Abstract
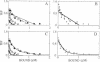
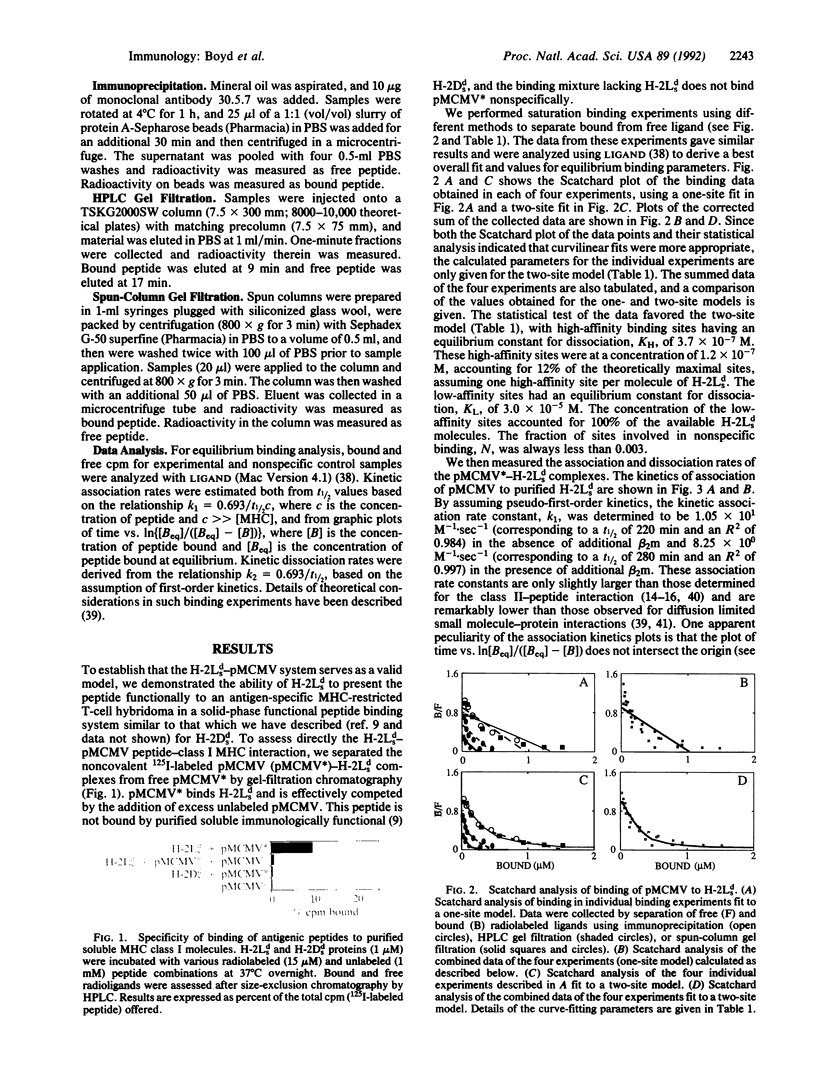
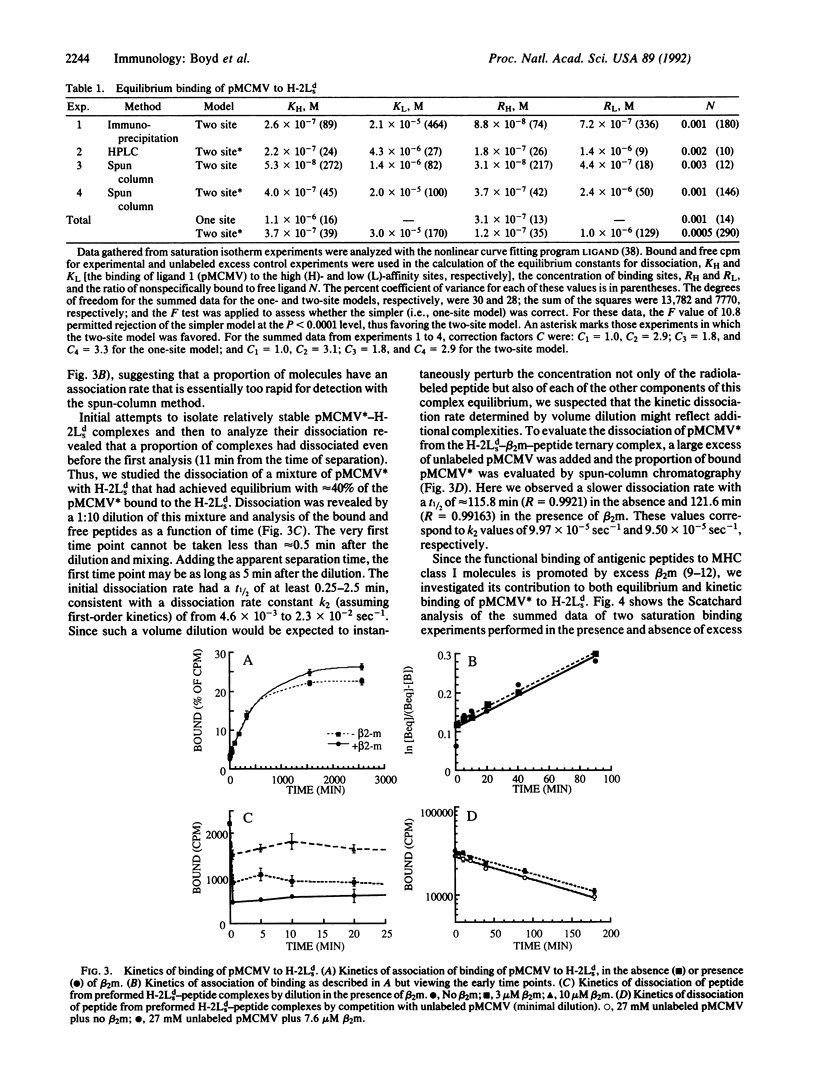
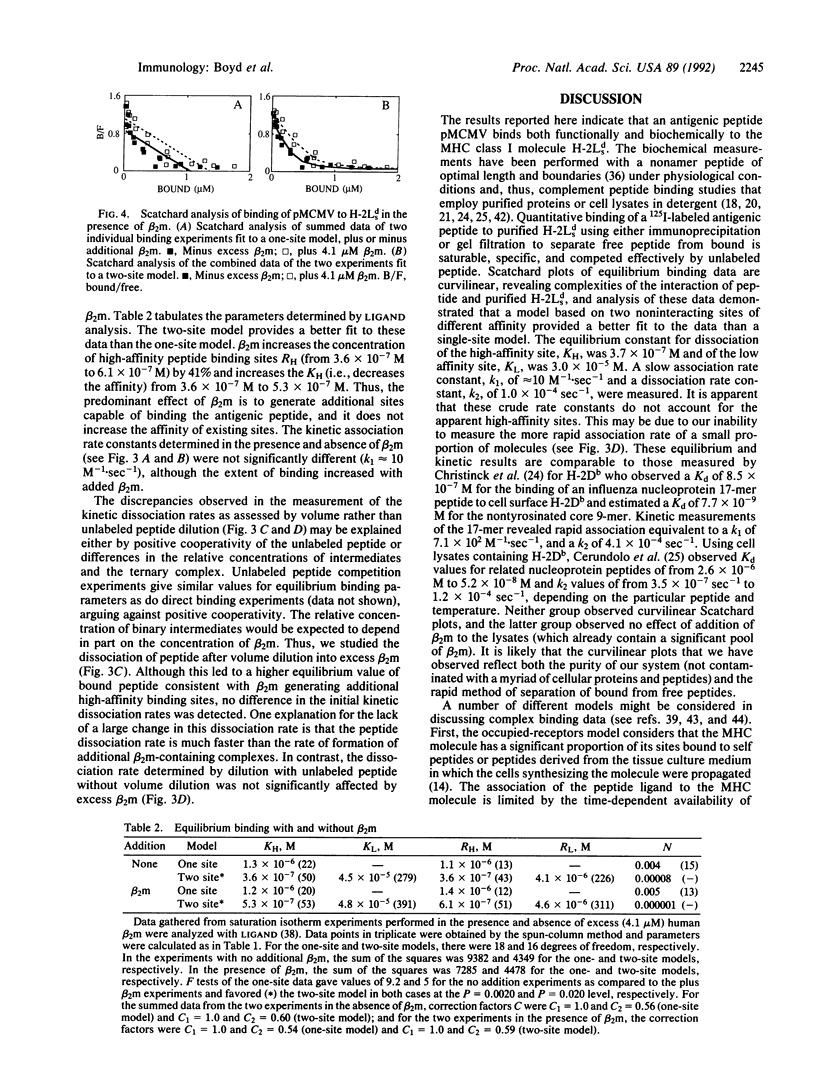
The major histocompatibility complex-encoded class I molecule, a noncovalent dimer of a polymorphic 45-kDa heavy chain and a nonpolymorphic 12-kDa beta 2-microglobulin (beta 2m) light chain, binds peptide antigen prior to its interaction with T-cell antigen receptors. We report here that the binding in aqueous solution at 37 degrees C of a soluble purified murine major histocompatibility complex class I protein, H-2Lds (a soluble analogue of H-2Ld consisting of the alpha 1 and alpha 2 domains of H-2Ld, the alpha 3 domain and the C terminus of Q10b), to an antigenic peptide is controlled by the light-chain subunit beta 2m. Analysis of the equilibrium binding data favors a model in which two classes of peptide binding sites exist, the high-affinity class having an equilibrium constant for dissociation, KH, of 3.7 x 10(-7) M and accounting for 12% of the theoretically available sites. Studies of binding in the presence of excess beta 2m indicate that this increases the concentration of available high-affinity sites. These data are consistent with a ternary model in which high-affinity sites are generated by the interaction of beta 2m with the peptide-binding class I heavy chain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Benjamin R. J., Madrigal J. A., Parham P. Peptide binding to empty HLA-B27 molecules of viable human cells. Nature. 1991 May 2;351(6321):74–77. doi: 10.1038/351074a0. [DOI] [PubMed] [Google Scholar]
- Bouillot M., Choppin J., Cornille F., Martinon F., Papo T., Gomard E., Fournie-Zaluski M. C., Levy J. P. Physical association between MHC class I molecules and immunogenic peptides. Nature. 1989 Jun 8;339(6224):473–475. doi: 10.1038/339473a0. [DOI] [PubMed] [Google Scholar]
- Busch R., Rothbard J. B. Detection of peptide-MHC class II complexes on the surface of intact cells. J Immunol Methods. 1990 Nov 6;134(1):1–22. doi: 10.1016/0022-1759(90)90107-7. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Carreno B. M., Anderson R. W., Coligan J. E., Biddison W. E. HLA-B37 and HLA-A2.1 molecules bind largely nonoverlapping sets of peptides. Proc Natl Acad Sci U S A. 1990 May;87(9):3420–3424. doi: 10.1073/pnas.87.9.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerundolo V., Alexander J., Anderson K., Lamb C., Cresswell P., McMichael A., Gotch F., Townsend A. Presentation of viral antigen controlled by a gene in the major histocompatibility complex. Nature. 1990 May 31;345(6274):449–452. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- Cerundolo V., Elliott T., Elvin J., Bastin J., Rammensee H. G., Townsend A. The binding affinity and dissociation rates of peptides for class I major histocompatibility complex molecules. Eur J Immunol. 1991 Sep;21(9):2069–2075. doi: 10.1002/eji.1830210915. [DOI] [PubMed] [Google Scholar]
- Chen B. P., Parham P. Direct binding of influenza peptides to class I HLA molecules. Nature. 1989 Feb 23;337(6209):743–745. doi: 10.1038/337743a0. [DOI] [PubMed] [Google Scholar]
- Chen B. P., Rothbard J., Parham P. Apparent lack of MHC restriction in binding of class I HLA molecules to solid-phase peptides. J Exp Med. 1990 Sep 1;172(3):931–936. doi: 10.1084/jem.172.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin J., Martinon F., Gomard E., Bahraoui E., Connan F., Bouillot M., Lévy J. P. Analysis of physical interactions between peptides and HLA molecules and application to the detection of human immunodeficiency virus 1 antigenic peptides. J Exp Med. 1990 Sep 1;172(3):889–899. doi: 10.1084/jem.172.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christinck E. R., Luscher M. A., Barber B. H., Williams D. B. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature. 1991 Jul 4;352(6330):67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- De Lean A., Stadel J. M., Lefkowitz R. J. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980 Aug 10;255(15):7108–7117. [PubMed] [Google Scholar]
- Frelinger J. A., Gotch F. M., Zweerink H., Wain E., McMichael A. J. Evidence of widespread binding of HLA class I molecules to peptides. J Exp Med. 1990 Sep 1;172(3):827–834. doi: 10.1084/jem.172.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Gorga J. C., Busch R., Rothbard J., Strominger J. L., Wiley D. C. Peptide binding to HLA-DR1: a peptide with most residues substituted to alanine retains MHC binding. EMBO J. 1990 Jun;9(6):1797–1803. doi: 10.1002/j.1460-2075.1990.tb08304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane K. P., Vitiello A., Sherman L. A., Mescher M. F. Cytolytic T-lymphocyte response to isolated class I H-2 proteins and influenza peptides. Nature. 1989 Jul 13;340(6229):157–159. doi: 10.1038/340157a0. [DOI] [PubMed] [Google Scholar]
- Kozlowski S., Takeshita T., Boehncke W. H., Takahashi H., Boyd L. F., Germain R. N., Berzofsky J. A., Margulies D. H. Excess beta 2 microglobulin promoting functional peptide association with purified soluble class I MHC molecules. Nature. 1991 Jan 3;349(6304):74–77. doi: 10.1038/349074a0. [DOI] [PubMed] [Google Scholar]
- Lie W. R., Myers N. B., Connolly J. M., Gorka J., Lee D. R., Hansen T. H. The specific binding of peptide ligand to Ld class I major histocompatibility complex molecules determines their antigenic structure. J Exp Med. 1991 Feb 1;173(2):449–459. doi: 10.1084/jem.173.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie W. R., Myers N. B., Gorka J., Rubocki R. J., Connolly J. M., Hansen T. H. Peptide ligand-induced conformation and surface expression of the Ld class I MHC molecule. Nature. 1990 Mar 29;344(6265):439–441. doi: 10.1038/344439a0. [DOI] [PubMed] [Google Scholar]
- Margulies D. H., Ramsey A. L., Boyd L. F., McCluskey J. Genetic engineering of an H-2Dd/Q10b chimeric histocompatibility antigen: purification of soluble protein from transformant cell supernatants. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5252–5256. doi: 10.1073/pnas.83.14.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey J., Boyd L., Foo M., Forman J., Margulies D. H., Bluestone J. A. Analysis of hybrid H-2D and L antigens with reciprocally mismatched aminoterminal domains: functional T cell recognition requires preservation of fine structural determinants. J Immunol. 1986 Dec 15;137(12):3881–3890. [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Rothbard J. B., Koszinowski U. H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature. 1989 Feb 16;337(6208):651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- Reyes V. E., Lu S., Humphreys R. E. Binding of radioiodinated influenza virus peptides to class I MHC molecules and to other cellular proteins as analyzed by gel filtration and photoaffinity labeling. Mol Immunol. 1991 Apr-May;28(4-5):341–348. doi: 10.1016/0161-5890(91)90146-b. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L., Benacerraf B. Reassociation with beta 2-microglobulin is necessary for Db class I major histocompatibility complex binding of an exogenous influenza peptide. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):301–304. doi: 10.1073/pnas.88.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock K. L., Rothstein L. E., Gamble S. R., Benacerraf B. Reassociation with beta 2-microglobulin is necessary for Kb class I major histocompatibility complex binding of exogenous peptides. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7517–7521. doi: 10.1073/pnas.87.19.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., McConnell H. M. A kinetic intermediate in the reaction of an antigenic peptide and I-Ek. Nature. 1989 Jan 19;337(6204):274–276. doi: 10.1038/337274a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Immune response (Ir) genes of the murine major histocompatibility complex. Adv Immunol. 1986;38:31–201. doi: 10.1016/s0065-2776(08)60006-1. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. L., Parker K. C., Wiley D. C. Reconstitution by MHC-restricted peptides of HLA-A2 heavy chain with beta 2-microglobulin, in vitro. Nature. 1991 Apr 18;350(6319):619–622. doi: 10.1038/350619a0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Vitiello A., Potter T. A., Sherman L. A. The role of beta 2-microglobulin in peptide binding by class I molecules. Science. 1990 Dec 7;250(4986):1423–1426. doi: 10.1126/science.2124002. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Brian A. A., Kappler J. W., Marrack P., McConnell H. M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7564–7568. doi: 10.1073/pnas.81.23.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., McConnell H. M. High-affinity fluorescent peptide binding to I-Ad in lipid membranes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9660–9664. doi: 10.1073/pnas.83.24.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreggett K. A., De Léan A. The ternary complex model. Its properties and application to ligand interactions with the D2-dopamine receptor of the anterior pituitary gland. Mol Pharmacol. 1984 Sep;26(2):214–227. [PubMed] [Google Scholar]