Abstract
Twenty years ago, there was profound, international interest in developing oral human, bovine, or chicken egg–derived immunoglobulin (Ig) for the prevention and nutritional treatment of childhood malnutrition and gastrointestinal disease, including acute diarrhea and necrotizing enterocolitis. Although such Ig products were shown to be effective, with both nutritional and antidiarrheal benefits, interest waned because of their cost and because of the perceived risk of bovine serum encephalitis (BSE). BSE is no longer considered a barrier to use of oral Ig, because the WHO has declared the United States to be BSE-free since the early 2000s. Low-cost bovine-derived products with high Ig content have been developed and are regulated as medical foods. These new products, called serum bovine Igs (SBIs), facilitate the management of chronic or severe gastrointestinal disturbances in both children and adults and are regulated by the US Food and Drug Administration. Well-established applications for use of SBIs include human immunodeficiency virus (HIV)-associated enteropathy and diarrhea-predominant irritable bowel syndrome. However, SBIs and other similar products could potentially become important components of the treatment regimen for other conditions, such as inflammatory bowel disease, by aiding in disease control without immunosuppressive side effects. In addition, SBIs may be helpful in conditions associated with the depletion of circulating and luminal Igs and could potentially play an important role in critical care nutrition. The rationale for their use is to facilitate intraluminal microbial antibody coating, an essential process in immune recognition in the gut which is disturbed in these conditions, thereby leading to intestinal inflammation. Thus, oral Ig may emerge as an important “add-on” therapy for a variety of gastrointestinal and nutritional problems during the next decade.
Keywords: Ig, serum bovine Ig, inflammatory bowel disease, irritable bowel syndrome, malnutrition, gut barrier function, enteropathy, colitis, diarrhea
Introduction
Previous studies have looked at the effects of several products, including human purified Igs given orally, human and bovine colostrum (BC)4, hyperimmune egg yolk (HEY), and porcine and bovine plasma-derived protein concentrates (PPC), for the prevention and nutritional treatment of childhood malnutrition and gastrointestinal disease. Two decades ago, a study focused on colostrum or Ig derived from colostrum as primary or adjunctive treatments for human diseases (1–4). BC contains between 3% and 25% IgG, compared with human colostrum, which contains ∼2% IgG (5).
Highly purified Ig preparations from bovine or porcine sources have recently begun to be intensely investigated for the nutritional management of several human diseases. PPCs are commonly added to the diets of several breeds of domestic piglets and calves to prevent weanling diarrhea (6–8). PPC products are prepared from blood obtained in abattoirs, concentrated by filtration and ultrafiltration, exposed to an anticoagulant, and processed to yield a spray-dried powder (9). Serum bovine Ig (SBI) is a newer product, purified via a series of shifts in pH and specific salt additions aimed to increase the concentration of Igs (10, 11). A recent meta-analysis showed that PPCs added to animal feed increased weight gain, food intake, and weight gain per calorie consumed in several domestic species, specifically pigs, cows, and turkeys (12).
SBI.
One commercial PPC preparation, intended for human use, was the subject of several recent clinical trials. This SBI product contains >50% IgG, 5–7% IgM, but <1% IgA (13, 14). Each packet of this SBI product provides 5 g of Ig, some of which can be recovered in active form from the stool of most patients who take it, indicating its stability in the human digestive tract (15). For the purpose of comparison, an 80–90-kg human secretes ∼4.5 g IgA/d into the intestine. The US FDA regulates this commercially produced SBI for the physician-directed management of enteropathy in patients with HIV-associated enteropathy and diarrhea-predominant irritable bowel syndrome (16). Mechanisms of action of these products that could potentially therapeutically benefit adults and children with critical illnesses are discussed in the next section.
Colostrum.
Colostrum has also been a popular Ig product in alternative medicine. Oral BC from hyper-immunized cattle protected children against rotavirus infection in one study (17). Lactobin, an Ig concentrate pooled from normal colostrum, was found to have antibody activity against surface antigens such as flagellin from Yersinia enterocolitica and cell wall antigens from Campylobacter jejuni (18). Lactobin was initially reported to cure a prolonged cryptosporidial diarrheal illness in a 4 y old with HIV infection (19). Furthermore, BC has been extensively studied in preclinical trials in preterm piglets and was shown to have beneficial effects on formula-fed piglets, with improved intestinal mucosal architecture and permeability (20). Clinically, BC prevented necrotizing enterocolitis (NEC) in preterm piglets (21). In addition, BC appeared to reduce diarrheal severity in children infected with enterohemorrhagic Escherichia coli (22). However, since 2000, to our knowledge, there have been few published studies of colostrum for the prevention of NEC or treatment of diarrheal diseases. The quality and manufacturing of colostral products are not regulated as medical foods. Ig content of BC is quite variable; it may reach concentrations as high as 30 g/L IgG, but only if collected in the first 24 h postpartum (23). BC is listed by manufacturers as possibly effective for HIV-associated diarrhea, but there is insufficient evidence for its use as an enema for ulcerative colitis (UC) (24). Most online sources advertise this supplement for “gastrointestinal health,” “immune function,” and “athletic performance.”
Mechanism of Action
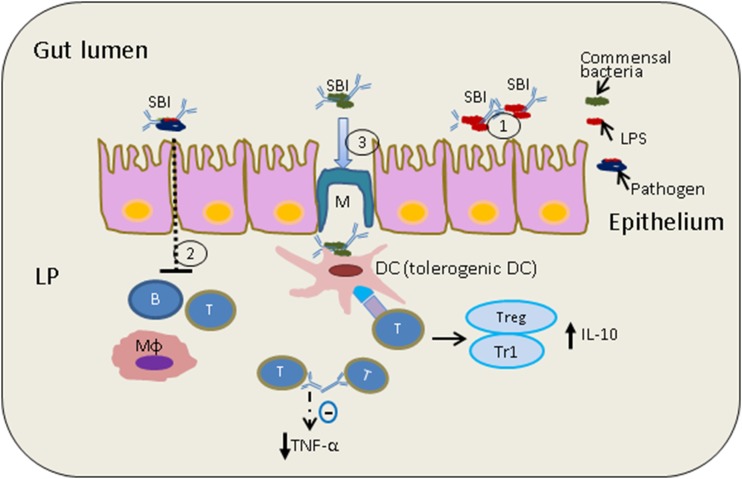
Proposed mechanisms of action for oral Ig products are shown in Figure 1.
FIGURE 1.
Proposed mechanisms of action of oral Ig SBI. (1) SBI binds luminal bacteria and their endotoxins (LPS), providing a level of immune exclusion. (2) Reduced transepithelial antigen absorption across the small and/or large intestine has been linked to reduced immune activation, including effects on B cells, T cells, and macrophages. (3) SBI may interact with healthy commensals to induce tolerogenic DCs. Shown is a tolerogenic DC signaling to CD4+ helper T cells, which are known to communicate with Treg/Tr1 cells to produce anti-inflammatory cytokine IL-10. Immune homeostasis may reduce production of pro-inflammatory cytokines such as TNF-α and would increase production of IL-10. CD4+, cluster of differentiation 4; DC, dendritic cell; LP, lamina propria; SBI, serum-derived bovine Ig; Treg, Foxp3+ regulatory T cell; Tr1, Foxp3− IL10-producing regulatory T cell.
Pathogen elimination, toxin exclusion, and gut barrier function.
A previous study by Hunt et al. (25) investigated a PPC product in piglets infected with the protozoan Cryptosporidium parvum, a major enteric pathogen in humans with HIV infection. In infected animals receiving oral bovine serum concentrate (BSC), peak diarrheal volume and intestinal permeability were improved by 33%; fewer cryptosporidial oocysts were shed; and villous surface area returned to normal more rapidly after infection (all P = 0.05 compared with infected controls) (25).
In another study, weaned pigs were challenged with enterotoxigenic E. coli K88, to investigate whether SBI could improve growth, enhance immune defense, and reduce intestinal inflammation (26). When compared with those on a diet based on fish protein, enterotoxigenic E. coli–infected pigs fed PPC showed higher daily weight gain, less intestinal mucosal damage, milder inflammatory cell infiltration, and reduced expression of proinflammatory cytokines. On the other hand, in a study of human infants with E. coli–associated diarrhea, oral administration of bovine milk concentrate from cattle immunized with E. coli did not alter stool output, even though this preparation had been found to be effective in preventing E. coli–associated diarrhea (27).
Mechanistic studies of SBI have looked at its effects on the permeability of the small intestine. After exposure to Staphylococcus aureus enterotoxin B, rat gut permeability to horseradish peroxidase (molecular weight 40 kDa) was reduced by dietary addition of SBI (28). Piglet studies have also shown gut barrier improvement after PPC feeding. In those studies, PPCs reduced lamina propria white blood cell numbers in the colon as well as tissue levels of IFN-γ and TNF-α. The reduction in inflammatory mediators was associated with reduced ileal and colonic permeability (29). In an in vitro model of intestinal barrier restitution measured by epithelial cell wounding and migration, we found that cultured intestinal cells migrated to cover ulcerated surfaces much more rapidly when SBI was added to the culture medium (30). However, to our knowledge, improvement in gut barrier function has not been investigated in human randomized controlled trials.
Anti-inflammatory effects in the intestine.
Children with Ig deficiencies are prone to infections that can be the precursors of chronic inflammatory conditions. Examples include celiac disease in patients with selective IgA deficiency (31) and UC in children with common variable immunodeficiency (32). On a similar note, a majority of children with very early–onset inflammatory bowel disease (IBD) (beginning in the first 6 y of life) have been found to have immune defects (33).
In a model of immunodeficiency-mediated IBD induced by a knockout of the MDR1a gene, mice fed SBI had reduced gut permeability, increased gut E-cadherin and zonula occludens-1 expression, and reduced tissue levels of TNF-α and IFN-γ (34). The immune exclusion occurred as SBI (≤50 g/L) bound free antigen in a dose-dependent manner. In addition, SBI was found to bind these proinflammatory bacterial cell wall components (LPS and Lipid A) and prevent immune activation in an intestinal coculture model (35).
In a mouse model of colitis induced by E. coli dextran sodium sulfate, mice were supplemented with either SBI or hydrolyzed collagen (as a control). As inflammation developed in the gut, SBI was found to improve histological inflammation, cytokine (IL-6) levels, and chemokine chemokine (C-C motif) ligand 5 levels in the cecum. In addition, the authors found an improvement in the circulating concentration of intestinal fatty acid binding protein-1, an index of damage to the villus tips of the ileum and cecum (36).
In humans, SBI improved duodenal mucosal cluster of differentiation cell surface markers 4 (CD4) T helper lymphocyte counts and duodenal absorptive function in patients with HIV enteropathy (37).
Shifts in the gut microbiota and their metabolic products.
Active investigation is just beginning to focus on whether oral Igs affect the intestinal microbiota composition. Secretory IgA is known to protect the intestinal epithelium against colonization and/or invasion by binding antigens on pathogens or commensals in the luminal mucus layer. In fact, about 50% of the human resident microbiota is coated with host antibody (38). The microorganism is thereby surrounded by a hydrophilic shell, which is repelled by the epithelial glycocalyx, providing immune exclusion of bacteria. Immune exclusion not only protects the epithelium from invading pathogens, but it also plays an important role in maintaining gut homeostasis by preventing overgrowth of commensal microflora (Figure 1) (39).
The relation between our human microbial community and the secretory antibodies that we produce against them is collaborative and bidirectional. Germ-free animals have a 90% reduction in mucosal IgA-secreting cells, whereas IgA-deficient hosts have a pathological overgrowth of organisms that drive inflammation in the intestine, raising Ig levels and producing systemic autoimmunity (40). As an example, the “activation-induced cytidine deaminase knockout” mouse model has been used to demonstrate that secretory IgA is critical for regulating bacterial communities in the gut lumen. The absence of IgA in the lumen of activation-induced cytidine deaminase knockout mice leads to an excessive anaerobic expansion in all segments of the small intestine, and among the expanded anaerobes, aggressive proinflammatory segmented filamentous bacteria were predominant (41).
To date, we are aware of only one study of the effect of SBI on the fecal microbiota in humans. In a group of patients with HIV-associated enteropathy who were treated with SBI, proinflammatory γ-proteobacteria decreased from 0.70% to 0.12% of the total microbial population. Clostridium (genus) decreased from 6.5% to 3.4% in the stool and correlated with duodenal cluster of differentiation cell surface markers CD3+/CD4+ cell density in the lamina propria of biopsy samples. Ruminococcus and the bacteroidetes-to-firmicutes ratio increased in most SBI-treated subjects. Changes in the gut microbiota found in this HIV study also correlated with local lymphocyte populations, which increased significantly with short-term SBI administration over 8 wks (42).
Uses of Oral Igs in Adult Gastrointestinal Disorders
Human studies of oral Igs are summarized in Table 1.
TABLE 1.
Human studies of oral Ig1
| Author, y (reference) | Product | Disease | Type | N | Result |
| Asmuth et al., 2013 (37) | SBI | HIV enteropathy | Open label | 8 | Reduced stools, improved consistency |
| Wilson et al., 2013 (43) | SBI | Irritable bowel syndrome-diarrhea | RCT | 66 | Improved abdominal pain and gastrointestinal symptoms |
| Lembcke et al., 1997 (44) | BSC | Infants with malnutrition | RCT | 10 | Increased fat absorption |
| Bégin et al., 2008 (45) | BSC | Infants with malnutrition | RCT | 259 | No nutritional benefit |
| Shafran et al., 2015 (46) | SBI | IBD (Crohn disease, ulcerative colitis) | Retrospective case series | 83 | Improved symptom scores |
| Good and Panas, 2015 (47) | SBI | IBD | Case series | 7 | Clinical improvement in 75% |
| Eibl et al., 1988 (1) | Human IgA + IgG | NEC prevention | RCT | 88 | Reduced NEC |
| Rubaltelli et al., 1991 (3) | Human IgG | NEC prevention | RCT | 132 | Reduced NEC |
| Lawrence et al., 2001 (48) | Human IgG | NEC prevention | RCT | 768 | No change in NEC incidence |
| Guarino et al., 1994 (2) | Human Ig | Rotavirus diarrhea | RCT | 71 | Reduced diarrhea, viral shedding |
| Sarker et al., 1998 (4) | Bovine colostrum | Rotavirus diarrhea | RCT | 80 | Reduced diarrhea, viral shedding |
| Sarker et al., 2001 (49) | Egg yolk Ig (hyperimmune egg yolk) | Rotavirus diarrhea | RCT | 79 | Reduced diarrhea, viral shedding |
| Sarker et al., 2013 (50) | Hyperimmune llama antibody (antirotavirus protein 1) | Rotavirus diarrhea | RCT | 176 | Reduced diarrhea |
| Young et al., 2007 (51) | Hyperimmune bovine colostrum | Clostridium difficile diarrhea | Phase 1 safety trial | 77 | Safe |
| Bölke et al., 2002 (52) | Pooled colostral Ig | Abdominal surgery | RCT | 40 | Ineffective, but reduced endotoxemia |
| Bölke et al., 2002b (53) | Pooled colostral Ig | Coronary artery bypass | RCT | 60 | Outcome unchanged, but C-reactive protein levels lower |
BSC, bovine serum concentrate; IBD, Inflammatory bowel disease; NEC, necrotizing enterocolitis; RCT, randomized controlled trial; SBI, serum-derived bovine Ig.
HIV enteropathy.
Oral Ig treatment has been shown to be useful in the management of HIV enteropathy. An open-label study of 8 subjects with HIV-associated enteropathy, who took 2.5 g SBI twice daily for 8 wk after a 4-wk washout period, showed that SBI was associated with important reductions in the number of bowel movements per day, improvement in stool consistency, and increase in intestinal mucosal CD4 lymphocyte counts. Although serum levels of intestinal-fatty acid binding protein, a marker of enterocyte damage, first increased in 7 of 8 subjects after 8 wk of treatment, it ultimately declined to less than the baseline level in 4 of 5 patients who opted to continue taking the SBI during an optional 9-mo extension study (37).
IBD.
The potential utility of oral SBI in the management of IBD has also recently been suggested. A 2015 single-center retrospective chart review of 45 adults with Crohn disease and 38 with UC, with symptoms refractory to traditional pharmacologic IBD treatment, looked at outcomes for individuals who received 5 g SBI/d as a nutritional adjunct for 12 wk. Shafran et al. (46) reported that 49% of patients had clinical improvement at week 1 and 76% after 12 wk of treatment. No side effects were reported. These patients were 3 times more likely to report improvement in symptom scores with SBI added to their management. Similarly, a smaller case review of 7 patients with IBD, 3 with UC, and 4 with Crohn disease, with inadequate response to conventional pharmacologic treatment, reported that the UC patients taking SBI had resolution of rectal bleeding, urgency, and/or nocturnal incontinence, whereas the Crohn disease patients had weight improvements, decreased ileostomy output, and/or reductions in the requirement for other drugs (47). Another case report describes 2 patients with refractory colitis (56-y-old female with ischemic colitis, 26-y-old male with UC), who had symptom improvement after 4–8 wk of SBI being added to their treatment regimens, with endoscopic resolution of colitis lasting at least 1 y following the addition of SBI (54). Note that none of these studies were large, multicenter, or placebo controlled.
Following major surgery.
The concentrated bovine colostral product Lactobin was studied as a treatment for patients after abdominal surgery (gastrectomy or pancreatic resection) to see if they had improved recovery or reduced endotoxin levels in the blood (52). The study did not show a clinical benefit, although inflammatory biomarker C-reactive protein levels were lower in treated patients compared with the placebo-treated controls. Another study of the same product in 60 adults who had coronary artery bypass surgery, showed that postoperative endotoxemia was not reduced by Ig-enriched colostrum, although C-reactive protein levels were again lower in the colostrum-treated group (53).
Potential Applications of Oral Ig in Pediatric Gastrointestinal Diseases and Critical Care Therapy
Malnutrition.
One pediatric study suggested that SBI can aid in recovery from severe malnutrition. In a 1997 study (44), 10 young Peruvian children (9–25 mo) recovering from severe protein-energy malnutrition were given 3 sequential, randomly ordered masked study diets, each lasting 7 d. In the 2 test diets, BSC replaced either 25% or 50% of the milk protein of the control diet (rice, milk, vegetable oil, sugar). The fractional absorption of dietary lipid and total energy of BSC-treated infants increased significantly in association with the percentage of BSC ingested. There was a trend toward improved nitrogen and carbohydrate absorption with increasing amounts of BSC (16, 55).
In a 2008, randomized, controlled, interventional study of 259 low-income, peri-urban Guatemalan children (aged 6–7 mo upon study entry and observed for 8 mo), a diet containing BSC did not affect growth velocity, rates of infection, or hemoglobin concentration (45). Potential explanations were that the majority of the infants in the study were breastfed and that BSC may be of more benefit to those not already receiving breast milk or that a higher dose of BSC might be necessary to improve infection rates (16, 55).
NEC.
NEC is the most common and severe gastrointestinal illness of prematurity, affecting up to 50% of very low birth weight infants (<1000 g). To our knowledge, there are no published reports of oral serum-derived bovine Ig use in the prevention of NEC in infants. However, earlier randomized trials investigated enterally administered human-derived oral Ig for this purpose in low birth weight and premature infants. In 1988, Eibl and colleagues (1) found no cases of NEC in the first 28 d of life in 88 infants weighing 800–2000 g who received from birth 600 mg human-derived oral IgA-IgG/d. There were 6 cases of NEC in 91 control infants not receiving treatment (P = 0.01). In a subsequent study of 132 newborns with a birth weight ≤1500 g or a gestational age ≤34 wk, Rubaltelli and colleagues (3) reported, during the first 15 d of life, 4 of 67 cases of NEC in the control group compared with 0 of 65 in the treatment group given 500 mg monomeric IgG orally during the first 2 wk of life. It came as a surprise when a subsequent placebo-controlled, masked trial of infants with a birth weight <1500 g, receiving enteral human IgG upon initiation of enteral feeding, reported that 43 of 768 infants developed NEC, similar to 41 of 761 receiving albumin placebo (P = NS) (48).
As a consequence, a Cochrane review (56) showed no substantial reduction in the incidence of definite or suspected NEC, a need for surgery, or death from NEC in babies receiving human Ig orally. However, these studies were subject to differences in Ig dosing, onset, and duration of treatment, weight, and gestational age, all of which could have affected outcome variables. Interestingly, Wolf and Eibl (57) found in cultured human monocytes incubated with a human IgA-IgG preparation, downregulation of TNF-α and IL-6, important inflammatory mediators in the pathogenesis of NEC. Studies of purified human IgA (individually, but not with IgG) were similarly effective, raising the question about whether the effect seen in the earlier trial by Eibl and colleagues (1) was secondary to IgA rather than IgG.
Immunodeficiency.
Parenteral Igs are commonly given to critically ill children with various disorders associated with either primary or secondary immune deficiency or immune dysregulation (58). Although these children are commonly affected by feeding intolerance, diarrhea, bacterial overgrowth, and inflammatory conditions, there are surprisingly few promising cost-effective, supportive therapies available for clinicians to treat these conditions. Nutritional supplementation with Igs to reduce intestinal inflammation and proinflammatory cytokine production could be quite beneficial. In theory, oral administration of Igs to modulate gut luminal and mucosal immunity would be preferable to parenteral administration. For example, orally administered Igs could potentially have 1) a lower risk of stimulating a systemic allergic response, 2) a decreased risk of causing human blood-related diseases such as hepatitis, and 3) a higher capacity to inhibit endotoxin. Total parenteral nutrition in severely ill children increases susceptibility to endotoxemia and sepsis as a consequence of increased gut permeability, whereas oral Igs may improve gut barrier function and decrease the absorption of endotoxins (57, 59).
Severe viral diarrhea.
Rotavirus-related acute gastroenteritis (AGE) constitutes a major health care burden in children, especially in developing countries where the new vaccines are less effective and less available. In severe cases, infection often requires hospitalization and critical care support. A study by Payne et al. (60) revealed that laboratory-confirmed rotavirus infections accounted for 50% of all AGE hospitalizations and emergency department visits. Children with rotavirus-related AGE exhibited symptoms (e.g., diarrhea, vomiting, fever, and lethargy) with significantly greater frequency and intensity than did those with non–rotavirus-related AGE (60). Protein losing enteropathy (PLE) has been reported in children with rotavirus (61) and cytomegalovirus infections (61, 62), often resulting in Ig deficiency. Although the mechanism for PLE development remains unclear, Wang et al. described the mechanisms by which rotavirus infection led to enteropathy (63).
Use of Ig-based enteral therapy in rotavirus AGE has been previously reported. A 1994 prospective, double-blind, placebo-controlled study of oral human serum Ig given as a single dose (300 mg/kg body weight) to 36 of 71 children with acute rotavirus enteritis admitted to a hospital unit resulted in both significantly reduced duration of diarrhea (76 compared with 131 h for placebo, P < 0.01) and reduced viral excretion (114 compared with 180 h for placebo, P < 0.01) (47). Similar results were seen in a study of 80 children with rotavirus diarrhea randomly assigned to receive immunized BC (4).
Sarker and colleagues (49) performed a randomized, double-blind study of 79 children with rotavirus diarrhea. The treatment group was assigned to receive either 10 g HEY in 4 equally divided doses daily for 4 d (HEY group) or a similar preparation obtained from nonimmunized chicken (placebo group). The daily stool frequency and amount, oral rehydration solution intake, and presence of rotavirus in the stool were monitored for 4 d. Treatment with HEY improved the symptoms of diarrhea and aided the clearance of rotavirus from the stool (49). Recently, the same investigators assessed the efficacy of a llama-derived, heavy-chain antibody fragment called antirotavirus protein (ARP1), in modifying the severity and duration of diarrhea in 176 male infants with rotavirus infection. The infants were randomly assigned to groups given oral ARP1 (15–30 mg ⋅ kg−1 ⋅ d−1, n = 88) or placebo (maltodextrin, n = 88) for a maximum of 5 d. The primary outcomes were severity (i.e., stool output) and duration of diarrhea and fecal excretion of rotavirus. There was a significant reduction in the rate of stool output (in g ⋅ kg−1 ⋅ d−1) in the ARP1 group compared with the placebo group (61%; P = 0.002) (50).
A Cochrane review looked at the use of oral Ig preparations in low birth weight infants for either treating or preventing rotavirus infection. However, only one study met the criteria, and the authors suggested no benefit in this study population (64). Although most pediatric studies focused on rotavirus enteritis, there were several studies that showed benefit of oral Igs in other intestinal infections, including cryptosporidiosis and shigellosis (65). In a study of adults with HIV infection and chronic cryptosporidiosis, oral bovine Ig reduced diarrheal stool weight by 50% (P = 0.03) (66).
C. difficile colitis.
The rising incidence of C. difficile–associated diarrhea among children has resulted in an increased number of associated hospital admissions and length of stay (67, 68). Although the underlying evidence is not particularly strong, current treatment guidelines suggest that in case of multiple recurrences or refractory disease, probiotics, Ig, or steroids should be considered as therapeutic options (69). However, a recent policy statement by the American Academy of Pediatrics did not comment on these therapeutic options, likely due to paucity of evidence (70). The use of oral Igs could potentially be promising in treating this disease. In animals, oral Ig derived from eggs of immunized leghorn hens effectively neutralized toxins A and B in a hamster model (71). A human study using bovine Ig concentrate prepared from colostrum of cattle immunized against toxin A demonstrated that the antibody preparation retains the ability to neutralize C. difficile toxin A (72). To our knowledge, there has been only one study investigating oral Ig therapy in humans for efficacy. This study was stopped prematurely, but the available data showed oral Ig therapy to be well tolerated and approximately as effective as metronidazole (51).
Chemotherapy-related gut barrier dysfunction.
Chemotherapeutic agents are very effective treatments for some forms of childhood cancers, such as acute lymphoblastic leukemia, and have been shown to improve event-free survival rate. Although exerting their antitumorigenic effect, these agents have toxic effects on rapidly dividing cells, including those found in the intestinal mucosa. For example, a recent study by Meng et al. (73) measured the concentration of plasma endotoxin and diamine oxidase as a marker for gut permeability in children with acute lymphoblastic leukemia, and it showed that high-dose methotrexate was associated with increased intestinal permeability and gut dysfunction (73). Gastrointestinal mucositis associated with chemotherapy is caused by apoptosis, immune dysfunction, and microbiome alterations. A recent animal study by Bateman et al. (74) showed that twice daily oral gavage of SBI reduced the incidence, severity, and duration of irinotecan-induced mucositis. SBI was associated with less pronounced changes in inflammatory cell levels and tissue damage in the colon and jejunum (74). These preliminary data suggest a role for oral Ig in attenuating the effects of chemotherapy-induced gut dysfunction and require further exploration.
Sepsis and multiorgan system failure.
Increased intestinal permeability due to intestinal mucosal injury as a result of gram-negative sepsis and other causes is considered a central factor for the development of multiple organ failure (75, 76). In an animal study, endotoxemia-induced sepsis was associated with reduced mesenteric blood flow and adversely affected the permeability and metabolic function of the small intestine (77). It appears logical that therapies targeted toward preservation of gut barrier and function would be beneficial. However, SBI has not been studied in this setting.
PLE with Fontan circulation.
The Fontan procedure is used to separate the systemic and pulmonary circulations in children with single ventricle physiology resulting from several types of congenital heart disease. A substantial number of patients exhibit high central venous pressures and develop PLE after the Fontan procedure, manifested by a decrease in serum proteins, increased stool α-1 antitrypsin levels, and loss of circulating lymphocytes. Although this gut dysfunction is thought to be related to heart failure, some reports suggest that infectious or inflammatory processes may also be involved in this phenomenon (78, 79). These children, who have lymphopenia and hypogammaglobulinemia, have an increased incidence of infections. During critical illness, they often receive parenteral Ig for replacement, but the role of oral Ig has not been assessed in this population. Inasmuch as no adequate long-term solutions are available for these patients, it is our opinion that oral Ig supplementation should be tested in this group to see if there is a potential role in preventing infection or in improving levels of serum proteins.
Potential Side Effects of Oral Ig Therapy
The most commonly reported adverse effects of oral Ig therapy include mild nausea, constipation, stomach cramps, headache, and increased urination. The incidence of these has been seen in 2–5% of clinical studies of SBI (B Burnett, EnteraHealth, personal communication, 2016; J Marc Rhoads, unpublished results, 2016). Concerns for potential allergic reactions and bovine serum encephalitis (BSE) as harmful side effects of oral Ig preparations warrant further attention.
Allergy.
To our knowledge, there are no published reports of oral Ig preparations provoking allergic or anaphylactic reactions. Of note, a study of children who were allergic to cow milk reported that 16 of 22 had circulating IgE directed against bovine milk–derived Igs (80). Also, in postmarketing surveillance of SBI, there have been 3 reported cases of mild pruritus and 2 of urticarial reactions, although it is not clear from the reports whether these reactions were clearly due to SBI or to other changes in the patients’ diets, underlying disease processes, or medical regimens.
Bovine serum encephalitis.
Currently, the risk of BSE appears negligible, but it is indeed monitored. The WHO declared the United States to be BSE-free in the early 2000s. The USDA has controls in place to mitigate any risk for BSE in the US cattle herd. Plasma collected from each animal must pass both ante- and postmortem inspection in USDA-inspected and approved facilities to be eligible for use in products for human consumption. The current commercially available product, SBI, is derived from only bovine plasma collected from young cattle in an effort to further reduce any such risk.
Conclusions
Luminal gamma globulins have important effects on intestinal pathogen elimination, inflammation, and most likely microbial community structure. The mucosal, immune, and microbial effects of orally administered serum-derived Ig isolates have multiple clinical implications, and their use may facilitate the management of various human gastrointestinal conditions involving impaired gut mucosal integrity and immune dysregulation by providing for a distinctive nutritional requirement in the binding and neutralization of microbial antigens, which contribute to the etiology of these diseases. Recent studies emphasize the need for randomized clinical trials of such preparations in the management of IBD (46, 47, 54). Also, several studies highlight the need for additional studies on the potential use of a preparation of oral Ig in the prevention of NEC in premature high-risk infants. We also suggest that they may be beneficial in children with congenital immunodeficiency diseases or severe protein-losing enteropathy.
In developing countries, the use of oral Ig is limited by the issues of cost and availability, whereas in developed countries, vaccination of children is protective against the previously most important diarrheal disease, rotavirus diarrhea. Furthermore, in developed countries, severe diarrhea leading to hospital admission and/or malnutrition is uncommon. Nevertheless, when children develop chronic diarrhea (lasting >2 wk), or become nutritionally compromised, they may benefit from oral Ig. The authors recognize that certain religious groups and those with dietary preferences may have reservations about the consumption of products derived from blood. However, we suggest that PPC products are worthy of consideration and further investigation of their use in specific intestinal inflammatory disorders, protein-losing enteropathies, and gut-related life-threatening conditions when current treatment options are limited.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AGE, acute gastroenteritis; ARP, antirotavirus protein; BC, bovine colostrum; BSC, bovine serum concentrate; BSE, bovine serum encephalitis; CD4, cluster of differentiation cell surface markers 4; HEY, hyperimmune egg yolk; IBD, Inflammatory Bowel Disease; MDR1a, multidrug resistance gene 1-α; NEC, necrotizing enterocolitis; PLE, protein-losing enteropathy; PPC, purified protein concentrate; SBI, serum-derived bovine Ig; UC, ulcerative colitis.
References
- 1.Eibl MM, Wolf HM, Furnkranz H, and Rosenkranz A. Prevention of necrotizing enterocolitis in low-birth-weight infants by IgA-IgG feeding. N Engl J Med 1988;319:1–7. [DOI] [PubMed] [Google Scholar]
- 2.Guarino A, Canani RB, Russo S, Albano F, Canani MB, and Ruggeri FM, Donelli G, Rubino A. Oral immunoglobulins for treatment of acute rotaviral gastroenteritis. Pediatrics 1994;93:12–6. [PubMed] [Google Scholar]
- 3.Rubaltelli FF, Benini F, and Sala M. Prevention of necrotizing enterocolitis in neonates at risk by oral administration of monomeric IgG. Dev Pharmacol Ther 1991;17:138–43. [DOI] [PubMed] [Google Scholar]
- 4.Sarker SA, Casswall TH, Mahalanabis D, Alam NH, Albert MJ, Brussow H, Fuchs GJ, and Hammerstrom L. Successful treatment of rotavirus diarrhea in children with immunoglobulin from immunized bovine colostrum. Pediatr Infect Dis J 1998;17:1149–54. [DOI] [PubMed] [Google Scholar]
- 5.Rawal P, Gupta V, Thapa BR. Role of colostrum in gastrointestinal infections. Indian J Pediatr 2008;75:917–21. [DOI] [PubMed] [Google Scholar]
- 6.Nofrarías M, Manzanilla EG, Pujols J, Gibert X, Majo N, Segales J, Gasa J. Effects of spray-dried porcine plasma and plant extracts on intestinal morphology and on leukocyte cell subsets of weaned pigs. J Anim Sci 2006;84:2735–42. [DOI] [PubMed] [Google Scholar]
- 7.Pierce JL, Cromwell GL, Lindemann MD, Russell LE, Weaver EM. Effects of spray-dried animal plasma and immunoglobulins on performance of early weaned pigs. J Anim Sci 2005;83:2876–85. [DOI] [PubMed] [Google Scholar]
- 8.Torrallardona D, Conde MR, Badiola I, Polo J, Brufau J. Effect of fishmeal replacement with spray-dried animal plasma and colistin on intestinal structure, intestinal microbiology, and performance of weanling pigs challenged with Escherichia coli K99. J Anim Sci 2003;81:1220–6. [DOI] [PubMed] [Google Scholar]
- 9.Borg BS, Campbell JM, Russel LE, Rodriguez C, Rodenas J. Evaluation of the chemical and biological characteristics of spray-dried plasma protein collected from various locations around the world. Kansas City (MO): Proc Am Assoc Swine Vet; 2002. p. 97–100. [Google Scholar]
- 10.FDA [Internet]. Silver Spring (MD): US FDA; c2015 [updated 2015 Nov 30; cited 2016 Mar 15]. Available from: http://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=255.
- 11.FDA [Internet]. Bovine globulin-agency response letter GRAS Notice No. GRN 000255. Silver Spring (MD): US FDA; c2008 [cited 2016 Mar 15]. Available from: http://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=255.
- 12.Kuchibhatla R, Petschow BW, Odle J, Weaver EM. Nutritional impact of dietary plasma proteins in animals undergoing experimental challenge and implications for patients with inflammatory bowel disorders: a meta-analysis. Adv Nutr 2015;6:541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EnteraGam Package Insert, 2014. [cited 2016 Mar 15]. Available from: http://www.enteragam.com.
- 14.Entera Health [Internet]. Cary (NC): Entera Health, Inc. [cited 2016 Mar 15]. Available from: http://www.enterahealth.com/enteragam.
- 15.Jasion VS, Burnett BP. Survival and digestibility of orally-administered immunoglobulin preparations containing IgG through the gastrointestinal tract in humans. Nutr J 2015;14:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petschow BW, Blikslager AT, Weaver EM, Campbell JM, Polo J, Shaw AL, Burnett BP, Klein GL, Rhoads JM. Bovine immunoglobulin protein isolates for the nutritional management of enteropathy. World J Gastroenterol 2014;20:11713–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson GP, Whyte PB, Daniels E, Franklin K, Nunan H, McCloud PI, Moore AG, Moore DJ. Passive immunisation of children with bovine colostrum containing antibodies to human rotavirus. Lancet 1989;2:709–12. [DOI] [PubMed] [Google Scholar]
- 18.Lissner R, Thurmann PA, Merz G, Karch H. Antibody reactivity and fecal recovery of bovine immunoglobulins following oral administration of a colostrum concentrate from cows (Lactobin) to healthy volunteers. Int J Clin Pharmacol Ther 1998;36:239–45. [PubMed] [Google Scholar]
- 19.Shield J, Melville C, Novelli V, Anderson G, Scheimberg I, Gibb D, Milla P. Bovine colostrum immunoglobulin concentrate for cryptosporidiosis in AIDS. Arch Dis Child 1993;69:451–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen RL, Thymann T, Ostergaard MV, Stoy AC, Krych L, Nielsen DS, Lauridsen C, Hartmann B, Holst JJ, Burrin DG, et al. Early gradual feeding with bovine colostrum improves gut function and NEC resistance relative to infant formula in preterm pigs. Am J Physiol Gastrointest Liver Physiol 2015;309:G310–23. [DOI] [PubMed] [Google Scholar]
- 21.Jensen ML, Sangild PT, Lykke M, Schmidt M, Boye M, Jensen BB, Thymann T. Similar efficacy of human banked milk and bovine colostrum to decrease incidence of necrotizing enterocolitis in preterm piglets. Am J Physiol Regul Integr Comp Physiol 2013;305:R4–12. [DOI] [PubMed] [Google Scholar]
- 22.Huppertz HI, Rutkowski S, Busch DH, Eisebit R, Lissner R, Karch H. Bovine colostrum ameliorates diarrhea in infection with diarrheagenic Escherichia coli, shiga toxin-producing E. coli, and E. coli expressing intimin and hemolysin. J Pediatr Gastroenterol Nutr 1999;29:452–6. [DOI] [PubMed] [Google Scholar]
- 23.Kelly GS. Bovine colostrums: a review of clinical uses. Altern Med Rev 2003;8:378–94. [PubMed] [Google Scholar]
- 24.Bovine colostrum [Internet]. Stockton (CA): Natural Medicines Comprehensive Database Consumer Version; c2005–16 [cited 2016 Mar 15]. Available from: http://www.webmd.com/vitamins-supplements/ingredientmono-785-bovine%20colostrum.aspx?activeingredientid=785&activeingredientname=bovine%20colostrum.
- 25.Hunt E, Fu Q, Armstrong MU, Rennix DK, Webster DW, Galanko JA, Chen W, Weaver EM, Argenzio RA, Rhoads JM. Oral bovine serum concentrate improves cryptosporidial enteritis in calves. Pediatr Res 2002;51:370–6. [DOI] [PubMed] [Google Scholar]
- 26.Bosi P, Casini L, Finamore A, Cremokolini C, Merialdi G, Trevisi P, Nobili F, Mengheri E. Spray-dried plasma improves growth performance and reduces inflammatory status of weaned pigs challenged with enterotoxigenic Escherichia coli K88. J Anim Sci 2004;82:1764–72. [DOI] [PubMed] [Google Scholar]
- 27.Casswall TH, Sarker SA, Faruque SM, Weintraub A, Albert MJ, Fuchs GJ, Alam NH, Dahlstrom AK, Link H, Brussow H, et al. Treatment of enterotoxigenic and enteropathogenic Escherichia coli-induced diarrhoea in children with bovine immunoglobulin milk concentrate from hyperimmunized cows: a double-blind, placebo-controlled, clinical trial. Scand J Gastroenterol 2000;35:711–8. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Bosque A, Amat C, Polo J, Campbell JM, Crenshaw J, Russell L, Moreto M. Spray-dried animal plasma prevents the effects of Staphylococcus aureus enterotoxin B on intestinal barrier function in weaned rats. J Nutr 2006;136:2838–43. [DOI] [PubMed] [Google Scholar]
- 29.Peace RM, Campbell J, Polo J, Crenshaw J, Russell L, Moeser A. Spray-dried porcine plasma influences intestinal barrier function, inflammation, and diarrhea in weaned pigs. J Nutr 2011;141:1312–7. [DOI] [PubMed] [Google Scholar]
- 30.Rhoads JM, Chen W, Gookin J, Wu GY, Fu Q, Blikslager AT, Rippe RA, Argenzio RA, Cance WG, Weaver EM, et al. Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut 2004;53:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang N, Shen N, Vyse TJ, Anand V, Gunnarson I, Sturfelt G, Rantapaa-Dahlqvist S, Elvin K, Truedsson L, Andersson BA, et al. Selective IgA deficiency in autoimmune diseases. Mol Med 2011;17:1383–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comunoglu N, Kara S, Kepil N. Inflammatory bowel disease-like colitis pathology in a patient with common variable immune deficiency. BMJ Case Rep 2015;2015:pii:bcr2014207177. [DOI] [PMC free article] [PubMed]
- 33.Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, Ouahed J, Wilson DC, Travis SP, Turner D, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014;147:990–1007.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez-Bosque A, Miro L, Maijo M, Polo J, Campbell J, Russell L, Crenshaw J, Weaver E, Moreto M. Dietary intervention with serum-derived bovine immunoglobulins protects barrier function in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 2015;308:G1012–8. [DOI] [PubMed] [Google Scholar]
- 35.Detzel CJ, Horgan A, Henderson AL, Petschow BW, Warner CD, Maas KJ, Weaver EM. Bovine immunoglobulin/protein isolate binds pro-inflammatory bacterial compounds and prevents immune activation in an intestinal co-culture model. PLoS One 2015;10:e0120278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson AL, Brand MW, Darling RJ, Maas KJ, Detzel CJ, Hostetter J, Wannemuehler MJ, Weaver EM. Attenuation of colitis by serum-derived bovine immunoglobulin/protein isolate in a defined microbiota mouse model. Dig Dis Sci 2015;60:3293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asmuth DM, Ma ZM, Albanese A, Sandler NG, Devaraj S, Knight TH, Flynn NM, Yotter T, Garcia JC, Tsuchida E, et al. Oral serum-derived bovine immunoglobulin improves duodenal immune reconstitution and absorption function in patients with HIV enteropathy. AIDS 2013;27:2207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Rhoads J. Communication between B-cells and microbiota for the maintenance of intestinal homeostasis. Antibodies (Basel) 2013;2:535–53. [Google Scholar]
- 39.Slack E, Balmer ML, Fritz JH, Hapfelmeier S. Functional flexibility of intestinal IgA—broadening the fine line. Front Immunol 2012;3:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endt K, Stecher B, Chaffron S, Slack E, Tchitchek N, Benecke A, Van ML, Sirard JC, Mueller AJ, Heikenwalder M, et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog 2010;6:e1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science 2002;298:1424–7. [DOI] [PubMed] [Google Scholar]
- 42.Asmuth D, Stombaugh J, Ma ZM, Albanese A, Hodzic E, Troia-CAncio P, Flynn N, Yotter T, Miller C, Knight R. Changes in stool microbiota, bacterial translocation and mucosal immunity after oral serum-derived bovine immunoglobulin (SBI) afministration. The 20th Conference on REtroviruses and Opportunistic Infections (CROI). 2013 Mar 3–6; Atlanta, Georgia. Atlanta: Conference on REtroviruses and Opportunistic Infections; 2013. p. B-186.
- 43.Wilson D, Evans M, Weaver E, Shaw AL, Klein GL. Evaluation of serum-derived bovine immunoglobulin protein isolate in subjects with diarrhea-predominant irritable bowel syndrome. Clin Med Insights Gastroenterol 2013;6:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lembcke JL, Peerson JM, Brown KH. Acceptability, safety, and digestibility of spray-dried bovine serum added to diets of recovering malnourished children. J Pediatr Gastroenterol Nutr 1997;25:381–4. [DOI] [PubMed] [Google Scholar]
- 45.Bégin F, Santizo MC, Peerson JM, Torun B, Brown KH. Effects of bovine serum concentrate, with or without supplemental micronutrients, on the growth, morbidity, and micronutrient status of young children in a low-income, peri-urban Guatemalan community. Eur J Clin Nutr 2008;62:39–50. [DOI] [PubMed] [Google Scholar]
- 46.Shafran I, Burgunder P, Wei D, Young HE, Klein G, Burnett BP. Management of inflammatory bowel disease with oral serum-derived bovine immunoglobulin. Therap Adv Gastroenterol 2015;8:331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Good L, Panas R. Case series investigating the clinical practice experience of serum-derived bovine immunoglobulin/protein isolate (SBI) in the clinical management of patients with inflammatory bowel disease. J Gastrointest Dig Syst 2015;5:268. [Google Scholar]
- 48.Lawrence G, Tudehope D, Baumann K, Jeffery H, Gill A, Cole M, Drew J, McPhee A, Ratcliffe J, Reynolds G, et al. Enteral human IgG for prevention of necrotising enterocolitis: a placebo-controlled, randomised trial. Lancet 2001;357:2090–4. [DOI] [PubMed] [Google Scholar]
- 49.Sarker SA, Casswall TH, Juneja LR, Hoq E, Hossain I, Fuchs GJ, Hammarstrom L. Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J Pediatr Gastroenterol Nutr 2001;32:19–25. [DOI] [PubMed] [Google Scholar]
- 50.Sarker SA, Jakel M, Sultana S, Alam NH, Bardhan PK, Chisti MJ, Salam MA, Theis W, Hammarstrom L, Frenken LG. Anti-rotavirus protein reduces stool output in infants with diarrhea: a randomized placebo-controlled trial. Gastroenterology 2013;145:740–8. [DOI] [PubMed] [Google Scholar]
- 51.Young KW, Munro IC, Taylor SL, Veldkamp P, van Dissel JT. The safety of whey protein concentrate derived from the milk of cows immunized against Clostridium difficile. Regul Toxicol Pharmacol 2007;47:317–26. [DOI] [PubMed] [Google Scholar]
- 52.Bölke E, Jehle PM, Hausmann F, Daubler A, Wiedeck H, Steinbach G, Storck M, Orth K. Preoperative oral application of immunoglobulin-enriched colostrum milk and mediator response during abdominal surgery. Shock 2002;17:9–12. [DOI] [PubMed] [Google Scholar]
- 53.Bölke E, Orth K, Jehle PM, Schwarz A, Steinbach G, Schleich S, Ulmer C, Storck M, Hannekum A. Enteral application of an immunoglobulin-enriched colostrum milk preparation for reducing endotoxin translocation and acute phase response in patients undergoing coronary bypass surgery–a randomized placebo-controlled pilot trial. Wien Klin Wochenschr 2002;114:923–8. [PubMed] [Google Scholar]
- 54.Awad AJ. Use of a nutritional therapy, serum-derived bovine immunoglobulin/protein isolate (SBI) to achieve improvement in two different cases of colitis. J Gastrointest Dig Syst 2015;5:274. [Google Scholar]
- 55.Petschow BW, Burnett BP, Shaw AL, Weaver EM, Klein GL. Dietary requirement for serum-derived bovine immunoglobulins in the clinical management of patients with enteropathy. Dig Dis Sci 2015;60:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foster J, Cole M. Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth-weight neonates. Cochrane Database Syst Rev 2004;CD001816. [DOI] [PubMed] [Google Scholar]
- 57.Wolf HM, Eibl MM. The anti-inflammatory effect of an oral immunoglobulin (IgA-IgG) preparation and its possible relevance for the prevention of necrotizing enterocolitis. Acta Paediatr Suppl 1994;396:37–40. [DOI] [PubMed] [Google Scholar]
- 58.Galal NM. Pattern of intravenous immunoglobulins (IVIG) use in a pediatric intensive care facility in a resource limited setting. Afr Health Sci 2013;13:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf HM, Fischer MB, Puhringer H, Samstag A, Vogel E, Eibl MM. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood 1994;83:1278–88. [PubMed] [Google Scholar]
- 60.Payne DC, Staat MA, Edwards KM, Szilagyi PG, Gentsch JR, Stockman LJ, Curns AT, Griffin M, Weinberg GA, Hall CB, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics 2008;122:1235–43. [DOI] [PubMed] [Google Scholar]
- 61.Russo T, Costa C, Crujo C, Lopes AI. Protein-losing gastropathy associated with cytomegalovirus infection in a child. BMJ Case Rep 2012;2012:pii: bcr0120125679. [DOI] [PMC free article] [PubMed]
- 62.Iwanaga M, Zaitsu M, Ishii E, Nishimura Y, Inada S, Yoshiki H, Okinami S, Hamasaki Y. Protein-losing gastroenteropathy and retinitis associated with cytomegalovirus infection in an immunocompetent infant: a case report. Eur J Pediatr 2004;163:81–4. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Dennehy PH, Keyserling HL, Tang K, Gentsch JR, Glass RI, Jiang B. Rotavirus infection alters peripheral T-cell homeostasis in children with acute diarrhea. J Virol 2007;81:3904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pammi M, Haque KN. Oral immunoglobulin for the prevention of rotavirus infection in low birth weight infants. Cochrane Database Syst Rev 2011;CD003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steele J, Sponseller J, Schmidt D, Cohen O, Tzipori S. Hyperimmune bovine colostrum for treatment of GI infections: a review and update on Clostridium difficile. Hum Vaccin Immunother 2013;9:1565–8. [DOI] [PubMed] [Google Scholar]
- 66.Greenberg PD, Cello JP. Treatment of severe diarrhea caused by Cryptosporidium parvum with oral bovine immunoglobulin concentrate in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol 1996;13:348–54. [DOI] [PubMed] [Google Scholar]
- 67.Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001–2006. Pediatrics 2008;122:1266–70. [DOI] [PubMed] [Google Scholar]
- 68.Nylund CM, Goudie A, Garza JM, Fairbrother G, Cohen MB. Clostridium difficile infection in hospitalized children in the United States. Arch Pediatr Adolesc Med 2011;165:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis 2005;5:549–57. [DOI] [PubMed] [Google Scholar]
- 70.Schutze GE, Willoughby RE. Clostridium difficile infection in infants and children. Pediatrics 2013;131:196–200. [DOI] [PubMed] [Google Scholar]
- 71.Anosova NG, Cole LE, Li L, Zhang J, Brown AM, Mundle S, Zhang J, Ray S, Ma F, Garrone P, et al. A combination of three fully human toxin A- and toxin B-specific monoclonal antibodies protects against challenge with highly virulent epidemic strains of Clostridium difficile in the hamster model. Clin Vaccine Immunol 2015;22:711–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warny M, Fatimi A, Bostwick EF, Laine DC, Lebel F, LaMont JT, Pothoulakis C, Kelly CP. Bovine immunoglobulin concentrate-clostridium difficile retains C difficile toxin neutralising activity after passage through the human stomach and small intestine. Gut 1999;44:212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng Y, Zhang Y, Liu M, Huang YK, Zhang J, Yao Q, Zhao YL, Xiong JJ. Evaluating intestinal permeability by measuring plasma endotoxin and diamine oxidase in children with acute lymphoblastic leukemia treated with high-dose methotrexate. Anticancer Agents Med Chem 2016;16:387–92. [DOI] [PubMed] [Google Scholar]
- 74.Bateman E, Weaver E, Klein G, Wignall A, Wozniak B, Plews E, Mayo B, White I, Keefe D. Serum-derived bovine immunoglobulin/protein isolate in the alleviation of chemotherapy-induced mucositis. Support Care Cancer 2016;24:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnston JD, Harvey CJ, Menzies IS, Treacher DF. Gastrointestinal permeability and absorptive capacity in sepsis. Crit Care Med 1996;24:1144–9. [DOI] [PubMed] [Google Scholar]
- 76.MacFie J, Reddy BS, Gatt M, Jain PK, Sowdi R, Mitchell CJ. Bacterial translocation studied in 927 patients over 13 years. Br J Surg 2006;93:87–93. [DOI] [PubMed] [Google Scholar]
- 77.Navaratnam RL, Morris SE, Traber DL, Flynn J, Woodson L, Linares H, Herndon DN. Endotoxin (LPS) increases mesenteric vascular resistance (MVR) and bacterial translocation (BT). J Trauma 1990;30:1104–13. [DOI] [PubMed] [Google Scholar]
- 78.Cheung YF, Tsang HY, Kwok JS. Immunologic profile of patients with protein-losing enteropathy complicating congenital heart disease. Pediatr Cardiol 2002;23:587–93. [DOI] [PubMed] [Google Scholar]
- 79.Lenz D, Hambsch J, Schneider P, Hausler HJ, Sauer U, Hess J, Tarnok A. Protein-losing enteropathy in patients with Fontan circulation: is it triggered by infection? Crit Care 2003;7:185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bernhisel-Broadbent J, Yolken RH, Sampson HA. Allergenicity of orally administered immunoglobulin preparations in food-allergic children. Pediatrics 1991;87:208–14. [PubMed] [Google Scholar]