Abstract
The present systematic review critically examines the available scientific literature on risk factors for malnutrition in the older population (aged ≥65 y). A systematic search was conducted in MEDLINE, reviewing reference lists from 2000 until March 2015. The 2499 papers identified were subjected to inclusion criteria that evaluated the study quality according to items from validated guidelines. Only papers that provided information on a variable’s effect on the development of malnutrition, which requires longitudinal data, were included. A total of 6 longitudinal studies met the inclusion criteria and were included in the systematic review. These studies reported the following significant risk factors for malnutrition: age (OR: 1.038; P = 0.045), frailty in institutionalized persons (β: 0.22; P = 0.036), excessive polypharmacy (β: −0.62; P = 0.001), general health decline including physical function (OR: 1.793; P = 0.008), Parkinson disease (OR: 2.450; P = 0.047), constipation (OR: 2.490; P = 0.015), poor (OR: 3.30; P value not given) or moderate (β: −0.27; P = 0.016) self-reported health status, cognitive decline (OR: 1.844; P = 0.001), dementia (OR: 2.139; P = 0.001), eating dependencies (OR: 2.257; P = 0.001), loss of interest in life (β: −0.58; P = 0.017), poor appetite (β: −1.52; P = 0.000), basal oral dysphagia (OR: 2.72; P = 0.010), signs of impaired efficacy of swallowing (OR: 2.73; P = 0.015), and institutionalization (β: −1.89; P < 0.001). These risk factors for malnutrition in older adults may be considered by health care professionals when developing new integrated assessment instruments to identify older adults’ risk of malnutrition and to support the development of preventive and treatment strategies.
Keywords: nutritional condition, malnutrition, older population, risk factors, longitudinal studies
Introduction
Older adults (aged ≥65 y) tend to be more prone to nutritional deficiencies (1), because aging may come with an accumulation of diseases and impairments. These include cognitive and physical decline, depressive symptoms, emotional variations (2), and poor oral health (3), along with socioeconomic changes (1). All of these factors may directly influence the balance between nutritional needs and intake (2). Even in cases of adequate nutrient and energy intake, the nutritional status of older adults can be challenged by a compromised nutrient metabolism (such as absorption, distribution, storage, utilization, and excretion), drug–nutrient interactions, or altered nutrient needs (4).
The prevalence of malnutrition in Europe and North America is 1–15% in noninstitutionalized older adults, 25–60% for older adults in geriatric care facilities, and 35–65% in older adults in hospitals (5). Between 2010 and 2050, with a predicted global increase in life expectancy, the population over the age of 80 y will grow from 11.5% to 21.0% worldwide and from 9.0% to 19.0% in the developed countries (6). This will result in an increase of older adults at risk of malnourishment (7).
Malnutrition is related to a decline in general functional status and to decreased bone mass, immune dysfunction, delayed postsurgery recovery, high hospitalization and readmission rates, and increased mortality (8), among other problems. Although malnutrition is a prognostic factor associated with morbidity, mortality, and costs of care (9, 10), nutritional problems in older adults often remain undetected or unaddressed (11). One-fourth of the patients who are nutritionally at risk do not receive nutritional support or counseling, despite having been in contact with health care professionals (12). This suggests that the condition of older adults at risk of malnutrition should be investigated and improved forthwith. For this, identification of prognostic determinants of malnutrition is required. Several studies analyzed factors associated with malnutrition. Most of these studies, however, had a cross-sectional design, whereby causality cannot be established.
This systematic review therefore aims to critically review the available scientific literature with a focus on studies with a longitudinal design on risk factors for malnutrition in the older population. Evaluation of the evidence for such risk factors is needed to facilitate the development of an assessment instrument that enables health care professionals to identify older adults’ risk of malnutrition and to support the development of preventive strategies.
Methods
Data sources and search strategy.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13, 14) and the guidelines described by the Cochrane Community (15) were used to plan, to conduct, and to report this systematic review. Potential studies were identified by searching the MEDLINE database (National Library of Medicine and National Institutes of Health) by using the PubMed interface. The following MeSH terms and operators were used: malnutrition OR malnourished AND risk factor AND (the following PubMed filters) full text AND “2000/01/01”:“2015/03/30” AND Humans AND English AND aged 65+ y. The authors also reviewed the reference lists from the review articles reported in the PubMed search to identify possible additional articles for inclusion.
Selection of studies and data synthesis.
All papers written in English and published between 1 January 2000 and 30 March 2015 were evaluated for inclusion if they presented data about risk factors for malnutrition in older adults (≥65 y). Studies of all types of populations (community dwelling, institutionalized, hospitalized, rural or urban) were included.
A 2-step screening process was used. In step 1, one investigator scanned the titles and abstracts of studies identified by the search for their eligibility. At step 2, full-text articles were screened by one investigator for eligibility. To be included in the present review, the study was required to meet the following criteria:
1. The study presented information about the nutritional status of older adults (≥65 y) based on data of validated measurements.
2. The study sample size was calculated based on a power analysis or included ≥100 subjects.
3. The study population was clearly specified and defined.
4. Key potential confounding variables (e.g., malnutrition/risk of malnutrition at baseline, age, sex, functional capacity, current health status, etc.) were measured and statistically adjusted for their impact on the relation between exposure(s) and outcome(s).
5. The study presented longitudinal data, implying that the comparable nutritional state data of ≥2 time points were measured in the same population and presented, enabling a relation of causality between the variables under investigation and the nutritional status.
6. The time frame between the measurements was appropriate to allow malnutrition to develop as a result of the potential risk factor. This time frame may have depended on the variable under investigation (e.g., a shorter time frame would be considered for acute illness compared to loneliness). The appropriateness of the time frames was discussed with all authors until a consensus was reached.
Data were first extracted from the longitudinal articles to an Excel table containing information such as title, authors, country where the study was performed, publication year and journal, information on how malnutrition was assessed, information about the population under study (age, number, setting), whether the study was interventional or not, the time frame between the measurements, the outcomes, the statistical analyses and results, and whether the results were corrected for possible bias identified by the authors. The data were then extracted from the Excel table into standardized tables by one of the investigators. In the tables, results of studies are reported only for the outcome measures interest. The results are reported as significant at P < 0.05, and no exclusions were made for type of statistical approach.
The concept of malnutrition was accepted as described by the authors of the included articles. The authors of only one paper explicitly defined malnutrition as a disorder of nutritional status resulting from reduced nutrient intake or impaired metabolism (16). The same applies for the concept of other variables analyzed by the longitudinal articles, such as polypharmacy, cognitive decline, low education, etc. The variables under investigation were considered risk factors when they correlated with the development of malnutrition between baseline and the time of reassessment. This implies that only longitudinal studies were eligible for inclusion in this systematic review.
Review of study strength and quality.
The strength and quality of the studies were determined by using items from Downs and Black (17) and the Newcastle scale (18), as well as the Cochrane (15) and PRISMA consort guidelines (13, 14). A review of strength and quality of the longitudinal studies, including risk of bias and appropriate statistical analysis, was assessed independently by 2 researchers (NCFM and CV). In case of disagreement, another researcher (SK-H) was consulted and participated in the discussion until agreement was reached. The decisions were then discussed with all co-authors until a consensus was reached.
Results
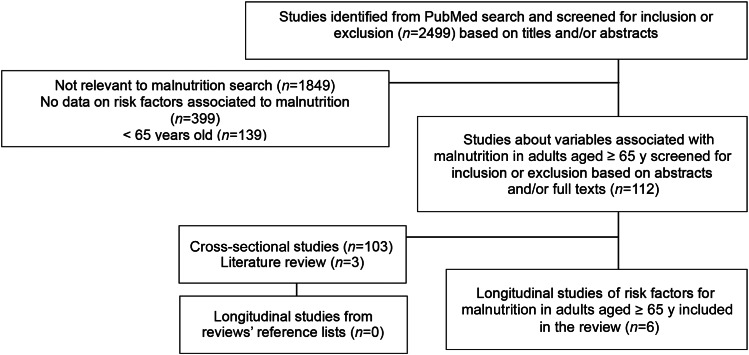
The search resulted in 2499 articles. After analyzing titles and abstracts, 1849 articles not related to malnutrition in older adults were excluded. From the 650 remaining articles, 112 reported on associations of malnutrition in older adults (65 of the studies were performed in European countries, 19 in Asia, 19 in North America, 6 in Oceania, and 3 in Africa). The other 538 articles were excluded because they did not report on associations with malnutrition (399 articles) or they reported on a population of <65 y of age (139 articles). Of the 112 articles reporting on associations of malnutrition in older adults, 103 cross-sectional studies and 3 literature reviews (19–21) were excluded. The reference lists from the 3 review studies previously identified in the PubMed search did not result in additional longitudinal studies. As a result, a final number of 6 longitudinal studies were included in the systematic review. The flow of articles through the review process is displayed in Figure 1.
FIGURE 1.
Flow diagram of the study selection for the review process.
There was no difference between the primary agreement that was established by the first reviewers (NCFM and CV) and the consensus that was reached by all authors on which papers to include. The strength and quality of studies were considered high, with all 6 longitudinal studies meeting the criteria defined by Downs and Black (17) and the Newcastle scale (18), as well as the Cochrane (15) and PRISMA consort guidelines (13, 14).
Nutritional status in the selected studies was assessed by using the following anthropometric measures: body weight (or percentage of initial body weight), weight loss (22), or BMI data (in kg/m2); or through the following validation methods or tools: Mini Nutritional Assessment (23), Mini Nutritional Assessment-Short Form (24, 25), or Elderly Nutrition Screening tool (26).
Table 1 presents the longitudinal studies sorted by publication year and also presents potential risk factors under investigation, population, malnutrition assessment instrument, and summarized results. In Table 2, the factors evaluated for their association with malnutrition (after correction for confounding factors) in the longitudinal studies are categorized into one of the following: physical, psychological, social, oral health-related, and eating-related factors. Table 2 also includes information on the malnutrition assessment instrument, the applied statistical methodology, and the results. The outcome column shows whether the potential risk factor under investigation was positively (+) or negatively (−) related to malnutrition or whether no association was found (0). The statistical analysis used to evaluate the association is also mentioned in Table 2.
TABLE 1.
Longitudinal studies on risk factors for malnutrition in older adults1
| Author, year (reference) | Potential risk factors | Population | Assessment method | Results |
| Shatenstein et al., 2001 (27) | Age | Baseline: 1529 community and 1174 institutionalized subjects | Percentage of initial body weight retained: | Multiple regression: for % of initial body weight2 |
| Cognitive function | Follow-up: 584 community and 237 institutionalized subjects | Prevalence analyses: | Institutionalized subjects: | |
| Study region | >95%, no risk of malnutrition | Frailty, β: −1.23; P = 0.039 | ||
| Ability to eat independently | 85–95%, low risk of malnutrition | Residence in Ontario, β: −9.02; P = 0.000 | ||
| Depression | <85%, moderate/severe risk of malnutrition | Residence in British Columbia, β: −5.62; P = 0.026 | ||
| Self-reported interest in life | Regression analyses: | Residence in Atlantic, β: −3.41; P = 0.225 | ||
| Loss of appetite | >95%, no risk of malnutrition | Residence in prairies, β: −4.54; P = 0.087 | ||
| Weight loss | ≤95%, risk of malnutrition | Community subjects: | ||
| Frailty | Ability to eat unaided, β: 4.24; P = 0.000 | |||
| For community subjects: | Reported sustained interest in life, β: 2.22; P = 0.013 | |||
| Ability to shop | Logistic regression: for malnutrition | |||
| Bereavement | Institutionalized subjects: | |||
| Frailty, β: 0.22; 95% CI: 1.01, 1.54; P = 0.036 | ||||
| Loss of interest in life, β: −0.63; 95% CI: 0.30, 0.93; P = 0.027 | ||||
| Community subjects: | ||||
| Loss of appetite, β: −1.52; 95% CI: 0.12, 0.42; P = 0.000 | ||||
| Loss of interest in life, β: −0.58; 95% CI: 0.34, 0.90; P = 0.017 | ||||
| Mamhidir et al., 2006 (28) | Underweight | Baseline: 719 institutionalized subjects | BMI < 22 and loss of 5% of body weight after 1 mo and 10% after 6 mo | Multiple logistic regression: for malnutrition |
| Weight loss | Follow-up: 503 institutionalized subjects | Dementia, OR: 2.139; 95% CI: 1.343, 3.407; P = 0.001 | ||
| Cognitive function | Parkinson disease, OR: 2.450; 95% CI: 1.006, 5.965; P = 0.047 | |||
| Depression | Eating dependencies, OR: 2.257; 95% CI: 1.676, 3.038; P = 0.001 | |||
| Functional impairment | Constipation, OR: 2.490; 95% CI: 1.185, 4.964; P = 0.015 | |||
| Age | Daily dental hygiene was associated with better weight status (values not given) | |||
| Sex | Logistic regression: predictive factors for malnutrition | |||
| Medical factors: vision problems, eating dependencies, constipation, heart failure, hip fracture, stroke, dementia, Parkinson disease, cancer | Cognitive capacity, OR: 1.844; 95% CI: 1.267, 2.683; P = 0.001 | |||
| Chewing and swallowing disorders | Functional decline, OR: 1.793; 95% CI: 1.163, 2.765; P = 0.008 | |||
| Mouth pain | Age, OR: 1.038; 95% CI: 1.001, 1.077; P = 0.045 | |||
| Complaints about the taste of the food | ||||
| Hunger | ||||
| Often leaves 25% of food uneaten | ||||
| Intake of dietary supplements | ||||
| Dental status | ||||
| Number of medications (last 7 d) | ||||
| Roberts et al., 2007 (29) | Sex | Baseline: 839 community subjects | ENS (low, moderate, or high risk of malnutrition classification) | Bivariate model: for malnutrition risk3 |
| Age at baseline | Follow-up: 779 community subjects | Sex (ref = female), OR: 0.83; 95% CI: 0.52, 1.32 | ||
| Highest level of education | Age (y), OR: 1.01; 95% CI: 0.95, 1.07 | |||
| Income satisfaction | Some high school education, OR: 1.00; 95% CI: 0.45, 2.20 | |||
| Medical conditions (chronic disease score) | High school education complete, OR: 1.49; 95% CI: 0.67, 3.32 | |||
| Measure of physical limitations | College or technical education, OR: 1.36; 95% CI: 0.60, 3.09 | |||
| Current health status, and status compared with the previous year | University education, OR: 1.21; 95% CI: 0.56, 2.62 | |||
| Psychological variables and distress | Income satisfaction (ref = no), OR: 0.60; 95% CI: 0.31, 1.14 | |||
| Type of housing | Physical limitations, OR: 1.12; 95% CI: 0.87, 1.44 | |||
| Number of cohabitants | ADL, OR: 1.26; 95% CI: 0.65, 2.45 | |||
| Marital status | IADL, OR: 1.61; 95% CI: 1.02, 2.55 | |||
| Perceived satisfaction with social support | Chronic disease score, OR: 1.02; 95% CI: 0.98, 1.07 | |||
| Stomach aches (ref = no), OR: 1.49; 95% CI: 0.89, 2.49; P value not given | ||||
| Dental problems (ref = no), OR: 1.39; 95% CI: 0.87, 2.22 | ||||
| Good current self-rated health (ref = excellent), OR: 1.66; 95% CI: 1.01, 2.75 | ||||
| Poor current self-rated health (ref = excellent), OR: 3.74; 95% CI: 1.65, 8.51 | ||||
| Worse self-rated health compared with the previous year (ref = the same), OR: 1.38; 95% CI: 0.62, 3.08 | ||||
| Better self-rated health compared with the previous year (ref = the same), OR: 0.55; 95% CI: 0.30, 0.99 | ||||
| Psychological distress (ref = low), OR: 1.35; 95% CI: 0.51, 3.60 | ||||
| Marital status (ref = not married), OR: 0.77; 95% CI: 0.48, 1.25 | ||||
| Satisfaction with social support (ref = no), OR: 0.56; 95% CI: 0.29, 1.08 | ||||
| 1 cohabitant (ref = 0), OR: 0.80; 95% CI: 0.49, 1.29 | ||||
| ≥2 cohabitants (ref = 0), OR: 1.61; 95% CI: 0.56, 4.63 | ||||
| Living in a house (ref = apartment), OR: 1.15; 95% CI: 0.69, 1.93 | ||||
| Living in a senior’s residence, OR: 1.45; 95% CI: 0.68, 3.10 | ||||
| Living in subsidized or nonprofit housing, OR: 0.94; 95% CI: 0.27, 3.33 | ||||
| Other kind of housing, OR: 3.29; 95% CI: 0.59, 8.47 | ||||
| Multivariable model: for malnutrition risk3 | ||||
| Sex (ref = female), OR: 0.93; 95% CI: 0.58, 1.51 | ||||
| Age (y), OR: 1.00; 95% CI: 0.94, 1.07 | ||||
| Good current self-rated health (ref = excellent), OR: 1.48; 95% CI: 0.87, 2.50 | ||||
| Poor current self-rated health (ref = excellent), OR: 3.30; 95% CI: 1.42, 7.67 | ||||
| Worse self-rated health compared with the previous year (ref = the same), OR: 1.09; 95% CI: 0.47, 2.50 | ||||
| Better self-rated health compared with the previous year (ref = the same), OR: 0.55; 95% CI: 0.30, 1.00 | ||||
| Jyrkkä et al., 2011 (30) | Polypharmacy status | Baseline: 294 community or institutionalized subjects | MNA-SF (≤11 = malnourished or at risk, ≥12 = well-nourished) | Linear mixed model: for decline in nutritional status (points compared with ref) |
| Residential status | Follow-up: 294 community or institutionalized subjects | Excessive polypharmacy (ref = nonpolypharmacy), β: −0.62; 95% CI: −0.98, −0.27; P = 0.001 | ||
| Self-reported health status | Age, β: −0.04; 95% CI: −0.08, −0.01; P = 0.016 | |||
| Nutritional status - MNA-SF | Institutionalized (ref = home), β: −1.89; 95% CI: −2.38, −1.39; P < 0.001 | |||
| Functional ability | Self-reported health status moderate (ref = good), β: −0.27; 95% CI: −0.49, −0.05; P = 0.016 | |||
| Cognitive capacity | Self-reported health status poor (ref = good), β: −1.05; 95% CI: −1.38, −0.73; P < 0.001 | |||
| Functional comorbidity index | Time of measurement 2005 (ref = 2004), β: −0.28; 95% CI: −0.50, −0.06; P = 0.011 | |||
| BMI > 30 | Time of measurement 2006 (ref = 2004), β: −0.42; 95% CI: −0.65, −0.20; P < 0.001 | |||
| Time of measurement 2007 (ref = 2004), β: −0.37; 95% CI: −0.60, −0.15; P = 0.001 | ||||
| Polypharmacy (ref = nonpolypharmacy), β: −0.12; 95% CI: −0.37, 0.13; P = 0.333 | ||||
| Male sex (ref = female), β: 0.11; 95% CI: −0.20, 0.42; P = 0.471 | ||||
| 0–6 y of education (ref = 7 y), β: −0.03; 95% CI: −0.32, 0.26; P = 0.823 | ||||
| Functional comorbidity index, β: 0.01; 95% CI: −0.08, 0.09; P = 0.950 | ||||
| Schilp et al., 2011 (16) | Education level | Baseline: 1120 subjects (98% living in the community) | BMI < 20 or self-reported involuntary weight loss ≥5% in the last 6 mo | Univariate model: for incidence of malnutrition3 |
| Cognitive functioning | Follow-up: 839 subjects | Light alcohol use, HR: 0.67; 95% CI: 0.46, 0.98 | ||
| Monthly household income | Female sex, HR: 1.40; 95% CI: 1.01, 1.92 | |||
| Depression | Loneliness, HR: 1.47; 95% CI: 1.06, 2.04 | |||
| Anxiety | No partner present, HR: 1.70; 95% CI: 1.24, 2.33 | |||
| Presence of chronic diseases (comorbidity) | Depressive symptoms, HR: 1.96; 95% CI: 1.32, 2.93 | |||
| Medication use | Anxiety symptoms, HR: 1.75; 95% CI: 1.11, 2.78; P value not given | |||
| Appetite during the last week | ≥2 chronic diseases, HR: 2.08; 95% CI: 1.31, 3.28 | |||
| Subjective pain | Poor appetite, HR: 1.99; 95% CI: 1.32, 3.00 | |||
| Problems biting and chewing | Limitations performing normal activities because of a health problem, HR: 1.76; 95% CI: 1.28, 2.43 | |||
| Limitation of normal activities because of a health problem | ≥3 medications, female, HR: 2.57; 95% CI: 1.50, 4.38 | |||
| Physical performance | Low physical performance test score (<75 y old), HR: 0.89; 95% CI: 0.81, 0.96 | |||
| Difficulty walking stairs | Difficulty walking stairs (<75 y old), HR: 2.50; 95% CI: 1.59, 3.91 | |||
| Smoking status | Pain data missing, female, HR: 1.62; 95% CI: 1.01, 2.61 | |||
| Alcohol use | Age ≥ 75 y, HR: 1.30; 95% CI: 0.95, 1.79 | |||
| Physical activity in the previous 2 wk | Medium education, HR: 0.78; 95% CI: 0.56, 1.09 | |||
| Visual or hearing impairment | High education, HR: 0.94; 95% CI: 0.56, 1.58 | |||
| Loneliness | Medium income, HR: 0.98; 95% CI: 0.66, 1.44 | |||
| Individuals without a partner inside or outside the household | High income, HR: 0.89; 95% CI: 0.57, 1.39 | |||
| Type of housing (independent and nonindependent living) | Missing income data, HR: 0.93; 95% CI: 0.54, 1.62 | |||
| Poor cognitive status, HR: 0.94; 95% CI: 0.49, 1.78 | ||||
| 1 chronic disease, HR: 1.23; 95% CI: 0.76, 2.00 | ||||
| 1–2 medications, male, HR: 0.47; 95% CI: 0.23, 0.95 | ||||
| 1–2 medications, female, HR: 0.36; 95% CI: 0.76, 2.41 | ||||
| ≥3 medications, male, HR: 1.51; 95% CI: 0.86, 2.66 | ||||
| Pain, male, HR: 1.29; 95% CI: 0.70, 2.37 | ||||
| Pain, female, HR: 1.37; 95% CI: 0.82, 2.27 | ||||
| Pain data missing, male, HR: 0.62; 95% CI: 0.29, 1.33 | ||||
| Frequent problems biting or chewing, HR: 1.81; 95% CI: 0.57, 1.16 | ||||
| Missing data on problems biting or chewing, HR: 0.83; 95% CI: 0.52, 1.32 | ||||
| Vision problems, HR: 1.00; 95% CI: 0.65, 1.52 | ||||
| Hearing problems, HR: 1.42; 95% CI: 0.93, 2.16 | ||||
| Low physical performance test score (≥75 y old), HR: 1.01; 95% CI: 0.92, 1.11 | ||||
| Difficulty walking stairs (≥75 y old), HR: 1.08; 95% CI: 0.67, 1.75 | ||||
| Former smoker, HR: 0.82; 95% CI: 0.50, 1.33 | ||||
| Current smoker, HR: 1.08; 95% CI: 0.73, 1.61 | ||||
| Moderate alcohol use, HR: 0.82; 95% CI: 0.52, 1.30 | ||||
| Excessive alcohol use, HR: 1.16; 95% CI: 0.52, 2.58 | ||||
| Physical activity, HR: 0.99; 95% CI: 0.997, 1.000 | ||||
| Independent housing, HR: 3.13; 95% CI: 0.44, 22.33 | ||||
| Multivariate model: for incidence of malnutrition3 | ||||
| Poor appetite, HR: 1.63; 95% CI: 1.02, 2.61 | ||||
| Difficulty walking stairs (<75 y old), HR: 1.91; 95% CI: 1.14, 3.22 | ||||
| 1–2 medications, female (interaction with sex), HR: 0.39; 95% CI: 0.18, 0.83 | ||||
| Female sex, HR: 0.73; 95% CI: 0.38, 1.39 | ||||
| Age ≥ 75 y, HR: 0.88; 95% CI: 0.29, 2.63 | ||||
| Depressive symptoms, HR: 0.89; 95% CI: 0.52, 1.52 | ||||
| Anxiety symptoms, HR: 1.26; 95% CI: 0.72, 2.21 | ||||
| 1 chronic disease, HR: 1.10; 95% CI: 0.64, 1.88 | ||||
| ≥2 chronic diseases, HR: 1.32; 95% CI: 0.75, 2.33 | ||||
| 1–2 medications, male, HR: 1.10; 95% CI: 0.60, 2.02 | ||||
| ≥3 medications, male, HR: 1.80; 95% CI: 0.99, 3.27 | ||||
| ≥3 medications, female, HR: 1.03; 95% CI: 0.54, 1.96 | ||||
| Limitations of normal activities due to a health problem, HR: 1.20; 95% CI: 0.81, 1.77 | ||||
| Low physical performance test score, age < 75 y, HR: 0.98; 95% CI: 0.89, 1.08 | ||||
| Low physical performance test score, age ≥ 75 y, HR: 1.06; 95% CI: 0.95, 1.18 | ||||
| Difficulty walking stairs (≥75 y old) (interaction with age), HR: 0.88; 95% CI: 0.51, 1.50 | ||||
| Light alcohol use, HR: 0.82; 95% CI: 0.55, 1.96 | ||||
| Moderate alcohol use, HR: 1.11; 95% CI: 0.67, 1.83 | ||||
| Excessive alcohol use, HR: 1.42; 95% CI: 0.58, 3.46 | ||||
| Loneliness, HR: 1.11; 95% CI: 0.75, 1.64 | ||||
| Partner present, HR: 1.37; 95% CI: 0.92, 2.02 | ||||
| Serra-Prat et al., 2012 (31) | Age | Baseline: 254 community subjects (69 subjects with OD and 185 without OD); Follow-up: 227 community subjects | MNA (>23.5 = well nourished, ≤23.5 = malnourished or at risk of malnutrition) | Logistic regression: for M/RM |
| Sex | Basal OD (on prevalence of M/RM), OR: 2.72; 95% CI: 1.25, 5.95; P = 0.010 | |||
| Education | Impaired efficacy of swallow (on prevalence of M/RM), OR: 2.73; 95% CI: 1.19, 6.26; P = 0.015 | |||
| Family support | Basal OD (on weight loss > 5%), OR: 1.33; 95% CI: 0.55, 3.24; P = 0.336 | |||
| Toxic habits | Impaired efficacy of swallow (on weight loss > 5%), OR: 1.30; 95% CI: 0.49, 3.46; P = 0.380 | |||
| Comorbidities | Effect adjusted by age, Barthel score, basal nutritional status: | |||
| Physical exploration (weight, height, waist circumference, and handgrip strength) | Impaired efficacy of swallow, OR: 2.31; 95% CI: 0.96, 5.57; P = 0.062 | |||
| Functional capacity | Age, OR: 1.03; 95% CI: 0.96, 1.08; P = 0.448 | |||
| Nutritional status | Barthel score, OR: 0.99; 95% CI: 0.95, 1.02; P = 0.443 | |||
| Frail condition | M/RM at baseline, OR: 0.70; 95% CI: 0.26, 1.89; P = 0.481 | |||
| Loss of >5% of initial handgrip strength, male, OR: 2.33; 95% CI: 1.02, 5.36; P = 0.043 |
All studies (16, 27–31) are from Europe, except Shatenstein et al. (27), which is from North America. ADL, activities of daily living; ENS, Elderly Nutrition Screening tool; IADL, instrumental activities of daily living; MNA, Mini Nutritional Assessment; MNA-SF, Mini Nutritional Assessment-Short Form; M/RM, malnutrition/risk of malnutrition; OD, oral dysphagia; ref, reference; β, standardized regression coefficient.
95% CI values not given.
P values not given.
TABLE 2.
Risk factors for malnutrition in older adults identified in the included longitudinal studies1
| Risk factor | Reference | Assessment method | Analysis | Statistics | Outcome2 | |
| Physical factors | Frailty (institutionalized subjects) | (27) | % of initial body weight | Multivariate model | β: −1.23; 95% CI: values not given; P = 0.039 | + |
| (27) | % of initial body weight | Logistic regression | β: 0.22; 95% CI: 1.01, 1.54; P = 0.036 | + | ||
| 1−2 medications | (16) | BMI | Multivariate model | Female (interaction with sex), HR: 0.39; 95% CI: 0.18, 0.83; P value not given | − | |
| (16) | BMI | Multivariate model | Male, HR: 1.10; 95% CI: 0.60, 2.02; P value not given | 0 | ||
| ≥3 medications | (16) | BMI | Multivariate model | Male, HR: 1.80; 95% CI: 0.99, 3.27; P value not given | 0 | |
| (16) | BMI | Multivariate model | Female, HR: 1.03; 95% CI: 0.54, 1.96; P value not given | 0 | ||
| Polypharmacy | (30) | MNA-SF | Linear mixed model | β: −0.12; 95% CI: −0.37, 0.13; P = 0.333 | 0 | |
| Excessive polypharmacy | (30) | MNA-SF | Linear mixed model | β: −0.62; 95% CI: −0.98, −0.27; P = 0.001 | + | |
| 1 chronic disease | (16) | BMI | Multivariate model | HR: 1.10; 95% CI: 0.64, 1.88; P value not given | 0 | |
| ≥2 chronic diseases | (16) | BMI | Multivariate model | HR: 1.32; 95% CI: 0.75, 2.33; P value not given | 0 | |
| Limitations of normal activities because of a health problem | (16) | BMI | Multivariate model | HR: 1.20; 95% CI: 0.81, 1.77; P value not given | 0 | |
| Low physical performance test score | (16) | BMI | Multivariate model | Age < 75 y, HR: 0.98; 95% CI: 0.89, 1.08; P value not given | 0 | |
| (16) | BMI | Multivariate model | Age ≥ 75 y, HR: 1.06; 95% CI: 0.95, 1.18; P value not given | 0 | ||
| Functional comorbidity index | (30) | MNA-SF | Linear mixed model | β: 0.01; 95% CI: −0.08, 0.09; P = 0.950 | 0 | |
| Functional decline | (28) | BMI | Logistic regression | OR: 1.793; 95% CI: 1.163, 2.765; P = 0.008 | + | |
| Barthel score | (31) | MNA | Logistic regression | OR: 0.99; 95% CI: 0.95, 1.02; P = 0.443 | 0 | |
| Difficulty walking stairs | (16) | BMI | Multivariate model | ≥75 y old (interaction with age), HR: 0.8; 95% CI: 0.51, 1.50; P value not given | 0 | |
| (16) | BMI | Multivariate model | <75 y old, HR: 1.91; 95% CI: 1.14, 3.22; P value not given | + | ||
| Light alcohol use | (16) | BMI | Multivariate model | HR: 0.82; 95% CI: 0.55, 1.96; P value not given | 0 | |
| Moderate alcohol use | (16) | BMI | Multivariate model | HR: 1.11; 95% CI: 0.67, 1.83; P value not given | 0 | |
| Excessive alcohol use | (16) | BMI | Multivariate model | HR: 1.42; 95% CI: 0.58, 3.46; P value not given | 0 | |
| Dementia | (28) | BMI | Multiple logistic regression | OR: 2.139; 95% CI: 1.343, 3.407; P = 0.001 | + | |
| Cognitive capacity | (28) | BMI | Logistic regression | OR: 1.844; 95% CI: 1.267, 2.683; P = 0.001 | + | |
| Parkinson disease | (28) | BMI | Multiple logistic regression | OR: 2.450; 95% CI: 1.006, 5.965; P = 0.047 | + | |
| Constipation | (28) | BMI | Multiple logistic regression | OR: 2.490; 95% CI: 1.185, 4.964; P = 0.015 | + | |
| Age (y) | (28) | BMI | Logistic regression | OR: 1.038; 95% CI: 1.001, 1.077; P = 0.045 | + | |
| (31) | MNA | Logistic regression | OR: 1.03; 95% CI: 0.96, 1.08; P = 0.448 | 0 | ||
| (30) | MNA-SF | Linear mixed model | β: −0.04; 95% CI: −0.08, −0.01; P = 0.016 | + | ||
| (29) | ENS | Multivariate model | OR: 1.00; 95% CI: 0.94, 1.07; P value not given | 0 | ||
| (16) | BMI | Multivariate model | ≥75 y old, HR: 0.88; 95% CI: 0.29, 2.63; P value not given | 0 | ||
| Loss of >5% of initial handgrip strength (male) | (31) | MNA | Logistic regression | OR: 2.33; 95% CI: 1.02, 5.36; P = 0.043 | + | |
| Sex | (29) | ENS | Multivariate model | Ref = female, OR: 0.93; 95% CI: 0.58, 1.51; P value not given | 0 | |
| (30) | MNA-SF | Linear mixed model | Male, β: 0.11; 95% CI: −0.20, 0.42; P = 0.471 | 0 | ||
| (16) | BMI | Multivariate model | Female, HR: 0.73; 95% CI: 0.38, 1.39; P value not given | 0 | ||
| Good current self-rated health | (29) | ENS | Multivariate model | Ref = excellent, OR: 1.48; 95% CI: 0.87, 2.50; P value not given | 0 | |
| Moderate current self-rated health | (30) | MNA-SF | Linear mixed model | β: −0.27; 95% CI: −0.49, −0.05; P = 0.016 | + | |
| Poor current self-rated health | (29) | ENS | Multivariate model | Ref = excellent, OR: 3.30; 95% CI: 1.42, 7.67; P value not given | + | |
| (30) | MNA-SF | Linear mixed model | β: −1.05; 95% CI: −1.38, −0.73; P < 0.001 | + | ||
| Worse self-rated health compared with the previous year | (29) | ENS | Multivariate model | Ref = the same, OR: 1.09; 95% CI: 0.47, 2.50; P value not given | 0 | |
| Better self-rated health compared with the previous year | (29) | ENS | Multivariate model | Ref = the same, OR: 0.55; 95% CI: 0.30, 1.00; P value not given | 0 | |
| Psychological factors | Reported loss of interest in life (institutionalized subjects) | (27) | % of initial body weight | Logistic regression | β: −0.63; 95% CI: 0.30, 0.93; P = 0.027 | + |
| Reported loss of interest in life (community subjects) | (27) | % of initial body weight | Logistic regression | β: −0.58; 95% CI: 0.34, 0.90; P = 0.017 | + | |
| Reported sustained interest in life (community subjects) | (27) | % of initial body weight | Multivariate model | β: 2.22; 95% CI values not given; P = 0.013 | − | |
| Depressive symptoms | (16) | BMI | Multivariate model | HR: 0.89; 95% CI: 0.52, 1.52; P value not given | 0 | |
| Anxiety symptoms | (16) | BMI | Multivariate model | HR: 1.26; 95% CI: 0.72, 2.21; P value not given | 0 | |
| Loneliness | (16) | BMI | Multivariate model | HR: 1.11; 95% CI: 0.75, 1.64; P value not given | 0 | |
| Partner present | (16) | BMI | Multivariate model | HR: 1.37; 95% CI: 0.92, 2.02; P value not given | 0 | |
| Oral health-related factors | Daily dental hygiene | (28) | BMI | Logistic regression | Not given | − |
| Basal OD (on prevalence of M/RM) | (31) | MNA | Logistic regression | OR: 2.72; 95% CI: 1.25, 5.95; P = 0.010 | + | |
| (29) | Weight loss > 5% | Logistic regression | OR: 1.33; 95% CI: 0.55, 3.24; P = 0.336 | 0 | ||
| Impaired efficacy of swallow (on prevalence of M/RM) | (29) | MNA | Logistic regression | OR: 2 0.73; 95% CI: 1.19, 6.26; P = 0.015 | + | |
| (29) | Weight loss > 5% | Logistic regression | OR: 1.30; 95% CI: 0.49, 3.46; P = 0.380 | 0 | ||
| Social factors | Residence in Ontario (institutionalized subjects) | (27) | % of initial body weight | Multivariate model | β: −1.23; 95% CI values not given; P = 0.039 | + |
| Residence in British Columbia (institutionalized subjects) | (27) | % of initial body weight | Multivariate model | β: −5.62, 95% CI values not given; P = 0.026 | + | |
| Residence in Atlantic (institutionalized subjects) | (27) | % of initial body weight | Multivariate model | β: −3.41, 95% CI values not given; P = 0.225 | 0 | |
| Residence in prairies (institutionalized subjects) | (27) | % of initial body weight | Multivariate model | β: −4.54, 95% CI values not given; P = 0.087 | 0 | |
| Institutionalized | (30) | MNA-SF | Linear mixed model | β: −1.89; 95% CI: −2.38, −1.39; P < 0.001 | + | |
| 0–6 y of education | (30) | MNA-SF | Linear mixed model | β: −0.03; 95% CI: −0.32, 0.26; P = 0.823 | 0 | |
| Eating-related factors | Loss of appetite (community-living subjects) | (27) | % of initial body weight | Logistic regression | β: −1.52; 95% CI: 0.12, 0.42; P = 0.000 | + |
| Poor appetite | (16) | BMI | Multivariate model | HR: 1.63; 95% CI: 1.02, 2.61; P value not given | + | |
| Eating dependency | (28) | BMI | Multiple logistic regression | OR: 2.257; 95% CI: 1.676, 3.038; P = 0.001 | + | |
| Ability to eat unaided (community-living subjects) | (27) | % of initial body weight | Multivariate model | β: 4.24, 95% CI values not given; P = 0.000 | − |
ENS, Elderly Nutrition Screening tool; MNA, Mini Nutritional Assessment; MNA-SF, Mini Nutritional Assessment-Short Form; M/RM, malnutrition/risk of malnutrition; OD, oral dysphagia; ref, reference; β, standardized regression coefficient.
+ indicates positive association with malnutrition; 0 indicates no association; and − indicates negative association.
In the 6 longitudinal studies, the following factors were found to statistically correlate with the development of malnutrition. Physical factors were frailty (for institutionalized people) (27), excessive polypharmacy (defined as taking ≥10 drugs) (30), functional decline (28, 30, 31), difficulty walking stairs (for persons <75 y old) (16), decline in cognitive capacity and dementia, Parkinson disease, constipation (28), loss of >5% of initial handgrip strength (31), and poor or moderate self-reported health status (29, 30).
Of the 5 studies that evaluated age, 2 (28, 30) presented this variable as a risk factor for malnutrition, whereas the others (16, 29, 30) did not observe an association. Excessive polypharmacy was identified as a risk factor for malnutrition in women, but not in men. Taking 1–2 drugs reduced the risk of malnutrition compared with taking no drugs in female participants (16).
Basal oral dysphagia and signs of impaired swallowing efficacy were statistically significant oral risk factors for malnutrition when assessed by the Mini Nutritional Assessment questionnaire but not when assessed by means of weight loss measurements (basal OD P = 0.010, impaired efficacy of swallow P = 0.015) (31). Moreover, daily oral hygiene was shown to lead to a better nutritional status (28). Poor appetite (16, 27) and needing assistance to eat (28) were statistically significant eating-related risk factors for malnutrition, whereas the ability to eat independently was related to the improvement of the nutritional status (27).
The only psychological factor related to the development of malnutrition was the loss of interest in life among institutionalized and community dwelling persons. A sustained interest in life was shown to predict a higher weight (27). Depressive symptoms, anxiety, loneliness, and not having a partner, as independent variables, were not related to the development of malnutrition (16).
Social factors demonstrated to be predictors for malnutrition were institutionalization (30) and residence in Ontario or British Columbia (27). Shatenstein et al. (27) looked at the risk of malnutrition in different regions in Canada, observing higher malnutrition incidence in Ontario or British Columbia compared with Quebec. Low educational level (defined as completion of 0–6 y of school) was not related to the progress of malnutrition over time (30).
The review articles (n = 3) and cross-sectional (n = 103) studies are presented as supplemental information (Supplemental Tables 1 and 2, respectively). All 103 cross-sectional studies were observational cohort studies, and no interventional studies were found. Of the 3 reviews identified, only Tamura et al. (19) performed a systematic literature review. The reviews conducted by Pauly et al. (20) and Bocock et al. (21) were not performed by using a systematic review approach. In the latter reviews, no rigid quality control was performed, resulting in a mere presentation of the identified papers.
Discussion
This systematic review presents information on potential risk factors for malnutrition in older adults, which allows the development of a malnutrition screening instrument that takes the multifactorial nature of malnutrition into account. Because a risk factor can only be identified if it causes an effect over time, the present systematic literature review includes only longitudinal studies in order to evaluate potential risk factors for malnutrition (16, 27–31).
When combining risk factors, the prevalence of malnutrition is higher in the older population than in younger adults (32). However, aging emerged as a risk factor for malnutrition in only 2 (28, 30) of the 5 longitudinal studies that included the effect of age, indicating that age as an isolated factor is not always confirmed as a risk factor for malnutrition (33, 34). Rather than age, the gradual deterioration of health status and body function caused by aging (35), also known as frailty, is suggested to be an important determinant for malnutrition among older individuals (36, 37). The concept of frailty denotes the multidimensional syndrome of the loss of reserves such as energy, physical ability, and cognition and an increase in vulnerability (38). As a result, a vast number of approaches have been used to assess frailty in the older population, which makes frailty a challenging parameter to discuss, especially because it is commonly defined based on variables that can be studied as isolated risk factors. Functional decline is an example of a physical performance measure of frailty, which is also identified as a significant risk factor for malnutrition (functional decline P = 0.008) (28), when defined as having difficulty walking stairs at < 75 y of age (16), loss of >5% of initial handgrip strength in men with oral dysphagia (31), or needing assistance to eat (27, 28). These results are in contrast to the findings of Jyrkkä et al. (30) and Serra-Prat et al. (31), showing no association between general physical performance or performance of daily life activities and the development of malnutrition. The conflicting observations may be due to the higher mean age and percentage of female participants in the Mamhidir et al. (28) study (85.8 y, 71.0% women) than in the studies by Jyrkkä et al. (30) (81.4 y, 69% female) and Serra-Prat et al. (31) (78.2 y, 46.5% female). Female sex as an isolated factor could not be identified as a risk factor for malnutrition (16) but, as well as older ages, is shown to be associated with greater overall prevalence of disability and functional limitation (39), which is likely to increase the probability of an association between functional impairment and malnutrition. Moreover, the Mamhidir et al. (28) study was conducted in individuals living in sheltered housing, in which the proportion of functionally disabled and malnourished subjects ≥65 y of age is expected to be higher (40) than in the general population in which the studies by Jyrkkä et al. (30) and Serra-Prat et al. (31) were performed.
Aging, and consequently frailty progress, can also be indirectly related to the development of malnutrition caused by health decline, which comes with onsets of physical and psychological diseases, increased medication intake (26), cognitive impairment, and dementia. Although one could expect a great number of diseases to be related to malnutrition development, only Parkinson disease, constipation (28), and basal oral dysphagia and signs of impaired swallowing (30) were observed to have a significant impact on the nutritional status because of the advanced age of the population included in the study (Parkinson disease P = 0.047, constipation P = 0.015, basal oral dysphagia P = 0.010, impaired efficacy of swallow P = 0.015). Some diseases are a challenge to investigate in advanced age because of the high mortality rates. In this context, a number of other diseases known to be risk factors for malnutrition in younger adults could be considered, such as head and neck (41) or gastric (42) oncology or congestive heart failure (43). The onset of Parkinson disease, on the other hand, often occurs at an older age (44), which enables investigation of the relation between this disease and malnutrition in this population. Parkinson disease is usually accompanied by severe motor symptoms (45–48), decreased mobility (49), reduced ability to carry out the activities of daily living (48, 50, 51), increased medication intake (45, 51), and cognitive impairment (52), all factors leading to a higher risk of developing malnutrition in the individuals with this condition (53–55). Constipation is also more prevalent in older adults because of slowing of the gastrointestinal transit (56), which is due to several factors such as increased rectal compliance, delayed colonic transit, low intake of dietary fiber, and neuromuscular disorders (57). Increased prevalence of dysphagia, on the other hand, is due to a vicious cycle in which dysphagia contributes to malnutrition and malnutrition contributes to further deterioration of functional capacity and muscle debilitation, which, in turn, favors dysphagia.
Cognitive decline and dementia were found to be statistically significant risk factors for malnutrition (28), which is consistent with numerous cross-sectional studies (dementia P = 0.001, cognitive decline P = 0.001) (5, 40, 58–70). The relation between cognitive impairment and nutritional risk seems to be a complex and reciprocal problem (71) because a variety of factors that were found to have an impact on malnutrition were also associated with a lower cognitive state, such as oral health-related problems (36, 70, 72–86), which was found to comply with the results of Mamhidir et al. (28), demonstrating that daily dental hygiene leads to a decrease in malnutrition prevalence over time.
Although many cross-sectional studies found an association between malnutrition and depression (19, 33, 34, 65–70, 87–91), anxiety (83, 92), and loneliness (81, 93–95), these factors were not identified as risk factors in the longitudinal study performed by Schilp et al. (16). However, poor or moderate self-reported health status was observed to be a significant risk factor for malnutrition (29, 30), whereas better self-rated health compared with the previous year was observed to be protective (poor P < 0.001, moderate P = 0.016) (29). These findings might be explained by the fact that those people who have a positive opinion about their general health are more alert and probably have an increased awareness of their nutritional needs (58). Poor or moderate self-reported health status was also related to loss of interest in life, the only psychological factor that was significantly correlated with increased weight loss in institutionalized and community-dwelling older adults (27).
As the occurrence of diseases that require pharmacologic treatment becomes more common with aging, higher age is accompanied by an increasing prevalence of (excessive) polypharmacy (96, 97). The extent of medication intake is a factor that influences, either directly or indirectly, the risk of malnutrition (16, 30). Whereas moderate medication intake seems to protect from malnutrition in older female participants (16), excessive medication intake (>10 drugs) has an inverse effect (30). All cross-sectional studies but one (34) also observed a positive correlation between polypharmacy and malnutrition. However, the findings from this systematic review regarding polypharmacy are difficult to compare because cross-sectional studies do not distinguish between various levels of polypharmacy (1, 34, 66, 82, 90, 98). Furthermore, the side effects of excessive polypharmacy can indirectly affect the development of malnutrition. Examples of such pharmacologic side effects are poor appetite or loss of appetite (56), also shown to be a risk factor for malnutrition (16, 27); physical and cognitive decline (56, 99, 100); dry mouth (hyposalivation and/or xerostomia) (101); nausea (102); and constipation (56).
Institutionalization of older adults was found to be a factor that contributes to the development of malnutrition in this specific population (28), which is in line with the available cross-sectional studies in the literature that comprehensively reports a decrease in nutritional status when moving to a long-term care institution (40, 64, 103–106). However, the reasons for this association should be carefully investigated. The hypothesis that poor care or care-related factors play a role in residents’ malnourishment was not confirmed in a study by Suominen et al. (40). Also other confounding variables in institutionalized older adults are shown to be related to nutritional deficiencies such as age, advanced dementia with immobility, functional dependence, and severe chewing/swallowing problems (19).
One limitation of this review concerns the relative heterogeneity in the variables analyzed, concept definitions, methodology, and populations among the 6 longitudinal studies identified. This complicates comparison of the studies and is likely to be the reason why some conflicting results were found.
The longitudinal nature of the studies included in this systematic review allows for determination of the impact of certain independent variables on the development of malnutrition over time. The identified risk factors for malnutrition were age, frailty in institutionalized persons, excessive polypharmacy, general health decline (including physical function and cognition), loss of interest in life, basal oral dysphagia and signs of impaired efficacy of swallowing, and institutionalization. The identification of these factors is crucial for being able to develop an integrated malnutrition assessment tool that takes the multifactorial nature of malnutrition into consideration. The current available screening instruments do not include all identified risk factors, which urges the development of an efficient comprehensive assessment instrument that will identify older adults’ risk of malnutrition and supports the development of preventive strategies. Because only longitudinal studies are able to detect causality, this feature was one of the inclusion criteria for this systematic review. Nevertheless, the numerous cross-sectional studies that reported significant associations between malnutrition and a variety of parameters are valuable, because they generate hypotheses for further longitudinal research on risk factors for malnutrition. For this reason, the cross-sectional studies are provided as Supplemental Tables 1 and 2.
Acknowledgments
JD conceived and designed the review, interpreted the results, and wrote the manuscript. NCFM designed and performed the review, interpreted the results, and wrote the manuscript. SKH, CM, CV, EV, AD, and GEB contributed to the review design, interpretation of the results, and to the manuscript revision. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
References
- 1.de Morais C, Oliveira B, Afonso C, Lumbers M, Raats M, de Almeida MDV. Nutritional risk of European elderly. Eur J Clin Nutr 2013;67:1215–9. [DOI] [PubMed] [Google Scholar]
- 2.van Bokhorst-de van der Schueren MA, Lonterman-Monasch S, de Vries OJ, Danner SA, Kramer MH, Muller M. Prevalence and determinants for malnutrition in geriatric outpatients. Clin Nutr 2013;32:1007–11. [DOI] [PubMed] [Google Scholar]
- 3.Mann T, Heuberger R, Wong H. The association between chewing and swallowing difficulties and nutritional status in older adults. Aust Dent J 2013;58:200–6. [DOI] [PubMed] [Google Scholar]
- 4.Howell S, Loeb M. Nutritional needs of the older adult. Gerontologist 1969;9:17–30. [Google Scholar]
- 5.Gil-Montoya JA, Ponce G, Sánchez Lara I, Barrios R, Llodra JC, Bravo M. Association of the oral health impact profile with malnutrition risk in Spanish elders. Arch Gerontol Geriatr 2013;57:398–402. [DOI] [PubMed] [Google Scholar]
- 6.United Nations. World population prospects, the 2012 revision [Internet]. 2012 [cited 2007 Jan 1]. Available from: http://esa.un.org/unpd/wpp/Documentation/pdf/WPP2012_Volume-II-Demographic-Profiles.pdf.
- 7.Zeanandin G, Molato O, Le Duff F, Guérin O, Hébuterne X, Schneider SM. Impact of restrictive diets on the risk of undernutrition in a free-living elderly population. Clin Nutr 2012;31:69–73. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed T, Haboubi N. Assessment and management of nutrition in older people and its importance to health. Clin Interv Aging 2010;5:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flodin L, Svensson S, Cederholm T. Body mass index as a predictor of 1 year mortality in geriatric patients. Clin Nutr 2000;19:121–5. [DOI] [PubMed] [Google Scholar]
- 10.Martyn CN, Winter PD, Coles SJ, Edington J. Effect of nutritional status on use of health care resources by patients with chronic disease living in the community. Clin Nutr 1998;17:119–23. [DOI] [PubMed] [Google Scholar]
- 11.NutritionDay Worldwide [Internet]. [cited 2015 Oct 14]. Available from: http://www.nutritionday.org/en/about-nday/what-is-nutritionday/index.html.
- 12.Orrevall Y, Tishelman C, Permert J, Cederholm T. Nutritional support and risk status among cancer patients in palliative home care services. Support Care Cancer 2009;17:153–61. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- 14.PRISMA. PRISMA guideline: Transparent reporting of systematic reviews and meta-analyses [Internet]. [cited 2015 Sep 30]. Available from: http://www.prisma-statement.org/Default.aspx.
- 15.Cochrane handbook for systematic reviews of interventions [Internet]. [cited 2015 Sep 30]. Available from: http://handbook.cochrane.org.
- 16.Schilp J, Wijnhoven HA, Deeg DJ, Visser M. Early determinants for the development of undernutrition in an older general population: Longitudinal Aging Study Amsterdam. Br J Nutr 2011;106:708–17. [DOI] [PubMed] [Google Scholar]
- 17.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses [Internet]. 2009. [cited 2015 Sep 30]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 19.Tamura BK, Bell CL, Masaki KH, Amella EJ. Factors associated with weight loss, low BMI, and malnutrition among nursing home patients: A systematic review of the literature. J Am Med Dir Assoc 2013;14:649–55. [DOI] [PubMed] [Google Scholar]
- 20.Pauly L, Stehle P, Volkert D. Nutritional situation of elderly nursing home residents. Z Gerontol Geriatr 2007;40:3–12. [DOI] [PubMed] [Google Scholar]
- 21.Bocock MA, Keller HH, Brauer PM. Defining malnutrition risk: For older home care clients. Can J Diet Pract Res 2008;69:171–6. [DOI] [PubMed] [Google Scholar]
- 22.Blackburn GL, Bistrian BR, Maini BS, Schlamm HT, Smith MF. Nutritional and metabolic assessment of the hospitalized patient. JPEN J Parenter Enteral Nutr 1977;1:11–22. [DOI] [PubMed] [Google Scholar]
- 23.Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev 1996;54:S59–65. [DOI] [PubMed] [Google Scholar]
- 24.Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: Developing the short-form Mini-Nutritional Assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001;56:M366–72. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, Thomas DR, Anthony P, Charlton KE, Maggio M, et al. Validation of the Mini Nutritional Assessment short-form (MNA®-SF): A practical tool for identification of nutritional status. J Nutr Health Aging 2009;13:782–8. [DOI] [PubMed] [Google Scholar]
- 26.Payette H, Cyr R. Les ressources communautaires en alimentation pour les personnes âgées : Étude des services offerts et des caractéristiques de la clientèle (rapport de recherche). [Community resources for nourishment for the elderly: study of the services and the characteristics of the customer (research report)]. Centre de recherche en gérontologie et gériatrie, Institut universitaire de gériatrie de Sherbrooke: Sherbrooke (Canada). 1996;p. 63 (in French).
- 27.Shatenstein B, Kergoat MJ, Nadon S. Weight change, nutritional risk and its determinants among cognitively intact and demented elderly Canadians. Can J Public Health 2001;92:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mamhidir AG, Ljunggren G, Kihlgren M, Kihlgren A, Wimo A. Underweight, weight loss and related risk factors among older adults in sheltered housing–a Swedish follow-up study. J Nutr Health Aging 2006;10:255–62. [PubMed] [Google Scholar]
- 29.Roberts KC, Wolfson C, Payette H. Predictors of nutritional risk in community-dwelling seniors. Can J Public Health 2007;98:331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jyrkkä J, Enlund H, Lavikainen P, Sulkava R, Hartikainen S. Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf 2011;20:514–22. [DOI] [PubMed] [Google Scholar]
- 31.Serra-Prat M, Palomera M, Gomez C, Sar-Shalom D, Saiz A, Montoya JG, Navajas M, Palomera E, Clavé P. Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: A population-based prospective study. Age Ageing 2012;41:376–81. [DOI] [PubMed] [Google Scholar]
- 32.Kyle UG, Unger P, Mensi N, Genton L, Pichard C. Nutrition status in patients younger and older than 60 y at hospital admission: A controlled population study in 995 subjects. Nutrition 2002;18:463–9. [DOI] [PubMed] [Google Scholar]
- 33.Iizaka S, Tadaka E, Sanada H. Comprehensive assessment of nutritional status and associated factors in the healthy, community-dwelling elderly. Geriatr Gerontol Int 2008;8:24–31. [DOI] [PubMed] [Google Scholar]
- 34.Smoliner C, Norman K, Wagner K-H, Hartig W, Lochs H, Pirlich M. Malnutrition and depression in the institutionalised elderly. Br J Nutr 2009;102:1663–7. [DOI] [PubMed] [Google Scholar]
- 35.Gobbens RJ, van Assen MA, Luijkx KG, Schols JM. Testing an integral conceptual model of frailty. J Adv Nurs 2012;68:2047–60. [DOI] [PubMed] [Google Scholar]
- 36.Griep MI, Mets TF, Collys K, Ponjaert-Kristoffersen I, Massart DL. Risk of malnutrition in retirement homes elderly persons measured by the “Mini-Nutritional Assessment.” J Gerontol A Biol Sci Med Sci 2000;55:M57–63. [DOI] [PubMed] [Google Scholar]
- 37.Cederholm T, Hellström K. Nutritional status in recently hospitalized and free-living elderly subjects. Gerontology 1992;38:105–10. [DOI] [PubMed] [Google Scholar]
- 38.Furukawa H, Tanemoto K. Frailty in cardiothoracic surgery: Systematic review of the literature. Gen Thorac Cardiovasc Surg 2015;63:425–33. [DOI] [PubMed] [Google Scholar]
- 39.Hairi NN, Bulgiba A, Cumming RG, Naganathan V, Mudla I. Prevalence and correlates of physical disability and functional limitation among community dwelling older people in rural Malaysia, a middle income country. BMC Public Health 2010;10:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suominen M, Muurinen S, Routasalo P, Soini H, Suur-Uski I, Peiponen A, Finne-Soveri H, Pitkala KH. Malnutrition and associated factors among aged residents in all nursing homes in Helsinki. Eur J Clin Nutr 2005;59:578–83. [DOI] [PubMed] [Google Scholar]
- 41.Jager-Wittenaar H, Dijkstra PU, Vissink A, Langendijk JA, van der Laan BFAM, Pruim J, Roodenburg JLN. Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head Neck 2011;33:863–70. [DOI] [PubMed] [Google Scholar]
- 42.Sachlova M, Majek O, Tucek S. Prognostic value of scores based on malnutrition or systemic inflammatory response in patients with metastatic or recurrent gastric cancer. Nutr Cancer 2014;66:1362–70. [DOI] [PubMed] [Google Scholar]
- 43.Özcan M, Öztürk GZ, Köse M, Emet S, Aydın S, Arslan K, Arman Y, Akkaya V, Tükek T. Evaluation of malnutrition with blood ghrelin and fecal elastase levels in acute decompensated heart failure patients. Turk Kardiyol Dern Arş 2015;43:131–7. [DOI] [PubMed] [Google Scholar]
- 44.Post B, Muslimovic D, van Geloven N, Speelman JD, Schmand B, de Haan RJ. Progression and prognostic factors of motor impairment, disability and quality of life in newly diagnosed Parkinson’s disease. Mov Disord 2011;26:449–56. [DOI] [PubMed] [Google Scholar]
- 45.Fargel M, Grobe B, Oesterle E, Hastedt C, Rupp M. Treatment of Parkinson’s disease: A survey of patients and neurologists. Clin Drug Investig 2007;27:207–18. [DOI] [PubMed] [Google Scholar]
- 46.Zach M, Friedman A, Sławek J, Derejko M. Quality of life in polish patients with long-lasting Parkinson’s disease. Mov Disord 2004;19:667–72. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord 2010;25:2493–500. [DOI] [PubMed] [Google Scholar]
- 48.Kleiner-Fisman G, Stern MB, Fisman DN. Health-related quality of life in Parkinson disease: Correlation between Health Utilities Index III and Unified Parkinson’s Disease Rating Scale (UPDRS) in U.S. male veterans. Health Qual Life Outcomes 2010;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjödahl Hammarlund C, Hagell P, Nilsson MH. Motor and non-motor predictors of illness-related distress in Parkinson’s disease. Parkinsonism Relat Disord 2012;18:299–302. [DOI] [PubMed] [Google Scholar]
- 50.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE. Predictors of deterioration in health-related quality of life in Parkinson’s disease: Results from the DATATOP trial. Mov Disord 2008;23:653–9. [DOI] [PubMed] [Google Scholar]
- 51.Behari M, Srivastava AK, Pandey RM. Quality of life in patients with Parkinson’s disease. Parkinsonism Relat Disord 2005;11:221–6. [DOI] [PubMed] [Google Scholar]
- 52.Gómez-Esteban JC, Zarranz JJ, Lezcano E, Tijero B, Luna A, Velasco F, Rouco I, Garamendi I. Influence of motor symptoms upon the quality of life of patients with Parkinson’s disease. Eur Neurol 2007;57:161–5. [DOI] [PubMed] [Google Scholar]
- 53.Sheard JM, Ash S, Silburn PA, Kerr GK. Prevalence of malnutrition in Parkinson’s disease: A systematic review. Nutr Rev 2011;69:520–32. [DOI] [PubMed] [Google Scholar]
- 54.Sheard JM, Ash S, Mellick GD, Silburn PA, Kerr GK. Malnutrition in a sample of community-dwelling people with Parkinson’s disease. PLoS One 2013;8:e53290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheard JM, Ash S, Silburn PA, Kerr GK. Nutritional status in Parkinson’s disease patients undergoing deep brain stimulation surgery: A pilot study. J Nutr Health Aging 2013;17:148–51. [DOI] [PubMed] [Google Scholar]
- 56.Pickering G. Frail elderly, nutritional status and drugs. Arch Gerontol Geriatr 2004;38:174–80. [DOI] [PubMed] [Google Scholar]
- 57.Prather C, Borum M. Gastrointestinal disorders (constipation). In: Beers MH, Berkow R, editors. Merck manual of geriatrics. 3rd ed. Whitehouse Station (NJ): Merck; 1995;2001–134.
- 58.Verbrugghe M, Beeckman D, Van Hecke A, Vanderwee K, Van Herck K, Clays E, Bocquaert I, Derycke H, Geurden B, Verhaeghe S. Malnutrition and associated factors in nursing home residents: A cross-sectional, multi-centre study. Clin Nutr 2013;32:438–43. [DOI] [PubMed] [Google Scholar]
- 59.Fagerström C, Palmqvist R, Carlsson J, Hellström Y. Malnutrition and cognitive impairment among people 60 years of age and above living in regular housing and in special housing in Sweden: A population-based cohort study. Int J Nurs Stud 2011;48:863–71. [DOI] [PubMed] [Google Scholar]
- 60.Isaia G, Mondino S, Germinara C, Cappa G, Aimonino-Ricauda N, Bo M, Isaia GC, Nobili G, Massaia M. Malnutrition in an elderly demented population living at home. Arch Gerontol Geriatr 2011;53:249–51. [DOI] [PubMed] [Google Scholar]
- 61.Ji L, Meng H, Dong B. Factors associated with poor nutritional status among the oldest-old. Clin Nutr 2012;31:922–6. [DOI] [PubMed] [Google Scholar]
- 62.Ferra A, Del Mar Bibiloni M, Zapata ME, Pich J, Pons A, Tur JA. Body mass index, life-style, and healthy status in free living elderly people in menorca island. J Nutr Health Aging 2012;16:298–305. [DOI] [PubMed] [Google Scholar]
- 63.Galesi LF, Leandro-Merhi VA, de Oliveira MRM. Association between indicators of dementia and nutritional status in institutionalised older people. Int J Older People Nurs 2013;8:236–43. [DOI] [PubMed] [Google Scholar]
- 64.Donini LM, Scardella P, Piombo L, Neri B, Asprino R, Proietti R, Carcaterra S, Cava E, Cataldi S, Cucinotta D, et al. Malnutrition in elderly: Social and economic determinants. J Nutr Health Aging 2013;17:9–15. [DOI] [PubMed] [Google Scholar]
- 65.Ferdous T, Kabir ZN, Wahlin A, Streatfield K, Cederholm T. The multidimensional background of malnutrition among rural older individuals in Bangladesh–a challenge for the Millennium Development Goal. Public Health Nutr 2009;12:2270–8. [DOI] [PubMed] [Google Scholar]
- 66.Kulnik D, Elmadfa I. Assessment of the nutritional situation of elderly nursing home residents in Vienna. Ann Nutr Metab 2008;52:51–3. [DOI] [PubMed] [Google Scholar]
- 67.Saka B, Kaya O, Ozturk GB, Erten N, Karan MA. Malnutrition in the elderly and its relationship with other geriatric syndromes. Clin Nutr 2010;29:745–8. [DOI] [PubMed] [Google Scholar]
- 68.Feldblum I, German L, Castel H, Harman-Boehm I, Bilenko N, Eisinger M, Fraser D, Shahar DR. Characteristics of undernourished older medical patients and the identification of predictors for undernutrition status. Nutr J 2007;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodríguez-Tadeo A, Wall-Medrano A, Gaytan-Vidana ME, Campos A, Ornelas-Contreras M, Novelo-Huerta HI. Malnutrition risk factors among the elderly from the us-Mexico Border: The “one thousand” study. J Nutr Health Aging 2012;16:426–31. [DOI] [PubMed] [Google Scholar]
- 70.Vanderwee K, Clays E, Bocquaert I, Gobert M, Folens B, Defloor T. Malnutrition and associated factors in elderly hospital patients: A Belgian cross-sectional, multi-centre study. Clin Nutr 2010;29:469–76. [DOI] [PubMed] [Google Scholar]
- 71.Lee KS, Cheong H-K, Kim EA, Kim KR, Oh BH, Hong CH. Nutritional risk and cognitive impairment in the elderly. Arch Gerontol Geriatr 2009;48:95–9. [DOI] [PubMed] [Google Scholar]
- 72.Avlund K, Holm-Pedersen P, Morse DE, Viitanen M, Winblad B. Tooth loss and caries prevalence in very old Swedish people: The relationship to cognitive function and functional ability. Gerodontology 2004;21:17–26. [DOI] [PubMed] [Google Scholar]
- 73.Samnieng P, Ueno M, Shinada K, Zaitsu T, Wright FAC, Kawaguchi Y. Oral health status and chewing ability is related to Mini-Nutritional Assessment results in an older adult population in Thailand. J Nutr Gerontol Geriatr 2011;30:291–304. [DOI] [PubMed] [Google Scholar]
- 74.De Marchi RJ, Hugo FN, Hilgert JB, Padilha DMP. Association between oral health status and nutritional status in south Brazilian independent-living older people. Nutrition 2008;24:546–53. [DOI] [PubMed] [Google Scholar]
- 75.Visvanathan R, Ahmad Z. Good oral health, adequate nutrient consumption and family support are associated with a reduced risk of being underweight amongst older Malaysian residents of publicly funded shelter homes. Asia Pac J Clin Nutr 2006;15:400–5. [PubMed] [Google Scholar]
- 76.Adebusoye LA, Ajayi IO, Dairo MD, Ogunniyi AO. Nutritional status of older persons presenting in a primary care clinic in Nigeria. J Nutr Gerontol Geriatr 2012;31:71–85. [DOI] [PubMed] [Google Scholar]
- 77.Saarela RKT, Soini H, Hiltunen K, Muurinen S, Suominen M, Pitkala K. Dentition status, malnutrition and mortality among older service housing residents. J Nutr Health Aging 2014;18:34–8. [DOI] [PubMed] [Google Scholar]
- 78.Tsai AC, Chang T-L. Association of dental prosthetic condition with food consumption and the risk of malnutrition and follow-up 4-year mortality risk in elderly Taiwanese. J Nutr Health Aging 2011;15:265–70. [DOI] [PubMed] [Google Scholar]
- 79.Soini H, Muurinen S, Routasalo P, Sandelin E, Savikko N, Suominen M, Ainamo A, Pitkala KH. Oral and nutritional status: Is the MNA a useful tool for dental clinics. J Nutr Health Aging 2006;10:495–501. [PubMed] [Google Scholar]
- 80.Chai J, Chu FCS, Chow TW, Shum NC, Hui WWH. Influence of dental status on nutritional status of geriatric patients in a convalescent and rehabilitation hospital. Int J Prosthodont 2006;19:244–9. [PubMed] [Google Scholar]
- 81.Cousson PY, Bessadet M, Nicolas E, Veyrune JL, Lesourd B, Lassauzay C. Nutritional status, dietary intake and oral quality of life in elderly complete denture wearers. Gerodontology 2012;29:e685–92. [DOI] [PubMed] [Google Scholar]
- 82.Amer MS, Mousa SM, Abdel Rahman TT, Saber HG. Malnutrition and its risk factors in nursing home residents in Cairo. J Am Geriatr Soc 2009;57:1716–8. [DOI] [PubMed] [Google Scholar]
- 83.Patel MD, Martin FC. Why don’t elderly hospital inpatients eat adequately? J Nutr Health Aging 2008;12:227–31. [DOI] [PubMed] [Google Scholar]
- 84.Kikutani T, Yoshida M, Enoki H, Yamashita Y, Akifusa S, Shimazaki Y, Hirano H, Tamura F. Relationship between nutrition status and dental occlusion in community-dwelling frail elderly people. Geriatr Gerontol Int 2013;13:50–4. [DOI] [PubMed] [Google Scholar]
- 85.Poulsen I, Rahm Hallberg I, Schroll M. Nutritional status and associated factors on geriatric admission. J Nutr Health Aging 2006;10:84–90. [PubMed] [Google Scholar]
- 86.Crogan NL, Corbett CF. Predicting malnutrition in nursing home residents using the minimum data set. Geriatr Nurs 2002;23:224–6. [DOI] [PubMed] [Google Scholar]
- 87.Dion N, Cotart JL, Rabilloud M. Correction of nutrition test errors for more accurate quantification of the link between dental health and malnutrition. Nutrition 2007;23:301–7. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimura K, Yamada M, Kajiwara Y, Nishiguchi S, Aoyama T. Relationship between depression and risk of malnutrition among community-dwelling young-old and old-old elderly people. Aging Ment Health 2013;17:456–60. [DOI] [PubMed] [Google Scholar]
- 89.Ülger Z, Halil M, Kalan I, Yavuz BB, Cankurtaran M, Güngör E, Arioĝul S. Comprehensive assessment of malnutrition risk and related factors in a large group of community-dwelling older adults. Clin Nutr 2010;29:507–11. [DOI] [PubMed] [Google Scholar]
- 90.Yap KB, Niti M, Ng TP. Nutrition screening among community-dwelling older adults in Singapore. Singapore Med J 2007;48:911–6. [PubMed] [Google Scholar]
- 91.Johansson L, Sidenvall B, Malmberg B, Christensson L. Who will become malnourished?: A prospective study of factors associated with malnutrition in older persons living at home. J Nutr Health Aging 2009;13:855–61. [DOI] [PubMed] [Google Scholar]
- 92.Ribeiro RSV, da Rosa MI, Bozzetti MC. Malnutrition and associated variables in an elderly population of Criciúma, SC. Rev Assoc Med Bras 2011;57:56–61. [PubMed] [Google Scholar]
- 93.Ramic E, Pranjic N, Batic-Mujanovic O, Karic E, Alibasic E, Alic A. The effect of loneliness on malnutrition in elderly population. Med Arh 2011;65:92–5. [PubMed] [Google Scholar]
- 94.Wham CA, Dyall L, Teh ROY, Kerse NM. Nutrition risk: Cultural aspects of assessment. Asia Pac J Clin Nutr 2011;20:632–8. [PubMed] [Google Scholar]
- 95.Aliabadi M, Kimiagar M, Ghayour-Mobarhan M, Shakeri MT, Nematy M, Ilaty AA, Moosavi A-R, Lanham-New S. Prevalence of malnutrition in free living elderly people in Iran: A cross-sectional study. Asia Pac J Clin Nutr 2008;17:285–9. [PubMed] [Google Scholar]
- 96.Jyrkkä J, Vartiainen L, Hartikainen S, Sulkava R, Enlund H. Increasing use of medicines in elderly persons: A five-year follow-up of the Kuopio 75+ Study. Eur J Clin Pharmacol 2006;62:151–8. [DOI] [PubMed] [Google Scholar]
- 97.Haider SI, Johnell K, Thorslund M, Fastbom J. Trends in polypharmacy and potential drug-drug interactions across educational groups in elderly patients in Sweden for the period 1992–2002. Int J Clin Pharmacol Ther 2007;45:643–53. [DOI] [PubMed] [Google Scholar]
- 98.Smoliner C, Fischedick A, Sieber CC, Wirth R. Olfactory function and malnutrition in geriatric patients. J Gerontol A Biol Sci Med Sci 2013;68:1582–8. [DOI] [PubMed] [Google Scholar]
- 99.Linjakumpu TA, Hartikainen SA, Klaukka TJ, Koponen HJ, Hakko HH, Viilo KM, Haapea M, Kivelä SL, Isoaho RE. Sedative drug use in the home-dwelling elderly. Ann Pharmacother 2004;38:2017–22. [DOI] [PubMed] [Google Scholar]
- 100.Cao Y-J, Mager DE, Simonsick EM, Hilmer SN, Ling SM, Windham BG, Crentsil V, Yasar S, Fried LP, Abernethy DR. Physical and cognitive performance and burden of anticholinergics, sedatives, and ACE inhibitors in older women. Clin Pharmacol Ther 2008;83:422–9. [DOI] [PubMed] [Google Scholar]
- 101.Schein OD, Hochberg MC, Muñoz B, Tielsch JM, Bandeen-Roche K, Provost T, Anhalt GJ, West S. Dry eye and dry mouth in the elderly: A population-based assessment. Arch Intern Med 1999;159:1359–63. [DOI] [PubMed] [Google Scholar]
- 102.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease: Donepezil Study Group. Neurology 1998;50:136–45. [DOI] [PubMed] [Google Scholar]
- 103.Lopez-Jornet P, Saura-Perez M, Llevat-Espinosa N. Effect of oral health dental state and risk of malnutrition in elderly people. Geriatr Gerontol Int 2013;13:43–9. [DOI] [PubMed] [Google Scholar]
- 104.Margetts BM, Thompson RL, Elia M, Jackson AA. Prevalence of risk of undernutrition is associated with poor health status in older people in the UK. Eur J Clin Nutr 2003;57:69–74. [DOI] [PubMed] [Google Scholar]
- 105.Vandewoude M, Van Gossum A. Nutritional screening strategy in nonagenarians: The value of the MNA-SF (Mini Nutritional Assessment short form) in NutriAction. J Nutr Health Aging 2013;17:310–4. [DOI] [PubMed] [Google Scholar]
- 106.Shum NC, Hui WWH, Chu FCS, Chai J, Chow TW. Prevalence of malnutrition and risk factors in geriatric patients of a convalescent and rehabilitation hospital. Hong Kong Med J 2005;11:234–42. [PubMed] [Google Scholar]