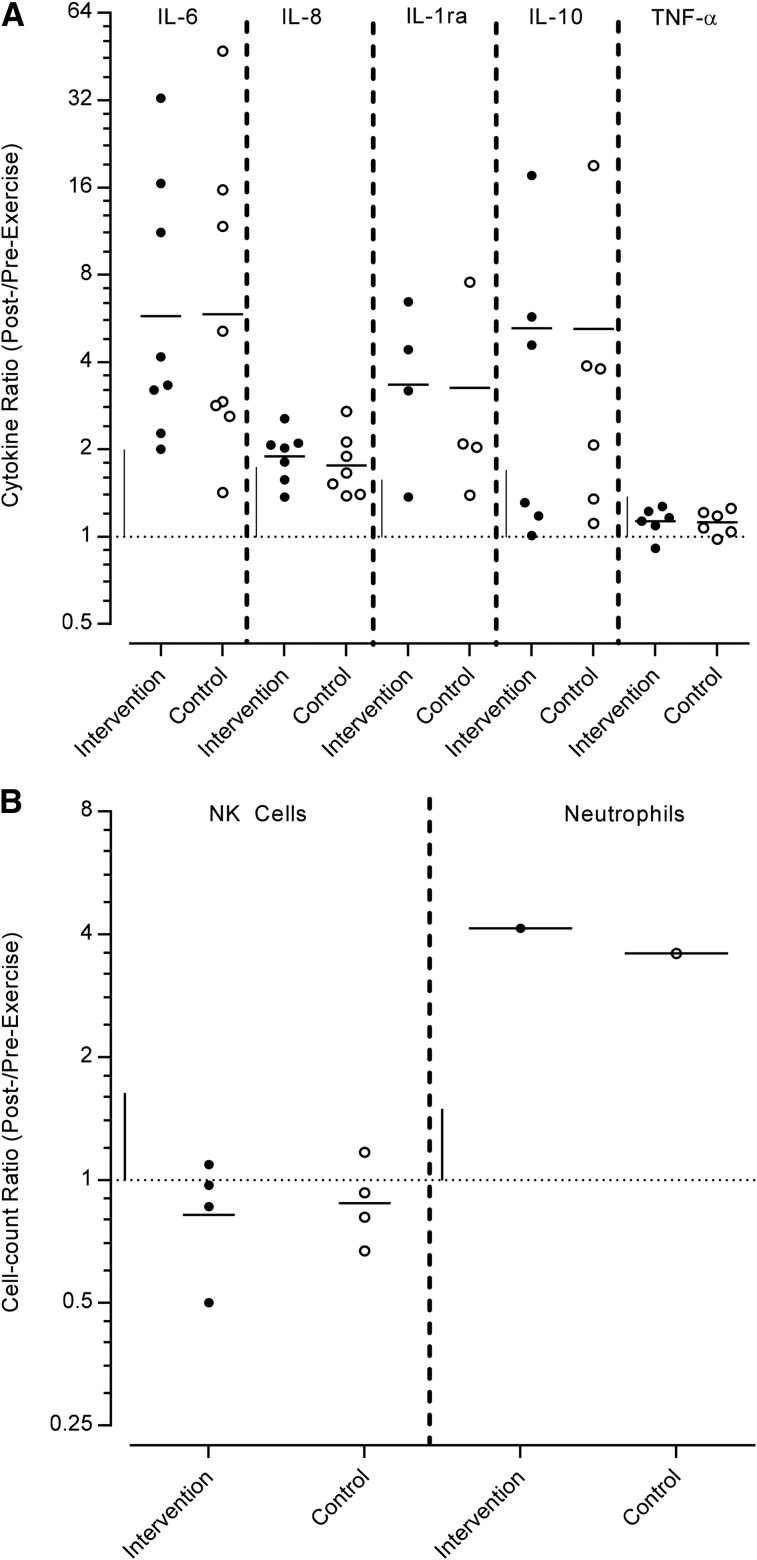
All cell and cytokine measures were raised after exercise, except NK cells, which decreased (Figure 5). The magnitudes of the change in the geometric means of the intervention measures were varied with extremely large (IL-6, IL-10, IL-1ra, and neutrophils), moderate (IL-8), and small (TNF-α and NK cell) changes. Although, according to the nonexercise data, the magnitude change in the intervention group was similar in the control group. No studies reported data before compared with after exercise for either CD4+ or CD8+ cells.
FIGURE 5.
Log scale scatter plot of the studies that investigated cytokine and cell concentrations before and after exercise, after a specified intervention duration, expressed as an after:before exercise ratio. The horizontal dotted line represents no change compared with baseline. The horizontal solid line represents the geometric mean, and the vertical solid line represents 1-factor SD. (A) Cytokine and (B) cell ratios of measures, defined by each study in a range of healthy trained or exercising adults. NK, natural killer.
Adverse effects.
Seven studies reported specific numbers of, or commented on, adverse effects (30, 37, 40, 41, 44, 45, 47). Four studies reported no adverse effects (30, 40, 41, 47), 2 reported equal numbers of adverse effects (44, 45), and one arm of the Huber et al. (37) study reported 18 side effects in the intervention group compared with 7 in the control group. The Huber et al. (37) arm that reported 18 adverse effects was an anticancer treatment and consequently contained cytotoxic lectins.
Discussion
To our knowledge, this is the first meta-analysis and systematic review conducted to examine the effect of flavonoids on URTIs and associated bio-immune markers in humans. The primary outcomes of this study are that flavonoids decrease URTI incidence by 33% compared with control with no increase in adverse effects and that a nonsignificant decrease was found in URTI severity and duration with the flavonoid intervention. Furthermore, trivial differences were found in the secondary outcomes (bio-immune markers throughout the duration of the intervention and before/after exercise) for participants in the intervention group compared with the control group. In addition, Nantz et al. (40, 41), the only studies to measure symptoms and severity of URTIs, reported statistically significant reductions in URTI symptoms with the flavonoid intervention and also reported a statistically significant reduction in days missed due to URTIs (40).
Six RCTs (n = 531) were identified that investigated the effect of flavonoids on URTI incidence. Participants taking the flavonoid intervention had a decreased URTI incidence compared with the control intervention, which is in agreement with theoretical evidence. Several secondary outcomes in this systematic review that could potentially explain the reduction of URTI incidence were also investigated. Previous research has suggested that flavonoids have an antiviral, anti-inflammatory, cytotoxic, antimicrobial, and antioxidant capacity to benefit the immune system (18). The current meta-analysis demonstrated only trivial differences between flavonoids and control for all immune biomarkers which suggests flavonoids do not attenuate URTI incidence by altering cytokine and immune cells in a beneficial way to the host. Because URTIs are primarily of viral origin, antimicrobial action will be of no potential benefit. From the data collected in this systematic review, it leads the researchers to propose that flavonoids decrease URTI incidence and symptoms by an antiviral mechanism. Currently, both observational and experimental data support flavonoids having antiviral activity (41). Kaul et al. (19) showed that particular flavonoids have different effects on viruses; quercetin and hesperetin have an antireplicative effect on RSV and parainfluenza virus type 3, whereas catechin and quercetin have an anti-infective effect on RSV. Quercetin was observed to have a dose-response effect, whereas naringin had no effect on the viruses (19).
When analyzing the immune function after exercise, all cytokines and cells increased or decreased with similar magnitude with or without flavonoid intervention. This suggests that flavonoids have no effect in moderating the change in bio-immune markers after exercise. Nieman et al. (54) reported that the “open window,” during which athletes had increased susceptibility, was 3–72 h after exercise. Given that all these studies reported measures immediately after exercise and not up to 72 h after exercise, this review is unable to deduce if flavonoids have an effect on the bio-immune markers in the open window.
Application
Further research is needed to quantify the optimal dose of flavonoids. Two clear moderators of the effect of flavonoids on URTI incidence are the dose and type, which also contribute to the practical application of a flavonoid intervention. We were only able to quantify the dose and/or type of flavonoid intervention in 3 trials included in this meta-analysis (36, 41, 42). As a result, data were insufficient to perform a subgroup analysis. This information is meaningful because it describes the optimal prescription of flavonoid intake to achieve the decrease in URTI incidence. Of the 3 studies that did provide the quantity of flavonoid, the intervention dose was greater than the average daily intake. It is therefore plausible that average daily intake of flavonoids is insufficient to reduce URTI incidence and that an increased intake is needed. Intake of flavonoids (∼1000 mg) can be achieved by diet through simple practices such as consuming green tea (250 mL), a glass of Shiraz (250 mL), blueberries (100 g), or some dark chocolate (100 g), which are all products high in flavonoid content (55). Similarly, the increased flavonoid intake could also be provided by a flavonoid supplement. The method of flavonoid increase will depend on which is of greater ease and accessibility to the general public.
This meta-analysis has quantified variation, and, as a result, a researcher can expect this magnitude of variation in the mean effect, irrespective of setting. This variance can be attributed to a few mechanisms, such as inadequate reporting of error and measures, unknown cofactors that also influence the incidence of URTIs and symptoms, and a meta-analytic model that fails to incorporate the full nature of reality, including nonlinear effects and interactions. In URTI incidence, the between-study variation interval means that researchers or practitioners are likely to see a benefit of flavonoid supplementation despite their differing settings. Conversely, the large interval of sick days suggests that researchers may not see a reduction in URTI sick days in their respective setting.
Finally, no increases in adverse effects were reported in the studies, except in 1 arm of the Huber et al. (37) study in which the intervention contained a cytotoxic lectin component. This is important when delivering a food-based health claim that could be used by the public. In this systematic review no notable increase was found in adverse effects; therefore, members of society could take flavonoid supplements with reasonable confidence that the flavonoid component will not result in increased adverse events.
Conclusion
The research presented in this systematic review demonstrates the essential role of flavonoids in the function of the respiratory immune system. To our knowledge, this is the first systematic review and meta-analysis to assess the effects of flavonoids on URTI incidence, severity and duration, bio-immune markers, and adverse effects. The findings demonstrate that flavonoids decrease URTI incidence compared with control with no increase in adverse effects. Although flavonoid intervention decreases URTI incidence, no conclusive evidence proves that flavonoids decrease duration or severity. We also identified that bio-immune marker measures have a similar magnitude of change over the intervention duration and after exercise in the intervention and control groups.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: IL-1ra, IL-1 receptor antagonist; NK, natural killer; RCT, randomized controlled trial; RSV, respiratory syncytial virus; URTI, upper respiratory tract infection.
References
- 1.Eccles MP, Grimshaw JM, Johnston M, Steen N, Pitts NB, Thomas R, Glidewell E, Maclennan G, Bonetti D, Walker A. Applying psychological theories to evidence-based clinical practice: identifying factors predictive of managing upper respiratory tract infections without antibiotics. Implement Sci 2007;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bramley TJ, Lerner D, Sarnes M. Productivity losses related to the common cold. J Occup Environ Med 2002;44:822–9. [DOI] [PubMed] [Google Scholar]
- 3.BMJ Best Practice [Internet]. Common cold. [cited 2015 Apr 15]. Available from: http://bestpractice.bmj.com/best-practice/monograph/252/basics/epidemiology.html.
- 4.Fields KB, Fricker PA. Medical problems in athletes. Hoboken (NJ): Blackwell Science; 1997. [Google Scholar]
- 5.Albers R, Antoine J, Bourdet-Sicard R, Calder PC, Gleeson M, Lesourd B, Samartín S, Sanderson IR, Van Loo J, Vas Dias FW. Markers to measure immunomodulation in human nutrition intervention studies. Br J Nutr 2005;94:452–81. [DOI] [PubMed] [Google Scholar]
- 6.Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol 2002;122:183–91. [DOI] [PubMed] [Google Scholar]
- 7.Mäkelä MJ, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, Kimpimäki M, Blomqvist S, Hyypiä T, Arstila P. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol 1998;36:539–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non–influenza-related viral respiratory tract infection in the United States. Arch Intern Med 2003;163:487–94. [DOI] [PubMed] [Google Scholar]
- 9.Engebretsen L, Soligard T, Steffen K, Alonso JM, Aubry M, Budgett R, Dvorak J, Jegathesan M, Meeuwisse WH, Mountjoy M, et al. Sports injuries and illnesses during the London Summer Olympic Games 2012. Br J Sports Med 2013;47:407–14. [DOI] [PubMed] [Google Scholar]
- 10.Engebretsen L, Steffen K, Alonso JM, Aubry M, Dvorak J, Junge A, Meeuwisse W, Mountjoy M, Renstrom P, Wilkinson M. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med 2010;44:772–80. [DOI] [PubMed] [Google Scholar]
- 11.Peters EM. Exercise, immunology and upper respiratory tract infections. Int J Sports Med 1997;18(Suppl 1):S69–77. [DOI] [PubMed] [Google Scholar]
- 12.Gleeson M, Walsh NP. The BASES expert statement on exercise, immunity, and infection. J Sports Sci 2012;30:321–4. [DOI] [PubMed] [Google Scholar]
- 13.Nieman DC, Johanssen LM, Lee JW, Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness 1990;30:316–28. [PubMed] [Google Scholar]
- 14.Haywood BA, Black KE, Baker D, McGarvey J, Healey P, Brown RC. Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. J Sci Med Sport 2014;17:356–60. [DOI] [PubMed] [Google Scholar]
- 15.Spencer JP. Flavonoids: modulators of brain function? Br J Nutr 2008;99(E Suppl 1):ES60–77–. [DOI] [PubMed] [Google Scholar]
- 16.Zamora-Ros R, Knaze V, Luján-Barroso L, Romieu I, Scalbert A, Slimani N, Hjartåker A, Engeset D, Skeie G, Overvad K. Differences in dietary intakes, food sources and determinants of total flavonoids between Mediterranean and non-Mediterranean countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr 2013;109:1498–507. [DOI] [PubMed] [Google Scholar]
- 17.Rathee P, Chaudhary H, Rathee S, Rathee D, Kumar V, Kohli K. Mechanism of action of flavonoids as anti-inflammatory agents: a review. Inflamm Allergy Drug Targets 2009;8:229–35. [DOI] [PubMed] [Google Scholar]
- 18.González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Fruit polyphenols, immunity and inflammation. Br J Nutr 2010;104(Suppl 3):S15–27. [DOI] [PubMed] [Google Scholar]
- 19.Kaul TN, Middleton E, Ogra PL. Antiviral effect of flavonoids on human viruses. J Med Virol 1985;15:71–9. [DOI] [PubMed] [Google Scholar]
- 20.Béládi I, Pusztai R, Mucsi I, Bakay M, Gábor M. Activity of some flavonoids against viruses. Ann N Y Acad Sci 1977;284:358–64. [DOI] [PubMed] [Google Scholar]
- 21.Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm 2007;2007:45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peluso I, Miglio C, Morabito G, Ioannone F, Serafini M. Flavonoids and immune function in human: a systematic review. Crit Rev Food Sci Nutr 2015;55:383–95. [DOI] [PubMed] [Google Scholar]
- 23.Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol 1995;33:1061–80. [DOI] [PubMed] [Google Scholar]
- 24.Hackett AM. The metabolism of flavonoid compounds in mammals. Prog Clin Biol Res 1986;213:177–94. [PubMed] [Google Scholar]
- 25.Kim SY, Chang YJ, Cho HM, Hwang YW, Moon YS. Non-steroidal anti-inflammatory drugs for the common cold. Cochrane Database Syst Rev 2013;CD006362. [DOI] [PubMed] [Google Scholar]
- 26.Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2013;CD000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartram T. Bartram’s encyclopedia of herbal medicine. London: Constable & Robinson; 2013. [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- 29.Knab AM, Nieman DC, Gillitt ND, Shanely RA, Cialdella-Kam L, Henson DA, Sha W. Effects of a flavonoid-rich juice on inflammation, oxidative stress, and immunity in elite swimmers: a metabolomics-based approach. Int J Sport Nutr Exerc Metab 2013;23:150–60. [DOI] [PubMed] [Google Scholar]
- 30.Luna LA Jr, Bachi AL, Novaes e Brito RR, Eid RG, Suguri VM, Oliveira PW, Gregorio LC, Vaisberg M. Immune responses induced by Pelargonium sidoides extract in serum and nasal mucosa of athletes after exhaustive exercise: modulation of secretory IgA, IL-6 and IL-15. Phytomedicine 2011;18:303–8. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. [cited 2015 May 15]. Available from: http://www.cochrane-handbook.org. The Cochrane Collaboration.
- 32.Yang M. A review of random effects modelling in SAS (release 8.2). Retrieved September. [cited 2015 Aug 10]. Available from http://www.bristol.ac.uk/media-library/sites/cmm/migrated/documents/rev-sas.pdf. 2003;5:2003. [Google Scholar]
- 33.Sheu CF, Suzuki S. Meta-analysis using linear mixed models. Behav Res Methods Instrum Comput 2001;33.2:102–7. [DOI] [PubMed] [Google Scholar]
- 34.Hopkins W. A New View on Statistics [Internet]. [cited 2015 Aug 1]. Available from: http://www.sportsci.org/resource/stats/. [Google Scholar]
- 35.Bell PG, Walshe IH, Davison GW, Stevenson E, Howatson G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014;6:829–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henson D, Nieman D, Davis J, Dumke C, Gross S, Murphy A, Carmichael M, Jenkins D, Quindry J, McAnulty S, et al. Post-160-km race illness rates and decreases in granulocyte respiratory burst and salivary IgA output are not countered by quercetin ingestion. Int J Sports Med 2008;29:856–63. [DOI] [PubMed] [Google Scholar]
- 37.Huber R, Lüdtke H, Wieber J, Beckmann C. Safety and effects of two mistletoe preparations on production of interleukin-6 and other immune parameters - a placebo controlled clinical trial in healthy subjects. BMC complementary and alternative medicine 2011;11.1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knab AM, Nieman DC, Gillitt ND, Shanely RA, Cialdella-Kam L, Henson D, Sha W, Meaney MP. Effects of a freeze-dried juice blend powder on exercise-induced inflammation, oxidative stress, and immune function in cyclists. Appl Physiol Nutr Metab 2014;39:381–5. [DOI] [PubMed] [Google Scholar]
- 39.McAnulty SR, McAnulty LS, Nieman DC, Dumke CL, Morrow JD, Utter AC, Henson DA, Proulx WR, George GL. Consumption of blueberry polyphenols reduces exercise-induced oxidative stress compared to vitamin C. Nutr Res 2004;24:209–21. [Google Scholar]
- 40.Nantz MP, Rowe CA, Muller CE, Creasy RA, Stanilka JM, Percival SS. Supplementation with aged garlic extract improves both NK and gammadelta-T cell function and reduces the severity of cold and flu symptoms: a randomized, double-blind, placebo-controlled nutrition intervention. Clin Nutr 2012;31:337–44. [DOI] [PubMed] [Google Scholar]
- 41.Nantz MP, Rowe CA, Muller C, Creasy R, Colee J, Khoo C, Percival SS. Consumption of cranberry polyphenols enhances human gammadelta-T cell proliferation and reduces the number of symptoms associated with colds and influenza: a randomized, placebo-controlled intervention study. Nutr J 2013;12:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieman DC, Henson DA, Gross SJ, Jenkins DP, Davis JM, Murphy EA, Carmichael MD, Dumke CL, Utter AC, McAnulty SR, et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med Sci Sports Exerc 2007;39:1561–9. [DOI] [PubMed] [Google Scholar]
- 43.Nieman DC, Henson DA, Davis JM, Angela Murphy E, Jenkins DP, Gross SJ, Carmichael MD, Quindry JC, Dumke CL, Utter AC, et al. Quercetin's influence on exercise-induced changes in plasma cytokines and muscle and leukocyte cytokine mRNA. J Appl Physiol (1985) 2007;103:1728–35. [DOI] [PubMed] [Google Scholar]
- 44.Riede L, Grube B, Gruenwald J. Larch arabinogalactan effects on reducing incidence of upper respiratory infections. Curr Med Res Opin 2013;29:251–8. [DOI] [PubMed] [Google Scholar]
- 45.Rowe CA, Nantz MP, Bukowski JF, Percival SS. Specific formulation of Camellia sinensis prevents cold and flu symptoms and enhances gamma,delta T cell function: a randomized, double-blind, placebo-controlled study. J Am Coll Nutr 2007;26:445–52. [DOI] [PubMed] [Google Scholar]
- 46.Ryan-Borchers TA, Park JS, Chew BP, McGuire MK, Fournier LR, Beerman KA. Soy isoflavones modulate immune function in healthy postmenopausal women. Am J Clin Nutr 2006;83:1118–25. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz E, Metzler J, Diedrich JP, Freudenstein J, Bode C, Bode JC. Oral administration of freshly expressed juice of Echinacea purpurea herbs fail to stimulate the nonspecific immune response in healthy young men: results of a double-blind, placebo-controlled crossover study. J Immunother 2002;25:413–20. [DOI] [PubMed] [Google Scholar]
- 48.Bai W, Wang C, Ren C. Intakes of total and individual flavonoids by US adults. Int J Food Sci Nutr 2014;65:9–20. [DOI] [PubMed] [Google Scholar]
- 49.Birt DF, Jeffery E. Flavonoids. Adv Nutr 2013;4:576–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beking K, Vieira A. An assessment of dietary flavonoid intake in the UK and Ireland. Int J Food Sci Nutr 2011;62:17–9. [DOI] [PubMed] [Google Scholar]
- 51.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr 2007;137:1244–52. [DOI] [PubMed] [Google Scholar]
- 52.Zamora-Ros R, Andres-Lacueva C, Lamuela-Raventós RM, Berenguer T, Jakszyn P, Barricarte A, Ardanaz E, Amiano P, Dorronsoro M, Larrañaga N, et al. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain). J Am Diet Assoc 2010;110:390–8. [DOI] [PubMed] [Google Scholar]
- 53.Hardcastle AC, Aucott L, Reid DM, Macdonald HM. Associations between dietary flavonoid intakes and bone health in a Scottish population. J Bone Miner Res 2011;26:941–7. [DOI] [PubMed] [Google Scholar]
- 54.Nieman DC, Pedersen BK. Exercise and immune function. Recent developments. Sports Med 1999;27:73–80. [DOI] [PubMed] [Google Scholar]
- 55.US Department of Agriculture. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1 (December 2013). Beltsville (MD): Nutrient Data Laboratory Beltsville Human Nutrition Research Center Agricultural Research Service; December 2013, Slightly revised, May 2014. [Google Scholar]