Abstract
Bone and heart health are linked through a variety of cellular, endocrine, and metabolic mechanisms, including the bidirectional effects of mineral-regulating hormones parathyroid hormone and fibroblast growth factor 23. Nutrition plays an important role in the development of both cardiovascular and bone disease. This review describes current knowledge on the relations between the cardiovascular system and bone and the influence of key nutrients involved in mineral metabolism—calcium, vitamin D, and phosphorus—on heart and bone health, as well as the racial/ethnic differences in cardiovascular disease and osteoporosis and the influence that nutrition has on these disparities.
Keywords: calcium, phosphorus, bone health, cardiovascular diseases, vitamin D
Introduction
Cardiovascular disease (CVD)7 and osteoporosis are common age-related diseases that are influenced by nutrition. In particular, calcium, vitamin D, and phosphorus have become prominent as nutrients that play roles in both heart and bone health. This review gives an overview of current knowledge of the cardioskeletal relation and explores aspects of the influence of calcium, vitamin D, and phosphorus on heart and bone, as well as how race and ethnicity might modify the influence of nutrition in CVD and osteoporosis.
The Cardio-Skeletal Relation
Vascular calcification and arteriosclerosis.
Vascular calcification was once thought of as only a passive process of dead and dying cells but is now being recognized as a highly regulated form of matrix mineralization (1). Deposition and egress of vascular calcium-containing salts (phosphates, phospholipids, and carbonates) are controlled by the integrated actions of circulating mineralization inhibitors, paracrine cues driving osteo/chondrogenic cell potential and microvesicle release, and vascular remodeling processes that impact both matrix and mineral. The relation between vascular calcification and arteriosclerotic disease has been increasingly appreciated since the 1850s when Virchow (2) described the histopathologic picture of atherosclerotic disease observed at autopsy as a lipid-laden, intimally oriented, lumen-deforming inflammatory lesion with bone-like sclerotic changes. Although disagreeing with Virchow on a number of other issues, von Rokitansky also noted bone-like morphologic features in advanced atherosclerotic lesions, as discussed by Mayerl et al. (3). Mönckeberg (4) highlighted a different type of vascular mineralization almost 5 decades later: a calcific sclerosis of the arterial tunica media that did not impinge upon the vessel lumen. This medial artery calcification was noted to be more prevalent in diabetes, in chronic kidney disease (CKD), and with aging (5). However, the clinical connections between vascular calcification and arteriosclerosis—the hardening of the conduit arteries that impairs conduit vessel function even in the absence of atherothrombosis or lumen stenosis (5, 6)—has been appreciated more recently. The experimental evidence that diastolic blood flow was substantially impaired by aortic stiffness was conceptually articulated by Frank in 1899 but was not widely available until the 1960s [reviewed by Westerhof (7)]. This Windkessel physiology—the capacity of elastic conduit vessels to store some kinetic energy as potential energy during systole and then release it as kinetic energy to drive perfusion during diastole (7)—has profound implications. While increasing systolic blood pressure and myocardial workload, vascular stiffening that impairs the arterial Windkessel reduces diastolic coronary and distal tissue perfusion (7). This can be well appreciated in the central nervous system, where white matter hyperintensity lesion volume (8), a marker of ischemic flow and progressive cognitive decline (9), tracks aortic stiffness independent of other risk factors including systolic blood pressure (8). Arterial stiffness/compliance is a composite of material properties (smooth muscle myosin-actin engagement; extracellular matrix accumulation, integrity, and cross-linking; mineral deposition, etc.) and geometric properties (wall thickness, lumen diameter) (5). For a variety of reasons, the interrelations between vascular calcium accrual and arterial stiffness are likely to be bidirectional. Indeed, in preclinical disease models of diabetic arteriosclerosis, genetic manipulations that increase or decrease arterial calcium accrual do increase and decrease, respectively, arterial stiffness (10, 11). However, as first highlighted by Engler et al. (12), the mechanocrine environment experienced by multipotent mesenchymal progenitors capable of osteochondrogenic fates markedly influences cellular differentiation. Stiffer matrices suppress myogenic potential while simultaneously enhancing osteogenic and fibrogenic potential of human multipotent mesenchymal cells. Thus, the propensity of proliferating vascular mesenchymal cells to adopt an osteogenic fate and thereby mineralize may represent a feed-forward mechanism reflecting increasing stiffness in the inflamed arterial microenvironment (13). Chen and Simmons (13) have convincingly demonstrated this principle using valve interstitial cells. This relation may explain why tibial artery calcification scores outperform ankle-brachial indexes in predicting the progression of limb ischemia to severity requiring amputation (14); arterial calcium load not only contributes to arteriosclerotic stiffening but also functions as a “biomarker” reflecting the degree of regional tissue stiffness and impaired conduit vessel Windkessel function (15). It remains to be determined the extent to which the enhancement of arterial calcium egress will restore arterial compliance and normal distal tissue perfusion.
Cellular mechanisms of vascular mineralization.
As mentioned above, advanced atherosclerotic lesions have bone-like tissue characteristics. In more recent studies of valve and vascular calcification, ∼15% of advanced lesions exhibit woven bone formation with marrow elements (16). The cellular contributions to true ectopic ossification in advanced lesions, however, may differ substantially from the early lesions forming during the initiation of vascular mineral deposition. Circulation progenitors capable of ectopically recapitulating the entire hematopoietic niche functions of bone may be required (17, 18). Boström et al. (19) first highlighted the role of regional progenitors, the mural pericyte population, as an oxylipid- and cytokine-activated osteogenic cell capable of initiating arterial mineral deposition in the absence of true ectopic bone formation. As in bone, alkaline phosphatase-positive and annexin-positive mineralizing phospholipid matrix vesicles initiate the extracellular calcium deposition with epitaxial growth (20). Importantly, although alkaline phosphatase/tissue nonspecific alkaline phosphatase (TNAP) reduces phospho-osteopontin and pyrophosphate mineralization inhibitors in the local milieu (21, 22) and vascular TNAP transgenesis is sufficient to drive arterial calcification (23), annexin-containing vesicles can nucleate mineralization without antecedent TNAP activity (24). The vascular smooth muscle cell (VSMC) has multiple important contributions. In response to elevated extracellular ionized calcium and phosphate, the VSMC lineage elaborates the extracellular vesicles capable of initiating mineralization (24). Moreover, viable VSMC can phagocytose these vesicles, thereby restricting the number of mineralizing foci nucleating deposition (20). Hyperphosphatemia-induced VSMC apoptosis substantially curtails this important vascular editing function. Furthermore, during dyslipidemia, an osteochondrogenic VSMC transdifferentiation occurs, responsible for ∼80% of the osteogenic cells accruing in vascular preclinical calcification models (25). Regional paracrine cues provided by adventitial (26) and circulating osteoprogenitors (27) have emerged as part of the vascular reprogramming. With diabetes, induction of the endothelial-mesenchymal transition may not only disrupt endothelial-VSMC interactions that preserve the VSMC phenotype but also generate substantial numbers of osteoprogenitors capable of contributing to vascular calcium load (28). As in bone repair following fracture, the relative contributions of each of these osteoprogenitor pools may differ dependent upon anatomic venue. Indeed, elegant studies from St. Hilaire et al. (29) demonstrate that lower extremity peripheral arterial calcification and VSMC-TNAP activity is actively restrained in humans by genetic programs distinct from those governing aortic and coronary calcification. However, VSMC activity of the master osteochondrogenic transcription factor, Runt related transcription factor 2 (Runx2/Cbfal), appears vitally important to all vascular sclerotic responses observed to date. Other transcription factors [mothers against decapentaplegia homolog 1/5 (Smad1/5), muscle segment homeobox homolog 1/2 (Msx1/2), osterix (Sp7/Osx), nuclear factor of activated T-cells (NFAT), sex determining region of the Y chromosome box 9 homolog (Sox9)] inductively regulated by the bone morphogenetic proteins and Wnt ligands that regionally coordinate matrix mineralization mediate their actions in great part via modulation of Runx2/Cbfa1-driven programs (30–32).
Features of vascular mineralization resemble that of calcifying granuloma, a component of the innate immune system that helps contain pulmonary mycobacterial and fungal infections (33). In a TNF-, oxylipid-, and bone morphogenetic protein-dependent fashion, pulmonary vascular mesenchymal cells, endothelial cells, T-cells, and monocyte/macrophage lineage cells direct a rigid, calcified extracellular matrix that physically restricts disease expansion. Intriguingly, the foreign lipids of these pathogens and oxylipids from LDL cholesterol activate Toll-like receptor signals that may hold promise for therapeutic intervention in arteriosclerotic disease (34). Moreover, as in the lung, cells of the monocyte/macrophage lineage program TNAP production by mineralizing progenitors, and recent data indicate that this lineage, too, can elaborate matrix vesicles to nucleate mineralization (35). Although T-cells are vital to the regulation of skeletal mineralization, their contributions to vascular mineral metabolism remain woefully understudied.
The bone-vascular connection in arteriosclerosis and arterial calcification: Clues from the “perfect storm” of CKD.
Bone never forms without vascular interactions. Indeed, during endochondral ossification, the mineralizing osteoprogenitor tracks along microvasculature that penetrates the initially avascular cartilaginous template (36). The osteoprogenitor niche of bone thereafter continues to reside in the perivascular sinusoidal marrow space (18). As discussed above, the macrovascular adventitia and media also create a “niche-like” venue for osteoprogenitors. Moreover, the vasculature provides the route for influx and egress of calcium and phosphate in response to metabolic, morphogenetic, and mechanical needs. Thus, the notion that active biomineralization is regulated in orthotopic and heterotopic venues via vascular cues is not surprising. However, the “perfect storm” of perturbed calcium phosphate homeostasis CKD and its accompanying mineral and bone disorder (CKD-MBD) has highlighted an emerging role for the skeleton in vascular health and homeostasis (37). The earliest changes in calcium phosphate metabolism noted with declining renal function encompass elevations in circulating fibroblast growth factor (FGF) 23, a bone-derived phosphaturic hormone that reduces parathyroid hormone (PTH) secretion and restricts renal calcitriol production (38). Actions in the kidney and the parathyroid require FGF receptor 1 and the coreceptor, Klotho. Loss of FGF23/Klotho signaling results in massive phosphate- and calcitriol-driven arterial calcification (39). High levels of endogenous, renally derived calcitriol, as occurs with FGF23 signaling-deficient mice (39), has direct procalcific actions in VSMC normally held in check by the PTH/PTH-related peptide receptor (PTH1R) (40). In the short term, the upregulation of FGF23 signaling with the phosphate retention of early CKD is a highly desirable homeostatic response, because 1) serum phosphate at elevated levels is a vascular toxin (41) that conveys substantial cardiovascular risk at any level of renal function (42), and 2) as long as glomerular filtration and the capacity for urine generation is preserved, net excretion of phosphate can be achieved to protect the vasculature and other tissues. However, FGF23 also induces myocardial hypertrophy of the left ventricle, independent of Klotho coreceptor actions (43). Because left ventricular hypertrophy predisposes to heart failure and sudden death in CKD, the long-term consequences of elevated bone-derived FGF23 become maladaptive, a state wherein abnormal bone endocrine signals elicit CVD (44). The extent to which this bone-vascular axis contributes to CVD in the absence of overt renal failure remains to be established. However, again, at any level of renal function, increased fasting phosphate levels portend increased CVD.
London et al. (45–47) have identified additional relations between bone homeostasis and vascular calcium load in CKD. In their early studies in humans, the presence of either atherosclerotic intimal calcification or medial artery calcification was shown to predict early mortality in dialysis patients as compared with the longevity enjoyed by the fortunate minority without vascular mineralization (45). Using dynamic histomorphometry, they showed that those individuals with low turnover bone disease exhibit the greatest arterial calcium load (47). Further stratification of vascular disease by ankle-brachial indexes revealed that the presence of peripheral arterial disease tracked impaired skeletal osteoblast synthetic function and apparent resistance to ambient PTH tone (46). Intermittent administration of the bone anabolic agonist PTH(1–34) can reduce arterial calcium deposition in preclinical models (48, 49), suggesting that maintaining normal skeletal osteoblast synthetic function is important for vascular health. Mathew et al. (50) made similar observations with another bone anabolic strategy. However, the arterial vasculature expresses abundant PTH1R, which is bioactive and directly regulates VSMC proliferation (51) and matrix metabolism (48). In the vasculature, paracrine PTH-related peptide vasodilatory signaling in response to mechanical stretch is likely most physiologically relevant (52). The extent to which skeletal compared with VSMC PTH1R signaling contributes to preservation of vascular health is an area of ongoing investigation.
Calcium phosphate nutritional regulation of bone-vascular interactions: Challenges, opportunities, and future directions.
As a consequence of dietary preferences, supplements, medications, and food additives, the intakes of calcium and phosphorus can vary dramatically in the population (53). Because of the direct effects of calcium and phosphate on VSMC mineral metabolism and indirect effects via calciotropic hormones with potent vasculotropic actions, a better understanding of the impact of nutrition and genomics is clearly needed. Declining renal function clearly perturbs the bone-vascular axis in great part via phosphate metabolism, and phosphate binders play an important role in controlling absorption in our phosphorus-laden diets (54). However, a didactic example of the problems faced in CKD-MBD management becomes apparent when calcium-based phosphate binders are implemented; as compared with a calcium-independent phosphate binder, calcium acetate substantially reduces PTH levels, concomitantly reduces bone mass, and increases vascular calcium load in patients on renal replacement therapy (55). The extent to which the declining renal function of aging or diabetes impacts dietary calcium and phosphorus recommendations and vascular health remains to be established (42). The optimal “set points” for PTH/PTH1R and FGF23/FGF receptor 1 signaling tone with respect to vascular health have yet to be established. As with most endocrine physiology, a biphasic relation appears to exist wherein excessive or deficient signaling in response to metabolic need creates challenges to health. In kl/kl mice (Klotho-deficient), a vascular senescence model driven in part by hyperphosphatemia in the setting of normal renal function, the premature vascular calcification tracks induction of Runx2/Cbfa1 and NFAT5 (56), where the latter is an osmoregulated member of the osteochondrogenic NFAT family. Ammonia accumulates in the cytosolic compartment and induces cell swelling that reduces NFAT5 expression. The addition of ammonium chloride to drinking water of kl/kl mice downregulates the osteo/chondrogenic program elicited by hyperphosphatemia, including Runx2 and TNAP, and reduces vascular calcification (56). Whether a similar strategy might prove useful in the treatment of patients with diabetes, dyslipidemia, or CKD-MBD has yet to be explored. However, these recent data and the impact of matrix stiffness on the osteogenic predilection of vascular progenitors (13) highlight the need to incorporate consideration for the role of mechanobiology in cardiovascular responses to metabolic and nutritional challenges. Emerging bone-vascular interactions are depicted in Figure 1.
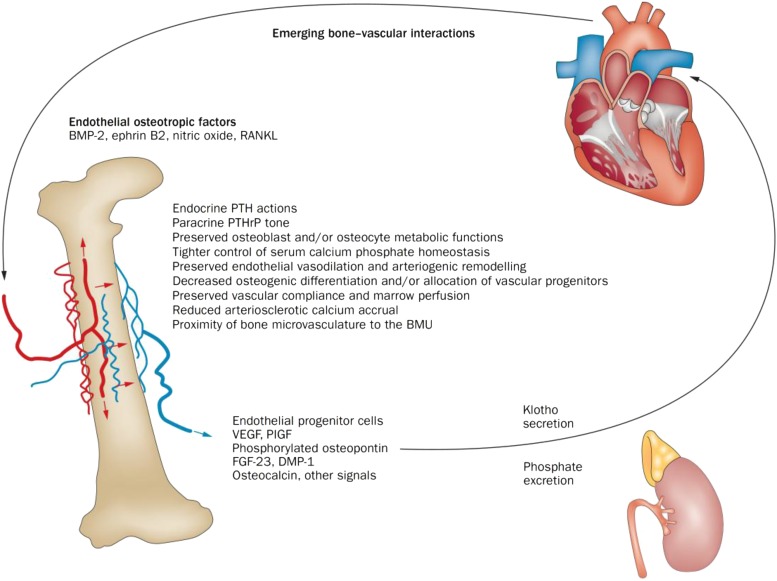
FIGURE 1.
Emerging bone-vascular interactions. A bidirectional endocrine relation exists between bone and the vasculature that mutually benefits bone and vascular health. The kidney is an important intermediary in this process via regulation of phosphate excretion (57) and expression of Klotho (58, 59). Importantly, PTH1R signaling maintains bone formation, sustains hematopoietic niche function (60) and endothelial progenitor cell mass (61), promotes intact osteoblast osteopontin 24 and osteocyte FGF23 (58, 62) secretion, supports renal Klotho production (58), and suppresses aortic osteofibrogenic Wnt/β-catenin signaling (40, 48) and vascular calcium accrual (48, 49). PTH1R signaling also reduces aortic (48) and skeletal (63) oxidative stress and maintains the proximity of the microvasculature to the BMU during bone formation (64). Declining renal function and tissue resistance to PTH1R signaling are key features in the perturbation of the bone-vascular axis in the setting of disease. Age-related changes in marrow composition and the vector of bone perfusion may also functionally perturb the bone-vascular axis. In addition, emerging data point to the role of circulating microvesicles—arising from endothelial cells, smooth muscle cells, and formed elements including platelets—in the endocrine regulation of bone-vascular interactions. BMP, bone morphogenic protein; BMU, basic multicellular unit; DMP-1, dentin matrix protein-1; FGF23, fibroblast growth factor 23; PlGF, placental growth factor; PTH, parathyroid hormone; PTHrP, parathyroid hormone related peptide; PTH1R, PTH/PTHrP receptor; RANKL, receptor activator of nuclear factor κB ligand; VEGF, vascular endothelial growth factor; Wnt, wingless-type mouse mammary tumor virus integration site family member. Reproduced from reference 6 with permission.
The Impact of Dietary Calcium and Vitamin D on the Heart and Bone
The vast majority, i.e., >99%, of calcium in the body resides in the skeleton. In the skeleton, increased calcium indicates more bone mineral, because calcium is a constant fraction of hydroxyapatite. Greater bone mass predicts reduced risk of fracture. Thus, stored calcium is a functional reserve. The <1% that is extraskeletal sustains life through a broad array of primary and secondary cell signaling functions and capacity to stabilize proteins. When excess calcium deposition in soft tissue occurs, tissue damage can occur, leading to chronic disease.
Studying calcium metabolism.
Calcium tracers can be used to determine distribution in the body and rates of transfer under different environmental conditions and disease states. Use of calcium tracers in various applications is reviewed by Weaver et al. (65). Calcium has a large number of useful isotopes that provide a wide array of applications. There are 2 radioisotopes, 45Ca and 47Ca, that are especially useful with animal models, although they both have been used in humans. There are 5 useful stable isotopes: 42Ca, 43Ca, 44Ca, 46Ca, and 48Ca, that can be used safely in any facility and individual. The availability of multiple isotopes allows different tracers to be given orally and intravenously so that complete kinetic analysis is possible. A long-lived isotope, 41Ca, is also available, and when measured by accelerator mass spectrometry, virtually atom quantities can be measured allowing detection of very early deposition into soft tissues.
Calcium tracers have been particularly useful in elucidating calcium absorption molecular mechanisms (66). Calcium absorption occurs by 2 pathways. Active calcium transport is activated when 1,25-dihydroxyvitamin D elicits transcription of transport proteins including transient receptor potential cation channel subfamily V member 6 (TRPV6), calbindin D9k, and PMCA 1b. This pathway is efficient and dominant in conditions of low calcium intake. Calcium absorption during conditions of higher calcium intakes is less dependent on active calcium absorption as the transport proteins become saturated. The non-vitamin D-dependent, passive calcium absorption pathway linearly increases with increased calcium loads over the physiologic range (67).
Factors affecting calcium absorption have also been characterized with calcium tracers. The double calcium isotopic tracer technique, in which one tracer is given orally and another intravenously, is the most sensitive indicator of calcium absorption. Characteristics of an individual such as age and sex steroid hormone deficiency (68) and external factors such as calcium load (69) and food matrix (70) have been identified by using isotopic tracer techniques.
Calcium and vitamin D benefits to bone.
Because calcium is integral to hydroxyapatite and bone continually remodels, a continual supply of calcium in the diet throughout life is essential. Needs are greater during periods of skeletal growth when bone is accruing. Serum calcium is tightly regulated to supply calcium to bone and all tissues. Calcitriol activates calcium absorption, enhances calcium retention, and stimulates osteoclast cells in the bone to release calcium from the bone mineral matrix during periods of low calcium intake, but vitamin D is not required to maintain bone health if calcium intake is sufficient (71). Bone loss by vitamin D receptor-null mice was reversed with dietary calcium and phosphorus (72). This suggests vitamin D is not required for bone health per se but rather is important for maintaining serum calcium. On the other hand, in the NHANES III population-based survey, higher calcium intake was associated (P = 0.005) with higher bone mineral density (BMD), but only in women with lower vitamin D status, i.e., 25-hydroxyvitamin D < 50 nmol/L (73).
The evidence for benefits of calcium and vitamin D supplementation and protection against fracture risk is mixed, likely because of research quality deficiencies such as lack of compliance, studying populations with already Adequate Intake, or use of studies that are too small or too short in duration compared with the development of disease. One meta-analysis reported that calcium and vitamin D supplementation in people aged ≥50 y reduced fracture risk by 12% and in studies with ≥80% compliance, the reduction was 24% (74). In the largest randomized controlled trial, the Women’s Health Initiative, hip BMD was improved with calcium and vitamin D in a subset of the population, but there was no improvement in hip fracture risk (75). However, in women not taking their own supplements who were ≥80% adherent to supplementation for ≥5 y, hip fracture HR was 0.24 (95% CI: 0.07, 0.84) (76). Calcium and vitamin D supplementation is standard therapy for osteoporosis treatment (77) but has not been endorsed by the US Preventative Services Task Force (78). This report has been criticized for not addressing persons with inadequate dietary intake (79). Recommended intakes, bioavailability, and usual intakes of calcium and vitamin D from supplements are addressed elsewhere (80).
The benefit to bone of a wide range of calcium and vitamin D intakes from adulthood through menopause was evaluated in a rat model where diets could be controlled for sufficiently long periods (81). Calcium and vitamin D independently increased tibial and femoral trabecular structures. Vitamin D increased tibial bone width and fracture resistance. Surprisingly few calcium and vitamin D interactions were observed—only for femur length and tibial calcium content. At high calcium and vitamin D intakes, calcium kinetic isotopic tracer analysis showed an increase in the soft tissue compartment. This finding suggests the possibility of soft tissue calcification with prolonged intakes of both calcium and vitamin that exceed recommended levels.
Concern over cardiovascular risks associated with calcium supplementation.
Traditionally, calcium supplementation was associated with only minimal adverse events, i.e., gastrointestinal upset and a small risk of kidney stones (78). Secondary analyses of randomized controlled trials aimed at bone outcome measures showed increased risk of CVD risk factors (82, 83). Fear over risk of heart attacks with supplement use has led to a decline in intake and stimulation of new research to examine the relation of calcium supplementation and CVD risk (84). The controversy over whether to recommend supplementation to reduce risk of osteoporosis or to not recommend it for fear of cardiovascular risk or lack of benefit to bone is not entirely resolved. No professional society or policy body has yet taken the stance that there is sufficient evidence to warn against supplementation to lower cardiovascular risk.
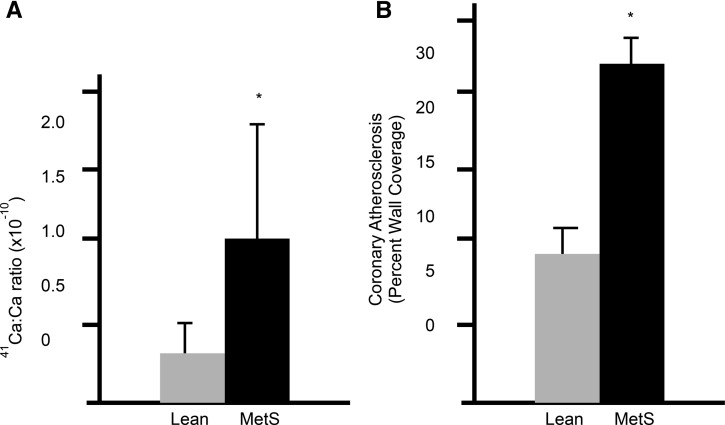
Epidemiologic studies in this area have been mixed. Michaëlsson et al. (85) found a U-shaped relation between calcium intake and cardiovascular mortality in a large Swedish cohort of women, particularly indicating increased risk in women who used calcium supplements on top of already high calcium intake (∼1400 mg/d). On the other hand, Van Hemelrijck et al. (86) found no association between dietary or supplemental calcium intake and cardiovascular mortality in an analysis of NHANES III. The evidence base suggesting a risk of CVD with calcium supplementation has suffered from lack of a clear underlying mechanism for calcium intake, no consistent dose-response relation, and insensitive methods to assess soft tissue calcification or accuracy and consistency of cardiovascular events. To help address these knowledge gaps, a study was conducted to specifically evaluate the impact of chronic high calcium intake on soft tissue calcification in an animal model relevant to humans by using a novel approach to determine early soft tissue calcification. The Ossabaw miniature swine fed an atherogenic diet developed metabolic syndrome with associated soft tissue calcification and CVD. A sensitive approach to measuring early soft tissue calcification was developed by using 41Ca administration and subsequently analyzing 41Ca:Ca ratios in coronary arteries. Coronary artery accumulation of 41Ca:Ca in pigs with metabolic syndrome was much higher than in healthy pigs. This approach was validated against percentage of wall coverage by ultrasound (Figure 2) and is capable of detecting earlier shifts in calcification (87).
FIGURE 2.
Coronary artery calcium uptake and level of atherosclerosis in Ossabaw miniature swine. (A) 41Ca:Ca ratio of right coronary artery samples of lean (n = 7) and MetS (n = 7) pigs (means ± SDs, P < 0.05). Values for 6 lean pigs and 2 MetS pigs were below the detection limit; the upper limit of detection was used for the pigs for calculating the means. (B) Coronary atheroma assessed by intravascular ultrasound. Percent wall coverage was obtained by pullback of the ultrasound catheter along the length of the coronary artery. Ca, calcium; MetS, metabolic syndrome. Adapted from reference 79 with permission.
To test the effect of high compared with recommended calcium intake, adult Ossabaw miniature swine were randomly assigned to high-calcium diets (2%, equivalent to the upper level in humans) from dairy or calcium carbonate and control diets (0.5%, recommended requirement for pigs) and fed for 6 mo until soft tissue calcium deposition had begun. High calcium intakes from neither source resulted in significant differences from control pigs in coronary artery 41Ca deposition (P > 0.05) or any traditional measure of cardiovascular function, plaque wall coverage, stroke volume and ejection fraction, or histologic assessment of calcification (88). Thus, this study suggests that calcium from diet or supplements up to the upper level does not appear to increase risk of CVD.
Calcium and vitamin D are essential nutrients. Current evidence supports that it is prudent to follow the Dietary Guidelines for Americans (89) and calcium and vitamin D recommendations from the Institute of Medicine (90) and to aim for intakes around the RDA but below the tolerable upper level. Whether or not calcium and vitamin D supplementation is beneficial to bones depends on whether an individual is deficient and other factors. Based on the study in pigs, calcium intakes up to the tolerable upper level are unlikely to increase risk of CVD. One group that remains vulnerable to bone and cardiovascular disorders is those with CKD, and this population requires further study. The impact of diet is not completely understood, but a study of calcium carbonate supplementation to control phosphorus with the use of calcium tracer kinetics suggested soft-tissue deposition may be occurring in patients with CKD (91).
The Role of Dietary Phosphorus in Cardiovascular and Bone Health
Phosphorus is an essential nutrient with a variety of functions in the body, including structure as a component of bone mineral, cell membranes, and nucleic acids; acid-base balance as an intracellular buffer and titratable acid buffer; and in energy metabolism as part of the compounds adenosine triphosphate, guanosine triphosphate, etc. The majority (∼85%) of the body’s phosphorus is found in bone mineral with calcium to form hydroxyapatite [Ca10(PO4)6(OH)2] (92). Thus, phosphorus deficiency manifests in osteomalacia or rickets. However, dietary phosphorus deficiency is rare because of its widespread presence in the food supply; it is found naturally in protein foods like meat, dairy, nuts, and seeds and also in grains and other plant foods. In addition, inorganic phosphates, which are highly absorbed, are added to many foods for a variety of purposes including emulsification, leavening, pH control, thickening, and increasing the shelf-life of products (93). Subsequently, concern has arisen over potential consequences of excessive intake of phosphorus on bone and cardiovascular health, particularly for those with CKD. In CKD, abnormal phosphate metabolism is central to the development of CKD-MBD, which is characterized by interrelated laboratory abnormalities in mineral metabolism, bone disease, and vascular calcification (44, 94). As a consequence of CKD-MBD, patients with end-stage renal disease have increased risk of cardiovascular mortality compared with the general population (95) and more than a 4-fold risk of fracture (96).
The ratio of calcium to phosphorus in the diet has been investigated related to bone health, with the hypothesis that the relative intakes of these nutrients determine their adequacy or excess. Optimal ratios have been estimated as 2:1 during infancy and 1.3–1.5:1 beyond. However, calcium balance studies in adults studied under a wide range of calcium:phosphorus conditions from 0.08:1 (>12× phosphorus compared with calcium) to 2.4:1 show no effect of ratio on calcium balance (97–99). High phosphorus stimulates PTH, and restriction of dietary phosphorus can lessen or correct secondary hyperparathyroidism. Thus, high dietary and serum phosphorus can influence bone disease, particularly in patients with CKD and end-stage renal disease through detrimental effects of high PTH on bone (100).
Relation between phosphorus and cardiovascular disease and mortality.
Serum phosphate has been associated with CVD and mortality consistently across numerous studies that included healthy people (101, 102), people with pre-existing heart disease (103), and patients with CKD (104, 105). It is notable that risk for CVD has been observed to increase even over a range of normal serum phosphate (102). Few studies, to our knowledge, have investigated the relation between dietary phosphorus intake and CVD and mortality, partially because of the challenges of assessing dietary intake (106). Chang et al. (107) found increased risk of all-cause mortality with high dietary phosphorus intakes in an analysis of NHANES III. Increased risk was reported to start at intake levels of ∼1400 mg/d; however, confidence intervals suggest that risk may not increase until high intakes are reached. Interestingly, another study of people with reduced renal function (estimated glomerular filtration rate) in NHANES III showed no relation between dietary phosphorus intake level and mortality (108).
One mechanism by which phosphorus may influence CVD and mortality is through the actions of FGF23 on left ventricular mass (43), as described above. Thus, it is plausible that high dietary phosphorus may affect cardiovascular risk indirectly through FGF23 effects. Supportive of this concept, Yamamoto et al. (109) have shown an association between higher dietary phosphorus intake and greater left ventricular mass in women, but not men. In addition, the direct effect of phosphate on VSMC transdifferentiation to osteoblastic-like cells capable of laying down collagen matrix and mineral is another plausible mechanism by which dietary phosphorus may affect CVD (110). Supportive of this, a study in CKD rats showed that restricting dietary phosphorus prevents the vascular calcification that is otherwise observed in rats fed a normal phosphorus diet (111). These associational and mechanistic studies indicate there is good reason for caution regarding excessive dietary phosphorus intake in the general population and particularly in special groups like people with CKD. However, further research is needed for more definitive recommendations regarding the potential dangers of phosphorus excess.
Racial Differences and the Impact of Nutrition in Osteoporosis and Cardiovascular Disease
Race and ethnicity.
Race and ethnicity are 2 different but related constructs that have a tremendous influence on the incidence of chronic diseases (112). Race encompasses biological/genetic characteristics, whereas ethnicity describes identification with cultural practices that include but are not limited to geographic region, including language, heritage, religion, tradition, and customs. Health disparities research investigates differences in health across disadvantaged populations including factors of race and ethnicity and determines whether they are attributable to decreased access to necessary health care services or caused by other factors such as greater occurrence of behavioral risk factors for a disease or condition (113). Differences in screening, treatment, adherence, and management of chronic diseases play an important role on health disparity between races. A complex framework exists for understanding the multitude of factors that exist and interact to cause health disparities (112). This section summarizes the role of nutrition in racial disparities with regard to osteoporosis and CVD.
Race disparity and bone health.
Low bone mass and osteoporosis greatly increase the susceptibility to bone fracture. The risk of osteoporosis and bone fracture increases linearly with age. However, several other factors are also related to bone disease including sex, race/ethnicity, and nutrition. Women carry a disproportionate burden of bone disorders related to aging; in fact, 1 in 3 women over the age of 50 y will experience a bone fracture in her lifetime (114).
According to national estimates from adults over the age of 50 y from NHANES 2005–2010, standardized to the population by using census data, ∼10% of the US population (10.2 million) and ∼44% (43.4 million) older adults have osteoporosis and low bone mass, respectively. However, differences exist in the prevalence of bone disorders among women by race (115). Approximately 7.7 million non-Hispanic white adults have osteoporosis compared with 0.5 million and 0.6 million non-Hispanic black and Mexican American adults, respectively (115). More importantly than prevalence estimates, non-Hispanic white women tend to have more hip fractures than women of other races/ethnicities (115). Another NHANES 2007–2008 analysis reveals that despite lower levels of physical activity, the prevalence of osteoporosis remains much lower in non-Hispanic black and Mexican Americans (n = 2819, aged 40–80 y than non-Hispanic white adults (116).
Calcium and vitamin D have a well-established role with regard to bone health; however, the exact mechanisms by which these nutrients interact with race are largely unknown. Across most age and race/ethnic groups, intakes of calcium and vitamin D are very low and well below recommendations (117, 118). The proportion of non-Hispanic black Americans with calcium and vitamin D intakes less than the estimated requirement was higher than all other race/ethnic groups in the United States (119). Differential intakes of milk and dairy products exist between race/ethnic groups and may be related to lactose tolerance; however, this relation is not well characterized, and a standard diagnostic criteria for lactose tolerance does not exist (120). Interestingly, despite lower intakes of bone relevant micronutrients and dairy in the diet, non-Hispanic black women have the lowest incidence of bone fracture of all the observed race/ethnic groups and higher BMD than non-Hispanic white females. Studies have shown that non-Hispanic blacks have lower calcium excretion and higher calcium retention (121, 122) and achieve 5–15% higher peak bone mass than non-Hispanic whites. Serum 25-hydroxyvitamin D concentrations are also lower among non-Hispanic black Americans than in other racial groups (123). The levels of vitamin D-binding proteins are stable (124–126) and do not change with the change in the levels of 25-hydroxyvitamin D, but the levels of vitamin D-binding proteins are relatively lower in non-Hispanic blacks than non-Hispanic whites (123, 127). PTH is one of the major hormones that influences bone resorption and calcium absorption in the intestine and impacts BMD. Studies have shown that the levels of PTH differ between race/ethnic groups and may be one of the possible mechanisms for the differences in bone health between race/ethnic groups (128). Asian-American females have similar BMD to non-Hispanic white women; however, they experience fewer falls and fractures (129). Thus, BMD alone may not be the most important component in understanding how race influences bone health and fracture risk. To our knowledge, very little is known about the bone health of Native Americans and Hispanics aside from Mexican Americans in the United States. In summary, racial differences in BMD and fracture risk in non-Hispanic black women appear to be independent of physical activity, PTH, and dietary intakes. Given that more women will experience a bone fracture than breast cancer, myocardial infarction, and coronary death in a given year, characterizing how race and ethnicity impact bone health and disease risk is critical.
Race disparity and CVD.
Obesity, being overweight, and diabetes mellitus are other relevant major health conditions in which race disparity is very profound. Analyses of data from NHANES 2009–2010 showed that non-Hispanic whites have the lowest rates of being overweight, while non-Hispanic blacks have the highest rates of obesity, with >13% having a BMI (in kg/m2) of ≥40 as compared with 6% among non-Hispanic whites (130). Weight status and diabetes are strong, independent risk factors for CVD. CVD is the leading cause of mortality among adults 65 y and older (131). Among types of CVD, coronary heart disease accounts for ∼48% of the deaths followed by stroke (16%) (131). Non-Hispanic black men and women have the highest prevalence of high blood pressure, myocardial infarction, stroke/transient ischemic attack, as well as death from these diseases when compared with other race/ethnic groups in the United States across multiple years of NHANES, whereas Mexican American men and women have the highest prevalence of elevated cholesterol (≥200 mg/dL) (132).
Diet and race interactions.
Diet and dietary patterns play an important role in the health of an individual and can be modifiable causes that contribute to race/ethnic disparities. Understanding how dietary differences interact with race is critical (133). Data from a multiethnic cohort (cohort includes >215,000 participants, including African American, Native Hawaiian, Japanese America, Latino, and Caucasian men and women, living in Hawaii and Los Angeles in 1993–1996) have demonstrated a complex interaction by race and sex for diet and health outcomes (134). For example, higher intakes of vegetables substantially reduced the risk of fatal stroke in African American women, whereas among Japanese women higher fruit intake and lower meat intake was associated with reduced risk of fatal stroke (134). It is interesting to note in this cohort that no significant associations by race were observed for males (P > 0.05).
Understanding the relation between diet and nutrition status, race, and chronic disease will provide insights to reduce health disparities. Health professionals need to understand that race and ethnicity are factors that should be considered in providing dietary advice to prevent, manage, or treat diseases. In public health, “cultural competency” refers to the attitudes, behaviors, and policies that guide health care systems, agencies, and personnel to work effectively in cross-cultural situations. Furthermore, cultural competency extends to include the ability of health care providers and systems to care for diverse patients in a manner that meets their social, cultural, and linguistic needs. Many more multidimensional studies are needed to identify appropriate interventions to reduce race disparity in health.
Conclusions
Cardiovascular and skeletal health are connected through interacting hormonal and cellular processes, and CKD presents conditions in which these relations are amplified and the risk of CVD and bone disease is greatly increased. Current evidence for the role of calcium, vitamin D, and phosphorus in the development of CVD and osteoporosis points toward a conservative approach of aiming for intakes around current recommended levels but cautions against excessive intakes, particularly in those with compromised renal function. It seems prudent to avoid excessive phosphorus intake that is facilitated through widespread natural sources and food additive use, as well as to avoid excessive calcium intake through high-dose supplements. Racial and ethnic disparities in the prevalence of CVD and osteoporosis exist, and there is evidence of race/ethnicity-nutrition interactions in disease susceptibility. Further investigation is needed to understand the role of nutrition in reducing these disparities.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: BMD, bone mineral density; Cbfa1, core-binding factor α 1; CKD, chronic kidney disease; CKD-MBD, chronic kidney disease-mineral bone disorder; CVD, cardiovascular disease; FGF, fibroblast growth factor; Msx1/2, muscle segment homeobox homolog 1/2; NFAT, nuclear factor of activated T-cells; Osx, osterix; PMCA 1b, plasma membrane Ca2+ ATPase 1b; PTH, parathyroid hormone; PTH1R, PTH/PTH-related peptide receptor; Runx2, Runt related transcription factor 2; Smad1/5, mothers against decapentaplegia homolog 1/5; Sox9, sex determining region of the Y chromosome box 9 homolog; Sp7, osterix; TNAP, tissue nonspecific alkaline phosphatase; VSMC, vascular smooth muscle cell; Wnt, wingless-type mouse mammary tumor virus integration site family member.
References
- 1.Demer LL, Tintut Y. Vascular calcification: Pathobiology of a multifaceted disease. Circulation 2008;117:2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virchow R. Cellular pathology: As based upon physiological and pathological histology: Lecture XVI–Atheromatous affection of arteries. 1858. Nutr Rev 1989;47:23–5. [DOI] [PubMed] [Google Scholar]
- 3.Mayerl C, Lukasser M, Sedivy R, Niederegger H, Seiler R, Wick G. Atherosclerosis research from past to present: On the track of two pathologists with opposing views, Carl von Rokitansky and Rudolf Virchow. Virchows Arch 2006;449:96–103. [DOI] [PubMed] [Google Scholar]
- 4.Mönckeberg JG. Über die reine mediaverkalkung der extremitätenarterien und ihr verhalten zur arteriosklerose. Virchows Arch Pathol Anat 1903;171:141–67. [Google Scholar]
- 5.Greenwald SE. Ageing of the conduit arteries. J Pathol 2007;211:157–72.17200940 [Google Scholar]
- 6.Thompson B, Towler DA. Arterial calcification and bone physiology: Role of the bone-vascular axis. Nat Rev Endocrinol 2012;8:529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput 2009;47:131–41. [DOI] [PubMed] [Google Scholar]
- 8.King KS, Chen KX, Hulsey KM, McColl RW, Weiner MF, Nakonezny PA, Peshock RM. White matter hyperintensities: Use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology 2013;267:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silbert LC, Dodge HH, Perkins LG, Sherbakov L, Lahna D, Erten-Lyons D, Woltjer R, Shinto L, Kaye JA. Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology 2012;79:741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng SL, Ramachandran B, Behrmann A, Shao JS, Mead M, Smith C, Krchma K, Bello Arredondo Y, Kovacs A, Kapoor K, et al. . Vascular smooth muscle LRP6 limits arteriosclerotic calcification in diabetic LDLR−/− mice by restraining noncanonical Wnt signals. Circ Res 2015;117:142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng SL, Behrmann A, Shao JS, Ramachandran B, Krchma K, Bello Arredondo Y, Kovacs A, Mead M, Maxson R, Towler DA. Targeted reduction of vascular Msx1 and Msx2 mitigates arteriosclerotic calcification and aortic stiffness in LDLR-deficient mice fed diabetogenic diets. Diabetes 2014;63:4326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006;126:677–89. [DOI] [PubMed] [Google Scholar]
- 13.Chen JH, Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: Critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res 2011;108:1510–24. [DOI] [PubMed] [Google Scholar]
- 14.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol 2008;51:1967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzman RJ, Bian A, Shintani A, Stein CM. Association of foot ulcer with tibial artery calcification is independent of peripheral occlusive disease in type 2 diabetes. Diabetes Res Clin Pract 2013;99:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohler ER III, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation 2001;103:1522–8. [DOI] [PubMed] [Google Scholar]
- 17.Fadini GP, Rattazzi M, Matsumoto T, Asahara T, Khosla S. Emerging role of circulating calcifying cells in the bone-vascular axis. Circulation 2012;125:2772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al. . Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007;131:324–36. [DOI] [PubMed] [Google Scholar]
- 19.Boström K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest 1993;91:1800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, Jahnen-Dechent W, Shanahan CM. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol 2005;16:2920–30. [DOI] [PubMed] [Google Scholar]
- 21.Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem 2000;275:20197–203. [DOI] [PubMed] [Google Scholar]
- 22.Narisawa S, Yadav MC, Millan JL. In vivo overexpression of tissue-nonspecific alkaline phosphatase increases skeletal mineralization and affects the phosphorylation status of osteopontin. J Bone Miner Res 2013;28:1587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheen CR, Kuss P, Narisawa S, Yadav MC, Nigro J, Wang W, Chhea TN, Sergienko EA, Kapoor K, Jackson MR, et al. . Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res 2015;30:824–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot D, Mayr M, et al. . Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res 2011;109:e1–12. [DOI] [PubMed] [Google Scholar]
- 25.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res 2009;104:733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 2005;115:1210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albiero M, Rattazzi M, Menegazzo L, Boscaro E, Cappellari R, Pagnin E, Bertacco E, Poncina N, Dyar K, Ciciliot S, et al. . Myeloid calcifying cells promote atherosclerotic calcification via paracrine activity and allograft inflammatory factor-1 overexpression. Basic Res Cardiol 2013;108:368. [DOI] [PubMed] [Google Scholar]
- 28.Yao J, Guihard P, Blazquez-Medela AM, Guo Y, Moon JH, Jumabay M, Bostrom KI, Yao Y. Serine protease activation essential for endothelial-mesenchymal transition in vascular calcification. Circ Res 2015;117:758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St. Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, et al. . NT5E mutations and arterial calcifications. N Engl J Med 2011;364:432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin ME, Chen T, Leaf EM, Speer MY, Giachelli CM. Runx2 expression in smooth muscle cells is required for arterial medial calcification in mice. Am J Pathol 2015;185:1958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heath JM, Sun Y, Yuan K, Bradley WE, Litovsky S, Dell’Italia LJ, Chatham JC, Wu H, Chen Y. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res 2014;114:1094–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raaz U, Schellinger IN, Chernogubova E, Warnecke C, Kayama Y, Penov K, Hennigs JK, Salomons F, Eken S, Emrich FC, et al. . Transcription factor Runx2 promotes aortic fibrosis and stiffness in type 2 diabetes mellitus. Circ Res 2015;117:513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol 2014;34:715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su X, Ao L, Shi Y, Johnson TR, Fullerton DA, Meng X. Oxidized low density lipoprotein induces bone morphogenetic protein-2 in coronary artery endothelial cells via Toll-like receptors 2 and 4. J Biol Chem 2011;286:12213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E. Macrophage-derived matrix vesicles: An alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res 2013;113:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 2010;19:329–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moe SM, Drueke T, Lameire N, Eknoyan G. Chronic kidney disease-mineral-bone disorder: A new paradigm. Adv Chronic Kidney Dis 2007;14:3–12. [DOI] [PubMed] [Google Scholar]
- 38.Kuro-o M. Overview of the FGF23-Klotho axis. Pediatr Nephrol 2010;25:583–90. [DOI] [PubMed] [Google Scholar]
- 39.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: Lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med 2006;12:298–305. [DOI] [PubMed] [Google Scholar]
- 40.Jono S, Nishizawa Y, Shioi A, Morii H. Parathyroid hormone-related peptide as a local regulator of vascular calcification: Its inhibitory action on in vitro calcification by bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 1997;17:1135–42. [DOI] [PubMed] [Google Scholar]
- 41.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, et al. . Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 2008;118:1748–57. [DOI] [PubMed] [Google Scholar]
- 42.Foley RN. Phosphate levels and cardiovascular disease in the general population. Clin J Am Soc Nephrol 2009;4:1136–9. [DOI] [PubMed] [Google Scholar]
- 43.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, et al. . FGF23 induces left ventricular hypertrophy. J Clin Invest 2011;121:4393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf M. Mineral (mal)adaptation to kidney disease-young investigator award address: American Society of Nephrology Kidney Week 2014. Clin J Am Soc Nephrol 2015;10:1875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 2003;18:1731–40. [DOI] [PubMed] [Google Scholar]
- 46.London GM, Marchais SJ, Guerin AP, de Vernejoul MC. Ankle-brachial index and bone turnover in patients on dialysis. J Am Soc Nephrol 2015;26:476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 2004;15:1943–51. [DOI] [PubMed] [Google Scholar]
- 48.Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, Towler DA. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/β-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res 2010;107:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sebastian EM, Suva LJ, Friedman PA. Differential effects of intermittent PTH(1–34) and PTH(7–34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone 2008;43:1022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathew S, Davies M, Lund R, Saab G, Hruska KA. Function and effect of bone morphogenetic protein-7 in kidney bone and the bone-vascular links in chronic kidney disease. Eur J Clin Invest 2006;36(Suppl 2):43–50. [DOI] [PubMed] [Google Scholar]
- 51.Song GJ, Fiaschi-Taesch N, Bisello A. Endogenous parathyroid hormone-related protein regulates the expression of PTH type 1 receptor and proliferation of vascular smooth muscle cells. Mol Endocrinol 2009;23:1681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raison D, Coquard C, Hochane M, Steger J, Massfelder T, Moulin B, Karaplis AC, Metzger D, Chambon P, Helwig JJ, et al. . Knockdown of parathyroid hormone related protein in smooth muscle cells alters renal hemodynamics but not blood pressure. Am J Physiol Renal Physiol 2013;305:F333–42. [DOI] [PubMed] [Google Scholar]
- 53.Heaney RP, Kopecky S, Maki KC, Hathcock J, Mackay D, Wallace TC. A review of calcium supplements and cardiovascular disease risk. Adv Nutr 2012;3:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amiri FS. Contemporary management of phosphorus retention in chronic kidney disease: A review. Clin Exp Nephrol 2015;19:985–99. [DOI] [PubMed] [Google Scholar]
- 55.Raggi P, James G, Burke SK, Bommer J, Chasan-Taber S, Holzer H, Braun J, Chertow GM. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res 2005;20:764–72. [DOI] [PubMed] [Google Scholar]
- 56.Leibrock CB, Alesutan I, Voelkl J, Pakladok T, Michael D, Schleicher E, Kamyabi-Moghaddam Z, Quintanilla-Martinez L, Kuro OM, Lang F. NH4Cl treatment prevents tissue calcification in Klotho deficiency. J Am Soc Nephrol 2015;26:2423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farrow EG, White KE. Recent advances in renal phosphate handling. Nat Rev Nephrol 2010;6:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López I, Rodriguez-Ortiz ME, Almaden Y, Guerrero F, de Oca AM, Pineda C, Shalhoub V, Rodriguez M, Aguilera-Tejero E. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int 2011;80:475–82. [DOI] [PubMed] [Google Scholar]
- 59.Moe SM, Radcliffe JS, White KE, Gattone VH 2nd, Seifert MF, Chen X, Aldridge B, Chen NX. The pathophysiology of early-stage chronic kidney disease-mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J Bone Miner Res 2011;26:2672–81. [DOI] [PubMed] [Google Scholar]
- 60.Kronenberg HM. PTH regulates the hematopoietic stem cell niche in bone. Adv Exp Med Biol 2007;602:57–60. [DOI] [PubMed] [Google Scholar]
- 61.Napoli C, William-Ignarro S, Byrns R, Balestrieri ML, Crimi E, Farzati B, Mancini FP, de Nigris F, Matarazzo A, D’Amora M, et al. . Therapeutic targeting of the stem cell niche in experimental hindlimb ischemia. Nat Clin Pract Cardiovasc Med 2008;5:571–9. [DOI] [PubMed] [Google Scholar]
- 62.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 2011;49:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell 2010;9:851–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prisby R, Guignandon A, Vanden-Bossche A, Mac-Way F, Linossier MT, Thomas M, Laroche N, Malaval L, Langer M, Peter ZA, et al. . Intermittent PTH(1–84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone-forming sites. J Bone Miner Res 2011;26:2583–96. [DOI] [PubMed] [Google Scholar]
- 65.Weaver C, Wastney M, Spence L. Quantitative clinical nutrition approaches to the study of calcium and bone metabolism. In: Holick M, Nieves J, editors. Nutrition and Bone Health. Totowa (NJ): Humana Press; 2015. p. 361–77. [Google Scholar]
- 66.Fleet JC, Hong J, Zhang Z. Reshaping the way we view vitamin D signalling and the role of vitamin D in health. Nutr Res Rev 2004;17:241–8. [DOI] [PubMed] [Google Scholar]
- 67.Weaver CM, Heaney RP, Teegarden D, Hinders SM. Wheat bran abolishes the inverse relationship between calcium load size and absorption fraction in women. J Nutr 1996;126:303–7. [DOI] [PubMed] [Google Scholar]
- 68.Heaney RP, Recker RR, Stegman MR, Moy AJ. Calcium absorption in women: Relationships to calcium intake, estrogen status, and age. J Bone Miner Res 1989;4:469–75. [DOI] [PubMed] [Google Scholar]
- 69.Heaney RP, Weaver CM, Fitzsimmons ML. Influence of calcium load on absorption fraction. J Bone Miner Res 1990;5:1135–8. [DOI] [PubMed] [Google Scholar]
- 70.Weaver CM, Proulx WR, Heaney R. Choices for achieving adequate dietary calcium with a vegetarian diet. Am J Clin Nutr 1999; 70(Suppl):543S–8S. [DOI] [PubMed] [Google Scholar]
- 71.Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-analysis. Lancet 2014;383:146–55. [DOI] [PubMed] [Google Scholar]
- 72.Masuyama R, Nakaya Y, Katsumata S, Kajita Y, Uehara M, Tanaka S, Sakai A, Kato S, Nakamura T, Suzuki K. Dietary calcium and phosphorus ratio regulates bone mineralization and turnover in vitamin D receptor knockout mice by affecting intestinal calcium and phosphorus absorption. J Bone Miner Res 2003;18:1217–26. [DOI] [PubMed] [Google Scholar]
- 73.Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, Orav JE, Li R, Spiegelman D, Dietrich T, Willett WC. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res 2009;24:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet 2007;370:657–66. [DOI] [PubMed] [Google Scholar]
- 75.Cauley JA. The Women’s Health Initiative: Hormone therapy and calcium/vitamin D supplementation trials. Curr Osteoporos Rep 2013;11:171–8. [DOI] [PubMed] [Google Scholar]
- 76.Prentice RL, Pettinger MB, Jackson RD, Wactawski-Wende J, Lacroix AZ, Anderson GL, Chlebowski RT, Manson JE, Van Horn L, Vitolins MZ, et al. . Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int 2013;24:567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bone Health and Osteoporosis: A Surgeon General's Report. US Public Health Service. 2004. Office of the Surgeon General (US). Bone health and osteoporosis: a report of the surgeon general. Rockville (MD): Office of the Surgeon General (US); 2004. [cited 2015 Jan 8]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK45513/. [Google Scholar]
- 78.Moyer VA. Force* USPST. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;158:691–6. [DOI] [PubMed] [Google Scholar]
- 79.Bauer DC. Clinical practice: Calcium supplements and fracture prevention. N Engl J Med 2013;369:1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phillips A, Lipkie T, Weaver C. Calcium and vitamin D: Nutrition role and the benefits and risks of dietary supplements in health promotion. In: Wallace T, editor. Dietary Supplements in Health Promotion. Boca Raton (FL): CRC Press; . 2015. p. 121–35. [Google Scholar]
- 81.Park CY, Lee WH, Fleet JC, Allen MR, McCabe GP, Walsh DM, Weaver CM. Calcium and vitamin D intake maintained from preovariectomy independently affect calcium metabolism and bone properties in Sprague Dawley rats. Osteoporos Int 2014;25:1905–15. [DOI] [PubMed] [Google Scholar]
- 82.Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, Gamble GD, Grey A, Reid IR. Vascular events in healthy older women receiving calcium supplementation: Randomised controlled trial. BMJ 2008;336:262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. BMJ 2010;341:c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weaver CM. Calcium supplementation: Is protecting against osteoporosis counter to protecting against cardiovascular disease? Curr Osteoporos Rep 2014;12:211–8. [DOI] [PubMed] [Google Scholar]
- 85.Michaëlsson K, Melhus H, Warensjo Lemming E, Wolk A, Byberg L. Long term calcium intake and rates of all cause and cardiovascular mortality: Community based prospective longitudinal cohort study. BMJ 2013;346:f228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Hemelrijck M, Michaelsson K, Linseisen J, Rohrmann S. Calcium intake and serum concentration in relation to risk of cardiovascular death in NHANES III. PLoS One 2013;8:e61037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wastney M, Lee W, Jackson GS, Alloosh M, Sturek M, Lachcik P, Peacock M, Martin B, Weaver CM. Soft tissue calcification in the Ossabaw miniature pig: Experimental and kinetic modeling studies. Osteoporos Int 2013;24:2123–6. [DOI] [PubMed] [Google Scholar]
- 88.Phillips-Eakley AK, McKenney-Drake ML, Bahls M, Newcomer SC, Radcliffe JS, Wastney ME, Van Alstine WG, Jackson G, Alloosh M, Martin BR, et al. . Effect of high-calcium diet on coronary artery disease in Ossabaw miniature swine with metabolic syndrome. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dietary Guidelines: Advisory Report [Internet]. Rockville (MD): Office of Disease Prevention and Health Promotion. 2015 [cited 2015 Jan 8]. Available from: http://www.health.gov/dietaryguidelines/2015-scientific-report/.
- 90.Ross AC, Taylor C, Yaktine A, Del Valle H. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): Institute of Medicine, National Academy Press; . 2011. [PubMed] [Google Scholar]
- 91.Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, Peacock M. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3–4 chronic kidney disease. Kidney Int 2013;83:959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aloia JF, Vaswani A, Yeh JK, Ellis K, Cohn SH. Total body phosphorus in postmenopausal women. Miner Electrolyte Metab 1984;10:73–6. [PubMed] [Google Scholar]
- 93.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, Kovesdy CP, Bross R, Shinaberger CS, Noori N, Hirschberg R, Benner D, Nissenson AR, et al. . Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010;5:519–30. [DOI] [PubMed] [Google Scholar]
- 94. Kidney Disease Improving Global Outcomes, C.K.D.M.B.D. Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009;76 (Suppl 113):S1–S130. [DOI] [PubMed] [Google Scholar]
- 95.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32(Suppl 3)S112–9. [DOI] [PubMed] [Google Scholar]
- 96.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, Wong C, Stehman-Breen C. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 2000;58:396–9. [DOI] [PubMed] [Google Scholar]
- 97.Heaney RP, Recker RR. Effects of nitrogen, phosphorus, and caffeine on calcium balance in women. J Lab Clin Med 1982;99:46–55. [PubMed] [Google Scholar]
- 98.Spencer H, Menczel J, Lewin I, Samachson J. Effect of high phosphorus intake on calcium and phosphorus metabolism in man. J Nutr 1965;86:125–32. [DOI] [PubMed] [Google Scholar]
- 99.Institute of Medicine. DRI: Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington (DC): National Academy Press; 1997. [PubMed] [Google Scholar]
- 100.Martin KJ, Gonzalez EA. Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: What is normal, when to start, and how to treat? Clin J Am Soc Nephrol 2011;6:440–6. [DOI] [PubMed] [Google Scholar]
- 101.Li JW, Xu C, Fan Y, Wang Y, Xiao YB. Can serum levels of alkaline phosphatase and phosphate predict cardiovascular diseases and total mortality in individuals with preserved renal function? A systemic review and meta-analysis. PLoS One 2014;9:e102276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007;167:879–85. [DOI] [PubMed] [Google Scholar]
- 103.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Cholesterol, Recurrent Events Trial I: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005;112:2627–33. [DOI] [PubMed] [Google Scholar]
- 104.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 2011;305:1119–27. [DOI] [PubMed] [Google Scholar]
- 105.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005;16:520–8. [DOI] [PubMed] [Google Scholar]
- 106.Hill Gallant KM. Studying dietary phosphorus intake: The challenge of when a gram is not a gram. Am J Clin Nutr 2015;102:237–8. [DOI] [PubMed] [Google Scholar]
- 107.Chang AR, Lazo M, Appel LJ, Gutierrez OM, Grams ME. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am J Clin Nutr 2014;99:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murtaugh MA, Filipowicz R, Baird BC, Wei G, Greene T, Beddhu S. Dietary phosphorus intake and mortality in moderate chronic kidney disease: NHANES III. Nephrol Dial Transplant 2012;27:990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamamoto KT, Robinson-Cohen C, de Oliveira MC, Kostina A, Nettleton JA, Ix JH, Nguyen H, Eng J, Lima JA, Siscovick DS, et al. . Dietary phosphorus is associated with greater left ventricular mass. Kidney Int 2013;83:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leopold JA. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med 2015;25:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moe SM, Chen NX, Seifert MF, Sinders RM, Duan D, Chen X, Liang Y, Radcliff JS, White KE, Gattone VH 2nd. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int 2009;75:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Report from the Strategic Planning Advisory Panel on Health Disparities: NIH Publication No. 99–4566. Bethesda (MD): National Institutes of Health; 2013. [Google Scholar]
- 113.Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. In: Smedley BDSA, Nelso AR, editors. Washington (DC): Institutes of Medicine; 2002. [PMC free article] [PubMed] [Google Scholar]
- 114.What Is Osteoporosis? [Internet]. Bethesda (MD): Osteoporosis and Related Bone Diseases National Resource Center, National Institutes of Health. 2011 [cited 2014 Feb 6]. Available from: http://www.niams.nih.gov/Health_Info/Bone/Osteoporosis/osteoporosis_ff.asp.
- 115.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 2014;29:2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vásquez E, Shaw BA, Gensburg L, Okorodudu D, Corsino L. Racial and ethnic differences in physical activity and bone density: National Health and Nutrition Examination Survey, 2007–2008. Prev Chronic Dis 2013;10:E216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 2010;140:817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bailey RL, Fulgoni VL 3rd, Keast DR, Lentino CV, Dwyer JT. Do dietary supplements improve micronutrient sufficiency in children and adolescents? J Pediatr 2012;161:837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wallace TC, Reider C, Fulgoni VL 3rd. Calcium and vitamin D disparities are related to gender, age, race, household income level, and weight classification but not vegetarian status in the United States: Analysis of the NHANES 2001–2008 data set. J Am Coll Nutr 2013;32:321–30. [DOI] [PubMed] [Google Scholar]
- 120.Bailey RK, Fileti CP, Keith J, Tropez-Sims S, Price W, Allison-Ottey SD. Lactose intolerance and health disparities among African Americans and Hispanic Americans: An updated consensus statement. J Natl Med Assoc 2013;105:112–27. [DOI] [PubMed] [Google Scholar]
- 121.Walker MD, Novotny R, Bilezikian JP, Weaver CM. Race and diet interactions in the acquisition, maintenance, and loss of bone. J Nutr 2008;138:1256S–60S. [DOI] [PubMed] [Google Scholar]
- 122.Braun M, Palacios C, Wigertz K, Jackman LA, Bryant RJ, McCabe LD, Martin BR, McCabe GP, Peacock M, Weaver CM. Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr 2007;85:1657–63. [DOI] [PubMed] [Google Scholar]
- 123.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, et al. . Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013;369:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lutsey PL, Michos ED, Misialek JR, Pankow JS, Loehr L, Selvin E, Reis JP, Gross M, Eckfeldt JH, Folsom AR. Race and vitamin D binding protein gene polymorphisms modify the association of 25-hydroxyvitamin D and incident heart failure: The ARIC (Atherosclerosis Risk in Communities) Study. JACC Heart Fail 2015;3:347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reis JP, Michos ED, Selvin E, Pankow JS, Lutsey PL. Race, vitamin D-binding protein gene polymorphisms, 25-hydroxyvitamin D, and incident diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2015;101:1232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sonderman JS, Munro HM, Blot WJ, Signorello LB. Reproducibility of serum 25-hydroxyvitamin D and vitamin D-binding protein levels over time in a prospective cohort study of black and white adults. Am J Epidemiol 2012;176:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ponda MP, McGee D, Breslow JL. Vitamin D-binding protein levels do not influence the effect of vitamin D repletion on serum PTH and calcium: Data from a randomized, controlled trial. J Clin Endocrinol Metab 2014;99:2494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Paik JM, Farwell WR, Taylor EN. Demographic, dietary, and serum factors and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int 2012;23:1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA 2005;293:2102–8. [DOI] [PubMed] [Google Scholar]
- 130.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA 2014;312:189–90. [DOI] [PubMed] [Google Scholar]
- 131.Center for Disease Control and Prevention. Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 10-year age groups, by race and sex: United States. 2013. [cited 2015 Jan 8]. Available from: http://www.cdc.gov/nchs/data/dvs/lcwk2_2013.pdf. [Google Scholar]
- 132.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. . Heart disease and stroke statistics–2014 update: A report from the American Heart Association. Circulation 2014;129:e28–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stamler J, Brown IJ, Yap IK, Chan Q, Wijeyesekera A, Garcia-Perez I, Chadeau-Hyam M, Ebbels TM, De Iorio M, Posma J, et al. . Dietary and urinary metabonomic factors possibly accounting for higher blood pressure of black compared with white Americans: Results of International Collaborative Study on macro-/micronutrients and blood pressure. Hypertension 2013;62:1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma S, Cruickshank JK, Green DM, Vik S, Tome A, Kolonel LN. Impact of diet on mortality from stroke: Results from the U.S. multiethnic cohort study. J Am Coll Nutr 2013;32:151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]