Plants contain three major growth-promoting plant hormones: auxin, gibberellins (GAs), and brassinosteroids (BRs), although other hormones also promote growth in certain circumstances. Possible interactions between the major three growth hormones have received much attention over the decades. In 2012, three articles proposed that the BRs and GAs can interact at the signaling level (Bai et al., 2012; Gallego-Bartolomé et al., 2012; Li et al., 2012), and since then, this has become an accepted model (Figure 1; Oh et al., 2014; Wang et al., 2014; Davière and Achard, 2016). This “signaling” model posits that DELLA proteins, which are negative regulators of GA signaling that are degraded by bioactive GA, physically interact with positive regulators of the BR response, BZR1 proteins (Bai et al., 2012; Gallego-Bartolomé et al., 2012; Li et al., 2012). The DELLA-BZR1 interaction interferes with the function of BZR1 proteins, thereby reducing growth.
Figure 1.
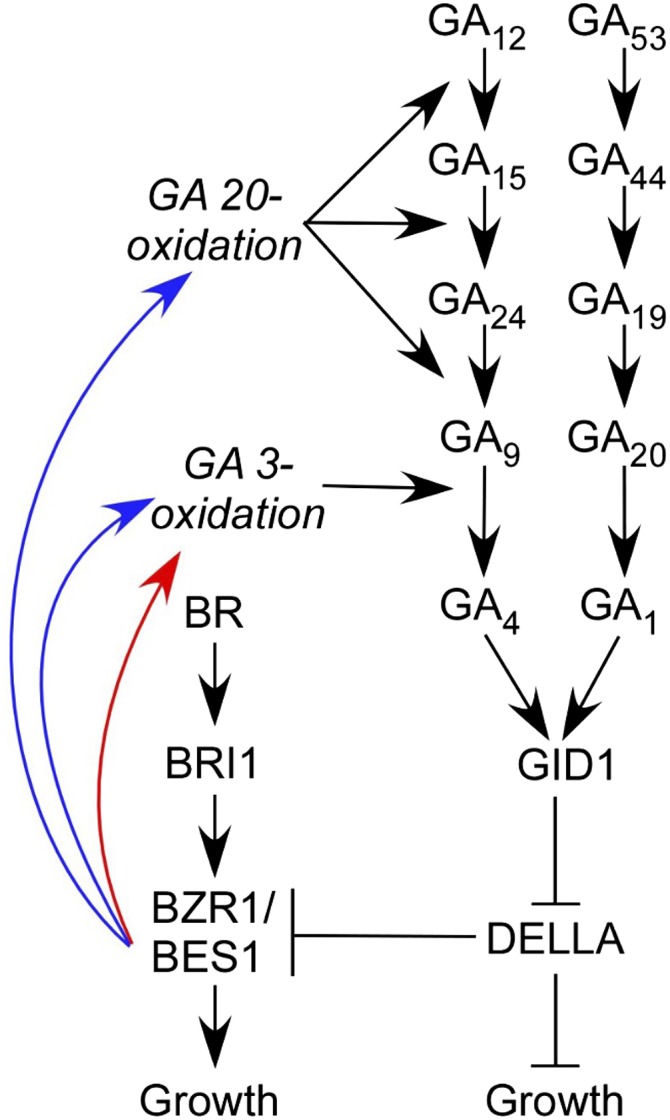
GA Biosynthesis Pathways and Models of BR-GA Interactions.
BZR1 is a key positive regulator of the BR growth response. According to the “GA signaling” model (Bai et al., 2012; Gallego-Bartolomé et al., 2012; Li et al., 2012), DELLA proteins (negative regulators of GA signaling that are degraded by bioactive GA) physically interact with BZR1, thereby reducing the BR growth response. The “GA synthesis” model of BR-GA interaction, shown in red (Tong et al., 2014) or blue (Unterholzner et al., 2015), suggests that, in addition to this, BRs are master regulators of GA biosynthesis and metabolism.
More recently, however, two articles have proposed a new theory, namely, that BRs can regulate plant growth by modulating GA levels (Tong et al., 2014; Unterholzner et al., 2015). These articles have generated considerable interest (e.g., Hofmann, 2015). For simplicity, we refer to this as the GA synthesis theory. There is no evidence to suggest that the synthesis and signaling models cannot coexist, but it now becomes important to compare their relative importance and to critically assess evidence put forward for the synthesis theory. The titles of the recent articles, “Brassinosteroid Regulates Cell Elongation by Modulating Gibberellin Metabolism in Rice” and “Brassinosteroids Are Master Regulators of Gibberellin Biosynthesis in Arabidopsis” give the impression that the synthesis model is of crucial importance, at least in certain circumstances.
The most visually striking evidence presented by Unterholzner et al. (2015) is the phenotypic rescue of a moderate Arabidopsis thaliana BR signaling mutant, bri1-301, by GA application or by the overexpression of a GA biosynthesis gene. Tong et al. (2014) also reported that moderate BR signaling mutants in rice (Oryza sativa; e.g., dlt) show normal or slightly enhanced sensitivity to GA. These observations are consistent with the idea that BRs can promote elongation growth by increasing GA levels. However, do they actually favor a key role for GA synthesis over the signaling model? The signaling model does not exclude a strong response to GA in moderate BR mutants. This is because in such mutants, we would still expect a functional (although reduced) level of BZR1 protein with which DELLAs can interact, and in fact there is experimental evidence (Tong et al., 2012) for the active form of BZR1 in one of the semi-dwarf lines, Go-2, used by Tong et al. (2014). The presence of some active BRZ1 protein might be sufficient for a strong GA response, in view of the markedly synergistic effects of BRs and GAs (Stewart Lilley et al., 2013). Therefore, the strong effect of GA on moderate BR mutants does not necessarily favor the GA synthesis theory.
Furthermore, if the GA synthesis theory applies generally in Arabidopsis, GA application should be highly effective not only on moderate BR mutants, but on severe BR mutants as well. However, Unterholzner et al. (2015) did not observe a strong response to GA in shoots of severely dwarfed BR mutants, such as bri1-1, and implicated the signaling model to explain that observation.
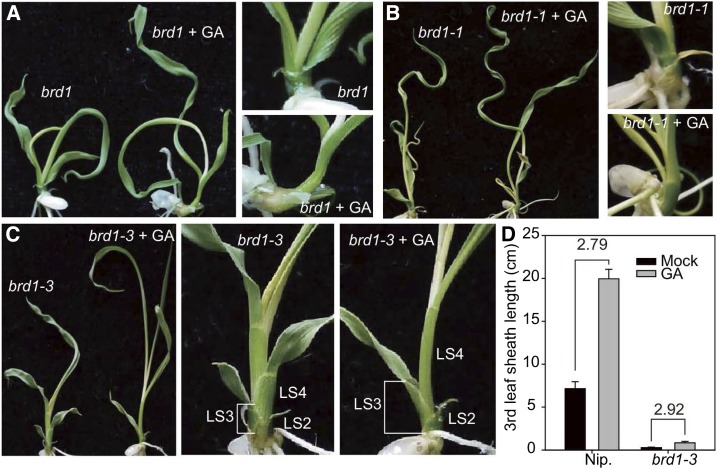
Tong et al. (2014) also found that GA did not rescue the most severe rice BR mutants (e.g., brd1-1; Figure 2). Nevertheless, these authors suggested that GA function does not require BR biosynthesis in their rice system, based on the GA response of another BRD1 mutant, brd1-3. Tong et al. (2014) claimed that the GA response in the third leaf sheath of this mutant was “normal,” resulting in a similar ratio of GA-treated sheath length to untreated sheath length in mutant and wild-type plants. However, it can be seen from Tong et al. (Supplemental Figure 7; Figure 2) that the response was in fact very weak, with GA-treated sheaths of the mutant reaching only ∼10% the length of untreated wild-type sheaths. The lack of GA responsiveness by brd1 alleles does not provide support for the GA synthesis model, but instead fits the signaling model, in which the GA response requires BR biosynthesis (Bai et al., 2012).
Figure 2.
GA Treatment Does Not Rescue the Phenotypes of BR-Deficient Mutants in Rice.
Little to no response to GA was evident in brd1 (A), brd1-1 (B), and brd1-3 (C), and GA did not restore the brd1-3 mutant to wild-type leaf sheath length ([D]; bars indicate sd, n = 5). (Figure reproduced from Tong et al. [2014], Supplemental Figure 7.)
EFFECTS OF BR STATUS ON GA CONTENT
Tong et al. (2014) and Unterholzner et al. (2015) support the GA synthesis theory by reporting effects of altered BR status on GA content. In rice, for example, a mutant with elevated BR levels (m107) contained 5 times more bioactive GA (GA1) and reduced levels of precursor GAs such as GA20 (Figure 1) compared with the wild type (Tong et al., 2014). The increase in GA1 content would be expected to alter growth, since a previous study (Oikawa et al., 2004) showed that a 2- to 3-fold increase in GA1 is sufficient to promote growth. On the other hand, the changes in GA content in some other rice BR mutants (e.g., d11-2) studied by Tong et al. (2014) were relatively slight and of uncertain statistical significance.
In Arabidopsis, a mutant with reduced BR signaling (ASKθoe), as well as other severe BR mutants, contained less bioactive GA than the wild type, although the statistical significance of the differences is not clear (Unterholzner et al., 2015). Some of these mutants showed only 2-fold reductions in GA content (e.g., bri1-1; Unterholzner et al., 2015). A comparison of known GA synthesis mutant phenotypes with their endogenous GA content (on the Columbia background; Plackett et al., 2012) indicates that a 2-fold drop might change the phenotype from a tall to a semi-dwarf stature, which would account for only part of the height phenotype of a severe BR mutant.
In other species, BR status does affect GA content, but without a consistent pattern. In pea (Pisum sativum), Jager et al. (2005) showed that mutants with either reduced BR levels or impaired BR signaling contained higher levels of GA20, while GA1 itself was unchanged, or in some circumstances elevated, compared with the wild type. Also, in sunflower (Helianthus annuus), treatment with BR reduced GA20 levels but did not alter those of GA1 (Kurepin et al., 2012).
In a more recent report of GA levels in BR mutants, Li et al. (2016) reported another variation: In tomato (Solanum lycopersicum), overexpression of a BR synthesis gene reduced the levels of both GA20 and GA1. Despite the reduction in GA1 content, elongation was enhanced in the overexpression lines, which is clearly inconsistent with the GA synthesis model of BR-GA interactions. Li et al. also reported that a mutant with reduced BR content (d^im) contained more GA20 than the wild type, although GA1 was unaffected. In fact, a similar result had been reported as early as 1988 (Nadhzimov et al., 1988), before the mutant concerned (another allele of the D gene) was shown to be BR deficient (Bishop et al., 1999). As noted above, a dwarf pea BR synthesis mutant also accumulates GA20 (Jager et al., 2005).
The picture that emerges is not one of a consistent effect of BRs on GA content, which we would expect if the latter hormones mediate the effects of the former. In fact, the effects of BRs on the GA pathway, across plant species, appear somewhat unpredictable. In addition, it appears that the same GA synthesis step can be up- or downregulated by BRs, depending on the species. For example, Unterholzner et al. (2015) (Figure 1) found that in Arabidopsis, GA 20-oxidation is upregulated by BR, confirming an earlier report (Bouquin et al., 2001). In pea, however, the Jager et al. (2005) results indicate a downregulation of GA 20-oxidation by BR. Tong et al. (2014) may provide an explanation for these apparently opposite effects. They report that the promoter of a rice GA 20-oxidase gene contains an element by which BR induces gene expression, as well as a different element by which BR represses expression. The same was true for a GA deactivation gene, GA2ox3, which was up- or downregulated by BR depending on the dose applied.
The question of BR effects on GA content is especially interesting given that there is already strong evidence that the third growth-promoting hormone, auxin, promotes elongation, at least in part by increasing GA levels. Indeed, in view of copious evidence that auxin regulates GA synthesis, in Arabidopsis and other species (Van Huizen et al., 1996; Ross et al., 2000; Wolbang and Ross, 2001; Wolbang et al., 2004; Frigerio et al., 2006; Chapman et al., 2012), including rice (Yin et al., 2007), we feel it is an exaggeration to say that BRs are “master regulators” of GA biosynthesis (Unterholzner et al., 2015). There is no evidence that BRs are more important than other regulators, such as auxin.
BR RESPONSES IN DELLA-DEFICIENT MUTANTS FAVOR THE SIGNALING MODEL
One way of differentiating between the signaling and GA synthesis models of BR-GA interactions is to observe the effects of exogenous BR on DELLA-deficient mutants. The two models predict opposite effects of this treatment. The signaling model posits that DELLAs effectively place a brake on the BR response and that in DELLA-deficient mutants, there should be an enhanced response to applied BR compared with the wild type. By contrast, the GA synthesis model predicts a reduced response to applied BR in DELLA-deficient mutants, in which the GA response is already more or less saturated. In other words, if BRs work mainly by increasing GA levels, DELLA-deficient mutants will not show a strong phenotypic BR response because these mutants are largely insensitive to increased GA content.
Bai et al. (2012) previously showed that a DELLA-deficient mutant of Arabidopsis does indeed respond markedly to applied BR. Tong et al. (2014) have now shown that this occurs in rice also, by reporting a very strong BR response in coleoptiles of the DELLA-deficient slr1 mutant. In fact, at an intermediate dose rate of BR, Tong et al. noted an increased BR response in this mutant compared with the wild type. Also consistent with the signaling model is the demonstration of a physical interaction in rice between BRZ1 and SLR1 proteins (Tong et al., 2014).
The strong BR response of DELLA-deficient mutants clearly favors the signaling model of BR-GA interactions (e.g., Bai et al., 2012). In fact, the articles by Tong et al. (2014) and Unterholzner et al. (2015) do not at this stage provide sufficient cause to replace the signaling model as the main BR-GA interaction. Furthermore, although in certain cases BR-induced GAs might contribute to the BR response, it is important to recognize that there is no consistent effect of BR status on GA content across plant species in general.
ACKNOWLEDGMENTS
We thank the Australian Research Council for financial assistance.
AUTHOR CONTRIBUTIONS
J.J.R. drafted the manuscript. L.J.Q. helped to assess the published articles, assisted with the writing, and prepared the figures.
Footnotes
Articles can be viewed online without a subscription.
References
- Bai M.-Y., Shang J.-X., Oh E., Fan M., Bai Y., Zentella R., Sun T.P., Wang Z.-Y. (2012). Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 14: 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G.J., Nomura T., Yokota T., Harrison K., Noguchi T., Fujioka S., Takatsuto S., Jones J.D.G., Kamiya Y. (1999). The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 96: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouquin T., Meier C., Foster R., Nielsen M.E., Mundy J. (2001). Control of specific gene expression by gibberellin and brassinosteroid. Plant Physiol. 127: 450–458. [PMC free article] [PubMed] [Google Scholar]
- Chapman E.J., Greenham K., Castillejo C., Sartor R., Bialy A., Sun T.-P., Estelle M. (2012). Hypocotyl transcriptome reveals auxin regulation of growth-promoting genes through GA-dependent and -independent pathways. PLoS One 7: e36210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davière J.-M., Achard P. (2016). A pivotal role of DELLAs in regulating multiple hormone signals. Mol. Plant 9: 10–20. [DOI] [PubMed] [Google Scholar]
- Frigerio M., Alabadí D., Pérez-Gómez J., García-Cárcel L., Phillips A.L., Hedden P., Blázquez M.A. (2006). Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol. 142: 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Bartolomé J., Minguet E.G., Grau-Enguix F., Abbas M., Locascio A., Thomas S.G., Alabadí D., Blázquez M.A. (2012). Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 109: 13446–13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann N.R. (2015). Taking hormone crosstalk to a new level: brassinosteroids regulate gibberellin biosynthesis. Plant Cell 27: 2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager C.E., Symons G.M., Ross J.J., Smith J.J., Reid J.B. (2005). The brassinosteroid growth response in pea is not mediated by changes in gibberellin content. Planta 221: 141–148. [DOI] [PubMed] [Google Scholar]
- Kurepin L.V., Joo S.H., Kim S.K., Pharis R.P., Back T.G. (2012). Interaction of brassinosteroids with light quality and plant hormones in regulating shoot growth of young sunflower and Arabidopsis seedlings. J. Plant Growth Regul. 31: 156–164. [Google Scholar]
- Li Q.-F., Wang C., Jiang L., Li S., Sun S.S.M., He J.-X. (2012). An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci. Signal. 5: ra72. [DOI] [PubMed] [Google Scholar]
- Li X.-J., et al. (2016). DWARF overexpression induces alteration in phytohormone homeostasis, development, architecture and carotenoid accumulation in tomato. Plant Biotechnol. J. 14: 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadhzimov U.K., Jupe S.C., Jones M.G., Scott I.M. (1988). Growth and gibberellin relations of the extreme dwarf dx tomato mutant. Physiol. Plant. 73: 252–256. [Google Scholar]
- Oh E., Zhu J.-Y., Bai M.-Y., Arenhart R.A., Sun Y., Wang Z.-Y. (2014). Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife 3: e03031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T., Koshioka M., Kojima K., Yoshida H., Kawata M. (2004). A role of OsGA20ox1, encoding an isoform of gibberellin 20-oxidase, for regulation of plant stature in rice. Plant Mol. Biol. 55: 687–700. [DOI] [PubMed] [Google Scholar]
- Plackett A.R.G., et al. (2012). Analysis of the developmental roles of the Arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and -3 are the dominant paralogs. Plant Cell 24: 941–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J.J., O’Neill D.P., Smith J.J., Kerckhoffs L.H.J., Elliott R.C. (2000). Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J. 21: 547–552. [DOI] [PubMed] [Google Scholar]
- Stewart Lilley J.L., Gan Y., Graham I.A., Nemhauser J.L. (2013). The effects of DELLAs on growth change with developmental stage and brassinosteroid levels. Plant J. 76: 165–173. [DOI] [PubMed] [Google Scholar]
- Tong H., Liu L., Jin Y., Du L., Yin Y., Qian Q., Zhu L., Chu C. (2012). DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell 24: 2562–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H., Xiao Y., Liu D., Gao S., Liu L., Yin Y., Jin Y., Qian Q., Chu C. (2014). Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 26: 4376–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner S.J., Rozhon W., Papacek M., Ciomas J., Lange T., Kugler K.G., Mayer K.F., Sieberer T., Poppenberger B. (2015). Brassinosteroids are master regulators of gibberellin biosynthesis in Arabidopsis. Plant Cell 27: 2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Huizen R., Ozga J.A., Reinecke D.M. (1996). Influence of auxin and gibberellin on in vivo protein synthesis during early pea fruit growth. Plant Physiol. 112: 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Bai M.-Y., Wang Z.-Y. (2014). The brassinosteroid signaling network-a paradigm of signal integration. Curr. Opin. Plant Biol. 21: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbang C.M., Chandler P.M., Smith J.J., Ross J.J. (2004). Auxin from the developing inflorescence is required for the biosynthesis of active gibberellins in barley stems. Plant Physiol. 134: 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbang C.M., Ross J.J. (2001). Auxin promotes gibberellin biosynthesis in decapitated tobacco plants. Planta 214: 153–157. [DOI] [PubMed] [Google Scholar]
- Yin C., Gan L., Ng D., Zhou X., Xia K. (2007). Decreased panicle-derived indole-3-acetic acid reduces gibberellin A1 level in the uppermost internode, causing panicle enclosure in male sterile rice Zhenshan 97A. J. Exp. Bot. 58: 2441–2449. [DOI] [PubMed] [Google Scholar]